Abstract
The excellent biocompatibility and unique inclusion capability as well as powerful functionalization capacity of cyclodextrins and their derivatives make them especially attractive for engineering novel functional materials for biomedical applications. There has been increasing interest recently to fabricate supramolecular systems for drug and gene delivery based on cyclodextrin materials. This review focuses on state of the art and recent advances in the construction of cyclodextrin-based assemblies and their applications for controlled drug delivery. First, we introduce cyclodextrin materials utilized for self-assembly. The fabrication technologies of supramolecular systems including nanoplatforms and hydrogels as well as their applications in nanomedicine and pharmaceutical sciences are then highlighted. At the end, the future directions of this field are discussed.
Keywords: Cyclodextrin, Amphiphile, Self-assembly, Supramolecular system, Nanoparticle, Nanomedicine, Hydrogel, Drug delivery, Gene therapy
1. Introduction
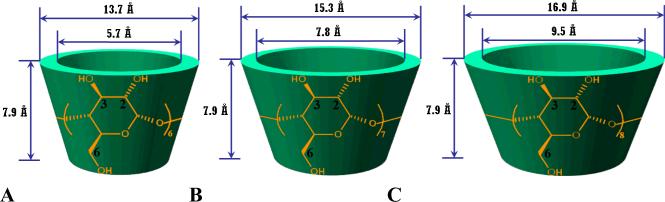
Cyclodextrins (CDs), a family of macrocyclic oligosaccharides linked by α-1,4 glycosidic bonds, have been extensively studied in diverse fields since their discovery by Villiers in 1891 [1-10]. Among them, α-, β-, and γ-CDs are the most common members, which are composed of 6, 7, and 8 glucose units, respectively. The truncated cone-shaped CDs possess a hollow, tapered cavity of 0.79 nm in depth, while both the top and bottom diameters are increased with the number of glucose units (Fig. 1) [2]. The hydrophobic cavity of CDs renders them inclusion capacity with a variety of compounds ranging from small molecules [11], ions [12], proteins [13], and oligonucleotides [14]. The biomedical applications of CDs are extremely attractive due to their low toxicity and low immunogenicity [15]. CDs have been extensively utilized to form inclusion complexes with drugs through host-guest interactions in pharmaceutical science and technologies. The related applications include increasing drug solubility and stability [16], enhancing drug absorption [17], masking odors and tastes [6], controlling drug release profiles [18], alleviating local and systemic toxicity [10], and improving drug permeability across biological barriers [19]. Pharmaceuticals formulated using CDs can be dosed by oral, nasal, ocular, rectal, and dermal delivery [17, 20-22]. To further improve the pharmaceutical features of native CDs with respect to solubility, inclusion capability, controlled drug delivery capacity, and toxicity, chemically modified CDs have been synthesized, including highly soluble [23], amphiphilic [24], and hydrophobic derivatives [25]. Currently, there are over 30 marketed pharmaceutical products based on CD complexes [26].
Fig. 1.
Molecular structures and dimensions of various CDs: A, α-CD; B, β-CD; and C, γ-CD. The positions 2, 3, and 6 are indicated with the numbers of 2, 3, and 6, respectively.
CDs and their derivatives have been successfully employed to construct supramolecular systems across length scales and to engineer novel functional materials, taking full advantage of host-guest interactions between the CD units and guest molecules [27-30]. Besides, CDs can be utilized as molecular valves to switch the ON/OFF release of payload from hybrid nanosystems [31, 32]. Recently, a broad spectrum of CD-containing polymers with versatile architectures have been synthesized to assemble functional platforms [33-37]. These assemblies have found wide applications in drug delivery [38], gene therapy [39], and medical imaging [36]. These comprehensive studies have showed the powerful capacity of CDs in the fabrication of novel supramolecular entities for biomedical applications. This review will focus on the current progress in the construction of CD-based supramolecular systems and their applications for drug and gene delivery. CD materials used for supramolecular self-assembly, their synthesis, and particulate delivery systems such as nanomicelles and nanoparticles will be summarized first. Supramolecular hydrogels will then be reviewed with emphasis on their drug delivery applications. Perspectives on the bench-to-bedside translation of newly developed CD delivery systems will be discussed at the end.
2. Cyclodextrin materials library
CDs have been structurally tailored with various charged groups, hydrophilic segments, hydrophobic moieties, or both hydrophilic and hydrophobic units to further elaborate their physicochemical properties and molecular recognition capability. Moreover, CDs have been conjugated onto polymers of various architectures to offer materials with unique features and excellent biocompatibility. This section will briefly summarize CD derivatives and CD-containing materials that have been employed to fabricate supramolecular systems for drug and gene delivery.
2.1. Amphiphilic cyclodextrins
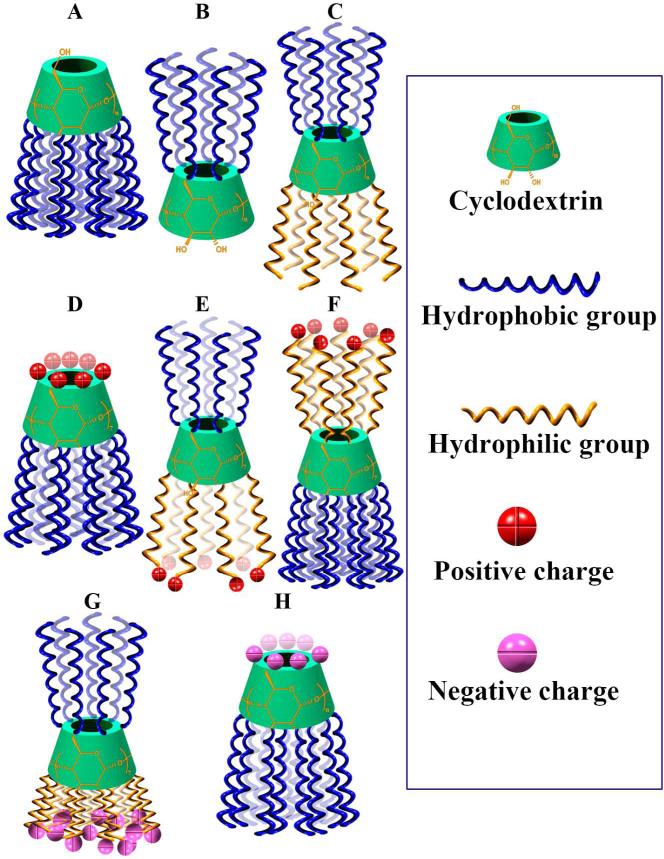
Amphiphilic CDs have been synthesized to increase interactions of CDs with biological membranes and to render self-assembly capacity in aqueous solutions [40]. According to the groups conjugated onto CDs, the resulting derivatives can be classified into neutral, cationic, and anionic CD amphiphiles (Fig. 2).
Fig. 2.
Schematic illustration of various amphiphilic CDs that have been synthesized so far. A, Neutral amphiphiles hydrophobically modified on the secondary side. B, Neutral amphiphiles hydrophobically decorated on the primary side. C, Neutral amphiphiles with hydrophobic moieties at the primary sites and hydrophilic segments on the secondary side. D, Cationic amphiphiles with charged groups on the primary side and hydrophobic moieties at the secondary sites. E, Cationic amphiphiles with charged moieties on the secondary side and hydrophobic moieties at the primary sites. F, Cationic amphiphiles with charged segments on the primary side and hydrophobic moieties at the secondary sites. G, Anionic amphiphiles with charged groups on the secondary side and hydrophobic moieties at the primary sites. H, Anionic amphiphiles with charged groups on the primary side and hydrophobic moieties at the secondary sites.
2.1.1. Neutral cyclodextrin amphiphiles
Neutral amphiphilic CDs with the primary hydroxyls as polar groups can be synthesized by selectively esterifying the secondary hydroxyls at positions 2 and 3, thus giving rise to CD amphiphiles with diacyl groups ranging from 2 to 14 carbons (Fig. 2A) [41-43]. Alternatively, persubstitution of CDs at the position 6 can be performed by bromide displacement of heptakis(6-bromo-6-deoxy)-β-CD using alkylthiolate with alkyl chains varying from 2 to 18 carbons, while the secondary hydroxyl groups remain unchanged (Fig. 2B) [44]. Neutral CD amphiphiles may also be obtained by conjugating with cholesterol derivatives [45, 46]. Recently, amphiphilic heptakis(6-alkylthio-6-deoxy)-β-CD 2-oligo(ethylene glycol) conjugates possessing oligo(ethylene glycol) substituent on the secondary side and alkyl groups on the primary face were synthesized by nucleophilic displacement of perbrominated CDs in combination with alkylation using ethylene carbonate (Fig. 2C) [47]. Besides amphiphiles with multiple substituents, mono-substituted amphiphilic CDs have been synthesized [45, 46, 48]. To provide additional functions to CD amphiphiles, various responsive linkages or targeting units have been incorporated. By using disulfide bonds to connect hydrophobic substituents to CDs, redox responsive CD amphiphiles were synthesized [49]. Functional ligands such as galactosyl and mannosyl residues can be conjugated onto the oligo(ethylene glycol) chains to render recognition capability and cell-targeting property [50, 51].
2.1.2. Cationic cyclodextrin amphiphiles
Cationic CD amphiphiles were initially synthesized for supramolecular assembly of soft materials with special surface chemistry and charge [52, 53]. Recent attention has been focused on their potential for gene delivery [54]. An easy way to synthesize cationic β-CD amphiphiles is to introduce amido groups on the primary face of CDs with alkyl chains at positions 2 and 3, thereby per-6-amino-β-CD 2,3-di-O-alkyl ethers were prepared (Fig. 2D) [52]. Cationic amphiphiles can also be synthesized by modifying oligo(ethylene oxide) containing amphiphiles with ω-amino groups on the oligomer chains (Fig. 2E) [53]. Recently, a series of amphiphilic β-CD polycations were synthesized by replacing the primary hydroxyl groups with various amino-containing moieties (Fig. 2F) [55]. By changing alkyl chains at positions 2 and 3, both the charge density and the hydrophobic-hydrophilic balance of resulting materials can be finely tuned.
2.1.3. Anionic cyclodextrin amphiphiles
Either carboxylated or sulfated amphiphilic CDs have been synthesized, in which the charged moieties can be on the primary face or on the secondary one (Fig. 2G-H). By introducing alkyl at the position 6 and carboxylmethyl groups at positions 2 and 3, carboxyls-containing CD amphiphiles were prepared (Fig. 2G) [56]. Sulfated amphiphilic CDs can be synthesized via sulfating the 6-hydroxyls of esterified CDs at positions 2 and 3 (Fig. 2H) [57-59].
2.2. Hydrophobic cyclodextrins
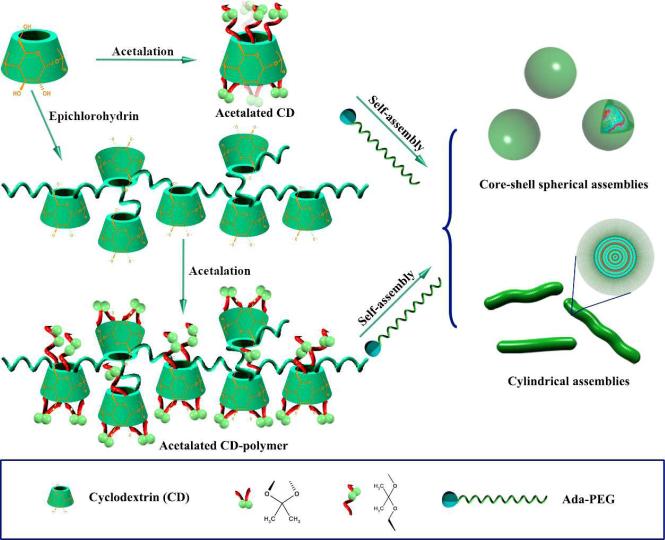
Hydrophobic modification of CDs has been made to develop sustained-release carriers. For this purpose, alkylated or acylated derivatives, such as heptakis(2,3,6-tri-O-ethyl)-β-CD and heptakis(2,3,6-tri-O-butyryl)-β-CD were synthesized [18]. More recently, hydrophobic CD derivatives were prepared by kinetically controlled acetalation of CDs using 2-methoxypropene as the acetonation reagent (Fig. 3) [60-62]. The acetalated materials can be easily dissolved in common solvents such as acetone, ethanol, dichloromethane, or THF. A pH-dependent hydrolysis was observed for the acetalated CD materials, with the degradation or hydrolysis rate dependent on pH and molecular structures. Elegant modulation of the degradation rate can be easily achieved by tailoring the acetal type and the degree of substitution, which may be implemented by controlling the reaction kinetics. More importantly, both in vitro and in vivo toxicological evaluations suggest that the acetalated CDs exhibit excellent biocompatibility and their nanoparticles can be safely delivered via local or systemic administration. Further more, acetalated CDs can form spherical or cylindrical assemblies in the presence of adamantyl-terminated polyethylene glycol (Ada-PEG), depending on their weight ratios. This indicated that the complexation capability of acetalated CDs is well retained.
