Abstract
Thiol oxidation is a probable outcome of cellular oxidative stress and is linked to degenerative disease progression. In addition, protein thiol redox reactions are increasingly identified as a mechanism to regulate protein structure and function. We assessed the effect of hypothiocyanous acid on the cytoskeletal protein tubulin. Total cysteine oxidation by hypothiocyanous and hypochlorous acids was monitored by labeling tubulin with 5-iodoacetamidofluorescein and by detecting higher molecular weight inter-chain tubulin disulfides by Western blot under nonreducing conditions. Hypothiocyanous acid induced nearly stoichiometric oxidation of tubulin cysteines (1.9 mol cysteine/mol oxidant) and no methionine oxidation was observed. Because disulfide reducing agents restored all the polymerization activity that was lost due to oxidant treatment, we conclude that cysteine oxidation of tubulin inhibits microtubule polymerization. Hypothiocyanous acid oxidation of tubulin cysteines was markedly decreased in the presence of 4% glycerol, a component of the tubulin purification buffer. Due to its instability and buffer- and pH-dependent reactivity, hypothiocyanous acid studies require careful consideration of reaction conditions.
Keywords: cysteine oxidation, disulfide, hypothiocyanous acid, tubulin, hypochlorous acid
Introduction
Our in vitro work with microtubule proteins including tubulin, tau and microtubule-associated protein-2 (MAP-2) shows that cysteine oxidation to disulfides by peroxynitrite anion, hypochlorous acid (HOCl) and other oxidants is associated with decreased microtubule polymerization.[1,2,3] Tubulin, a heterodimer composed of similar 50 kDa α- and β-subunits, contains 20 reduced cysteines (12 in α-tubulin and 8 in β-tubulin).[4,5] Because some tubulin cysteine oxidation (~1–2 mol cys) by oxidants is tolerated before microtubule polymerization is compromised, microtubule protein thiols may protect other cellular targets from oxidation.[1,6] This hypothesis is reinforced by our studies showing that the disulfides in tubulin and microtubule-associated proteins are repaired by the thioredoxin and glutaredoxin reductase systems thereby restoring polymerization activity.[1,7]
Though present in all cells, tubulin constitutes 10–15% of total cellular protein in neurons.[8,9] Tubulin cysteine modifications including oxidation to disulfides, S-glutathionylation and S-nitrosation, have been identified in several proteomics studies using cell lines and tissue samples.[10,11,12,13] Recently, tubulin was identified as a target for thiol oxidation by HOCl and chloramines in endothelial cells.[14]
In this study, we examine the effects of hypothiocyanous acid (HOSCN) on purified porcine tubulin. HOSCN is a cellular oxidant formed from thiocyanate ion (SCN−) and H2O2 by peroxidases including myeloperoxidase (MPO) and eosinophil peroxidase.[15,16] HOSCN, like HOCl, oxidizes protein thiols and, if produced in cells, will likely affect tubulin cysteines.[17,18] Our current interest in HOSCN is two-fold: 1) HOSCN is more selective for cysteines than other oxidants tested. Our published work over the past decade has included oxidants that cause additional types of damage – for example, methionine oxidation, S-nitrosation and tyrosine nitration.[3,6,19] While Angeli’s salt is largely a cysteine oxidant, presumably via release of HNO, it produces nitrite as a byproduct which could yield nitrosation of cysteines. 2) MPO is aberrantly expressed in Alzheimer’s disease brain.[20,21] Moreover, MPO immunoreactivity co-localized with neurofibrillary tangles in neurons of Alzheimer’s disease brain and, 3-chlorotyrosine, a marker of HOCl oxidation was detected. Based on these findings, it is reasonable to hypothesize that HOSCN and HOCl could be formed by MPO in AD neurons.
Our focus on tubulin allows us to categorize oxidants with respect to their specificity for cysteine rather than other amino acids. Total cysteine oxidation and effects on microtubule polymerization by HOSCN and previously characterized oxidants, HOCl, chloramines and Angeli’s salt, an HNO donor, are presented.[3]
Materials and Methods
Materials
Porcine brains were obtained from Smithfield Packing Company in Smithfield, Virginia. Angeli’s salt was from Cayman Chemicals (Ann Arbor, MI). Bicinchoninic acid (BCA) protein assay reagent, West Pico chemiluminescence detection system, Tris(2-carboxyethyl)phosphine (TCEP) and 5-iodoacetomido-fluorescein (IAF) were from Thermo Pierce. The mouse anti-β-tubulin antibody (clone TUB 2.1) and the goat anti-mouse secondary antibody HRP conjugate were from Sigma. All other chemicals were from Fisher or Sigma. The concentration of HOCl was determined by measuring the absorbance at 292 nm (ε292 = 350 M−1 cm−1) in 0.1 M NaOH.[22] A solution of Angeli’s salt was prepared immediately prior to use in 0.01 M NaOH and stored on ice. Glycine chloramine was prepared as described.[3]
Preparation of hypothiocyanous acid
HOSCN was synthesized enzymatically in 0.1 M phosphate buffer pH 6.4. A typical reaction (250 μl) contained 12.5 μg LPO, 1.2 mM KSCN and H2O2. After 15 min at 22 °C, the reaction was quenched with 500 U catalase. HOSCN was separated from LPO and catalase using an Amicon Ultra centrifuge filter with a 10K cutoff. HOSCN was stored on ice and its concentration was determined using thionitrobenzoic acid (TNB). Under these conditions, the concentration of HOSCN was 225 – 240 μM.
Purification of porcine brain tubulin
Tubulin was purified from porcine brain by two cycles of temperature-dependent polymerization and depolymerization and subsequent phosphocellulose chromatography as described.[3]
Labeling of tubulin cysteines with IAF
Tubulin (6 μM, 120 μM cys) was diluted in either 0.1 M PB pH 7.4 or PME buffer pH 6.9 and then treated with each oxidant for 10 min at 22 °C in a total reaction volume of 10–20 μl. Either methionine or dichlorodimedone (200 μM) was added to quench high HOCl concentrations. IAF (1.2 mM) in DMF was added to achieve a 10-fold molar excess relative to protein cys and samples were incubated at 37 °C for an additional 30 min. Proteins were resolved by SDS-PAGE on 7.5% gels under reducing conditions and gel images were captured using a Bio-rad Chemi-doc XRS imaging system. The intensity of the fluorescein-labeled protein bands was measured using Bio-rad Image Lab software. Alternatively, IAF-labeled tubulin was precipitated with 80% ethanol, incubated on ice for 20 min and the protein pellet was collected at 16,000 x g for 20 min. Pellets were washed twice with 80% ethanol and then resuspended in 3 M guanidine HCl in 0.1 M Tris pH 8.8. Fluorescein in each protein sample was quantitated at 490 nm relative to a fluorescein standard curve prepared in 3 M guanidine HCl in 0.1 M Tris pH 8.8.
