Abstract
Benefits and risks of antithrombotic agents remain unclear in the hemodialysis population. We aimed to determine variation in antithrombotic agent use, rates of major bleeding events, and to determine factors predictive of stroke and bleeding to allow for risk stratification, enabling more rational decisions about using antithrombotic agents.
The sample included 48,144 patients in 12 countries in the Dialysis Outcomes and Practice Patterns Study Phase I–IV. Antithrombotic agents included oral anticoagulants (OAC), ASA and anti-platelet agents (APA). OAC prescription, comorbidities and vascular access were assessed at study entry; data on clinical events including hospitalization due to bleeding were collected every four months during follow-up.
There was wide variation in OAC (0.3–18%), APA (3–25%) and ASA use (8–36%), and major bleeding rates (0.05–0.22 events/year) among countries. Rates of all-cause mortality, cardiovascular mortality, and bleeding events requiring hospitalization were elevated in patients prescribed OAC across adjusted models. The CHADS2 score predicted the risk of stroke in atrial fibrillation patients. Gastrointestinal bleeding in the past 12 months was highly predictive of major bleeding events; for patients with previous gastrointestinal bleeding, the rate of bleeding exceeded the rate of stroke by at least 2-fold across categories of CHADS2 score.
Prescription of antithrombotic agents varied greatly. The CHADS2 score and a history of gastrointestinal bleeding were predictive of stroke and bleeding events, respectively, with bleeding rates substantially exceeding stroke rates in all groups including patients at high stroke risk. Appropriate risk stratification and a cautious approach should be considered before OAC use in the dialysis population.
Introduction
For patients without end-stage renal disease (ESRD), antithrombotic agents such as oral anticoagulants (OAC) are often indicated for prevention of stroke in atrial fibrillation, and antiplatelet agents (APA) for the secondary prevention of myocardial infarction and cardiovascular death1. The degree of benefit varies with bleeding risk: in patients at high bleeding risk, increased mortality from bleeding exceeds any reduction in cardiovascular risk from OAC/APA use2,3. For this reason, several stroke and bleeding risk scores have been developed and validated in the general population to help select patients likely to benefit from therapy4–7. For example the CHADS2 score predicts the rate of stroke in patients with atrial fibrillation and the modified outpatient bleeding risk index (mORBI), predicts the rate of major bleeding as a function of age, renal function, anemia, history of stroke, history of gastrointestinal (GI) bleed, diabetes mellitus, and atrial fibrillation 4,8. A combination of validated tools such as these permits assessment of the risk-to-benefit ratio of anticoagulant use and aids in guiding therapeutic decisions.
In contrast, in ESRD patients the risk-to-benefit trade-off with antithrombotic agents is unclear and may be unfavorable in many patients. Hemodialysis (HD) patients are at higher risk of serious bleeding due to several factors including uremic platelet dysfunction, anemia, and heparin use during dialysis9–12. The rate of clinically important bleeding events, however, is not well defined in the literature, ranging from 2.5–54% per year depending on the definition of bleeding used13. There is also considerable uncertainty about the risk of major bleeding in subgroups of HD patients receiving OAC or APA. Bleeding risk scores developed for the general non-dialysis population have not been validated in dialysis patients. This makes assessment of bleeding risk, and calculation of a risk-to-benefit ratio for antithrombotic agents, difficult. At the same time, there is little direct evidence of benefit for antithrombotic agents in prevention of stroke, cardiovascular events, or vascular access thrombosis in dialysis patients14–24.
Given these uncertainties, it is not clear whether the indications for antithrombotic agents in dialysis patients can be extrapolated from data in the general population. Despite these concerns, in current practice, OAC and APA are frequently prescribed in dialysis patients for the same indications and with the same expectation of benefit as in the general population.
The objectives of this study are to determine variation in use of antithrombotic agents, rates of major bleeding events, the associations of antithrombotic agents with clinical outcomes, and factors predictive of stroke and bleeding, to better allow for risk stratification and informed decision-making with antithrombotic agents.
Results
Regional distribution of OAC, APA and ASA use
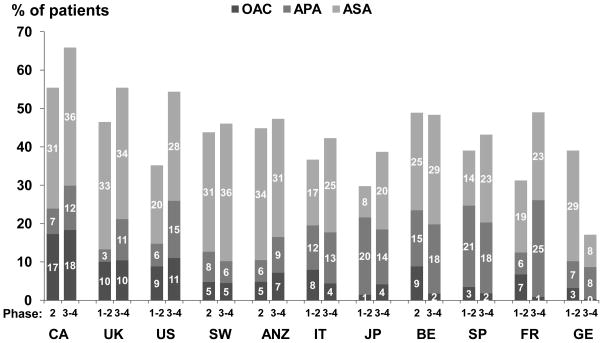
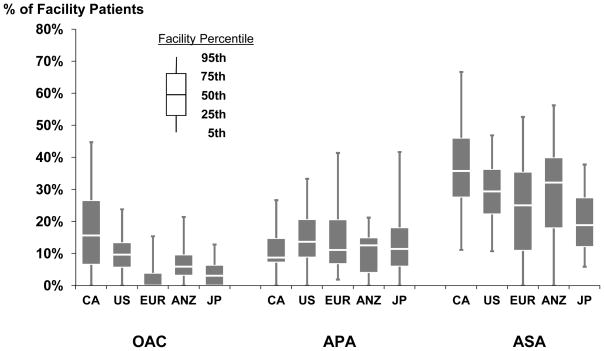
OAC prescription was the highest in Canada followed by the U.S. and lowest in Germany (Figure 1). Sweden and Canada had the highest acetylsalicylic acid (ASA) prescriptions with Germany having the least. There was a high degree of variability among dialysis facilities for prescribing antithrombotic agents (Figure 2). For example, Canadian facilities ranged from 0–45% of HD patients on OAC, and European and Japanese facilities ranged from 0–42% on APA. Low molecular weight heparin use was low overall (3%).
Figure 1.
Use of antithrombotic agents, by DOPPS phase and country. APA -other than aspirin (clopidogrel, ticlopidine, dipyridamole, pentoxifylline); OAC includes warfarin (85% of total OAC) and a few other products (e.g. fluindione in France); DOPPS study period: phases 1–2 (1998–2004) and 3–4 (2005–10) with N=48,144 patients.
Figure 2.