Fig. 3.
A schematic showing the synthesis of pH-sensitive acetalated CDs and CD-containing polymers by kinetically controlled acetalation in the presence of 2-methoxypropene, and their self-assembly in the presence of Ada-PEG.
2.3. Cyclodextrin-containing polymers
CD-containing polymers of various structures have been synthesized to obtain materials with multiple recognition sites for molecular self-assembly, to enhance biocompatibility of polymers for biomedical applications, and to produce functional materials for controlled drug delivery and gene therapy. These polymers possess diverse architectures varying from linear, grafted, block, branched, to hyperbranched and dendritic, while the CD units can be either covalently linked in the main chains or conjugated as flanking side groups.
2.3.1. Polymers with cyclodextrins in the main chain
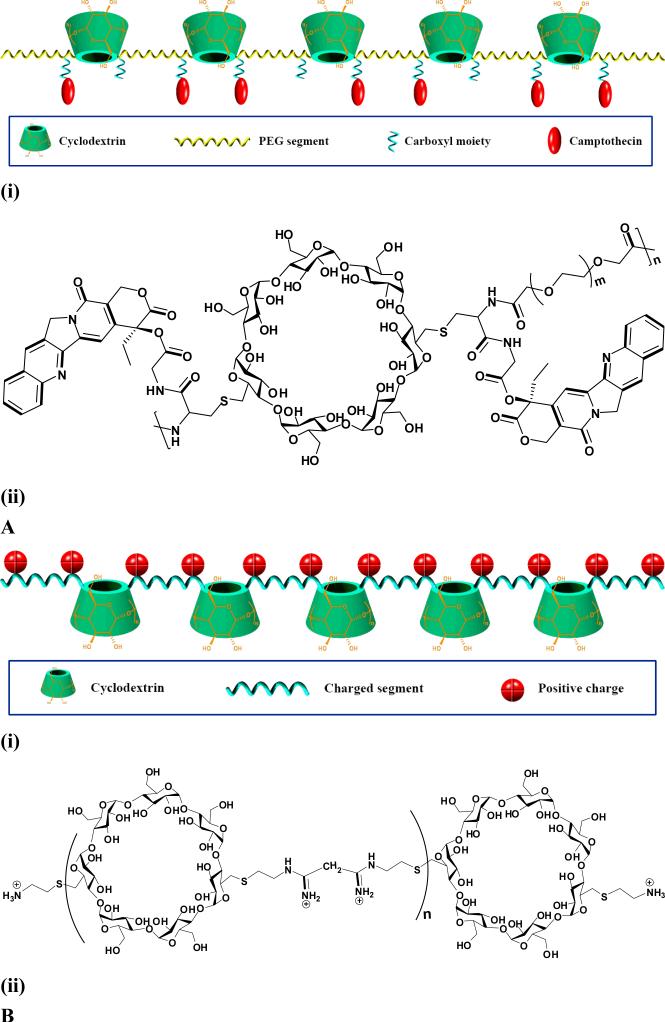
A facile approach to prepare CD-containing polymers is to polycondense CDs with epichlorohydrin in the alkali solution (Fig. 3) [63-65]. Hydrophobic modification on this type of CD-polymers can be performed by kinetically controlled acetonation to give rise to pH-sensitive polymers [60]. The acetalated CD-polymers can efficiently encapsulate drugs by both hydrophobic and host-guest interactions. By introducing other functional monomers such as charged compounds in the reaction mixture of CD-epichlorohydrin, CD-based polycations can be obtained by a similar polycondensation reaction [66]. Compared with nonionic counterparts, charged CD polymers have additional electrostatic interactions with oppositely charged guest molecules to achieve a synergetic effect [67]. This type of CD-based polymers has been widely employed to construct nanospheres, nanogels or hydrogels, and nanocapsules, via host-guest interaction mediated self-assembly in the presence of guest molecules including hydrophobic drugs, hydrophobically modified hydrophilic polymers, and hydrophobic polymers [68-74]. Nevertheless, polymers thus obtained generally exhibit branched structure and broad molecular weight distribution. Davis's group has designed and synthesized a series of linear polymers containing β-CDs in their main chains, which have been intensively studied for drug and gene delivery [10]. For drug delivery, β-CD based linear polymers (CDPs) with flanking carboxylic groups were synthesized by the polycondensation of diamino-β-CD derivative with difunctionalized PEG comonomer [75]. These polymers are extremely soluble in aqueous solutions and exhibit very low toxicity to cultured cells. Camptothecin (CPT), a highly potent antineoplastic agent, can be covalently conjugated onto CDPs via its 20-OH functionalized derivative (Fig. 4A). By copolymerization of diamino-functionalized β-CD monomers with other difunctionalized comonomers such as dimethyl suberimidate or dithiobis(succinimidyl propionate), a series of linear, cationic, β-CD-containing polymers (βCDPs) were also synthesized as non-viral vectors (Fig. 4B) [76-78]. CD-backboned cationic polymers can also be efficiently synthesized via “click polymerization”. To this end, acetylated-diazido-β-CD and α,ω-dipropargylated oligoethyleneimines were prepared and then Cu(I)-catalyzed azide-alkyne 1,3-dipolar cycloaddition was carried out to polymerize them to obtain linear polymers with high molecular weight, and their potential for plasmid DNA (pDNA) delivery was explored [79]. In order to develop polyethyleneimine (PEI)-based gene vehicles with enhanced transfection efficiency and reduced cytotoxicity, CD-containing polycations based on low molecular weight PEI were prepared by using CDs such as (2-hydroxypropyl)-β-CD and (2-hydroxypropyl)-γ-CD as cross-linking agents [80, 81]. These CD cross-linked polycations can be further functionalized by conjugating peptide ligands to achieve active targeting or by incorporating anticancer drugs to implement dual delivery for synergistic treatment of tumors [82-84].
Fig. 4.
Linear CD-polymers for drug and gene delivery: A, Schematic illustration (i) and molecular structure of CPT-conjugated CDP; and B, Schematic illustration (i) and structure (ii) of β-CD based linear cationic polymer. Both schemes and structures were made according to references [75, 78].
In addition, CDs have been utilized as core moieties to produce star-shaped molecules for drug and gene delivery as well as medical imaging. For examples, β-CD-centered amphiphilic copolymers were synthesized as nanocarriers for drug delivery, in which drugs can be loaded by physical encapsulation or covalent conjugation [85-87]. On the other hand, per(6-guanidino-6-deoxy)-CDs, per(6-amino-6-deoxy)-CDs, and per(6-guanidinoalkylamino-6-deoxy)-CDs were prepared and examined as in vitro transfection agents for pDNA expressing the green fluorescent protein [88]. Taking advantage of the Cu(I)-catalyzed “click reaction” between acetylated perazido-β-CD and alkyne terminated oligo ethyleneimines protected with tert-butoxycarbonyl groups, a series of polycationic β-CD “click clusters” have been developed, which were found to be effective vectors with low cytotoxicity [89]. By coupling seven alkyne-functionalized diethylenetriaminetetraacetic acid chelates to a per-azide-β-CD, a macromolecular contrast agent for magnetic resonance imaging has been developed [90].
2.3.2. Cyclodextrin-conjugated polymers
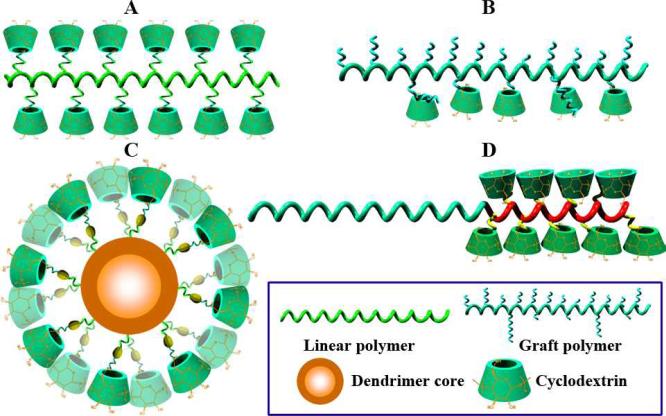
CDs have been covalently conjugated onto various polymers to modify physicochemical properties, to improve biocompatibility, to enhance drug/gene delivery capabilities, or to impart macromolecular hosts with multiple binding sites. Linear, branched, hyperbranched, block, and dendritic polymers have been modified with CD units (Fig. 5). These CD-containing polymers were utilized to fabricate supramolecular assemblies across nano, micro, and macro-scales. Table 1 lists representative CD-conjugated polymers that have been investigated for applications in biomedical fields, involving either biodegradable or nonbiodegradable polymers of synthetic or natural sources. Generally, two approaches are employed to synthesize CD-pendant polymers. One is the direct polymerization of CD-containing monomers alone or copolymerization with other monomers, which is frequently used to prepare CD-pendant acrylate polymers [91-95]. Another strategy is frequently carried out by coupling functionalized CDs to the target polymers or their derivatives, which is generally applied to polymers with functional groups [96-98]. While most of the studied CD-flanking polymers are homopolymers or random copolymers in which CD units are randomly distributed, block-structured polymers characterized by tandem alignment of a PEG block and a block bearing β-CD units on the side chain have been developed recently to construct assemblies for versatile drug delivery [35]. First, a PEG-based diblock copolymer with a polyaspartamide block containing ethylenediamine (EDA) units (PEG-b-PEDA) was synthesized. β-CD was then covalently linked to PEG-b-PEDA via nucleophilic reaction between mono-6-tosyl β-CD and EDA units to obtain CD-pendant block copolymer PEG-b-PCD. α-CD conjugated block copolymers can be synthesized via a similar procedure. As a more efficient approach, β-CD units can be conjugated via the copper(I)-catalyzed “click” reaction [99]. To do this, PEG-b-polyaspartamide with propargyl side groups were synthesized, onto which mono-6-azido-β-CD was conjugated via Huisgen [3+2] dipolar cycloaddition between alkyne and azide.
Fig. 5.
Schematic illustration of the typical structures of CD-pendent polymers studied for biomedical applications: A, CD-grafted linear polymer; B, CD-pendant graft copolymer; C, CD-conjugated dendrimer; and D, CD-flanking block copolymer.
Table 1.
CD-conjugated polymers for biomedical applications.
| Type of polymer | CD type | Synthesis method | Biomedical applications | References |
|---|---|---|---|---|
| Dextran and its derivatives | α-CD, β-CD | Conjugating to the polymers or oxidized products | Drug delivery | [199-201] |
| Hyperbranched PEI | β-CD | Coupling to the preformed polymer via “click chemistry” | Biomimetic biological membranes or solubilization | [202-204] |
| Hyperbranched polyglycerol | β-CD | Conjugating to the preformed polymer via nucleophilic reaction | Drug delivery | [205, 206] |
| Copolymer of oligo-(ethylene glycol) methyl ether acrylate, N-isopropylacrylamide, and acrylate | β-CD | Conjugating to the preformed copolymers via “click chemistry” | Drug delivery | [207] |
| Polyaspartamide | β-CD | Conjugating β-CD to the preformed polymers via nucleophilic reaction or “click chemistry” | Drug delivery | [98, 99] |
| 8-Arm star PEG | β-CD | Conjugating to the preformed polymer | Drug delivery | [195] |
| Chitosan and its derivatives | β-CD or its derivatives | Coupling or nucleophilic reaction between CD and chitosan | Drug delivery, gene delivery | [208-211] |
| Polyamidoamine (PAMAM) dendrimer | α-, β-, and γ-CD | Conjugating CDs to the preformed polymers | Drug delivery, gene delivery | [139, 212] |
| Linear and branched PEI | β-CD | Conjugating CDs to the preformed polymers | Drug delivery, drug/gene codelivery, gene delivery, and medical imaging | [142, 165, 168, 213-215] |
| Poly(ε-lysine) | β-CD | Conjugating CDs to the preformed polymers | Gene delivery | [143] |
| Hyperbranched poly(amido amine) | β-CD | Michael addition copolymerization | Gene delivery | [216] |
3. Nanomedicines assembled by cyclodextrin derivatives
3.1. Supramolecular therapeutics
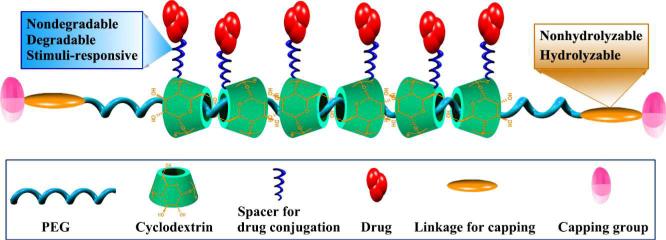
CD-based polypseudorotaxanes (CDPRs) are a family of supramolecular polymers with CDs threading onto the polymer chains. Since their discoveries by several groups [100-102], there has been great interest in engineering novel controlled drug delivery systems from these novel polymers. Because the inclusion of CD units onto polymer chains provides abundant hydroxyls in the resulting materials, a plethora of therapeutics may be conjugated on the polymers to produce supramolecular prodrugs. Furthermore, additional functions such as targeting to specific tissues, cells, or molecules, can be implemented by incorporating recognizable units such as carbohydrate ligands, peptides, proteins, apatamers or other nucleic acids, and antibodies and their derivatives. For drug delivery applications, biodegradable CDPRs have been developed that can be dissociated by hydrolyzing the terminal moieties (Fig. 6). These CDPRs generally consist of α-CD, PEG, and a biodegradable moiety [103]. The biodegradable moiety exists at both terminals of the PEG chain. As a conceptual proof study, Yui and coworkers synthesized biodegradable CDPRs based on α-CD and PEG, which are capped with L-phenylalanine (L-Phe) via a peptide spacer. The water solubility of these CDPRs can be improved by hydroxypropylation of CDs [104]. Enzymatic cleavage of the peptide linkage by papain or α-chymotrypsin may lead to disassociation of supramolecular structure and complete release of threaded CD units [105]. Drugs can be conjugated onto CDPRs by either nondegradable spacers or degradable linkages. For instance, theophylline, an anti-asthma drug, was introduced onto a hydroxypropylated α-CD units of α-CD/PEG polyrotaxane capped with L-Phe [106]. A complete release of theophylline-immobilized α-CDs could be achieved, as a result of the dissociation of supramolecular structure triggered by the papain-mediated terminal hydrolysis. The release rate is closely related to the association profiles of drug-CDPR conjugates in aqueous solution, which is dependent on interactions among drug molecules and/or the terminal moiety in the conjugate. The antitumor agent doxorubicin (Dox) was conjugated to a tyrosine capped CDPR via hydrolysable ester linkages, and the cytotoxic drug could be released upon hydrolysis [107]. In vitro release study demonstrated that this supramolecular polymer-Dox conjugate yielded a sustained release of Dox over 4 days in PBS (pH 7.4). Furthermore, a cell-penetrating peptide could be attached to the two termini of the polymer chain to facilitate intracellular uptake by tumor cells, which was substantiated by fluorescence imaging. For drugs conjugated to polyrotaxanes via degradable bonds, their release is largely determined by the hydrolytic characteristics of the linkages. Accordingly, selective delivery to intracellular organelles may be achieved by using acid-labile spacers or reducible linkages, since these endocytic vacuoles possess low pH and high reduction potential [108, 109].