Detection of interchain disulfides by Western Blot
Following treatment with oxidants as described above, tubulin species (10–20 μg protein per lane) were separated by SDS-PAGE on 7.5% polyacrylamide gels under nonreducing conditions. Proteins were transferred to PVDF membranes, blocked with 3% milk for 30 min and probed with a mouse monoclonal anti-β-tubulin antibody (1:2000) for two hours. The β-tubulin/antibody complex was detected using a goat anti-mouse HRP conjugate (1 hr, 1:10,000) and Pierce West Pico chemiluminescent substrate. Chemiluminescence was captured using the Bio-rad Chemi-doc XRS imaging system.
Microtubule polymerization assays
Purified tubulin, diluted with PME or PB, was treated with up to 75 μM oxidant, for 10 min at 25 °C (50 μl, 25 μM tubulin, 500 μM cys). For repair assays, 1 mM DTT or TCEP was added for an additional 10 min at 22 °C. GTP (1 mM final) was added to induce polymerization and the samples were incubated at 37 °C for 20–25 min. Microtubule polymer was collected by centrifugation at 16,000 x g for 20 min. Control polymerization activity was set at 100% for those samples containing GTP but no oxidant. Controls without GTP were used to establish 0% activity. Supernatant protein concentrations were determined by the BCA protein assay. Protein supernatants were analyzed by SDS-PAGE with Coomassie Blue staining. Protein pellets were dissolved in 6 M guanidine-HCl and the absorbance was measured at 275 nm.[1]
CNBr cleavage to detect methionine oxidation
Tubulin (12.5 μM, 250 μM cys, 325 μM methionine) was treated with each oxidant as described for IAF labeling above. Following acidification to pH 2.5 with 70% formic acid, samples were treated with 35–40 mM CNBr O.N. Samples were neutralized to pH 7.4 – 7.6 with NH4OH and subjected to SDS-PAGE under reducing conditions on a 7.5% polyacrylamide gel. Proteins were transferred to PVDF, blocked with 3% milk and incubated with mouse anti-β-tubulin (1:2000) for 2 hr. The tubulin-antibody complex was visualized using a goat anti-mouse HRP conjugate (1:10000, 1 hr) and a chemiluminescent substrate.
RESULTS AND DISCUSSION
IAF labeling of tubulin cysteines
To assess oxidation of tubulin cysteines, we used the thiol-specific reagent, iodoacetamido-fluorescein (IAF). Because IAF reacts with reduced cysteines only, tubulin labeling will decrease as the dose of oxidant increases. Previous work in our laboratory showed that all 20 cysteines of tubulin, 12 in α- and 8 in β-tubulin, can be labeled and are accessible without denaturants.[1] Figure 1A shows that labeling of both α- and β-tubulin decreased as the concentration of oxidant, HOSCN or HOCl, increased. This is typical of the oxidants we have studied and no subunit specificity has been observed.[3,6,19]