Variation in facility use of antithrombotic agents, by geographic region. N=20,475 patients from DOPPS phases 3 and 4 (2005–2010) in facilities with at least 7 patients with medication use records.
OAC, APA, ASA use and patient characteristics
For OAC, the most commonly prescribed medication was warfarin (85%), and for APA, the most commonly prescribed medications were: ticlopidine (36%), clopidogrel (35%), and dipyridamole (14%), and pentoxifylline (14%, medication not mutually exclusive). In general, patients on antithrombotic agents were older with more vascular disease compared to those not receiving agents (Table 1). Patients on OAC more commonly dialyzed using a central vascular catheter (CVC), had a lower serum albumin and a slightly higher Kt/V than the comparators. Of patients on OAC, 34% had atrial fibrillation, 14% had deep vein thrombosis, 38% had a CVC (categories not mutually exclusive), and 34% had none of these indications.
Table 1.
Baseline characteristics of DOPPS participants stratified by prescription of OAC, APA, ASA or no antithrombotic agent.
| OAC | APA | ASA | None | |
|---|---|---|---|---|
|
|
||||
| Mean (SD) or % | n=2513 (6.6%) | n=4122 (10.9%) | n=8086 (21.3%) | n=23177 (61.2%) |
| Percent of patients on antithrombotic agents a | ||||
| % on OAC | 100 | 0 | 0 | 0 |
| % on APA | 7 | 100 | 0 | 0 |
| % on ASA | 16 | 26 | 100 | 0 |
|
| ||||
| Age (years) 65.3 (13.9) ** | 65.4 (12.4) ** | 65.2 (13.0) ** | 59.7 (15.5) | |
| Male (%) | 55 | 61 ** | 60 ** | 57 |
| Black | 14* | 7 ** | 11 ** | 15 |
| Time on dialysis (years) | 3.5 (4.7) ** | 4.3 (5.5)* | 3.2 (4.6) ** | 3.7 (5.4) |
| BMI | 26.4 (6.5)* | 24.1 (5.3)* | 25.5 (5.6) ** | 24.3 (5.6) |
| Catheter Use (%) | 38* | 19* | 26 ** | 25 |
| Hemoglobin (g/dL) | 11.13 (1.63) ** | 10.85 (1.73) ** | 11.07 (1.65) ** | 10.68 (1.72) |
| Albumin (g/dL) | 3.59 (0.51)* | 3.69 (0.49)* | 3.65 (0.51)* | 3.68 (0.55) |
| Calcium (mg/dL) | 9.14 (0.93)* | 9.13 (0.91)* | 9.16 (0.91) ** | 9.07 (1) |
| Phosphorous (mg/dL) | 5.31 (1.77) ** | 5.32 (1.68) ** | 5.44 (1.73) ** | 5.67 (1.87) |
| PTH | 296 (415)* | 249 (340) ** | 273 (397) ** | 306 (419) |
| Dialysis dose (spKt/V) | 1.42 (0.33) | 1.38 (0.32) | 1.40 (0.33) | 1.37 (0.33) |
| Comorbid Conditions: (%) | ||||
| CAD | 58.0 ** | 53.2 ** | 61.6 ** | 35.4 |
| CHF | 46.8 ** | 37.5 ** | 42.1 ** | 29.9 |
| Cerebrovascular disease | 24.8 ** | 27.8 ** | 22.0 ** | 11.8 |
| PVD | 34.5 ** | 39.0 ** | 32.7 ** | 18.7 |
| Atrial Fibrillation | 34.1 ** | 10.2 ** | 12.4 ** | 7.7 |
| Other cardiac disease | 40.4 ** | 33.6 ** | 33.0 ** | 24.6 |
| Hypertension | 80.4 | 79.4 ** | 84.3 ** | 78.0 |
| Cancer (excluding skin) | 14.9* | 10.2 | 12.3 | 11.2 |
| Diabetes | 42.0 ** | 46.0 ** | 47.4 ** | 33.3 |
| GI bleed | 6.7 | 6.0 | 5.6* | 6.5 |
| HIV/AID | 0.4* | 0.3* | 0.3 ** | 1.4 |
| Lung disease | 16.6 ** | 13.0 ** | 13.7 ** | 9.6 |
| Neurological disorder | 11.9 | 10.5* | 10.6 | 9.5 |
| Psychiatric disease | 23.2 | 15.4 | 19.1 | 17.8 |
| Recurrent cellulitis/gangrene | 12.7 ** | 11.0 ** | 9.0 ** | 6.1 |
| Deep vein thrombosis | 14.0 ** | 4.0 ** | 3.8 | 2.9 |
| CHADS2 Score: (%) | ||||
| CHADS2 Low (score=0) | 7.4* | 9.5 ** | 5.7 ** | 12.5 |
| CHADS2 Moderate (score=1) | 20.7 ** | 20.7 ** | 21.8 ** | 35.1 |
| CHADS2 High (score ≥ 2) | 71.9 ** | 69.8 ** | 72.6 ** | 52.4 |
| mORBI Score: (%) | ||||
| mORBI Low (score=0,1) | 56.5 ** | 51.7 ** | 56.2 ** | 68.6 |
| mORBI Moderate (score=2) | 33.3 ** | 36.2 ** | 34.7 ** | 25.7 |
| mORBI High (score=3,4) | 10.2 ** | 12.1 ** | 9.1 ** | 5.8 |
| Eventsb: | ||||
| Major Bleeding | 0.078* | 0.062 | 0.063 | 0.049 |
| Stroke | 0.033 | 0.034* | 0.025 | 0.021 |
| CV Mortality | 0.111* | 0.081* | 0.082 | 0.059 |
| All-Cause Mortality | 0.240* | 0.165* | 0.174 | 0.132 |
| Follow up time, years | 1.47 (0.98) | 1.57 (0.90) | 1.57 (0.98) | 1.59 (1.01) |
Based on data from DOPPS phase 1, 2, and 3 (1996–2008) Adjusted for phase and region; accounted for facility clustering; p-values from logistic or mixed models with no-antithrombotic agent category as reference. Type 3 overall tests were significant at p<0.01 for all variables (indicating that patients were not all the same across all four categories) except for dialysis dose (spKt/V) p=0.75, neurologic disorder p=0.15, and psychiatric disorder p=0.10.