Fig. 6.
Schematic illustration of supramolecular therapeutics based on CD-based polypseudorotaxane.
3.2. Cyclodextrin-based nanoparticles for drug delivery
The well-recognized biocompatibility and inclusion capability of CDs and their derivatives make them extremely attractive in developing therapeutic nanoparticles. Nanoentities such as nanospheres, nanogels, micelles, and vesicles can form from CD-based materials including amphiphilic CDs, CD-polymers, CD-pendant polymers, and CD-based polyrotaxanes (Fig. 7) [35, 40, 110]. Nanoassemblies thus formed exhibit multiple hydrophilic/hydrophobic domains and recognition sites, and therefore are potential nanocarriers for both hydrophilic and hydrophobic bioactive molecules.
Fig. 7.
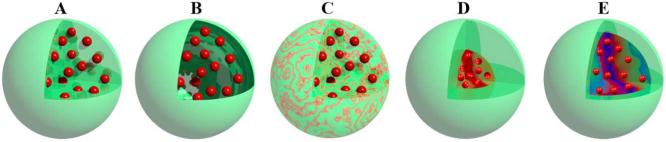
CD-based nanoassemblies studied for drug delivery. A, Nanosphere; B, Vesicle; C, Nanogel; D, Micelle; and E, Micelle-like core-shell structured assembly.
Hydrophobic interaction mediated self-assembly of neutral amphiphilic CDs bearing fatty acyl groups at positions 2 and 3 produced nanospheres with size from tens to hundreds of nanometers [111]. These nanocarries can load both water-soluble and insoluble drugs such as metronidazole, Dox hydrochloride, progesterone, and bifonazole, via both hydrophobic and host-guest interactions between drug and CD units [112, 113]. These nanoformulations were considered to be useful in oral, ocular and intravenous administration, due to their favorable stability at pH from 1 to 11 and lower hemolytic activity [113]. Using amphiphilic β- or γ-CDs with decanoic alkyl chains at the secondary side, which were synthesized through one-step transesterification of CDs by vinyl-acyl fatty esters catalyzed using thermolysin, nanoassemblies of 70-220 nm can be obtained [114]. These nanostructures can serve as efficient nanoplatforms to deliver artemisinin (ART), an antimalarial lipophilic drug. ART-loaded nanosystems can be further decorated by PEG fatty acid esters through complexation between fatty and CD units. In vitro study using both multi-resistant K1 and susceptible 3D7 strains demonstrated the effective antimalarial activity of assembled nanomedicines. Depending on the hydrophobic chains, self-assembly of cationic amphiphilic β-CDs substituted with alkyl groups at the primary side and ω-amino-oligo-PEG segments at the secondary side may form bilayer vesicles or nanoparticles [53]. For the hexyl substituted β-CD amphiphile, it may form nanoparticles that can efficiently load anionic 5,10,15,20-tetrakis(4-sulfonatophenyl)-21H,23H-porphyrin (TPPS) [115]. The photodynamic properties of the entrapped photoactive TPPS were well preserved. In vitro studies based on HeLa cells evidenced the photodynamic efficacy of TPPS-loaded nanomedicines, resulting in significant cell death upon illumination with visible light. Furthermore, biomodal photodynamic nanomedicines simultaneously photogenerating nitric oxide (NO) and singlet oxygen (1O2), can be fabricated by encapsulating both TPPS and a NO photodonor into the nanoassemblies wherein hydrophobic, electrostatic, and inclusion interactions may be involved [116]. Photodelivery of NO and 1O2 from the nanoassembly upon visible light excitation was evidenced by detecting these transient species. Cell culture experiments indicated amplified level of cancer cell mortality, and therefore confirmed the dual photodynamic action of these multifunctional nanomedicines integrated with dual therapeutics and imaging capability. For cationic CD derivatives, electrostatic attraction mediated self-assembly may form nanoparticles in the presence of anionic starch, in which hydrophobic molecules can be included by CD cavities [117].
To address challenges related to clinical development of CPT such as poor solubility, insufficient in vivo stability of the active form, and toxicity, CPT was covalently conjugated to CDPs, which could assemble into nanoparticles with a diameter smaller than 100 nm. Through this strategy, the solubility of CPT can be increased by more than 3 orders of magnitude. The inclusion complexation between β-CD and CPT has been well elaborated [118]. Both hydrophobic and host-guest interactions are responsible for the formation of nanoassemblies. Pharmacokinetics and biodistribution studies in rats and tumor-bearing mice indicated that intravenous administration of the optimized CPT-conjugated nanomedicine (IT-101) gave prolonged plasma half-life and enhanced CPT distribution in the tumor tissue when compared to CPT alone [119]. Preclinical efficacy evaluations in several mouse xenografts indicated that IT-101 displayed good tolerability and antitumor activity [120]. More importantly, both preclinical and clinical data confirmed that this novel nanopharmaceutical is able to address not only solubility, stability, toxicity, and pharmacokinetic issues associated with the CPT delivery, and also can impart unique biological properties that enhance therapeutic efficacy of CPT [121]. IT-101 is now in phase I/II clinical development for the treatment of cancer and solid tumor [122]. Utilizing similar CDPs, antiproliferative peptide tubulysin can be covalently conjugated through a disulfide linker to obtain nanomedicines with potent antitumor activity and dramatically enhanced maximum tolerable dose [123].
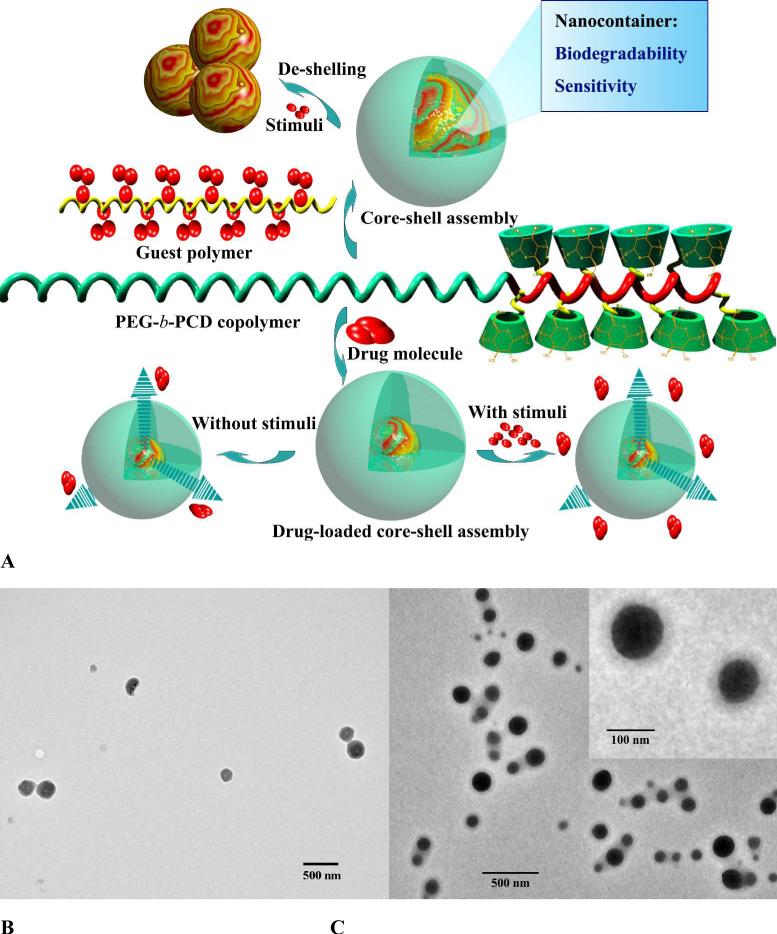
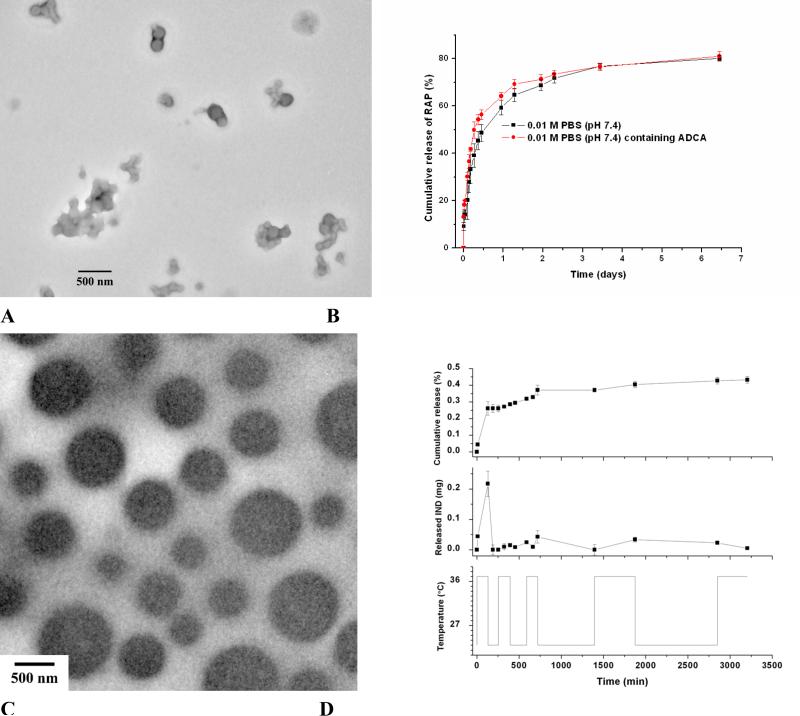
In order to construct versatile nano-assembled drug carriers, diblock copolymer PEG-b-PCD with a PEG block and a block bearing β-CD units on the side chain has been developed recently (Fig. 5D and Fig. 8A) [98]. Host-guest complexation between PEG-b-PCD and hydrophobic guest molecules allowed for the formation of core-shell structured host-guest nanoassemblies. In the presence of small guest molecules, spontaneous formation of nanoparticles concomitant with cargo loading was directly observed by transmission electron microscopy (TEM) and atomic force microscopy (AFM) [124]. Through this approach, hydrophobic drugs such as ibuprofen (IBU), indomethacin (IND), and dexamethasone (DMS) can be efficiently formulated into nanoparticles with significantly improved solubility. Even for guest molecules like rapamycin (RAP) that has been considered to be thermodynamically unfavorable to complex with β-CD can be successfully loaded to form nanoassemblies (Fig. 8B). The presence of multiple recognition sites and their synergistic effect should have contributed to the enhanced complexation ability of PEG-b-PCD to guest molecules with a larger molecular volume. Similarly, hydrophobic polymers such as poly(β-benzyl L-aspartate) (PBLA) and poly(D,L-lactide) can serve as guest molecules to drive the formation of nanoassemblies. For the nanoparticles with PBLA as the hydrophobic building component, they exhibited well-defined spherical shape as observed by TEM, AFM, and SEM. Fluorescence and 1H NMR measurements together with TEM observation suggested the core-shell structure (Fig. 8C). The rigid core is mainly composed of PBLA chains, while palisade PEG chains constitute the hydrophilic shell, which endows the particles with colloidal stability. These assemblies were stable during long term storage and could also be successfully reconstituted after freeze-drying [125]. The size of these nanoparticles can be modulated by the composition of the host PEG-b-PCD copolymer, the molecular weight of PBLA, and the weight ratio of PBLA/PEG-b-PCD. For example, significant increase in size was observed when the PBLA content was increased. Since for the micelle-like assemblies, the release profile of payload is largely controlled by the physicochemical properties of core that is dramatically affected by the constituent materials [126-128], PEG-b-PCD-based nanoassemblies offer customer-made control over in vitro pharmacokinetics simply by changing the guest materials for nanoformulation.