Figure 1. Modification of tubulin cysteines by HOSCN and other oxidants A).
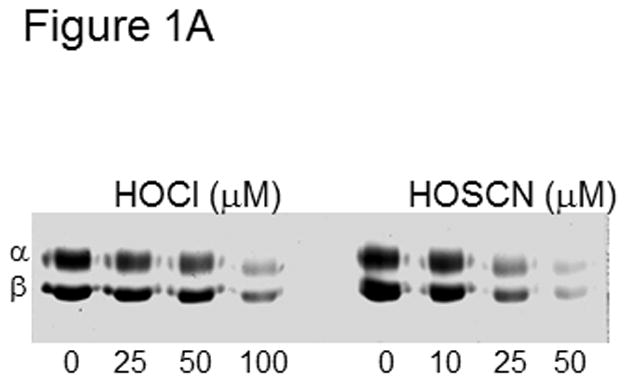
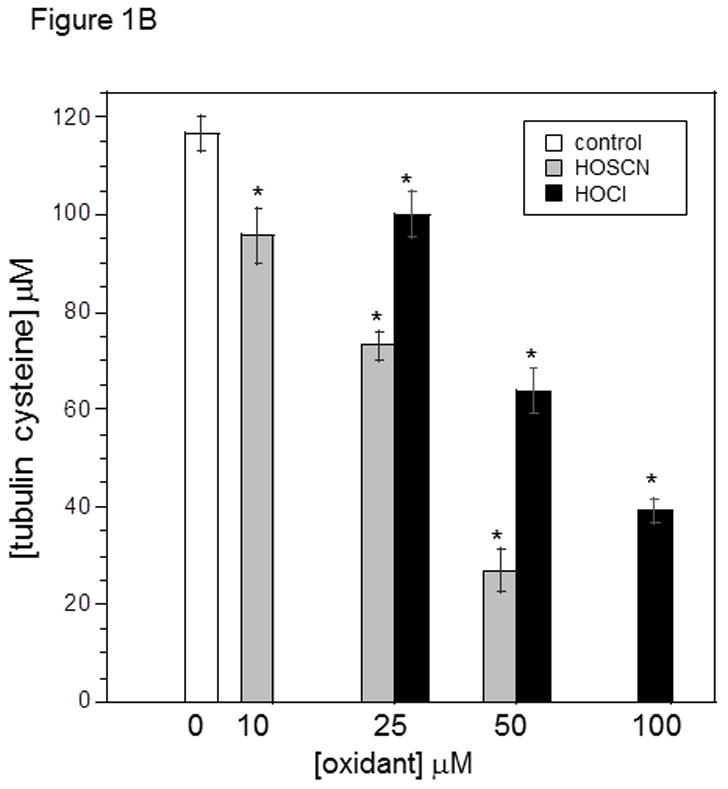
Tubulin (6 μM, 120 μM cys) in 0.1 M phosphate pH 7.4 was treated with 25, 50 and 100 μM HOCl or 10, 25, and 50 μM HOSCN for 10 min. at 22 °C. After treatment with 1.2 mM IAF for 30 min at 37 °C, samples were subjected to SDS-PAGE under reducing conditions on 7.5% polyacrylamide gels. Gel images were captured using Bio-rad Chemi-doc XRS imaging system. B) Reaction conditions were identical to A. Tubulin samples were precipitated in 80% ethanol and fluorescein incorporation was quantitated at 490 nm. These data represent the mean ± standard error of three independent experiments. One-way ANOVA with Tukey post hoc test showed that each concentration of HOSCN and HOCl assayed was significant p< .001
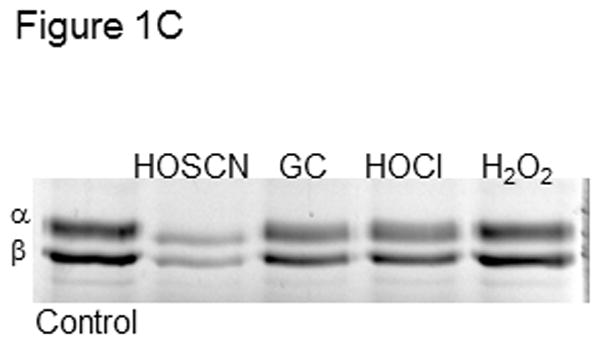
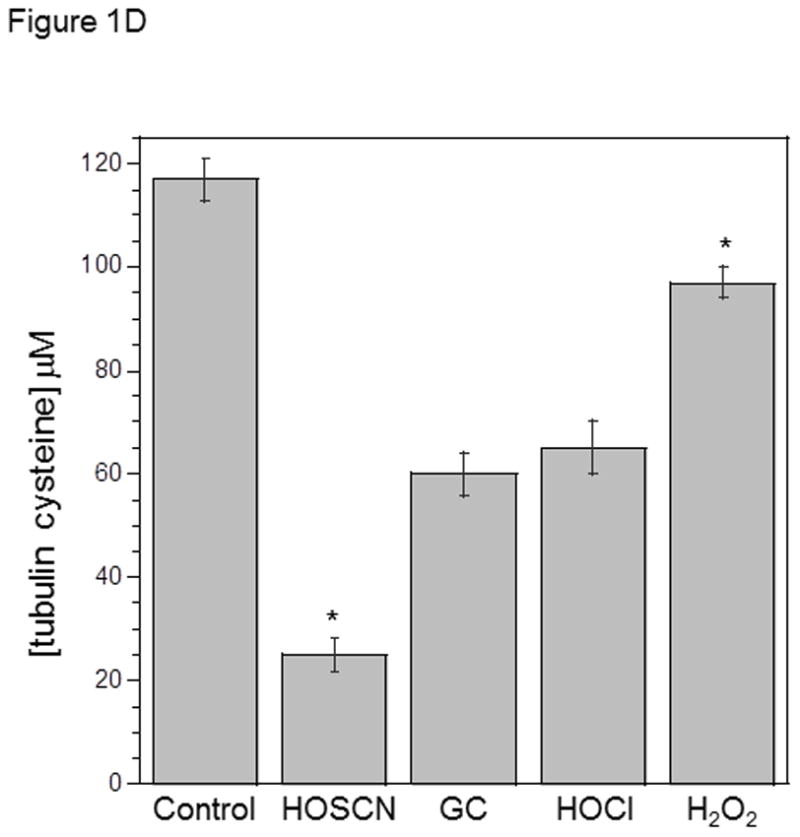
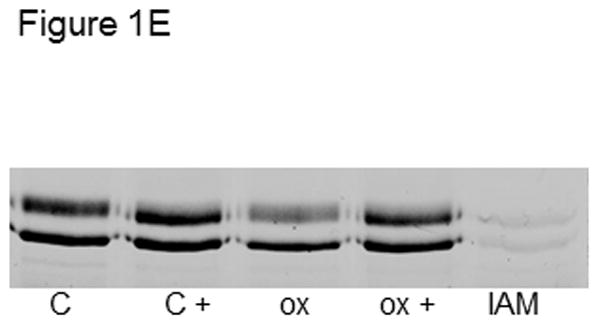
C) Tubulin (6 μM, 120 μM cys) in 0.1 M phosphate pH 7.4 was treated with 50 μM HOSCN, GC, HOCl or H2O2 for 10 min at 22 °C. After treatment with 1.2 mM IAF for 30 min at 37 °C, samples were subjected to SDS-PAGE under reducing conditions on 7.5% polyacrylamide gels. D) Reaction conditions were identical to C. Tubulin samples were precipitated in 80% ethanol and fluorescein incorporation was quantitated at 490 nm. These data represent the mean ± standard error of three independent experiments. One-way ANOVA with Tukey post hoc tests showed that all oxidants decreased IAF labeling relative to control P< .001. HOSCN was most effective and HOCl and GC were equivalent. E) Tubulin (6 μM, 120 μM cys) in 0.1 M phosphate pH 7.4 was treated with oxidant or TCEP for 10 min at 22 °C. Lane 1, control; lane 2, control + 5 mM TCEP; lane 3, 25 μM HOSCN; lane 4, 25 μM HOSCN followed by 5 mM TCEP; lane 5) 1.2 mM IAM for 30 min. After treatment with 1.2 mM IAF for 30 min at 37 °C, samples were subjected to SDS-PAGE under reducing conditions on 7.5% polyacrylamide gels.
The tubulin preparation used in Figures 1 & 2 was desalted to remove small molecules present during purification including unbound GTP, glycerol, EGTA and Mg2+. This tubulin was exchanged into PB pH 7.4 because this buffer does not react with oxidants. These methods, including the buffers and ratios of oxidant to tubulin cys are consistent with those we have performed in our studies of tubulin oxidation by ONOO−, NO donors, H2O2, HOCl and chloramines[1,3,19,23,24]
Figure 2. Detection of higher molecular weight tubulin disulfides by Western blot and Coomassie stain A).