p < 0.05
p < 0.0001 with None as the referent
OAC (warfarin [85% of total OAC], dicumarol, phenprocoumon, fluindione); APA-other than aspirin (ticlopidine [36%], clopidogrel [35%], dipyridamole [14%], pentoxifylline [14%] medication not mutually exclusive)
events during follow up; not mutually exclusive; rate per patient year;
Rates of bleeding events and stroke by country
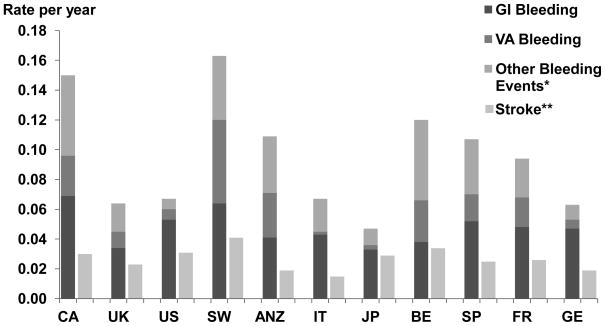
Overall, rate of total bleeding events requiring hospitalization was 0.08 per year. Sweden had the highest rate at 0.16 per year followed by Canada at 0.15, and the lowest was in Japan at 0.05 (Figure 3). Gastrointestinal and vascular access-related bleeding events were highest in Sweden and Canada. Sweden and Belgium had the highest stroke rate (0.041/year and 0.034/year respectively) and Italy had the lowest (0.015/year). Subdural hematomas occurred in 155 (0.4%) patients, 68 (44%) of whom died. Rates of bleeding and stroke events were somewhat higher among patients with versus without atrial fibrillation.
Figure 3.
Rates of bleeding events and stroke by country. GI=gastrointestinal, VA=vascular access. N=39,440 patients from DOPPS phases 1,2 and 3. *Other bleeding events include epistaxis, subdural hematoma, evacuation of hematoma, hemoptysis and hematuria. **Stroke includes hospitalization or death due to stroke.
Antithrombotic agents influenced the bleeding rates, and bleeding rates were generally higher for patients using medication combinations. Examining the influence of antithrombotic agents on bleeding events hierarchically, the bleeding rate for patients on no agents was 0.049, on ASA only 0.063, on APA (+/− ASA) 0.062, and OAC (+/− ASA+/−APA) 0.078 per year (see Table 1).
Associations of OAC, APA and ASA use with clinical outcomes
In the adjusted model, OAC use was positively associated with any bleeding event (adjusted hazard ratio (HR) =1.30, [95% confidence interval: 1.11–1.52]), all-cause mortality (1.16, [1.07–1.26]) and cardiovascular mortality (1.14, [1.01–1.29]) (Table 2) (Supplemental Figure A). APA use was positively associated with stroke, cardiovascular mortality and all-cause death, as well as a non-significant association with bleeding events. No clear pattern concerning the associations of ASA use with clinical outcomes was evident. With progressive adjustments in the statistical models, the associations of OAC and APA use with higher hazard rates were attenuated but generally remained statistically significant (Table 2). Associations for ASA use were brought to the null or reversed direction with progressive adjustments. The association of major bleeding events with antithrombotic agent use was not modified by use of H2 Blockers or proton pump inhibitors (model not shown, p-values for interaction >0.4), and there was no association of major bleeding events and H2 Blocker use or proton pump inhibitor use (when added to the full model individually, p-values >0.2).
Table 2.
Cox models for association of OAC, APA and ASA prescription with major bleeding events, stroke, all-cause and cardiovascular mortality, by different levels of adjustment
| Level 1 | Level 2 | Level 3 | |
|---|---|---|---|
|
|
|||
| No adjustments, stratified | Level 1 + demographics + comorbidities | Level 2 + labs | |
|
|
|||
| HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| Major bleeding events: | |||
| OAC | 1.39 (1.20–1.61) | 1.28 (1.10–1.50) | 1.30 (1.11–1.52) |
| APA | 1.25 (1.10–1.42) | 1.10 (0.97–1.26) | 1.12 (0.98–1.27) |
| ASA | 1.13 (1.02–1.26) | 1.06 (0.96–1.18) | 1.07 (0.96–1.19) |
| Stroke: | |||
| OAC | 1.51 (1.23–1.86) | 1.12 (0.90–1.40) | 1.12 (0.90–1.40) |
| APA | 1.79 (1.52–2.12) | 1.21 (1.02–1.44) | 1.22 (1.02–1.45) |
| ASA | 1.17 (1.01–1.36) | 0.89 (0.76–1.04) | 0.89 (0.76–1.04) |
| All-cause mortality: | |||
| OAC | 1.60 (1.48–1.73) | 1.13 (1.04–1.23) | 1.16 (1.07–1.26) |
| APA | 1.52 (1.42–1.64) | 1.09 (1.02–1.17) | 1.12 (1.04–1.20) |
| ASA | 1.25 (1.19–1.32) | 0.99 (0.93–1.05) | 0.99 (0.94–1.05) |
| CV mortality: | |||
| OAC | 1.66 (1.48–1.86) | 1.11 (0.98–1.25) | 1.14 (1.01–1.29) |
| APA | 1.75 (1.58–1.94) | 1.17 (1.05–1.29) | 1.19 (1.07–1.32) |
| ASA | 1.35 (1.25–1.46) | 0.99 (0.91–1.08) | 0.99 (0.92–1.08) |
Table displays results from 12 separate models (3 levels of adjustment for each of the 4 listed outcomes). Referent in all models was the use of no OAC, APA or ASA. Bolded HR( 95% CI) indicates p<0.05. CI- confidence interval; OAC- oral anticoagulant; APA- anti-platelet agent; ASA- acetylsalicyclic acid.
Level 1: unadjusted, stratified by phase and region, and accounting for facility-level clustering
Level 2: adjusted for demographics (age, race, sex, vintage, BMI), catheter use, deep vein thrombosis, atrial fibrillation, and co-morbidities (CAD, cancer, CVA, CHF, DM, other cardiac disease, GI bleed, HIV/AIDS, hypertension, lung disease, neurological disease, psychiatric disease, PVD, recurrent cellulitis/gangrene).
Level 3: Level 2 + laboratory values (albumin, Hgb, Ca, PO4, PTH) and Kt/V.
In models comparing OAC vs. APA, OAC vs. ASA, and APA vs. ASA, OAC users had higher adjusted rates of stroke, bleeding, cardiovascular-, and all-cause mortality than ASA users (p=0.05 for stroke, p<0.03 for others), whereas OAC and APA users had generally similar event rates (p > 0.10 for each). APA users had higher stroke, cardiovascular-, and all-cause mortality rates than ASA users (p<0.002) while bleeding rates were similar (p=0.54) (data not shown).