Fig. 8.
Core-shell structured responsive nanoparticles assembled by PEG-b-PCD and guest molecules. A, Schematical illustration of the formation of nanoassemblies and their responsive behaviors. B, TEM image of nanoparticles based on PEG-b-PCD/RAP. C, TEM image showing the core-shell structured nanoparticles formed by PEG-b-PCD and PBLA.
It was found that coassembly of β-CD polymers (polyβCD) and hydrophobically modified dextrans led to gel-like nanoassemblies of ~200 nm [72]. The host-guest complexation between β-CD units and alkyl groups is responsible for the spontaneous formation of supramolecular nanoassemblies. Further tailoring of physicochemical properties of nanoassemblies such as disassembling and swelling characters can be achieved by changing the hydrophobic groups conjugated onto dextran [129]. These nanogels can efficiently load hydrophobic compounds such as benzophenone and tamoxifen, by including them into CD cavities of polyβCD and/or solubilizing them into the hydrophobic microdomains formed by the modified dextran. Accordingly, these assemblies are potentially useful in pharmaceutics and cosmetic field [68].
Besides above strategies, the formation of CD-based supramolecular nanostructures in aqueous solutions can be realized through self-assembly driven by CD threading onto specific polymer chains of homopolymers or copolymers. Zhang et al. reported a facile one-pot method to assemble polymer micelles from α-CD and poly(ε-caprolactone) homopolymer. The core-shell structured micelles with a hydrophobic core can efficiently load prednisone acetate and sustain its release for 700 h [130]. Via inclusion complexation of α-CD and PEG, cytocompatible vesicles can be assembled by PEG-containing block or graft copolymers [131-134]. These hollow nanospheres may encapsulate enzymes and other hydrophilic drugs, and the drug-loaded assemblies exhibited better in vitro efficacy compared with the free therapeutics. In these cases, inter-molecular aggregation, resulting from the threading of α-CD molecules onto PEG chains, mediates the assembling process and the formation of assemblies.
3.3. Supramolecular systems for gene delivery
Recently, CDs and their derivatives as well as CD-based polymers have attracted great attention for gene delivery [39]. It has been found that CDs and their derivatives can bind with nucleic acids and increase their stability against nuclease as well as improve cellular uptake, and accordingly have been utilized as absorption enhancers of both viral and nonviral vectors for oligonucleotide delivery [135-137]. Positive charge-modified CDs have been explored as novel vectors for gene delivery [88, 89, 138]. In addition, CD units have also been covalently linked to various polycations to increase their transfection efficiency and decrease their toxicity [79, 139-143]. This section highlights supramolecular polycations and CD-materials derived assemblies that have been developed for gene delivery.
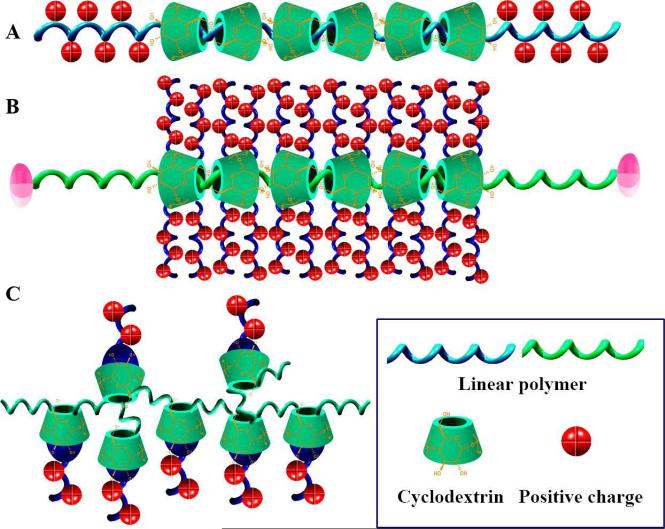
Threading CD units to polymer chains has demonstrated to be a facile and effective approach to develop novel supramolecular functional materials [29, 38, 144]. Linear PEI (LPEI) has been found capable of forming polypseudorotaxane with α- or γ-CD, and the complexation is dependent on pH (Fig. 9A) [145]. Utilizing the polypseudorotaxane consisted of LPEI (Mn =2.2kDa) and γ-CD, polyplexes of pDNA encoding luciferase can be formed [146]. Polyplexes based on this supramolecular polycation showed increased cellular uptake, high gene expression, and low cytotoxicity even at high N/P ratios. Based on this type of supramolecules, polycations with charged moieties on the side chains can also be developed. Ooya et al. fabricated a biocleavable cationic polyrotaxane [147], in which dimethylaminoethyl-modified α-CDs are threaded onto a 4 kDa-PEG chain capped with benzyloxycarbonyl tyrosine via disulfide linkages. This supramolecular polycation can effectively condense pDNA to form polyplexes as evidenced by AFM observation together with gel electrophoresis and ξ-potential measurements. The cleavage of disulfide linkages under reducible conditions in cytosolic milieu facilitated the decondensation and intracellular trafficking of pDNA payload. In vitro transfection revealed that the assembled polyplexes could rapidly escape from endosome/lysosome and deliver pDNA to the nucleus. Li and coworkers designed similar supramolecular vectors composed of multiple oligoethylenimine-grafted β-CDs threaded on a poly(ethylene oxide)-b-poly(propylene oxide)-b-poly(ethylene oxide) (PEO-PPO-PEO) chain (Fig. 9B) [148]. These cationic polyrotaxanes with different oligoethylenimines could condense pDNA into nanoparticles with low cytotoxicity against cultured L929 and HEK 293 cells, and displayed effective gene transfection with efficiency comparable to those of branched PEI (25 kDa). Through a similar strategy, anthryl-modified-β-CD was threaded onto polypropylene glycol (PPG) to prepare supramolecules that showed good binding abilities towards calf thymus DNA [149]. Another strategy to construct CD-based supra-polycations is complexing charged molecules with CD-polymers, utilizing the host-guest interactions between guest molecules and β-CD units (Fig. 9C). This has been demonstrated by using Ada cationic derivatives and β-CD polymers [150]. The mentioned host-guest supramolecules can form polyplexes with pDNA as indicated by gel retardation, ζ-potential, and surface enhanced Raman spectroscopy experiments. The stability of assembled polyplexes was closely related to the chemical structure of guest molecules. In the presence of a fusogenic peptide, the optimized polyplexes exhibited transfection efficincy comparable to that of DOTAP, a commercialized agent.
Fig. 9.
CD-based supramolecular polycations for gene delivery. A, Threading of CD units onto positively charged polymers. B, CD-based polyrotaxane modified with positively charged moieties. C, Supramolecular polycations formed by the complexation of CD-polymer and positively charged molecules.
Besides suprapolycations, CD-originated supramolecular assemblies have been found effective nanovehicles for gene therapy. This has been corroborated by nanosystems fabricated using cationic CD-amphiphiles and CD-polymers. As extensively demonstrated, CD-amphiphiles may form thermotropic liquid crystals and lyotropic assemblies including micelles, nanoparticles, and monolayer/bilayer vesicles [40]. However, it was found only recently that positively charged assemblies formed by CD amphiphiles might function as efficient vectors for gene delivery. For this purpose, amphiphilic β-CDs installed with fatty acyl chains at the secondary positions and positive moieties on the primary side were synthesized and utilized to form assemblies [151]. The assembled nanoentities showed superior DNA complexing and delivery capabilities. High resolution TEM observation suggested an onion-like structure for the pDNA-containing nanoparticles, in which CD amphiphiles and pDNA molecules were alternately arranged to form the lamellar architecture [55]. By carefully tailoring the molecular structure of CD amphiphiles, both the amphiphilicity and charge density can be finely tuned, which in turn can modulate transfection efficiency and cytotoxicity. The optimized nanosystems displayed transfection efficiencies significantly higher and toxicities lower than those of PEI (25 kDa)-based polyplexes. Besides the amphiphilic nature and cationic clusters, the presence of hydrogen-bonding centers, which were considered to be also responsible for cooperative and reversible complexation with anionic DNA chains, is crucial to attain high transgene expression with a relatively low cytotoxicity. On the other hand, Davis and coworkers have utilized βCDPs to produce polyplexes with size of <200 nm [76]. These low toxic polycations possess in vitro transfection efficiencies comparable to those of PEI (25 kDa) and Lipofectamine. The transfection activity and cell toxicity are mainly determined by the comonomer structure, charge center type as well as the CD type and functionalization [77, 152-154]. Additionally, the colloidal stability of these nanoplexes can be further enhanced by surface decoration using Ada-conjugated PEG (PEG-Ada), which is achieved through the host-guest interactions between β-CD and Ada group. Furthermore, active targeting may be implemented by introducing transferrin (Tf) onto the PEG shell of nanoassemblies, using a Tf-PEG-Ada conjugate [155]. In vivo transfection experiments in both murine models and non-human primates showed that the Tf-functionalized nanoparticles can efficiently deliver the gene payload to tumor tissues, and perform their pharmacological action after intravenous injections [156-158]. Recent clinical results further confirmed the effectiveness and safety of these non-viral nanoparticles for systemic administrations [159].
3.4. Nanosystems for simultaneous drug and gene delivery
Combination therapy has long been recognized as a highly efficient strategy to establish new regimens for the chemotherapy of infectious diseases and cancer as well as antiviral chemotherapy [160]. Nanoparticles have been demonstrated to be effective platforms to load two or more synergistically active drugs and concurrently deliver them in a spatiotemporally controlled manner to promote synergism and suppress drug resistance [161]. There is increasing interest to combine drug delivery and gene therapy in one nanoparticle to enhance the transfection efficiency, achieve a synergistic effect, or to ameliorate the toxicity related to gene delivery [162-164].
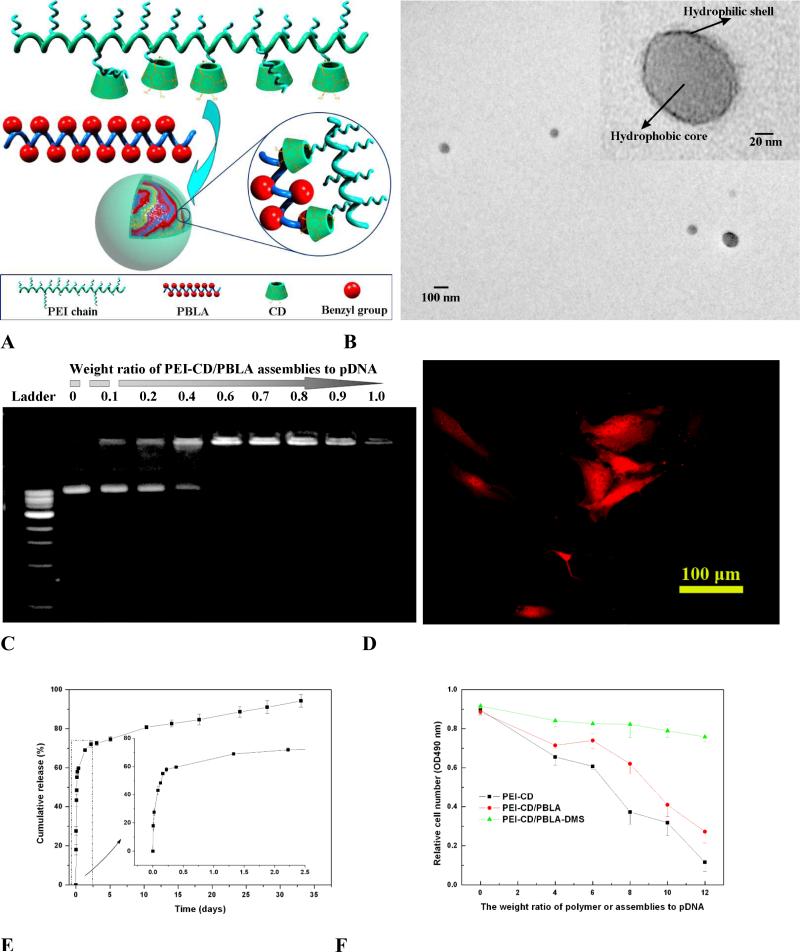
To develop a multifunctional polymer nanocarrier via host-guest interactions for simultaneous drug and gene delivery, β-CD units were conjugated onto branched PEI (PEI-CD) to synthesize a host polymer (Fig. 5B) [165]. Using PBLA as a macromolecular guest, nanoassemblies with diameter <200 nm can be constructed (Fig. 10A). Observation using TEM, AFM, and SEM revealed the assembled nanoparticles to be spherical. TEM study together with fluorescence, zeta-potential, and NMR measurements as well as titration study suggested that the spheres had a core-shell structure (Fig. 10B). The core was mainly composed of hydrophobic PBLA chains, while the shell comprised PEI segments. The cationic shell can effectively condense pDNA to form nanoscaled polyplexes (Fig. 10C). Cell culture experiments showed that pDNA encoding red fluorescence protein can be transfected and expressed in osteoblast cells, using the polyplexes based on the host-guest nanoparticles (Fig. 10D). On the other hand, the hydrophobic core may serve as a nanocontainer to accommodate hydrophobic drugs. DMS, a potent steroidal anti-inflammatory drug can be efficiently loaded and released in a sustained manner (Fig. 10E). Moreover, introducing DMS into the polyplexes can increase the transfection efficiency as well as dramatically reduce the cytotoxicity of assemblies (Fig. 10F).