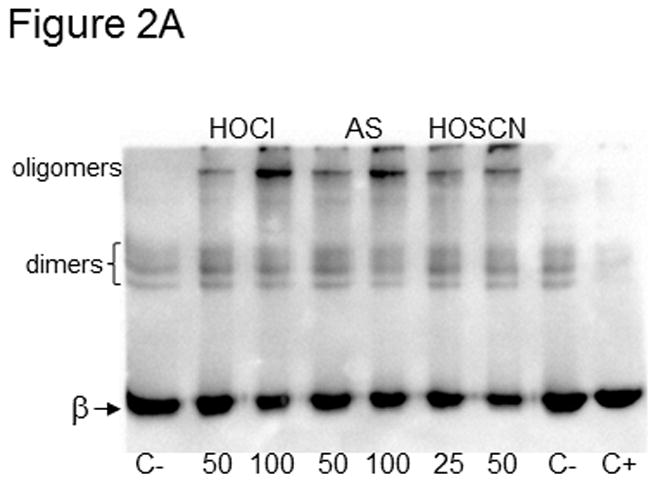
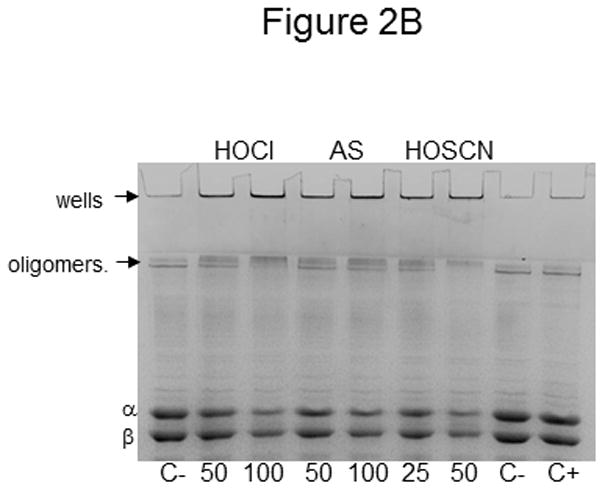
Tubulin (10 μM, 200 μM cys) was treated with 50 and 100 μM HOCl and Angeli’s salt or 25 and 50 μM HOSCN for 10 min at 22 °C. C- is control tubulin without a reducing agent whereas C+ contained βME. For Western blot detection, oxidized tubulin species were separated by SDS-PAGE under nonreducing conditions, transferred to PVDF and probed with anti-β-tubulin. Monomeric β-tubulin (50 kDa) is labeled as well as dimers and oligomers. B) Tubulin samples were identical to those in A. Prior to SDS-PAGE under nonreducing conditions, samples were treated with 50 mM iodoacetamide. Protein bands were stained with Coomassie blue and photographed.
In Figure 1A, tubulin (120 μM cys) was treated with increasing concentrations of HOCl and HOSCN. As the concentration of oxidant increased, labeling with IAF decreased. Neither thiocyanate ion alone nor decomposed HOSCN affected IAF labeling (Figure 1S). The concentrations of HOCl used were greater than those of HOSCN and yet, more oxidation was observed with HOSCN. To ensure that no oxidant remained, we performed time-course experiments with HOSCN and the reaction was essentially complete after 5 min (Supplemental Data Figure 2S). In the case of 100 μM HOCl treatment, 200 μM methionine or monochlorodimedone was added to scavenge any remaining oxidant.
Our data is consistent with published work showing that HOSCN is a more selective cysteine oxidant than HOCl.[17,18] The highest concentration of HOSCN used was 50 μM and given the tubulin cysteine concentration (120 μM) and the ratio of 2 cys oxidized per HOSCN, lane 8 in Figure 1A shows nearly stoichiometric cysteine oxidation. We and others have determined that all cysteines of tubulin are accessible to thiol reagents without addition of denaturants.[23,24] Fluorescein incorporation into precipitated tubulin pellets was quantitated at 490 nm vs. a fluorescein standard curve (Figure 1B). Using this method and the same concentrations of tubulin and oxidants, 25 and 50 μM HOSCN oxidized 46 and 95 μM tubulin cys, respectively which is consistent with the IAF labeling in Figure 1A. Given that HOSCN was always substoichiometric with respect to tubulin cysteines (50 μM HOSCN and 120 μM cys), it is unlikely that tryptophan residues of tubulin were oxidized.[25] Given the 1.9:1 stoichiometry (mol cys/mol HOSCN) that is observed, residual RS-SCN would not be expected.
HOSCN was compared to other oxidants that we have examined in addition to HOCl. Figure 1C shows oxidation of tubulin (120 μM cys) by 50 μM HOSCN, glycine chloramine (GC), HOCl and H2O2. The concentration of oxidant used was substoichiometric relative to protein cys and no HOSCN, HOCl or GC remained in solution after 10 min. In addition to time course experiments, we determined that no oxidant remained using TNB. When TNB was added to the reactions after 10 min, no TNB absorbance change was observed. In the case of H2O2, catalase was added to ensure that no oxidant remained.
HOSCN was the most effective cysteine oxidant followed by GC/HOCl and H2O2 (Figures 1C&D). This is consistent with our previous work showing that GC was similar in reactivity to HOCl with tubulin cysteines.[3] However, we did observe that taurine chloramine was more selective for tubulin cysteines than GC.[3] Peskin and Winterbourn observed that taurine chloramine was a more selective cysteine oxidant than HOCl.[26,27] This ranking of oxidants was confirmed using absorbance data for identical oxidant-treated and IAF-labeled samples that were precipitated with ethanol and resuspended in guanidine HCl (Figure 1D). Analysis of the data in Figure 1D showed no statistical difference between GC and HOCl.
IAF is specific for cysteines and no other amino acids are labeled under the conditions employed (Figures 1E & supplemental Data Figure 3S). The oxidant-induced decrease in IAF labeling that we observed in Figures 1A–1D was reversible when the disulfide reducing agent TCEP was added after oxidant treatment. IAF labeling of oxidized tubulin was restored to control levels by TCEP (Figure 1E). We chose to use TCEP because it is a phosphine, not a thiol-based reducing agent like DTT. Therefore, it can be present during the IAF labeling step.
In addition, pretreatment of tubulin with iodoacetamide (IAM) abolished IAF labeling (Figure 1E – lane 5) thereby confirming that IAF is specific for thiols and does not bind nonspecifically to tubulin. An additional IAM-blocking experiment and the corresponding Coomassie-stained gel image are presented in the Supplemental Data (Figure 3S).
Western blot detection of tubulin inter-chain disulfides
In addition to IAF labeling, we employed a Western blot method to assess cysteine oxidation of tubulin. Oxidation of tubulin cysteines yields higher molecular weight disulfide-linked species that can be resolved by SDS-PAGE under non-reducing conditions. Tubulin dimers and tetramers are formed as well as larger oligomers that do not enter the separating gel. Previous work from our laboratory confirmed that the addition of reducing agents such as β-mercaptoethanol, DTT and TCEP abolish all higher molecular weight tubulin species including those in the sample wells.[1,6]
In Figure 2A, tubulin (200 μM cys) was treated with 50 and 100 μM HOCl and Angeli’s salt, an HNO donor, and 25 and 50 μM HOSCN. As tubulin was oxidized to higher molecular weight species, the β-tubulin band at 50 kDa decreased in intensity. Likewise the bands observed near the top of the blot increased. This upper band is near the interface of the stacking and separating gels. This top band was not as apparent for tubulin treated with 50 μM HOSCN (lane 6) even though the 50 kDa β-tubulin band is the lightest on this blot. Additional blots, in which any tubulin in the sample wells was transferred, showed that the greatest β-tubulin antibody reactivity was in the wells of those samples treated with HOSCN.