Bleeding risk and mORBI score
Bleeding rates according to mORBI bleeding risk categories were calculated. The majority of patients (>50%) were low risk independent of type or presence of anti-thrombotic agent. The proportion of patients deemed high risk was highest among those on APA (12.1%) followed by OAC (10.2%) (see Table 1). Among all patients on OACs, as the score increased there was an incremental increase in the bleeding rate per patient year (low-risk=0.07, intermediate risk=0.09 and high risk=0.13). Similar increases in bleeding rates were observed among patients prescribed OAC with a history of atrial fibrillation (n=852; Risk groups: low=0.07, intermediate=0.10 and high=0.14 per year, data not shown). Among the components of the mORBI score, a history of GI bleed within the past 12 months was much more strongly associated with bleeding events (HR=2.7 [2.4,3.1]) than any of the other factors (age ≥ 65: HR=1.3 [ 1.2,1.4]; history of stroke or transient ischemic attack (TIA): HR=1.2 [1.1,1.3], history of myocardial infarction in the last three months: HR= 0.9 [0.7,1.3], diabetes: HR=1.0 [0.9,1.1]; anemia: HR= 1.3 [1.2,1.4]).
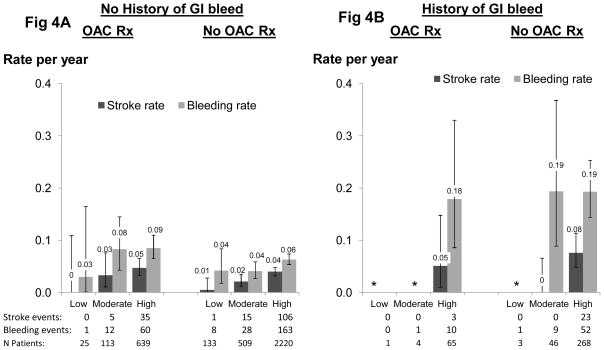
Event rates by CHADS2 score and history of GI bleed
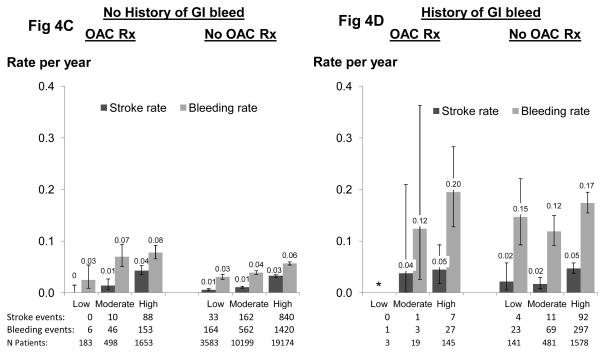
Rates of stroke by CHADS2 score and a history of GI bleed in patients with and without OAC prescription are shown in patients with documented atrial fibrillation (Figure 4A and B), as well as in all patients (Figure 4C and D) to allow for confirmation of findings in a much larger sample. Among atrial fibrillation patients with no history of GI bleeding, the stroke and bleeding rates increased with higher CHADS2 score and were modestly higher for OAC-treated patients (Figure 4A). Bleeding rates were dramatically higher in patients with a history of prior GI bleeding (Figure 4B) and exceeded stroke rates by at least 2-fold. Similar patterns were observed among all patients (Figure 4C and 4D).
Figure 4.
Stroke rate and bleeding rate by CHADS2 score and history of GI bleed. Stroke risk categories are based on CHADS2 categories used commonly in the non-dialysis population: 0=low, 1=moderate, ≥2=high. N=37,657 (N=4,026 atrial fibrillation) patients from DOPPS phases 1, 2 and 3. NOTE: Patient medication usage was classified hierarchically (OAC >APA >ASA for patients taking ≥one medication class. See text for details).
Discussion
In this large international HD cohort, there was wide variation in prescription of antithrombotic agents and major bleeding events across geographic regions. OAC use was associated with an increase in major bleeding events, and all-cause, and cardiovascular mortality. As previously reported, the CHADS2 score successfully stratified stroke risk in the HD population25. Additionally, we found that a history of recent GI bleeding was a strong predictor of serious future bleeding events. Rates of major bleeding substantially exceeded rates of stroke for all patient groups, even those at high stroke risk. These findings raise concern about the potential safety of OAC use in the HD population and should serve to caution clinicians about the high risk of bleeding in the dialysis population. Employing CHADS2 for stroke risk stratification plus consideration of recent bleeding history may provide a more rational guide for decision making regarding OAC therapy.
Compared to the general population where the guidelines for antithrombotic agent usage is well established, there is a paucity of literature reporting use of antithrombotic agents in the HD population12–13, 23, 25–29. In the largest study of 41,000 U.S. HD patients, prescriptions rates were 8.3%, 10%, and 30.4% for OAC, clopidogrel, and ASA respectively29. In a prospective, multicentre Italian HD cohort, OAC and APA use was 10.6% and 36% respectively21. In addition to regional variation, we reported striking variation between facilities in the prescription patterns of antithrombotic agents within geographic regions. OAC prescriptions among Canadian facilities ranged from 0–45% while APA use varied from 0–42% within Japanese and European facilities. The high variability is not surprising as there is a lack of direct evidence to guide therapeutic decision making, and thereby, choices are influenced by regional policies, practice patterns, and clinician preferences.
Rates of stroke varied considerably among countries with a greater than 2-fold difference between low and high stroke rate nations. The lowest stroke rate was in Italy (0.018/year) whereas the highest was in Sweden (0.04/year). Reasons for this variability could be related to reporting error in a relatively rare event, even in a large cohort such as the Dialysis Outcomes and Practice Patterns Study (DOPPS). Other than regional variations in underlying risk (due to genetics, diet, lifestyle, etc.), numerous dialysis-specific factors may contribute to the observed variation in stroke risk including length of time on dialysis30, blood pressure control31, vascular access32, hemoglobin targets33, and inter-dialytic weight gain34, all of which vary between countries. In general, the stroke rate among HD patients considerably exceeded that observed in the general population. In the U.S., adjusted hospitalization rates for ischemic stroke were 4 to 10-fold greater than the general population.26
The rate of major bleeding events in our study was lower than previously reported in the HD population. A systematic review reported a bleeding rate of 0.1 to 0.54 events/year in the ESRD population on warfarin compared to our rate of 0.078 events/year13.This considerable variation can be explained by most previous studies14,16–18,21,29 having differences in the definitions of major bleeding, study design (retrospective vs. prospective), small sample size, and concomitant APA and ASA use. Due to the large population size and heterogeneity of our cohort, we believe that our study provides a more robust estimate of major bleeding rates in the ESRD population, with or without exposure to antithrombotic agents, and allows for more accurate ascertainment of the risk for major bleeding. For example, based on our findings, the rate of major bleeding events in HD patients on OAC (0.078 per year) is roughly ten times the rate of non-ESRD patients on OAC.