Fig. 10.
Core-shell structured nanoassemblies formed by β-CD-conjugated PEI (PEI-CD) and PBLA for simultaneous drug and gene delivery. A, Schematic showing the formation of nanoassemblies. B, TEM image of PEI-CD/PBLA assemblies after staining with phosphotungstic acid. C, Gel retardation assay of PEI-CD/PBLA assemblies and pDNA. D, Transfected cells were viewed by fluorescence microscopy 48 h after transfection. Excitation was performed with green light. Polyplexes with the weight ratio of PEI-CD/PBLA assemblies to pDNA of 8 were employed. E, In vitro DMS release profile from DMS containing PEI-CD/PBLA assemblies. F, Cytotoxicity of PEI-CD/PBLA assemblies and DMS containing assemblies against osteoblast cells. After incubation for 24 h with the solutions of different polyplexes, the cell viability was detected.
It is known that nanoparticle-based drug combinations may address multidrug resistance associated with the overexpression of P-glycoproteins (Pgps) by codelivery of chemo drugs with Pgp inhibitors or siRNA, or by distinct mechanisms of action due to changed delivery pathways [166, 167]. However, precisely controlling the delivery pathway and on-demand releasing the payload at the diseased sites remain challenging in terms of developing desirable nanovehicles. To circumvent this issue, Dox, a commonly used anticancer drug, was conjugated to a water-soluble polycation of β-CD crosslinked low molecular weight PEI (600 Da) to render a prodrug polycation (PC-Dox) [84]. PC-Dox can effectively condense a tumor suppressor gene p53, giving rise to nanocomplexes. In vitro studies of mRNA expression and Western blot demonstrated that PC-Dox/p53 nanoparticles could significantly suppress the proliferation of multi-drug resistant cancer cells to achieve the synergistic effects. In vivo investigation further substantiated the desirable therapeutic outcome with enhanced efficacy and alleviated side effects. Alternatively, a host-guest assembly approach can be adopted to construct nanoparticles for combination therapy [168]. Ada group modified Dox was synthesized, which can form a cationic supramolecular prodrug (PEI-CD/Ada-Dox) in the presence of β-CD crosslinked PEI (PEI-CD). By forming host-guest nanoparticles, this drug-containing supramolecular polycation functioned as an efficacious vector for human tumor necrosis factor-related apoptosis-inducing ligand-encoding plasmid gene. Pharmacodynamic evaluation indicated that these supramolecular nanoassemblies effectively inhibited tumor growth and significantly prolonged the survival time of tumor-bearing mice.
3.5. Responsive nanoparticles based on cyclodextrin derivatives
Stimuli-responsive nanoparticles have been recognized as promising carriers that may enhance the therapeutic efficacy and minimize side effects by selectively releasing the payload at the right location and in a well-controlled profile upon triggering by diseases-associated pathophysiological signals or specific transporting pathways. CDs and their derivatives are among the most popular organic materials that have been utilized to engineer responsive nanovehicles. In one way, the reversibility of host-guest interactions between CDs and guest molecules provides an easy way to achieve the chemical-responsiveness. In another way, CDs may serve as easily functionalizable scaffolds to introduce additional responsive moieties by supramolecular recognition or covalent conjugation to attain chemical and/or physical stimuli-sensitivity.
As aforementioned, core-shell structured nanoassemblies can be formed by the host copolymer PEG-b-PCD and various guest molecules. These nanoparticles displayed chemical-sensitivity due to the reversible complexation between CD units and guest molecules. As for PEG-b-PCD/PBLA nanoassemblies, in the presence of small molecule stimuli such as potassium iodide, benzyl alcohol or cetyltriethylammonium bromide, significant inter-particle aggregates were formed as shown in Fig. 11A [98]. This chemical-triggered aggregation is largely related to the deshelling effect resulting from the competition between small stimuli and PBLA to complex with β-CD units. The disassociation of PEG shell is beneficial to the intracellular trafficking of nanoparticles, and thereby improving the efficiency of intracellular drug and gene delivery [169, 170]. Stimuli-responsive nanoparticles can also be formed by small molecules and PEG-b-PCD copolymers [124]. For pyrene/PEG-b-PCD nanoassemblies, the excimer intensity was decreased when adamantane-1-carboxylic acid (ADCA) or β-CD was introduced to the aqueous solutions, suggesting the chemical-triggered release of pyrene from assemblies. More intuitive results were observed in release studies using two hydrophobic drugs, i.e., IND and RAP. The presence of various chemical stimuli, including benzyl alcohol and ADCA, accelerated the release rate of model drugs in the initial stage, which is independent of drug structure (Fig. 11B). Interestingly, increase in the RAP release was also observed in the presence of another hydrophobic drug IBU. These results demonstrated that the cargo release from PEG-b-PCD-based assemblies could be triggered to some extent by competiting compounds against guest molecules. Additionally, free β-CD may serve as a competitor to the host copolymer to spring and speed the release of drug payload. Temperature responsive nanoparticles can also be fabricated using PEG-b-PCD copolymers. This was implemented by using poly(N-isopropylacrylamide) (PNIPAm), a well-known thermosensitive polymer, as a guest polymer [171]. Spontaneous formation of spherical nanoparticles can be achieved by co-assembly of PEG-b-PCD/PNIPAm in aqueous solutions (Fig. 11C). Measurements based on dynamic light scattering (DLS), SEM, TEM, and NMR evidenced that these PNIPAm-containing nanoparticles exhibited thermosensitive phase transition behavior. In vitro drug release study using IND as a model drug showed that drug release rate could be switched between high and low in an On/Off fashion by temperature (Fig. 11D).
Fig. 11.
Responsive nanoplatforms assembled from PEG-b-PCD and guest molecules. A, De-shelling of PEG-b-PCD/PBLA assemblies in the presence of potassium iodide (0.05 M). B, In vitro release profiles of PEG-b-PCD/RAP nanoassemblies in the absence or presence of a chemical stimulant ADCA. C, TEM image of assemblies formed by PEG-b-PCD/PNIPAm at a weight ratio of 10:5. D, In vitro release profiles of IND from PEG-b-PCD/PNIPAm (10:5) assemblies in response to temperature switching between 23 and 37°C.
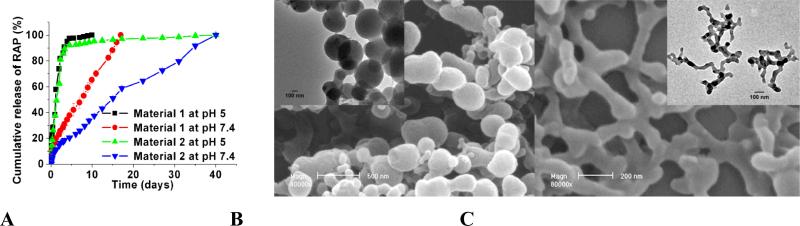
Responsive nanosystems can also be produced by functionalized CDs. Acetalated CDs or CD-polymers are a new generation of biomaterials that hydrolyze in a pH sensitive fashion [60-62]. RAP-containing nanoparticles based on either acetalated β-CD or β-CD polymers exhibited pH-responsive release profiles (Fig. 12A). Assembly of acetalated β-CD materials and Ada-PEG offered spherical or cylindrical assemblies, as imaged using SEM and TEM (Fig. 12B-C). These aggregates possess multiple sensitivity, considering the presence of host-guest interactions and pH-labile acetal moieties. On the other hand, redox-responsive vesicles or nanoparticles can be obtained using amphiphilic CDs with their hydrophobic substituents linked by disulfide bonds. These nanoassemblies may disintegrate in the presence of the disulfide reducing agent dithiothereitol, leading to the release of hydrophobic guest molecules [49].
Fig. 12.
pH-responsive nanoparticles based on acetalated CDs. A, In vitro release profiles of RAP-containing nanospheres based on acetalated α-CD (material 1) or β-CD (material 2) at pH 5 or 7.4. B-C, Core-shell particles (B) and cylindrical assemblies (C) originated from acetalated β-CD by self-assembly. The weight ratio of Ada-PEG/acetalated β-CD was 2:10 (B) and 10:10 (C).
Another approach to construct responsive CD-assemblies is to introduce stimuli-sensitive moieties using host-guest recognition mediated supramolecular assembly. Amphiphilic CDs with an alkyl chain at the position 6 and oligo-PEG segments at the positions 2 and 3 can form bilayer vesicles (CDVs) in aqueous solutions [172, 173]. Taking advantage of host-guest interactions, CDVs can be peripherally decorated with an Ada modified peptide [174]. In comparison to CDVs without peptide decoration, the modified ones displayed pH-triggered release of cargo molecules, where the release rate was accelerated at pH 5. The morphological transition from spherical vesicles to fibers, resulting from the secondary structural transitions of the octapeptide from random coil to β-sheet domains as a function of pH, may be responsible for the significantly varied release behaviors since this effect has been observed for other supramolecular assemblies [175]. As a result, this supramolecular nanosystem can function as a pH-responsive drug delivery carrier.
4. Supramolecular hydrogels based on cyclodextrins and their derivatives
Supramolecular hydrogels are particularly interesting for drug delivery and tissue engineering. The complexation capability and versatile functionalibility of CDs and their derivatives make them attractive to construct supramolecular hydrogels or even hydrogel assemblies with excellent biocompatibility, stimuli-sensitivity, and self-healing capacity [176-180].
4.1. Hydrogels based on inclusion complexes
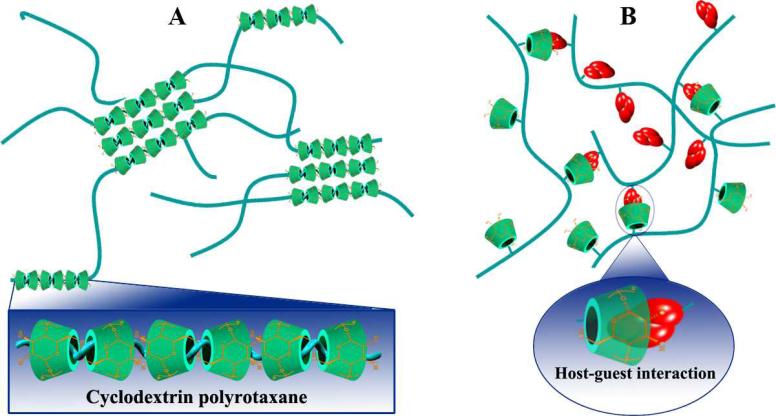
It was found by Li et al. that high molecular weight PEGs (or PEOs) are able to form inclusion complexes with α-CD in an aqueous solution to produce hydrogels [181]. Since their report, threading CD units onto polymer chains to form polypseudorotaxanes as physical crosslinkers has become the most extensively studied approach to engineer supramolecular hydrogels based on CDs or their derivatives (Fig. 13A) [29, 34]. So far, physical hydrogels have been fabricated by inclusion complexation between α-CD and PEO homopolymer or its block/graft copolymers, α-CD and PEI or poly(ε-lysine) grafted copolymers, β-CD or its hydrophilic derivatives (such as hydroxypropyl-β-CD and partially methylated-β-CD) and PPO block/graft copolymers, γ-CD and PEI graft copolymers [34, 182]. The physicochemical properties, biocompatibility, biodegradability, and sensitivity of these supramolecular hydrogels can be elegantly tailored by carefully selecting appropriate guest polymers for assembling. Through this approach, hydrogels with various functions, such as temperature-, photon-, chemical-, redox-, or pH-sensitivity, have been prepared for drug delivery applications [183-185].