Control tubulin in lane 7 of Figure 2A did not contain a reducing agent whereas control tubulin in lane 8 was treated with DTT. Control tubulin stored in the absence of a reducing agent always contains trace amounts of dimers due to air-oxidation (lane 7).[6] The two faint dimer bands run at slightly different sizes because brain tubulin is composed of multiple α- and β-tubulin gene products yielding a heterogeneous mixture of proteins.[28]
The greatest extent of tubulin cysteine oxidation to higher molecular weight disulfides was observed with HOSCN even though lower concentrations of HOSCN were used relative to HOCl or Angeli’s salt. The HOCl results are consistent with the IAF labeling results in Figures 1A–1D. Because changes in the β-tubulin band intensity at 50 kDa only represent inter-subunit disulfides, it is not possible to quantify total cysteine oxidation based on the Western blot results. Our qualitative data comparing HOSCN and HOCl by this method and our quantitative data by IAF labeling demonstrate that HOSCN is a more selective oxidant of cysteine residues in tubulin than HOCl.
Coomassie staining of oxidized tubulin
Because the Western blot in Figure 2A only showed oxidation of β-tubulin, and because Western blots with α-tubulin displayed considerable smearing, we have developed another method to assess tubulin cysteine oxidation. Furthermore, we were concerned about antibody binding and effective transfer to PVDF given the formation of very high molecular weight disulfide-linked species following HOSCN treatment.
Tubulin samples were oxidized, treated with IAM to modify any free cysteines and separated by SDS-PAGE under nonreducing conditions. The Coomassie-stained samples in Figure 2B are identical to those in Figure 2A. In addition to the α- and β-tubulin bands which decreased in intensity as oxidation increased, tubulin was detected in the wells of those lanes that contained oxidant-treated tubulin (lanes 1–6). In addition, tubulin was observed at the interface between the stacking and separating gels. This is different from the controls in lanes 7 & 8 where MAP-2, a minor microtubule-associated protein contaminant was detected. Because both the α- and β-tubulin bands in Figure 2B decreased as oxidation increased, both subunits must comprise the higher molecular weight, disulfide-linked tubulin species detected by Western blot in Figure 2A. Given that the mobility of the MAP2 contaminant also changed, this result suggests that it too is oxidized to higher molecular weight species.
Effect of oxidants on tubulin polymerization
To assess the effect of oxidants on tubulin polymerization, we oxidized protein samples and then induce polymerization with GTP at 37 °C. Polymerization assays require a much higher concentration of tubulin (25 μM, 500 μM cys) relative to the oxidation studies in Figures 1 & 2 (120 – 200 μM cys). Furthermore, due to the instability of HOSCN and its relatively low concentration following synthesis, the highest dose of HOSCN and HOCl tested was 75 μM. The ratio of oxidant to tubulin cys is important because, in previous work, we determined that some cysteine oxidation is tolerable before polymerization activity is compromised.[1,6]
Tubulin polymerization yields a microtubule polymer that can be collected by centrifugation. If polymerization is inhibited by oxidants, the tubulin concentration in the supernatant will increase. In addition, equal volumes of supernatants were subjected to SDS-PAGE with Coomassie staining to determine the extent of polymerization. This is especially important if polymerization samples contain reducing agents because they interfere with the BCA assay. Lastly, the amount of microtubule protein in the pellet was measured at 275 nm and compared to control samples to determine polymerization activity.
Table 1 shows that tubulin treated with 75 μM HOSCN and HOCl resulted in decreased polymerization activity. Inhibition of polymerization by HOSCN was greater than by HOCl at both 50 and 75 μM. However, the difference was minimal with 71% of control activity in those samples treated with 75 μM HOSCN vs 76% of control activity in the 75 μM HOCl samples. SDS-PAGE and Coomassie staining of the resulting supernatants consistently showed more protein in the HOSCN samples compared to the HOCl samples. As expected, the protein pellets obtained for the HOSCN-treated samples contained slightly more protein than the HOCl-treated samples.
Table 1. Effect of HOSCN and HOCl on cysteine oxidation and microtubule polymerization.
Polymerization assays contained 25 μM tubulin (500 μM cys) and 75 μM oxidant in PME-tubulin. Cysteine oxidation of PB-tubulin was assessed by IAF labeling as described in Figure 1 using 100 – 150 μM tubulin cys and up to 75 μM oxidant. PME-tubulin was diluted to the same ratio of tubulin cys to oxidant. Band intensities were used to calculate mol cys/mol oxidant.
| Oxidant | % polymerization activity | cysteine oxidation (mol cys/mol oxidant) | |
|---|---|---|---|
| PB-tubulin | PME-tubulin | ||
| HOSCN | 71 ± 3 | 1.9 ± 0.1 | 1.2 ± 0.2 |
| HOCl | 76 ± 2 | 0.9 ± 0.2 | 1.0 ± 0.2 |
A particular challenge in this work was the comparison of HOSCN and HOCl given that HOSCN was synthesized enzymatically in phosphate buffer at pH 6.4. The lower pH was required to maximize synthesis of HOSCN; however, tubulin polymerization in vitro is typically performed in PME buffer pH 6.9.[18] Controls showed that polymerization was reduced in phosphate buffer at all pH values tested (6.4, 6.9 and 7.4). In addition, the concentration of HOSCN was typically 225 – 240 μM and therefore, relatively large volumes of HOSCN in PB pH 6.4 were combined with tubulin in PME pH 6.9. To correct for these buffer differences, HOCl was diluted in PB pH 6.4 and control polymerization assays contained mixtures of PME and PB.
Of note, samples treated with the oxidants and then with DTT prior to the addition of GTP, contained the same amount of pellet protein as controls that did not contain oxidants and DTT. This is important because it shows that reduction of disulfides restored all polymerization activity and that cysteine oxidation was responsible for inhibition. In previous work from our laboratory, higher doses of HOCl yielded greater cysteine oxidation and lost polymerization activity was not fully restored with a reducing agent.[3] We hypothesized that oxidation and reduction altered protein structure such that some fraction of tubulin was rendered nonfunctional. Thiocyanate ion alone did not inhibit polymerization nor did decomposed HOSCN.
Given the results in Figures 1 and 2 confirming that HOSCN is a much more effective cysteine oxidant than HOCl, the polymerization results in Table 1 were perplexing. Careful consideration of the tubulin preparations used in Figures 1 & 2 vs. Table 1 suggested a possible explanation. Tubulin is purified from brain tissue by cycles of temperature-dependent polymerization and depolymerization followed by phosphocellulose column chromatography to remove microtubule-associated proteins. In addition to PME buffer, some GTP and glycerol is present following purification. It is this PME-tubulin that was used for polymerization assays whereas portions of PME-tubulin were desalted into phosphate buffer pH 7.4 for the assays in Figures 1 & 2 (PB-tubulin).