The use of antithrombotic agents was associated with an increase in mortality in our study. In an analysis of U.S. HD patients, Chan et al also demonstrated an increased adjusted risk of mortality with OAC (HR=1.3, CI 1.2, 1.4), clopidogrel (HR=1.2, CI 1.1,1.4), and ASA (HR=1.1 CI 1.01.1)28. Our findings are comparable, except ASA use was not associated with mortality in our fully adjusted models (HR=1.0, CI 0.9, 1. 1) (Table 2). Cardiovascular mortality was also higher with OAC and APA use in our cohort. This present study is consistent with the findings of Chan et al and strengthens the evidence that OAC and APA use are associated with an increase in mortality. These findings should be interpreted with caution due to the possibility of confounding by indication as an alternative explanation for study findings; namely do patients prescribed antithrombotic agents have higher mortality because the drug increases mortality or because of higher underlying mortality risk? Differences between those with any anti-thrombotic agent(s) and those with none are apparent in Table 1: individuals prescribed no agents on average were younger, with fewer comorbidities, and lower CHADS2 and mORBI score. All these factors were associated with differences in survival.
Accurate determination of the bleeding risk in the HD population has been difficult due to a lack of data. The mORBI score was derived and validated in a general outpatient population with diverse indications for OAC therapy4,7. Among mORBI components, we found that only a history of GI bleeding was strongly associated with major bleeding events (HR=2.7 [2.4,3.1]). In the original mORBI validation cohort, the lowest risk group (score 0) had a 10-fold lower risk of bleeding at one year than the highest score (score 3–4) (3% to 30%). In contrast, the mORBI score calibrates bleeding risk less effectively in the HD population with rates that ranged 2-fold (.066 to .133 per year) from lowest to highest risk score. This suggests that the mORBI is not a useful tool in ESRD patients. In lieu of the mORBI, we have found that GI bleeding in the prior 12 months, which is easy to ascertain clinically, readily stratifies bleeding risk. Whether other factors add additional predictive value (e.g. a dialysis-specific bleeding risk score) requires additional study.
The decision to initiate OAC for a HD patient can be difficult and requires individualized risk-benefit determination35. A previous DOPPS analysis25 examined the rate of stroke in patients with atrial fibrillation. By comparison of the crude event rates from stroke and major bleeding, we have now found that the overall rate of bleeding is much higher than the stroke rate in all geographic regions, ranging from 1.6-fold higher in Japan to 5.7-fold higher in Australia/New Zealand. This crude estimate of risk-benefit among patients overall can be refined by incorporation of the CHADS2 score and a history of GI bleeding to risk-stratify patients individually8. Among atrial fibrillation patients with no history of GI bleeding and categorization of the CHADS2 score as low, intermediate, and high risk, major bleeding rates were roughly 2-fold or greater than stroke rates (Figure 4). Among patients with a history of GI bleeding, the rate of subsequent bleeding was high (approaching 0.2 events/year) and substantially exceeded the stroke rate, even for patients at high stroke risk based on CHADS2 score. These findings were corroborated when examined among all patients, with greater precision due to the much larger sample size (Figure 4).
In our opinion, the choice to use OAC to potentially reduce stroke risk in atrial fibrillation should be made with caution as the risk of major bleeding is substantially higher than the risk of stroke, even among individuals deemed at high stroke risk (CHADS score ≥2). In particular, a recent history of GI bleeding raises the likelihood of bleeding so significantly that this alone may be considered a contraindication to OAC use in some cases. Although risk/benefit comparisons such as these can aid in individual decision, other issues need to be considered36. Stroke-related mortality and morbidity is dependent on the type of stroke (for example lacunar versus hemorrhagic), and recent retrospective studies suggest the mortality risk with GI bleed is higher than previously believed24, 37,38. Individual decisions regarding anticoagulation should consider the patient’s wishes, beliefs, and preferences. Lastly, other concerns regarding the complications of OAC should be considered including accelerated vascular calcification, and calcific uremic arteriopathy12, 39–43.
Hemorrhagic strokes may be caused by OAC and portend a poor prognosis. Most hemorrhagic strokes (other than stroke events associated with “subdural hemorrhage” or “evacuation of hematoma”) would have been identified as ‘stroke,’ but not specifically ‘hemorrhagic stroke,’ in the DOPPS. As such, the rates of major bleeding that we report underestimate the true rate of major bleeding, and the stroke rates that we report overestimate the rate of thromboembolic stroke. For this reason, the true ratio of major bleeding to thromboembolic stroke rate (i.e., the true risk to benefit ratio for OAC use) is likely higher than we report.
Our study is strengthened by being a large prospective cohort study with substantial international variation in the prescription patterns for antithrombotic agents. Also, we employed an objective definition of a major bleeding event, namely the requirement for hospitalization.
There are limitations to our study. As an observational study, residual confounding cannot be ruled out as an alternative explanation for our findings. Data on international normalized ratio measurements, specific indications for antithrombotic agent use, and intracranial hemorrhage were unknown. Medications in our cohort were captured based on prescription, and adherence or duration of past exposure could not be verified. OAC may be prescribed with varying international normalized ratio measurement targets which directly impact the subsequent risk of bleeding2,7. In selecting our definition of bleeding as a major bleeding event requiring hospitalization, we may have missed occasional serious bleeding events, e.g. chronic bleeding leading to transfusions in the outpatient setting, although we expect this would have been uncommon. As previously reported, the CHADS2 score has several components that may not be strongly predictive of stroke in dialysis patients, and additional studies to better tailor CHADS2 to the ESRD population are warranted25. We did not study other agents such as low molecular weight heparin use due to limited use but this may represent an area of future investigation as its usage becomes more widespread.