Fig. 13.
Schematic illustration of two typical approaches to engineer supramolecular hydrogels based on CDs and their derivatives. A, Fabrication of hydrogels based on inclusion complexes between CDs and various polymers that can thread into CD cavities. B, Supramolecular hydrogels formed by physical cross-linking through host-guest interactions between CD-containing polymers and hydrophobically modified polymers.
Hydrogels based on inclusion complexes have been extensively investigated as injectable drug delivery systems for a broad spectrum of bioactive agents including small molecular drugs, proteins, vaccines or pDNA [177]. Since these hydrogels are formed by supramolecular self-assembly in an aqueous solution without the use of chemical crosslinkers, in situ loading of drug payload can be achieved at room temperature and under mild conditions, which is especially beneficial to biomacromolecular drugs [186-189]. Depending on the characteristics of guest polymer used, the laden drugs may be released in a rapid, sustained, triggered, or a combined manner. Under certain circumstances, drugs can be incorporated into hydrogels by covalent conjugation to modulate their release kinetics. For example, heparin (a powerful anticoagulant) was modified by PEGylation [190]. By forming α-CD/PEG inclusion complexes under mild conditions, the assembling of supramolecular hydrogels and loading of PEGylated heparin could be achieved simultaneously. Similarly, hydrophobic drugs can be entrapped into hydrogels by synthesizing polymeric prodrugs via PEGylation [191]. Compared with their free counterparts, PEGylated drugs have different diffusion rate within hydrogel, and therefore may result in varied release kinetics. In addition, the released PEGylated drug may have changed in vivo circulation and biodistribution, which in turn lead to different therapeutic significance [192].
4.2. Host-guest hydrogels
Co-assembly of CDs or its derivatives with hydrophobically modified polymers is another widely explored strategy to self-assemble hydrogels (Fig. 13B). The formation of these supramolecular hydrogels is largely driven by inclusion complexation between the CD units and hydrophobic groups. Hydrogels can be instantaneously formed at room temperature by mixing β-CD polymers or β-CD conjugated polymers with hydrophobically modified hydrophilic polymers such as PEG, dextran, chitosan, and hyaluronic acid, which can sustain the release of proteins or hydrophobic drugs [74, 97, 193-195]. Based on host-guest recognition between β-CD and cholesterol inclusion, Hennink's group fabricated self-assembling hydrogels utilizing 8-arm star shaped PEG modified with either β-CD or cholesterol via a succinyl linker that is hydrolytically cleavable. The resulting hydrogels are thermally reversible upon heating and cooling cycles, and their properties can be tailored by a plethora of parameters, such as temperature, polymer concentration, β-CD/cholesterol stoichiometry, and the molecular weight of the star shaped PEG. In vitro degradation results suggested a surface erosion mechanism, and the degradation behavior is mainly controlled by network swelling stresses and the initial cross-linking density. Consistent with the degradation profile, the entrapped proteins like lysozyme and bovine serum albumin can be released from the hydrogels in continuous, nearly zero-order patterns. It was also found that the combination of free β-CD and cholesterol-derivatized 8-arm or linear PEG in an aqueous solution may form elastic hydrogels [196]. In general, pharmacologically active cargoes varying from small molecules to proteins are loaded into these hydrogels by physical entrapment during the self-assembling process. On the other hand, drugs can be charged by conjugating to materials that may serve as or interact with the components of hydrogels. By using photoresponsive groups such as azobenzene as the host moieties, responsive supramolecular hydrogels were developed, which can achieve light controlled cargo release [197, 198].
5. Concluding remarks
Whereas CDs and their small molecular derivatives have already been successfully used as excipients in the pharmaceutical field, the use of their derivatives for constructing supramolecular assemblies for biomedical applications has emerged only recently. Previous comprehensive studies on CDs for the creation of new formulations for water-insoluble drugs have addressed critical issues related to their pharmaceutical significance, safety, and limitations, especially for α- and β-CDs. However, the use of parent CDs, particularly β-CD, is limited to oral and topical formulations because of their renal toxicity resulting from the accumulation of CD crystals or CD-cholesterol complexes due to their low water solubility. This has been partly circumvented by developing two proprietary modified β-CDs, i.e. hydroxypropyl- and sulfobutylether-β-CD, which have dramatically increased water solubility. Currently, parent CDs and their modifiers have been utilized to formulate arrays of lipophilic drugs to deliver by dermal, buccal, oral, sublingual, rectal, ophthalmic, intramuscular, and intravenous routes. These CD-based formulations, however, cannot achieve spatiotemporally controlled payload delivery according to the anatomical and pathological features of various diseases and the disease development state, due to their intrinsic limitations.
Nanoscaled supramolecular systems have been considered to be the most promising carriers for drug and gene delivery as well as other biomedical applications, in view of their successful clinical performances for tumor therapy and diagnosis of cardiovascular diseases. With the delicate design and well-controlled manipulation, the resulting nanoparticles may have many advantages. They could have prolonged circulation, broad payload spectrum, unique size and shape characteristics for tissue penetration and passive targeting as well as specific cellular/subcellular trafficking pathways, easy to tailor for active targeting at different levels, and facile control of cargo release by sophisticated materials engineering. Introducing CDs into supramolecular platforms may render enhanced biocompatibility, functionalizing flexibility, and recognition capability for both the drug payload and the therapeutic target, as partly demonstrated by intensive studies on nanomedicines assembled from CD-containing polymers. Recent advances in cyclodextrin chemistry and self-assembly make it possible to engineer diverse assemblies such as general nanoparticles, micelles, vesicles, nanogels, nanopolyplexes, supramolecular hydrogels as well as highly complex superstructures and multifunctional systems simply by a combinatorial strategy, taking full advantages of host-guest interactions and other noncovalent forces by utilizing CD-materials. Nevertheless, most of these studies are in the proof-of-concept stage, and only a few therapeutic nanosystems have been comprehensively investigated. The successful translation of these laboratory innovations to clinical reality remains challenging. First, more efficient synthetic approaches that can be easily scaled up need to be developed to produce small molecule CD derivatives with high purity and a desirable yield, and to give rise to CD-containing polymers with finely controlled topology, structure, and molecular weight as well as its distribution. Second, whereas myriad of CD modifiers and CD-containing polymers have been created for drug delivery applications, the majority of them lacks systemic biocompatibility evaluation and comprehensive toxicological study with the exception of a few commercially available products. The establishment of the nonclinical safety data package is necessary for clinical trials of new pharmaceutical products. Third, much more comprehensive and intensive studies need to be carried out for promising delivery systems with candidate therapeutics of clinical significance. Last, the cost is another factor that limits the successful translation of new delivery systems. The high costs are justified only when the new pharmaceuticals can overcome the inability of traditional formulations to deliver a drug or to perform an important function. One should always bear in mind that development of new therapeutics based on the newly created supramolecular systems is often a long way to a therapy, considering its success probability, clinical outcomes, and market popularity. In spite of these challenges, the future of CD-based supramolecular systems in drug and gene delivery is promising, in view of the notable clinical success of new pharmaceuticals based on parent CDs, their small molecule derivatives, and CD-containing polymers as well as other controlled delivery systems.
Acknowledgements
We gratefully acknowledge the financial support from the NIH (NIDCR DE022327 & DE015384, NHLBI HL114038), DOD (W81XWH-12-2-0008), and NSF (DMR-1206575). JXZ acknowledges the financial support from the National Natural Science Foundation of China (Nos. 81271695 & 21004077).
References
- 1.Villiers A. Sur la fermentation de la fe cule par l'action du ferment butyrique. Compt. Rend. Acad. Sci. 1891;112:536–538. [Google Scholar]
- 2.Li S, Purdy WC. Cyclodextrins and their applications in analytical chemistry. Chem. Rev. 1992;92:1457–1470. [Google Scholar]
- 3.Saenger W. Cyclodextrin inclusion compounds in research and industry. Angew. Chem. Int. Ed. 1980;19:344–362. [Google Scholar]
- 4.Villalonga R, Cao R, Fragoso A. Supramolecular chemistry of cyclodextrins in enzyme technology. Chem. Rev. 2007;107:3088–3116. doi: 10.1021/cr050253g. [DOI] [PubMed] [Google Scholar]
- 5.Takahashi K. Organic reactions mediated by cyclodextrins. Chem. Rev. 1998;98:2013–2033. doi: 10.1021/cr9700235. [DOI] [PubMed] [Google Scholar]
- 6.Uekama K, Hirayama F, Irie T. Cyclodextrin drug carrier systems. Chem. Rev. 1998;98:2045–2076. doi: 10.1021/cr970025p. [DOI] [PubMed] [Google Scholar]
- 7.Breslow R, Dong SD. Biomimetic reactions catalyzed by cyclodextrins and their derivatives. Chem. Rev. 1998;98:1997–2011. doi: 10.1021/cr970011j. [DOI] [PubMed] [Google Scholar]
- 8.Lipkowitz KB. Applications of computational chemistry to the study of cyclodextrins. Chem. Rev. 1998;98:1829–1873. doi: 10.1021/cr9700179. [DOI] [PubMed] [Google Scholar]
- 9.Singh M, Sharma R, Banerjee UC. Biotechnological applications of cyclodextrins. Biotechnol. Adv. 2002;20:341–359. doi: 10.1016/s0734-9750(02)00020-4. [DOI] [PubMed] [Google Scholar]
- 10.Davis ME, Brewster ME. Cyclodextrin-based pharmaceutics: past, present, and future. Nat. Rev. Drug Discov. 2004;3:1023–1035. doi: 10.1038/nrd1576. [DOI] [PubMed] [Google Scholar]
- 11.Rekharsky MV, Inoue Y. Complexation thermodynamics of cyclodextrins. Chem. Rev. 1998;98:1875–1917. doi: 10.1021/cr970015o. [DOI] [PubMed] [Google Scholar]
- 12.Fuchs R, Habermann N, Klufers P. Multinuclear sandwich-type complexes of deprotonated β-cyclodextrin and copper(II) ions. Angew. Chem. Int. Ed. 1993;32:852–854. [Google Scholar]
- 13.Irie T, Uekama K. Cyclodextrins in peptide and protein delivery. Adv. Drug Deliv. Rev. 1999;36:101–123. doi: 10.1016/s0169-409x(98)00057-x. [DOI] [PubMed] [Google Scholar]