To address this discrepancy, we treated PME-tubulin, used for polymerization assays, with HOSCN and HOCl using the same protein to oxidant ratio as employed in our polymerization studies (Table 1). The IAF labeling results showed that both HOSCN and HOCl oxidized cysteines; however, the differences between the two oxidants are not as striking as in Figures 1A – 1D). Quantitation of band intensities showed that 75 μM HOSCN oxidized 1.2 mol cys/mol oxidant whereas 75 μM HOCl oxidized 1.0 mol cys/mol oxidant.
Buffer and glycerol effects on HOSCN oxidation of tubulin
To determine which component of the PME-tubulin preparation interfered with cysteine oxidation by HOSCN, we systematically added the buffer components: PIPES, Mg2+, and EGTA to PB-tubulin prior to the addition of HOSCN. In addition, we tested the ability of GTP and glycerol to infer with tubulin cysteine oxidation by HOSCN.
Although a slight difference in oxidation was observed with PIPES vs. PB, the presence of as little as 1.5% glycerol decreased HOSCN oxidation of tubulin in PME. These results are shown in Figure 3A. The samples in lanes 1–4 contained tubulin in PB whereas those in lanes 5–8 contain tubulin in a mixture of phosphate and PIPES buffers. Greater oxidation of tubulin by HOSCN is observed in lane 6 for the PIPES/phosphate sample vs. the phosphate-only sample in lane 2 indicative of a buffer effect. This is not unexpected given that HOSCN is unstable and its half-life varies in a pH-dependent manner.[15,17,29] Tubulin samples in lanes 7 and 8 were identical to lane 6 except they contained 1.5 and 4% glycerol respectively. The much darker α- and β-tubulin bands are indicative of less tubulin oxidation to higher molecular weight species. For the tubulin samples oxidized by HOSCN in phosphate buffer (lanes 2–4), the 4 % glycerol sample showed decreased tubulin cysteine oxidation (lane 4) but the 1.5% sample had only a modest effect.
Figure 3. Effect of buffer and glycerol on tubulin cysteine oxidation by HOSCN and HOCl.
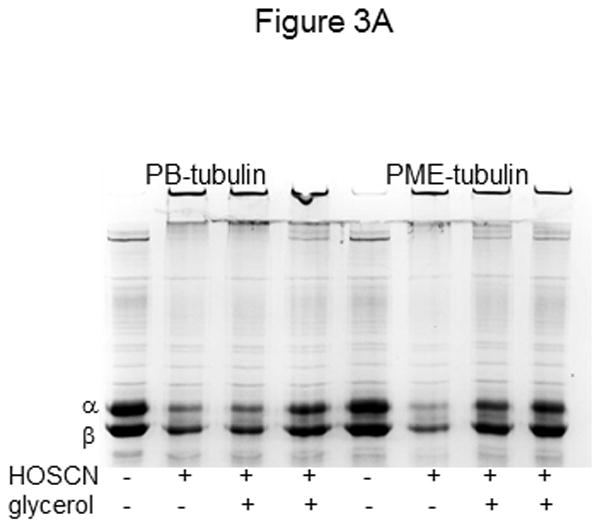
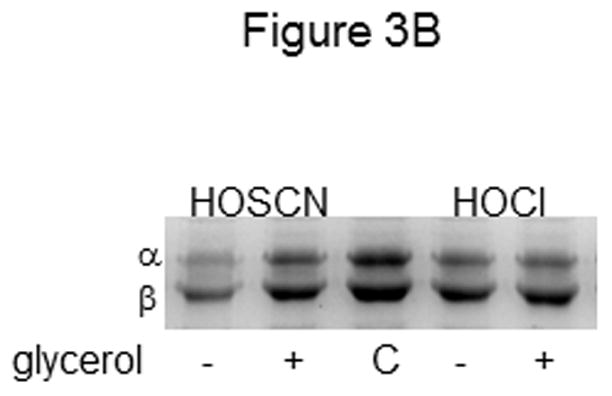
A) Tubulin (6 μM, 120 μM cys) was treated with 25 μM HOSCN in 0.1 M phosphate pH 7.4 (lanes 1–4) or phosphate plus PME (lanes 5–8) for 10 min at 22 °C. In addition to HOSCN, samples in lanes 3 and 7 contained 1.5% glycerol whereas those in lanes 4 and 8 contained 4% glycerol. Controls are shown in lanes 1 and 5. B) Tubulin (6 μM, 120 μM cys) was treated with 50 μM HOSCN (lane1) or HOCl (lane 4) in 0.1 M phosphate pH 7.4 for 10 min at 22 °C. Samples in lanes 2 and 5 contained 4% glycerol in addition to oxidant. C is control tubulin. Prior to SDS-PAGE under nonreducing conditions on 7.5% gels, samples were treated with 50 mM iodoacetamide. Protein bands were stained with Coomassie blue and photographed.
We hypothesized that glycerol decreased tubulin oxidation by HOSCN due to changes in solution viscosity and hydrogen-bonding capacity. Alterations in hydrogen-bonding networks on the surface of tubulin by glycerol could limit accessibility to thiols by oxidants and labeling reagents. Because HOSCN is a short-lived oxidant with a half-life of 42 min at pH 6.5, it is possible that glycerol impedes its interaction with tubulin cysteines.[17,29]
Experiments with TNB, a small molecule thiol, were performed to determine if glycerol decreased HOSCN oxidation. No glycerol effect was observed with up to 10% glycerol. However, 4% glycerol hindered control IAF labeling of cysteines in the model proteins, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and creatine kinase. IAF labeling of control GAPDH and creatine kinase decreased by 23% and 20%, respectively in the presence of 4% glycerol (Supplemental data Figure 4S). While it is clear that IAF labeling of unoxidized GAPDH and creatine kinase was affected by 4% glycerol, interpretation of changes in HOSCN oxidation are unclear.
To confirm a glycerol effect for HOSCN, but not for HOCl, we treated tubulin with each oxidant in the presence and absence of 4% glycerol. Figure 3B clearly shows the difference in tubulin oxidation by HOSCN when glycerol was present (lanes 1 vs. 2). However, no appreciable difference in tubulin oxidation by HOCl was observed in lanes 3 vs. 4. This result is consistent with the data in Table 1 showing nearly equivalent cysteine oxidation of PME-tubulin and correspondingly similar inhibition of tubulin polymerization by the two oxidants.
Cyanogen bromide cleavage assay to detect methionine oxidation
We had previously shown that HOCl and chloramines oxidized tubulin methionines using a cyanogen bromide (CNBr) cleavage assay.[3] Therefore we wanted to confirm that tubulin methionines were not oxidized by HOSCN. Controls were performed in the presence of excess thiocyanate to ensure that the ion did not interfere with CNBr cleavage. Tubulin treated with HOSCN was fragmented to the same extent as tubulin that had not been oxidized (Figure 4).