In conclusion, there is large variation in the prescription patterns of antithrombotic agents. The use of OAC and APA was associated with an elevated risk of all-cause and cardiovascular mortality. To aid with individual prescription decisions, a history of GI bleeding is highly predictive of major bleeding risk and should be considered directly along with the risk of stroke by CHADS2. Caution should be exercised in the use of OAC for HD patients, and treatment decisions should incorporate risk-benefit stratification at the individual patient level. Lastly, a well-designed multicenter clinical trial could help to gain clarity about the safety and effectiveness of antithrombotic agents in dialysis patients with conditions such as atrial fibrillation.
Methods
Study design and data sources
The DOPPS is a prospective cohort study of HD practices and outcomes. Details on the study design have been published44,45. Data from the DOPPS Phases I–IV (n=48,144 patients in 1235 facilities) were used for descriptive analyses. Analyses assessing the impact of OAC and APA on clinical outcomes included 37,898 patients enrolled in the DOPPS Phase I (1996–2001, 15,821 patients in 307 facilities from France, Germany, Italy, Spain, the United Kingdom, Japan and the U.S.), II (2002–2004, 11,767 patients in 320 facilities from the seven countries in DOPPS I, plus Australia, Belgium, Canada, New Zealand, and Sweden), or III ( 2005–2008, 10,310 patients in 300 facilities from the twelve countries in DOPPS II). DOPPS 4 was excluded due to insufficient follow-up time. Patients with mechanical heart valves (n=1782, 4%) were excluded because they have a clear indication for anticoagulation.
Baseline patient-level data including demographic characteristics, comorbidities46, laboratory values, and prescription drugs were abstracted from the medical record at time of entry into the DOPPS using standardized questionnaires. Drug data included name and dose.
Exposure and outcome variables
Exposure variables included prescription of OAC (warfarin, dicumarol, phenprocoumon, fluindione), and of APA (clopidogrel, ticlopidine, dipyridamole, and pentoxifylline). APA and ASA prescription was categorized separately from other APA due to reports of different bleeding rates in the non-ESRD population and limited direct evidence of bleeding risk in the ESRD population 47. Patients were categorized hierarchically in one of four categories: (1) OAC use, with or without APA or ASA use; (2) APA use, but no OAC use and with or without ASA use; (3) ASA use, but no OAC or APA use; or (4) no OAC, APA or ASA.
The CHADS2 score was calculated as the sum of two points for previous stroke or TIA, and one point each for history of CHF, diabetes, hypertension, and age over 75 years (range 0–6)8. Stroke risk categories were defined as low (CHADS2 score = 0), intermediate (CHADS2 = 1) and high (CHADS2 ≥ 2), as in the non-dialysis setting. The mORBI was calculated by adding one point for age > 65, history of stroke or TIA, history of GI bleeding, and one point for any one of the following, history of acute coronary syndrome, diabetes, anemia (hemoglobin<10 g/dl) (range 0 to 4)4. The mORBI and CHADS2 scores were calculated for patients with a prescription of OAC (n=2,513) and also among those with history of atrial fibrillation who were prescribed an OAC (n=852) or were not prescribed an OAC (n=1661).
Major bleeding events were defined by the requirement of hospitalization and categorized by subtype (epistaxis, GI bleed, subdural hematoma, evacuation of hematoma, hemoptysis, hematuria, vascular access bleeding). Of note, intracranial hemorrhage, other than subdural hematoma or hemorrhage requiring evacuation was not captured. Stroke events requiring hospitalization were captured; however, the type of stroke (ischemic or hemorrhagic) could not be differentiated unless the hemorrhagic stroke was due to a subdural hematoma or required evacuation. Other outcomes were time to any first reported bleeding event, death, all-cause and cardiovascular mortality (primary or secondary cause of death due to acute myocardial infarction, hyperkalemia, pericarditis, atherosclerotic heart disease, cardiomyopathy, cardiac arrhythmia, cardiac arrest, valvular heart disease, pulmonary edema due to exogenous fluid, congestive heart failure, or cerebrovascular accident including intracranial hemorrhage).
Observation time began when the patient enrolled in the DOPPS. End of follow-up was end of the study phase, transfer from facility, or death. Outcomes were identified by hospitalization diagnosis and procedure codes.
Statistical methods
Descriptive statistics were used to compare baseline characteristics of patients according to the medication categories. Prescription of OAC, APA and ASA, and major bleeding event rates were calculated by country and risk group scores. Cox regression models were used to determine the association of OAC, APA and ASA use with all-cause mortality, cardiovascular mortality and major bleeding events. Three Cox regression models were created with step-wise adjustments: level 1 was unadjusted; level 2 was adjusted for baseline demographics (including age, gender, race, body mass index, years with ESRD), CVC use, deep vein thrombosis, atrial fibrillation and 14 comorbidity classes (coronary artery disease, other cardiac disease, cerebrovascular disease, congestive heart failure, diabetes, GI bleeding, hypertension, peripheral vascular disease, lung disease, neurologic disorder, cancer (excluding skin), psychiatric disease, recurrent cellulitis, and HIV infection); and level 3 included the covariates from level 2 and was additionally adjusted for laboratory variables (serum albumin, serum calcium, serum phosphorous, parathyroid hormone, hemoglobin, single pool Kt/V). Cox models were stratified by DOPPS region and phase, and robust variance estimates (sandwich estimator) were used to account for facility-level clustering. Referent in all models was the use of no-medications (none of OAC, APA or ASA). All statistical analyses were performed using SAS v.9.2 (SAS Institute; Cary, NC, USA).
Supplementary Material
Acknowledgments
Sources of Support: The DOPPS is administered by Arbor Research Collaborative for Health and is supported by scientific research grants from Amgen (since 1996), Kyowa Hakko Kirin (since 1999, in Japan), Sanofi Renal (since 2009), Abbvie (since 2009), Baxter (since 2011), and Vifor Fresenius Renal Pharma (since 2011), without restrictions on publications.
Dr. Tentori is supported in part by NIDDK grant 1K01DK087762-01A1. Dr. Robinson has received speakers fees for Kyowa Hakko Kirin.
Footnotes
All other authors have no conflicts of interest to report.
Disclosure: The DOPPS is administered by Arbor Research Collaborative for Health and is supported by scientific research grants from Amgen (since 1996), Kyowa Hakko Kirin (since 1999, in Japan), Sanofi Renal (since 2009), AbbVie (since 2009), Baxter (since 2011), and Vifor Fresenius Renal Pharma (since 2011), without restrictions on publications.