- 14.Lysik MA, Wu-Pong S. Innovations in oligonucleotide drug delivery. J. Pharm. Sci. 2003;92:1559–1573. doi: 10.1002/jps.10399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Irie T, Uekama K. Pharmaceutical applications of cyclodextrins. III. toxicological issues and safety evaluation. J. Pharm. Sci. 1997;86:147–162. doi: 10.1021/js960213f. [DOI] [PubMed] [Google Scholar]
- 16.Brewster ME, Loftsson T. Cyclodextrins as pharmaceutical solubilizers. Adv. Drug Deliv. Rev. 2007;59:645–666. doi: 10.1016/j.addr.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 17.Carrier RL, Miller LA, Ahmed I. The utility of cyclodextrins for enhancing oral bioavailability. J. Control. Release. 2007;123:78–99. doi: 10.1016/j.jconrel.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 18.Hirayama F, Uekama K. Cyclodextrin-based controlled drug release system. Adv. Drug Deliv. Rev. 1999;36:125–141. doi: 10.1016/s0169-409x(98)00058-1. [DOI] [PubMed] [Google Scholar]
- 19.Loftsson T, Brewster ME. Pharmaceutical applications of cyclodextrins: effects on drug permeation through biological membranes. J. Pharm. Pharmacol. 2011;63:1119–1135. doi: 10.1111/j.2042-7158.2011.01279.x. [DOI] [PubMed] [Google Scholar]
- 20.Matsuda H, Arima H. Cyclodextrins in transdermal and rectal delivery. Adv. Drug Deliv. Rev. 1999;36:80–99. doi: 10.1016/s0169-409x(98)00056-8. [DOI] [PubMed] [Google Scholar]
- 21.Loftsson T, Jarvinen T. Cyclodextrins in ophthalmic drug delivery. Adv. Drug Deliv. Rev. 1999;36:59–79. doi: 10.1016/s0169-409x(98)00055-6. [DOI] [PubMed] [Google Scholar]
- 22.Merkus FWHM, Verhoef JC, Marttin E, Romeijn SG, van der Kuy PHM, Hermens WAJJ, Schipper NGM. Cyclodextrins in nasal drug delivery. Adv. Drug Deliv. Rev. 1999;36:41–57. doi: 10.1016/s0169-409x(98)00054-4. [DOI] [PubMed] [Google Scholar]
- 23.Szente L, Szejtli J. Highly soluble cyclodextrin derivatives: chemistry, properties, and trends in development. Adv. Drug Deliv. Rev. 1999;36:17–28. doi: 10.1016/s0169-409x(98)00092-1. [DOI] [PubMed] [Google Scholar]
- 24.Duchene D, Ponchel G, Wouessidjewe D. Cyclodextrins in targeting application to nanoparticles. Adv. Drug Deliv. Rev. 1999;36:29–40. doi: 10.1016/s0169-409x(98)00053-2. [DOI] [PubMed] [Google Scholar]
- 25.Sinha VR, Nanda A, Kumria R. Cyclodextrins as sustained-release carriers. Pharm. Technol. 2002;44:36–44. [Google Scholar]
- 26.Loftsson T, Duchene D. Cyclodextrins and their pharmaceutical applications. Int. J. Pharm. 2007;329:1–11. doi: 10.1016/j.ijpharm.2006.10.044. [DOI] [PubMed] [Google Scholar]
- 27.Harada A. Cyclodextrin-based molecular machines. Acc. Chem. Res. 2001;34:456–464. doi: 10.1021/ar000174l. [DOI] [PubMed] [Google Scholar]
- 28.Araki J, Ito K. Recent advances in the preparation of cyclodextrin-based polyrotaxanes and their applications to soft materials. Soft Matter. 2007;3:1456–1473. doi: 10.1039/b705688e. [DOI] [PubMed] [Google Scholar]
- 29.Li J, Loh XJ. Cyclodextrin-based supramolecular architectures: Syntheses, structures, and applications for drug and gene delivery. Adv. Drug Deliv. Rev. 2008;60:1000–1017. doi: 10.1016/j.addr.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 30.Yuen F, Tam KC. Cyclodextrin-assisted assembly of stimuli-responsive polymers in aqueous media. Soft Matter. 2010;6:4613–4630. [Google Scholar]
- 31.Ferris DP, Zhao YL, Khashab NM, Khatib HA, Stoddart JF, Zink JI. Light-operated mechanized nanoparticles. J. Am. Chem. Soc. 2009;131:1686–1688. doi: 10.1021/ja807798g. [DOI] [PubMed] [Google Scholar]
- 32.Liu R, Zhang Y, Feng PY. Multiresponsive supramolecular nanogated ensembles. J. Am. Chem. Soc. 2009;131:15128–15129. doi: 10.1021/ja905288m. [DOI] [PubMed] [Google Scholar]
- 33.Harada A, Hashidzume A, Takashima Y. Cyclodextrin-based supramolecular polymers. Adv. Polym. Sci. 2006;201:1–43. [Google Scholar]
- 34.Li J. Cyclodextrin inclusion polymers forming hydrogels. Adv. Polym. Sci. 2009;222:79–112. [Google Scholar]
- 35.Zhang JX, Ma PX. Host-guest interactions mediated nano-assemblies using cyclodextrin-containing hydrophilic polymers and their biomedical applications. Nano Today. 2010;5:337–350. doi: 10.1016/j.nantod.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van de Manakker F, Vermonden T, van Nostrum CF, Hennink WE. Cyclodextrin-based polymeric materials: Synthesis, properties, and pharmaceutical/biomedical applications. Biomacromolecules. 2009;10:3157–3175. doi: 10.1021/bm901065f. [DOI] [PubMed] [Google Scholar]
- 37.Zhou J, Ritter H. Cyclodextrin functionalized polymers as drug delivery systems. Polym. Chem. 2010;1:1552–1559. [Google Scholar]
- 38.Yui N, Katoono R, Yamashita A. Functional cyclodextrin polyrotaxanes for drug delivery. Adv. Polym. Sci. 2009;222:55–77. [Google Scholar]
- 39.Mellet CO, Fernandez JMG, Benito JM. Cyclodextrin-based gene delivery systems. Chem. Soc. Rev. 2011;40:1586–1608. doi: 10.1039/c0cs00019a. [DOI] [PubMed] [Google Scholar]
- 40.Sallas F, Darcy R. Amphiphilic cyclodextrins-advances in synthesis and supramolecular chemistry. Eur. J. Org. Chem. 2008:957–969. [Google Scholar]
- 41.Zhang P, Ling CC, Coleman AW, Parrot-Lopez H, Galons H. Formation of amphiphilic cyclodextrins via hydrophobic esterification at the secondary hydroxyl face. Tetrahedron Lett. 1991;32:2769–2770. [Google Scholar]
- 42.Zhang P, Parrot-Lopez H, Tchoreloff P, Baszkin A, Ling CC, de Rango C, Coleman AW. Self-organizing systems based on amphiphilic cyclodextrin diesters. J. Phys. Org. Chem. 1992;5:518–528. [Google Scholar]
- 43.Tchoreloff PC, Boissonnade MM, Coleman AW, Baszkin A. Amphiphilic monolayers of insoluble cyclodextrins at the water/air interface. surface pressure and surface potential studies. Langmuir. 1995;11:191–196. [Google Scholar]
- 44.Ling CC, Darcy R, Risse W. Cyclodextrin liquid crystals: Synthesis and self-organisation of amphiphilic thio-β-cyclodextrins. J. Chem. Soc., Chem. Commun. 1993:438–440. [Google Scholar]
- 45.Auzely-Velty R, Djedaini-Pilard F, Desert S, Perly B, Zemb T. Micellization of hydrophobically modified cyclodextrins. 1. Micellar structure. Langmuir. 2000;16:3727–3734. [Google Scholar]
- 46.Wang T, Chipot C, Shao XG, Cai WS. Structural characterization of micelles formed of cholesteryl-functionalized cyclodextrins. Langmuir. 2011;27:91–97. doi: 10.1021/la103288j. [DOI] [PubMed] [Google Scholar]
- 47.Mazzaglia A, Donohue R, Ravoo JB, Darcy R. Novel amphiphilic cyclodextrins: Graft-synthesis of heptakis(6-alkylthio-6-deoxy)-β-cyclodextrin 2-oligo(ethylene glycol) conjugates and their ω-halo derivatives. Eur. J. Org. Chem. 2001:1715–1721. [Google Scholar]
- 48.Silva OF, Fernandez MA, Pennie SL, Gil RR, de Rossi RH. Synthesis and characterization of an amphiphilic cyclodextrin, a micelle with two recognition sites. Langmuir. 2008;24:3718–3726. doi: 10.1021/la702962f. [DOI] [PubMed] [Google Scholar]
- 49.Nolan D, Darcy R, Ravoo BJ. Preparation of vesicles and nanoparticles of amphiphilic cyclodextrins containing labile disulfide bonds. Langmuir. 2003;19:4469–4472. [Google Scholar]
- 50.Mazzaglia A, Forde D, Garozzo D, Malvagna P, Ravoo BJ, Darcy R. Multivalent binding of galactosylated cyclodextrin vesicles to lectin. Org. Biomol. Chem. 2004;2:957–960. doi: 10.1039/b400988f. [DOI] [PubMed] [Google Scholar]
- 51.McNicholas S, Rencurosi A, Lay L, Mazzaglia A, Sturiale L, Perez M, Darcy R. Amphiphilic N-glycosyl-thiocarbamoyl cyclodextrins: Synthesis, self-assembly, and fluorimetry of recognition by lens culinaris lectin. Biomacromolecules. 2007;8:1851–1857. doi: 10.1021/bm070055u. [DOI] [PubMed] [Google Scholar]
- 52.Parrot-Lopez H, Ling CC, Zhang P, Baszkin A, Albrecht G, de Rango C, Coleman AW. Self-assembling systems of the amphiphilic cationic per-6-amino-β-cyclodextrin 2,3-Di-O-alkyl ethers. J. Am. Chem. Soc. 1992;114:5479–5480. [Google Scholar]
- 53.Donohue R, Mazzaglia A, Ravoo BJ, Darcy R. Cationic β-cyclodextrin bilayer vesicles. Chem. Commun. 2002:2864–2865. doi: 10.1039/b207238f. [DOI] [PubMed] [Google Scholar]
- 54.Mellet CO, Benito JM, Fernandez JMG. Preorganized, macromolecular, gene-delivery systems. Chem. Eur. J. 2010;16:6728–6742. doi: 10.1002/chem.201000076. [DOI] [PubMed] [Google Scholar]
- 55.Diaz-Moscoso A, Gourrierec LL, Gomez-Garcia M, Benito JM, Balbuena P, Ortega-Caballero F, Guilloteau N, Giorgio CD, Vierling P, Defaye J, Mellet CO, Fernandez JMG. Polycationic amphiphilic cyclodextrins for gene delivery: Synthesis and effect of structural modifications on plasmid DNA complex stability, cytotoxicity, and gene expression. Chem. Eur. J. 2009;15:12871–12888. doi: 10.1002/chem.200901149. [DOI] [PubMed] [Google Scholar]
- 56.Kraus T, Budesinsky M, Zavada J. General approach to the synthesis of persubstituted hydrophilic and amphiphilic β-cyclodextrin derivatives. J. Org. Chem. 2001;66:4595–4600. doi: 10.1021/jo010046q. [DOI] [PubMed] [Google Scholar]
- 57.Dubes A, Bouchu D, Lamartine R, Parrot-Lopez H. An efficient regio-specific synthetic route to multiply substituted acyl-sulphated β-cyclodextrins. Tetrahedron Lett. 2001;42:9147–9151. [Google Scholar]
- 58.Dubes A, Degobert G, Fessi H, Parrot-Lopez H. Synthesis and characterisation of sulfated amphiphilic α-, β- and γ-cyclodextrins: application to the complexation of acyclovir. Carbonhydrate Res. 2003;338:2185–2193. doi: 10.1016/s0008-6215(03)00356-2. [DOI] [PubMed] [Google Scholar]
- 59.Sukegawa T, Furuike T, Niikura K, Yamagishi A, Monde K, Nishimura SI. Erythrocyte-like liposomes prepared by means of amphiphilic cyclodextrin sulfates. Chem. Commun. 2002:430–431. doi: 10.1039/b110673b. [DOI] [PubMed] [Google Scholar]
- 60.Zhang JX, Jia Y, Li XD, Hu YQ, Li XH. Facile engineering of biocompatible materials with pH-modulated degradability. Adv. Mater. 2011;23:3035–3040. doi: 10.1002/adma.201100679. [DOI] [PubMed] [Google Scholar]
- 61.Chen HP, Liu XP, Dou Y, He BF, Liu L, Wei ZH, Li J, Wang CZ, Mao CD, Zhang JX, Wang GS. A pH-responsive cyclodextrin-based hybrid nanosystem as a nonviral vector for gene delivery. Biomaterials. 2013;34:4159–4172. doi: 10.1016/j.biomaterials.2013.02.035. [DOI] [PubMed] [Google Scholar]
- 62.He HM, Chen S, Zhou JZ, Dou Y, Song L, Che L, Zhou X, Chen X, Jia Y, Zhang JX, Li SH, Li XH. Cyclodextrin-derived pH-responsive nanoparticles for delivery of paclitaxel. Biomaterials. 2013 doi: 10.1016/j.biomaterials.2013.03.068. http://dx.doi.org/10.1016/j.biomaterials.2013.1003.1068. [DOI] [PubMed]
- 63.Solms J, Egli RH. Harze mit einschlusshohlrhumen von cyclodextrin-struktur. Helv. Chim. Acta. 1965;48:1225–1228. [Google Scholar]
- 64.Renard E, Barnathan G, Deratani A, Sebille B. Polycondensation of cyclodextrins with epichlorohydrin. influence of reaction conditions on the polymer structure. Macromol. Symp. 1997;122:229–234. [Google Scholar]
- 65.Renard E, Deratani A, Volet G, Sebille B. Preparation and characterization of water soluble high molecular weight β-cyclodextrin-epichlorohydrin polymers. Eur. Polym. J. 1997;33:49–57. [Google Scholar]
- 66.Guan Y, Qian L, Xiao H. Novel anti-microbial host-guest complexes based on cationic β-cyclodextrin polymers and triclosan/butylparaben. Macromol. Rapid Commun. 2007;28:2244–2248. [Google Scholar]