Figure 4. CNBr cleavage assay to detect methionine oxidation.

Tubulin (12.5 μM, 250 μM cys, 325 μM methionine) samples were treated as follows: Lane 1: control tubulin; lane 2: tubulin + formic acid and NH4OH; lane 3: tubulin + 500 μM KSCN, formic acid and NH4OH; lane 4: tubulin + formic acid, CNBr, and NH4OH; lane 5: tubulin + 100 μM HOSCN, formic acid, CNBr, and NH4OH. Samples were separated by SDS-PAGE under reducing conditions, transferred to PVDF and probed with anti-β-tubulin.
While it is apparent that HOSCN does oxidize tubulin cysteines, the kinetics and pH dependence of the reaction are complex. Kinetic data shows that HOSCN, not −OSCN, reacts with protein thiolates (RS−).[17] Given that the HOSCN pKa is ~5 (both 5.3 and 4.85 have been reported) and most thiols have pKa values of 8–9, pH, buffer choice and even ionic strength can have a great effect on HOSCN reactivity. [15,17] Furthermore, HOSCN is unstable and decomposes with pH-dependent half-life of 42.5 min at pH 6.5.[29]
All 20 cysteines of tubulin 12 in α- and 8 in β-tubulin, can be labeled by thiol reagents.[23,24] However, no detailed study of their pKa values has been reported and it is assumed that pKa values are typical of the amino acid cysteine (~8.5 – 9). Of note, Britto and coworkers have identified two classes of tubulin cysteines, slow- and fast-reacting but they could not be distinguished with iodoacetamide-based reagents. Further, Britto and coworkers examined the 3.5 A electron diffraction structure of tubulin and identified some positively charged amino acids in proximity to cysteines.[23] They hypothesize that such a positive charge could stabilize a thiolate thereby enhancing reactivity with electrophiles and possibly with oxidants.
Our results herein and in previous work do not support selective oxidation of tubulin cysteines. We routinely check for selectively with each oxidant by trypsin digestion of IAF-labeled tubulin. We observe that as the concentration of oxidant increases, all IAF labeled peptide peaks decrease suggesting partial oxidation, and therefore similar reactivity of tubulin cysteines.
HOSCN is the most selective tubulin cysteine oxidant we have identified to date. Of all the oxidants we have tested previously, only Angeli’s salt, an HNO donor, is specific for cysteine. However, the rate of HNO release is also dependent on reaction conditions and the half-life of Angeli’s salt is only 2.3 min at 37 °C in 0.1 M phosphate buffer pH 7.4.[30] Thus, the effective dose of HNO from Angeli’s salt is dependent on reaction conditions. Given that all the oxidants tested have the potential to form in cells, our findings are important in both understanding the outcome of cellular oxidative stress and in understanding tubulin reactivity in vitro.
Supplementary Material
Highlights.
Cysteines of both α- and β-tubulin were oxidized by hypothiocyanous acid.
Hypothiocyanous acid is more selective for tubulin cysteines than other oxidants tested.
Oxidation of cysteines by hypothiocyanous acid inhibited tubulin polymerization.
Buffer composition and glycerol affected hypothiocyanous acid oxidation of tubulin.
Acknowledgments
The authors acknowledge support from the National Institute of Neurological Disorders and Stroke (R15-NS38885 to LML).
ABBREVIATIONS
- AS
Angeli’s salt
- BCA
bicinchoninic acid
- DTT
dithiothreitol
- GC
glycine chloramine
- HNO
nitroxyl
- HOCl
hypochlorous acid
- IAF
5-iodoacetomido-fluorescein
- PB
phosphate buffer
- PME
0.1 M PIPES pH 6.9, 1 mM MgSO4, 2 mM EGTA
- TCEP
Tris(2-carboxyethyl)phosphine
- TNB
thionitrobenzoic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Landino LM, Iwig JS, Kennett KL, Moynihan KL. Repair of peroxynitrite damage to tubulin by the thioredoxin reductase system. Free Radical Biology and Medicine. 2004;36:497–506. doi: 10.1016/j.freeradbiomed.2003.11.026. [DOI] [PubMed] [Google Scholar]
- 2.Landino LM, Skreslet TE, Alston JA. Cysteine oxidation of tau and microtubule-associated protein-2 by peroxynitrite: modulation of microtubule assembly kinetics by the thioredoxin reductase system. Journal of Biological Chemistry. 2004;279:35101–35105. doi: 10.1074/jbc.M405471200. [DOI] [PubMed] [Google Scholar]
- 3.Landino LM, Hagedorn TD, Kim SB, Hogan KM. Inhibition of tubulin polymerization by hypochlorous acid and chloramines. Free Radical Biology and Medicine. 2011;50:1000–1008. doi: 10.1016/j.freeradbiomed.2011.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nogales E, Wolf SG, Downing KH. Structure of the αβ-tubulin dimer by electron crystallography. Nature. 1998;391:199–203. doi: 10.1038/34465. [DOI] [PubMed] [Google Scholar]
- 5.Luduena RF, Roach MC. Tubulin sulfhydryl groups as probes and targets for antimitotic and antimicrotubule agents. Pharmacological Therapeutics. 1991;49:133–152. doi: 10.1016/0163-7258(91)90027-j. [DOI] [PubMed] [Google Scholar]
- 6.Landino LM, Hasan R, McGaw A, Cooley S, Smith AW, Masselam K, Kim G. Peroxynitrite oxidation of tubulin sulfhydryls inhibits microtubule polymerization. Archives of Biochemistry and Biophysics. 2002;398:213–220. doi: 10.1006/abbi.2001.2729. [DOI] [PubMed] [Google Scholar]
- 7.Landino LM, Moynihan KL, Todd JV, Kennett KL. Modulation of the redox state of tubulin by the glutathione/glutaredoxin reductase system. Biochemical and Biophysical Research Communications. 2004;314:555–560. doi: 10.1016/j.bbrc.2003.12.126. [DOI] [PubMed] [Google Scholar]
- 8.Olmsted JB. Tubulin pools in differentiating neuroblastoma cells. Journal of Cell Biology. 1981;89:418–423. doi: 10.1083/jcb.89.3.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson PJ. The structure and amount of tubulin in cells and tissues. Journal of Biological Chemistry. 1979;254:2168–2171. [PubMed] [Google Scholar]