References
- 1.Fuster V, Ryden LE, Asinger RW, et al. ACC/AHA/ESC Guidelines for the Management of Patients With Atrial Fibrillation: Executive Summary A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines and Policy Conferences (Committee to Develop Guidelines for the Management of Patients With Atrial Fibrillation) Developed in Collaboration With the North American Society of Pacing and Electrophysiology. Circulation. 2001;104:2118–50. [PubMed] [Google Scholar]
- 2.Schulman S, Beyth RJ, Kearon C, et al. Hemorrhagic complications of anticoagulant and thrombolytic treatment: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133:257S–298S. doi: 10.1378/chest.08-0674. [DOI] [PubMed] [Google Scholar]
- 3.Singer DE, Albers GW, Dalen JE, et al. American College of Chest, Physicians Antithrombotic therapy in atrial fibrillation: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133:546S–592S. doi: 10.1378/chest.08-0678. [DOI] [PubMed] [Google Scholar]
- 4.Beyth RJ, Quinn LM, Landefeld CS. Prospective evaluation of an index for predicting the risk of major bleeding in outpatients treated with warfarin. Am J Med. 1998;105:91–9. doi: 10.1016/s0002-9343(98)00198-3. [DOI] [PubMed] [Google Scholar]
- 5.Gage BF, Yan Y, Milligan PE, et al. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF) Am Heart J. 2006;151:713–9. doi: 10.1016/j.ahj.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 6.Ruiz-Gimenez N, Suarez C, Gonzalez R, et al. Predictive variables for major bleeding events in patients presenting with documented acute venous thromboembolism. Findings from the RIETE Registry. Thromb Haemost. 2008;100:26–31. doi: 10.1160/TH08-03-0193. [DOI] [PubMed] [Google Scholar]
- 7.Palareti G, Cosmi B. Bleeding with anticoagulation therapy - who is at risk, and how best to identify such patients. Thromb Haemost. 2009;102:268–78. doi: 10.1160/TH08-11-0730. [DOI] [PubMed] [Google Scholar]
- 8.Gage BF, Waterman AD, Shannon W, et al. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864–70. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 9.Kaw D, Malhotra D. Platelet dysfunction and end-stage renal disease. Semin Dial. 2006;19:317–22. doi: 10.1111/j.1525-139X.2006.00179.x. [DOI] [PubMed] [Google Scholar]
- 10.Gangji AS, Sohal AS, Treleaven D, Crowther MA. Bleeding in patients with renal insufficiency: a practical guide to clinical management. Thromb Res. 2006;118:423–8. doi: 10.1016/j.thromres.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 11.Holden RM, Harman GJ, Wang M, et al. Major bleeding in hemodialysis patients. Clin J Am Soc Nephrol. 2008;3:105–10. doi: 10.2215/CJN.01810407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Limdi NA, Beasley TM, Baird MF, et al. Kidney function influences warfarin responsiveness and hemorrhagic complications. J Am Soc Nephrol. 2009;20:912–21. doi: 10.1681/ASN.2008070802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elliott MJ, Zimmerman D, Holden RM. Warfarin anticoagulation in hemodialysis patients: a systematic review of bleeding rates. Am J Kidney Dis. 2007;50:433–40. doi: 10.1053/j.ajkd.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 14.Kawamura M, Fijimoto S, Hisanaga S, et al. Incidence, outcome, and risk factors of cerebrovascular events in patients undergoing maintenance hemodialysis. Am J Kidney Dis. 1998;31:991–6. doi: 10.1053/ajkd.1998.v31.pm9631844. [DOI] [PubMed] [Google Scholar]
- 15.Fabbian F, Catalano C, Lambertini D, et al. Clinical characteristics associated to atrial fibrillation in chronic hemodialysis patients. Clin Nephrol. 2000;54:234–9. [PubMed] [Google Scholar]
- 16.Vazquez E, Sanchez-Perales C, Borrego F, et al. Influence of atrial fibrillation on the morbido-mortality of patients on hemodialysis. Am Heart J. 2000;140:886–90. doi: 10.1067/mhj.2000.111111. [DOI] [PubMed] [Google Scholar]
- 17.Abbott KC, Trespalacios FC, Taylor AJ, et al. Atrial fibrillation in chronic dialysis patients in the United States: risk factors for hospitalization and mortality. BMC Nephrol. 2003;4:1. doi: 10.1186/1471-2369-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Genovesi S, Pogliani D, Faini A, et al. Prevalence of atrial fibrillation and associated factors in a population of long-term hemodialysis patients. Am J Kidney Dis. 2005;46:897–902. doi: 10.1053/j.ajkd.2005.07.044. [DOI] [PubMed] [Google Scholar]
- 19.Ethier J, Bragg-Gresham JL, Piera L, et al. Aspirin prescription and outcomes in hemodialysis patients: the Dialysis Outcomes and Practice Patterns Study (DOPPS) Am J Kidney Dis. 2007;50:602–11. doi: 10.1053/j.ajkd.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 20.Quinn RR, Naimark DM, Oliver MJ, et al. Should hemodialysis patients with atrial fibrillation undergo systemic anticoagulation? A cost-utility analysis. Am J Kidney Dis. 2007;50:421–32. doi: 10.1053/j.ajkd.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 21.Genovesi S, Vincenti A, Rossi E, et al. Atrial fibrillation and morbidity and mortality in a cohort of long-term hemodialysis patients. Am J Kidney Dis. 2008;51:255–62. doi: 10.1053/j.ajkd.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 22.Wasse H. Catheter-related mortality among ESRD patients. Semin Dial. 2008;21:547–9. doi: 10.1111/j.1525-139X.2008.00500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Willms L, Vercaigne LM. Does warfarin safely prevent clotting of hemodialysis catheters? a review of efficacy and safety. Semin Dial. 2008;21:71–7. doi: 10.1111/j.1525-139X.2007.00381.x. [DOI] [PubMed] [Google Scholar]