- 10.Cumming RC, Andon NL, Haynes PA, Park M, Fischer WH, Schubert D. Protein disulfide bond formation in the cytoplasm during oxidative stress. Journal of Biological Chemistry. 2004;279:21749–21758. doi: 10.1074/jbc.M312267200. [DOI] [PubMed] [Google Scholar]
- 11.Pamplona R, Dalfo E, Ayala V, Bellmunt MJ, Prat J, Ferrer I, Portero-Otin M. Proteins in human brain cortex are modified by oxidation, glycoxidation, and lipoxidation. Journal of Biological Chemistry. 2005;280:21522–21530. doi: 10.1074/jbc.M502255200. [DOI] [PubMed] [Google Scholar]
- 12.Brennan JP, Wait R, Begum S, Bell JR, Dunn MJ, Eaton P. Detection and mapping of widespread intermolecular protein disulfide formation during cardiac oxidative stress using proteomics with diagonal electrophoresis. Journal of Biological Chemistry. 2004;279:41352–41360. doi: 10.1074/jbc.M403827200. [DOI] [PubMed] [Google Scholar]
- 13.Sparaco M, Gaeta LM, Tozzi G, Bertini E, Pastore A, Simonati A, Santorelli FM, Piemonte F. Protein glutathionylation in human central nervous system: potential role in redox regulation of neuronal defense against free radicals. Journal of Neuroscience Research. 2006;83:256–263. doi: 10.1002/jnr.20729. [DOI] [PubMed] [Google Scholar]
- 14.Summers FA, Quigley AF, Hawkins CL. Identification of proteins susceptible to thiol oxidation in endothelial cells exposed to hypochlorous acid and N-chloramines. Biochemical and Biophysical Research Communications. 2012;425:157–161. doi: 10.1016/j.bbrc.2012.07.057. [DOI] [PubMed] [Google Scholar]
- 15.Barrett TJ, Hawkins CL. Hypothiocyanous acid: benign or deadly? Chemical Research in Toxicology. 2011;25:263–273. doi: 10.1021/tx200219s. [DOI] [PubMed] [Google Scholar]
- 16.Lane AE, Tan JTM, Hawkins CL, Heather AK, Davies MJ. The myeloperoxidase-derived oxidant HOSCN inhibits protein tyrosine phosphatases and modulates cell signalling via the mitogen-activated protein kinase (MAPK) pathway in macrophages. Biochemical Journal. 2010;430:161–169. doi: 10.1042/BJ20100082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagy P, Jameson GNL, Winterbourn CC. Kinetics and mechanisms of the reaction of hypothiocyanous acid with 5-thio-2-nitrobenzoic acid and reduced glutathione. Chemical Research in Toxicology. 2009;22:1833–1840. doi: 10.1021/tx900249d. [DOI] [PubMed] [Google Scholar]
- 18.Skaff O, Pattison DI, Davies MJ. Hypothiocyanous acid reactivity with low-molecular-mass and protein thiols: absolute rate constants and assessment of biological relevance. Biochemical Journal. 2009;422:111–117. doi: 10.1042/BJ20090276. [DOI] [PubMed] [Google Scholar]
- 19.Landino LM, Koumas MT, Mason CE, Alston JA. Modification of tubulin cysteines by nitric oxide and nitroxyl donors alters tubulin polymerization activity. Chemical Research in Toxicology. 2007;20:1693–1700. doi: 10.1021/tx7001492. [DOI] [PubMed] [Google Scholar]
- 20.Green PS, Mendez AJ, Jacob JS, Crowley JR, Growdon W, Hyman BT, Heinecke JW. Neuronal expression of myeloperoxidase is increased in Alzheimer’s disease. Journal of Neurochemistry. 2004;90:724–733. doi: 10.1111/j.1471-4159.2004.02527.x. [DOI] [PubMed] [Google Scholar]
- 21.Maki RA, Tyurin VA, Lyon RC, Hamilton RL, DeKosky ST, Kagan VE, Reynolds WF. Aberrant expression of myeloperoxidase in astrocytes promotes phospholipid oxidation and memory deficits in a mouse model of Alzheimer Disease. Journal of Biological Chemistry. 2009;284:3158–3169. doi: 10.1074/jbc.M807731200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Winterbourn CC, Brennan SO. Characterization of the oxidation products of the reaction between reduced glutathione and hypochlorous acid. Biochemical Journal. 1997;326:87–92. doi: 10.1042/bj3260087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Britto PJ, Knipling L, McPhie P, Wolff J. Thiol-disulphide interchange in tubulin: kinetics and the effect on polymerization. Biochemical Journal. 2005;389:549–558. doi: 10.1042/BJ20042118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Britto PJ, Knipling L, Wolff J. The local electrostatic environment determines cysteine reactivity of tubulin. Journal of Biological Chemistry. 2002;277:29018–29027. doi: 10.1074/jbc.M204263200. [DOI] [PubMed] [Google Scholar]
- 25.Hawkins CL, Pattison DI, Stanley NR, Davies MJ. Tryptophan residues are targets in hypothiocyanous acid-mediated protein oxidation. Biochemical Journal. 2008;416:441–452. doi: 10.1042/BJ20070941. [DOI] [PubMed] [Google Scholar]
- 26.Peskin AV, Winterbourn CC. Kinetics of the reactions of hypochlorous acid and amino acid chloramines with thiols, methionine and ascorbate. Free Radical Biology and Medicine. 2001;30:572–579. doi: 10.1016/s0891-5849(00)00506-2. [DOI] [PubMed] [Google Scholar]
- 27.Peskin AV, Winterbourn CC. Taurine chloramine is more selective than hypochlorous acid at targeting critical cysteines and inactivating creatine kinase and glyceraldehyde-3-phosphate dehydrogenase. Free Radical Biology and Medicine. 2006;40:45–53. doi: 10.1016/j.freeradbiomed.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 28.Luduena RF. Multiple forms of tubulin: different gene products and covalent modifications. Internation review of cytology. 1998;178:207–275. doi: 10.1016/s0074-7696(08)62138-5. [DOI] [PubMed] [Google Scholar]
- 29.Nagy P, Alguindigue SS, Ashby MT. Lactoperoxidase-catalyzed oxidation of thiocyanate by hydrogen peroxide: a reinvestigation of hypothiocyanite by nuclear magnetic resonance and optical spectroscopy. Biochemistry. 2006;45:12610–12616. doi: 10.1021/bi061015y. [DOI] [PubMed] [Google Scholar]
- 30.Maragos CM, Morley D, Wink DA, Dunams TM, Saavedra JE, Hoffman A, Bove AA, Isaac L, Hrabie JA, Keefer LK. Complexes of .NO with nucleophiles as agents for the controlled biological release of nitric oxide. Vasorelaxant effects. Journal of Medicinal Chemistry. 1991;34:3242–3247. doi: 10.1021/jm00115a013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