- 24.Sozio SM, Armstrong PA, Coresh J, et al. Cerebrovascular disease incidence, characteristics, and outcomes in patients initiating dialysis: the choices for healthy outcomes in caring for ESRD (CHOICE) study. Am J Kidney Dis. 2009;54:468–77. doi: 10.1053/j.ajkd.2009.01.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wizemann V, Tong L, Satayathum S, et al. Atrial fibrillation in hemodialysis patients: clinical features and associations with anticoagulant therapy. Kidney Int. 2010;77:1098–106. doi: 10.1038/ki.2009.477. [DOI] [PubMed] [Google Scholar]
- 26.Seliger SL, Gillen DL, Longstreth WT, et al. Elevated risk of stroke among patients with end-stage renal disease. Kidney Int. 2003;64:603–9. doi: 10.1046/j.1523-1755.2003.00101.x. [DOI] [PubMed] [Google Scholar]
- 27.Phillips KW, Dobesh PP, Haines ST. Considerations in using anticoagulant therapy in special patient populations. Am J Health Syst Pharm. 2008;65:S13–21. doi: 10.2146/ajhp080241. [DOI] [PubMed] [Google Scholar]
- 28.Chan KE, Lazarus JM, Thadhani R, et al. Anticoagulant and antiplatelet usage associates with mortality among hemodialysis patients. J Am Soc Nephrol. 2009;20:872–81. doi: 10.1681/ASN.2008080824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chan KE, Lazarus JM, Thadhani R, et al. Warfarin use associates with increased risk for stroke in hemodialysis patients with atrial fibrillation. J Am Soc Nephrol. 2009;20:2223–33. doi: 10.1681/ASN.2009030319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tentori F, Zhang J, Li Y, et al. Longer dialysis session length is associated with better intermediate outcomes and survival among patients on in-center three times per week hemodialysis: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS) Nephrol Dial Transplant. 2012 Mar 19;2012 doi: 10.1093/ndt/gfs021. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robinson BM, Tong L, Zhang J, et al. Blood pressure levels and mortality risk among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study. Kidney Int. 2012 Sep;82(5):570–80. doi: 10.1038/ki.2012.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pisoni RL, Arrington CJ, Albert JM, et al. Facility hemodialysis vascular access use and mortality in countries participating in DOPPS: an instrumental variable analysis. Am J Kidney Dis. 2009 Mar;53(3):475–91. doi: 10.1053/j.ajkd.2008.10.043. [DOI] [PubMed] [Google Scholar]
- 33.McFarlane PA, Pisoni RL, Eichleay MA, Wald R, Port FK, Mendelssohn D. International trends in erythropoietin use and hemoglobin levels in hemodialysis patients. Kidney Int. 2010;78(2):215–23. doi: 10.1038/ki.2010.108. [DOI] [PubMed] [Google Scholar]
- 34.Hecking M, Karaboyas A, Saran R, et al. Predialysis Serum Sodium Level, Dialysate Sodium, and Mortality in Maintenance Hemodialysis Patients: The Dialysis Outcomes and Practice Patterns Study (DOPPS) Am J Kidney Dis. 2012 Feb;59(2):238–48. doi: 10.1053/j.ajkd.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 35.Sood MM, Komenda P, Sood AR, et al. The intersection of risk and benefit: is warfarin anticoagulation suitable for atrial fibrillation in patients on hemodialysis? Chest. 2009;136:1128–33. doi: 10.1378/chest.09-0730. [DOI] [PubMed] [Google Scholar]
- 36.Lo DS, Rabbat CG, Clase CM. Thromboembolism and anticoagulant management in hemodialysis patients: a practical guide to clinical management. Thromb Res. 2006;118:385–95. doi: 10.1016/j.thromres.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 37.Toyoda K, Fujii K, Fujimi S, et al. Stroke in patients on maintenance hemodialysis: a 22-year single-center study. Am J Kidney Dis. 2005;45:1058–66. doi: 10.1053/j.ajkd.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 38.Yang JY, Lee TC, Montez-Rath ME, et al. Trends in Acute Nonvariceal Upper Gastrointestinal Bleeding in Dialysis Patients. J Am Soc Nephrol. 2012;23(3):495–506. doi: 10.1681/ASN.2011070658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rockx MA, Sood MM. A necrotic skin lesion in a dialysis patient after the initiation of warfarin therapy: a difficult diagnosis. J Thromb Thrombolysis. 2010;29:130–3. doi: 10.1007/s11239-009-0325-3. [DOI] [PubMed] [Google Scholar]
- 40.Mazhar AR, Johnson RJ, Gillen D, et al. Risk factors and mortality associated with calciphylaxis in end-stage renal disease. Kidney Int. 2001;60:324–32. doi: 10.1046/j.1523-1755.2001.00803.x. [DOI] [PubMed] [Google Scholar]
- 41.Wilmer WA, Magro CM. Calciphylaxis: emerging concepts in prevention, diagnosis, and treatment. Semin Dial. 2002;15:172–86. doi: 10.1046/j.1525-139x.2002.00052.x. [DOI] [PubMed] [Google Scholar]
- 42.Holden RM, Booth SL. Vascular calcification in chronic kidney disease: the role of vitamin K. Nat Clin Pract Nephrol. 2007;3:522–3. doi: 10.1038/ncpneph0601. [DOI] [PubMed] [Google Scholar]
- 43.Pilkey RM, Morton AR, Boffa MB, et al. Subclinical vitamin K deficiency in hemodialysis patients. Am J Kidney Dis. 2007;49:432–9. doi: 10.1053/j.ajkd.2006.11.041. [DOI] [PubMed] [Google Scholar]
- 44.Pisoni RL, Gillespie BW, Dickinson DM, et al. The Dialysis Outcomes and Practice Patterns Study (DOPPS): design, data elements, and methodology. Am J Kidney Dis. 2004;44:7–15. doi: 10.1053/j.ajkd.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 45.Young EW, Goodkin DA, Mapes DL, et al. The Dialysis Outcomes and Practice Patterns Study (DOPPS): An international hemodialysis study. Kidney Int. 2000;57(suppl 74):S-74–S-81. [Google Scholar]
- 46.Miskulin D, Bragg-Gresham J, Gillespie BW, Tentori F, Pisoni RL, Tighiouart H, Levey AS, Port FK. Key Comorbid Conditions that Are Predictive of Survival among Hemodialysis Patients. Clin J Am Soc Nephrol. 2009 Nov;4(11):1818–26. doi: 10.2215/CJN.00640109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eikelboom JW, Hirsh J, Spencer FA, Baglin TP, Weitz JI. Antiplatelet drugs: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e89S–e119S. doi: 10.1378/chest.11-2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.