- 67.Smith RC, Riollano M, Leung A, Hammond PT. Layer-by-layer platform technology for small-molecule delivery. Angew. Chem. Int. Ed. 2009;48:8974–8977. doi: 10.1002/anie.200902782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Daoud-Mahammed S, Couvreur P, Bouchemal K, Cheron M, Lebas G, Amiel C, Gref R. Cyclodextrin and polysaccharide-based nanogels: Entrapment of two hydrophobic molecules, benhenone and tamoxifen. Biomacromolecules. 2009;10:547–554. doi: 10.1021/bm801206f. [DOI] [PubMed] [Google Scholar]
- 69.Gosselet NM, Beucler F, Renard E, Amiel C, Sebille B. Association of hydrophobically modified poly(N,N-dimethylacrylamide hydroxyethylmethacrylate) with water soluble β-cyclodextrin polymers. Colloids Surf. A. 1999;155:177–188. [Google Scholar]
- 70.Yallapu MM, Jaggi M, Chauhan SC. Poly(β-cyclodextrin)/curcumin self-assembly: A novel approach to improve curcumin delivery and its therapeutic efficacy in prostate cancer cells. Macromol. Biosci. 2010;10:1141–1151. doi: 10.1002/mabi.201000084. [DOI] [PubMed] [Google Scholar]
- 71.Fagui AE, Dalmas F, Lorthioir C, Wintgens V, volet G, Amiel C. Well-defined core-shell nanoparticles containing cyclodextrin in the shell: a comprehensive study. Polymer. 2011;52:3752–3761. [Google Scholar]
- 72.Gref R, Amiel C, Molinard K, Daoud-Mahammed S, Sebille B, Gillet B, Beloeil JC, Ringard C, Rosilio V, Poupaert J, Couvreur P. New self-assembled nanogels based on host–guest interactions: Characterization and drug loading. J. Control. Release. 2006;111:316–324. doi: 10.1016/j.jconrel.2005.12.025. [DOI] [PubMed] [Google Scholar]
- 73.Wintgens V, Layre AM, Hourdet D, Amiel C. Cyclodextrin polymer nanoassemblies: Strategies for stability improvement. Biomacromolecules. 2012;13:528–534. doi: 10.1021/bm201608n. [DOI] [PubMed] [Google Scholar]
- 74.Daoud-Mahammed S, Grossiord JL, Bergua T, Amiel C, Couvreur P, Gref R. Self-assembling cyclodextrin based hydrogels for the sustained delivery of hydrophobic drugs. J. Biomed. Mater. Res. 2008;86A:736–748. doi: 10.1002/jbm.a.31674. [DOI] [PubMed] [Google Scholar]
- 75.Cheng JJ, Khin KT, Jensen GS, Liu AJ, Davis ME. Synthesis of linear, β-cyclodextrin-based polymers and their camptothecin conjugates. Bioconjugate Chem. 2003;14:1007–1017. doi: 10.1021/bc0340924. [DOI] [PubMed] [Google Scholar]
- 76.Gonzalez H, Hwang SJ, Davis ME. New class of polymers for the delivery of macromolecular therapeutics. Bioconjugate Chem. 1999;10:1068–1074. doi: 10.1021/bc990072j. [DOI] [PubMed] [Google Scholar]
- 77.Popielarski SR, Mishra S, Davis ME. Structural effects of carbohydrate-containing polycations on gene delivery. 3. cyclodextrin type and functionalization. Bioconjugate Chem. 2003;14:672–678. doi: 10.1021/bc034010b. [DOI] [PubMed] [Google Scholar]
- 78.Davis ME. The first targeted delivery of siRNA in humans via a self-assembling, cyclodextrin polymer-based nanoparticle: From concept to clinic. Mol. Pharmaceutics. 2009;6:659–668. doi: 10.1021/mp900015y. [DOI] [PubMed] [Google Scholar]
- 79.Srinivasachari S, Reineke TM. Versatile supramolecular pDNA vehicles via “click polymerization” of β-cyclodextrin with oligoethyleneamines. Biomaterials. 2009;30:928–938. doi: 10.1016/j.biomaterials.2008.09.067. [DOI] [PubMed] [Google Scholar]
- 80.Huang HL, Tang GP, Wang QQ, Li D, Shen FP, Zhou J, Yu H. Two novel non-viral gene delivery vectors: low molecular weight polyethylenimine cross-linked by (2-hydroxypropyl)-b-cyclodextrin or (2-hydroxypropyl)-c-cyclodextrin. Chem. Commun. 2006:2382–2384. doi: 10.1039/b601130f. [DOI] [PubMed] [Google Scholar]
- 81.Tang GP, Guo HY, Alexis F, Wang X, Zeng S, Lim TM, Ding J, Yang YY, Wang S. Low molecular weight polyethylenimines linked by β-cyclodextrin for gene transfer into the nervous system. J. Gene Med. 2006;8:736–744. doi: 10.1002/jgm.874. [DOI] [PubMed] [Google Scholar]
- 82.Huang HL, Yu H, Tang GP, Wang QQ, Li J. Low molecular weight polyethylenimine cross-linked by 2-hydroxypropyl-gamma-cyclodextrin coupled to peptide targeting HER2 as a gene delivery vector. Biomaterials. 2010;31:1830–1838. doi: 10.1016/j.biomaterials.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 83.Lu X, Ping Y, Xu FJ, Li ZH, Wang QQ, Chen JH, Yang WT, Tang GP. Bifunctional conjugates comprising β-cyclodextrin, polyethylenimine, and 5-fluoro-2′-deoxyuridine for drug delivery and gene transfer. Bioconjugate Chem. 2010;21:1855–1863. doi: 10.1021/bc1002136. [DOI] [PubMed] [Google Scholar]
- 84.Lu X, Wang QQ, Xu FJ, Tang GP, Yang WT. A cationic prodrug/therapeutic gene nanocomplex for the synergistic treatment of tumors. Biomaterials. 2011;32:4849–4856. doi: 10.1016/j.biomaterials.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 85.Gou PF, Zhu WP, Shen ZQ. Synthesis, self-Assembly, and drug-loading capacity of well-defined cyclodextrin-centered drug-conjugated amphiphilic A(14)B(7) miktoarm star copolymers based on poly(epsilon-caprolactone) and poly(ethylene glycol) Biomacromolecules. 2010;11:934–943. doi: 10.1021/bm901371p. [DOI] [PubMed] [Google Scholar]
- 86.Gou PF, Zhu WP, Xu N, Shen ZQ. Synthesis and self-assembly of well-defined cyclodextrin-centered amphiphilic A(14)B(7) multimiktoarm star copolymers based on poly(epsilon-caprolactone) and poly(acrylic acid) J. Polym. Sci. Part A: Polym. Chem. 2010;48:2961–2974. doi: 10.1021/bm901371p. [DOI] [PubMed] [Google Scholar]
- 87.Adeli M, Zarnegar Z, Kabiri R. Amphiphilic star copolymers containing cyclodextrin core and their application as nanocarrier. Eur. Polym. J. 2008;44:1921–1930. [Google Scholar]
- 88.Mourtzis N, Paravaton M, Mavridis IM, Roberts ML, Yannakopoulou K. Synthesis, characterization, and remarkable biological properties of cyclodextrins bearing guanidinoalkylamino and aminoalkylamino groups on their primary side. Chem. Eur. J. 2008;14:4188–4200. doi: 10.1002/chem.200701650. [DOI] [PubMed] [Google Scholar]
- 89.Srinivasachari S, Fichter KM, Reineke TM. Polycationic β-cyclodextrin “click clusters”: Monodisperse and versatile scaffolds for nucleic acid delivery. J. Am. Chem. Soc. 2008;130:4618–4627. doi: 10.1021/ja074597v. [DOI] [PubMed] [Google Scholar]
- 90.Bryson JM, Chu WJ, Lee JH, Reineke TM. A β-cyclodextrin “click cluster” decorated with seven paramagnetic chelates containing two water exchange sites. Bioconjugate Chem. 2008;19:1505–1509. doi: 10.1021/bc800200q. [DOI] [PubMed] [Google Scholar]
- 91.Harada A, Furue M, Nozakura SI. Cyclodextrin-containing polymers. 1. Preparation of polymers. Macromolecules. 1976;9:701–704. [Google Scholar]
- 92.Munteanu M, Choi SW, Ritter H. Cyclodextrin methacrylate via microwave-assisted click reaction. Macromolecules. 2008;41:9619–9623. [Google Scholar]
- 93.Liu YY, Fan XD, Gao L. Synthesis and characterization of β-cyclodextrin based functional monomers and its copolymers with N-isopropylacrylamide. Macromol. Biosci. 2003;3:715–719. [Google Scholar]
- 94.Liu YY, Zhong YB, Nan JK, Tian W. Star polymers with both temperature sensitivity and inclusion functionalities. Macromolecules. 2010;43:10221–10230. [Google Scholar]
- 95.Wang J, Jiang M. Polymeric self-assembly into micelles and hollow spheres with multiscale cavities driven by inclusion complexation. J. Am. Chem. Soc. 2006;128:3703–3708. doi: 10.1021/ja056775v. [DOI] [PubMed] [Google Scholar]
- 96.Weickenmeier M, Wenz G. Cyclodextrin sidechain polyesters- synthesis and inclusion of adamantan derivatives. Macromol. Rapid Commun. 1996;17:731–736. [Google Scholar]
- 97.Charlot A, Auzely-Velty R. Synthesis of novel supramolecular assemblies based on hyaluronic acid derivatives bearing bivalent β-cyclodextrin and adamantane moieties. Macromolecules. 2007;40:1147–1158. [Google Scholar]
- 98.Zhang JX, Ma PX. Polymeric core-shell assemblies mediated by host-guest interactions: versatile nanocarriers for drug delivery. Angew. Chem. Int. Ed. 2009;48:964–968. doi: 10.1002/anie.200804135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang JX, Ellsworth K, Ma PX. Synthesis of β-cyclodextrin containing copolymer via “click” chemistry and its self-assembly in the presence of guest compounds. Macromol. Rapid Commun. 2012;33:664–671. doi: 10.1002/marc.201100814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ogata N, Sanui K, Wada J. Novel synthesis of inclusion polyamides. J. Polym. Sci. Polym. Lett. Ed. 1976;14:459–462. [Google Scholar]
- 101.Harada A, Kamachi M. Complex formation between poly(ethylene glycol) and α-cyclodextrin. Macromolecules. 1990;23:2821–2823. [Google Scholar]
- 102.Wenz G, Keller B. Threading cyclodextrin rings on polymer chains. Angew. Chem. Int. Ed. 1992;31:197–199. [Google Scholar]
- 103.Ooya T, Yui N. Polyrotaxanes: synthesis, structure, and potential in drug delivery. Crit. Rev. Ther. Drug Carrier Systems. 1999;16:289–330. doi: 10.1615/critrevtherdrugcarriersyst.v16.i3.20. [DOI] [PubMed] [Google Scholar]
- 104.Ooya T, Yui N. Synthesis and characterization of biodegradable polyrotaxane as a novel supramolecular-structured drug carrier. J. Biomater. Sci. Polym. Ed. 1997;8:437–455. doi: 10.1163/156856297x00371. [DOI] [PubMed] [Google Scholar]
- 105.Ooya T, Yui N. Supramolecular dissociation of biodegradable polyrotaxanes by enzymatic terminal hydrolysis. Macromol. Chem. Phys. 1998;199:2311–2320. [Google Scholar]
- 106.Ooya T, Yui N. Synthesis of theophylline-polyrotaxane conjugates and their drug release via supramolecular dissociation. J. Control. Release. 1999;58:251–269. doi: 10.1016/s0168-3659(98)00163-1. [DOI] [PubMed] [Google Scholar]
- 107.Moon C, Kwon YM, Lee WK, Park YJ, Yang VC. In vitro assessment of a novel polyrotaxane-based drug delivery system integrated with a cell-penetrating peptide. J. Control. Release. 2007;124:43–50. doi: 10.1016/j.jconrel.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mellman I, Fuchs R, Helenius A. Acidification of the endocytic and exocytic pathways. Ann. Rev. Biochem. 1986;55:663–700. doi: 10.1146/annurev.bi.55.070186.003311. [DOI] [PubMed] [Google Scholar]
- 109.Saito G, Swanson JA, Lee KD. D rug delivery strategy utilizing conjugation via reversible disulfide linkages: role and site of cellular reducing activities. Adv. Drug Deliv. Rev. 2003;55:199–215. doi: 10.1016/s0169-409x(02)00179-5. [DOI] [PubMed] [Google Scholar]
- 110.Bilensoy E, Hincal AA. Recent advances and future directions in amphiphilic cyclodextrin nanoparticles. Expert Opin. Drug Deliv. 2009;6:1161–1173. doi: 10.1517/17425240903222218. [DOI] [PubMed] [Google Scholar]
- 111.Skiba M, Wouessidjewe D, Puisieux F, Duchene D, Gulik A. Characterization of amphiphilic β-cyclodextrin nanospheres. Int. J. Pharm. 1996;142:121–124. [Google Scholar]
- 112.Skiba M, Duchene D, Puisieux F, Wouessidjewe D. Development of a new colloidal drug carrier from chemically-modified cyclodextrins: nanospheres and influence of physicochemical and technological factors on particle size. Int. J. Pharm. 1996;129:113–121. [Google Scholar]
- 113.Memisoglu E, Bochot A, Ozalp M, Sen M, Duchene D, Hincal AA. Direct formation of nanospheres from amphiphilic β-cyclodextrin inclusion complexes. Pharm. Res. 2003;20:117–125. doi: 10.1023/a:1022263111961. [DOI] [PubMed] [Google Scholar]
- 114.Yameogo JBG, Geze A, Choisnard L, Putaux JL, Gansane A, Sirima SB, Semde R, Wouessidjewe D. Self-assembled biotransesterified cyclodextrins as Artemisinin nanocarriers-I: Formulation, lyoavailability and in vitro antimalarial activity assessment. Eur. J. Pharm. Biopharm. 2012;80:508–517. doi: 10.1016/j.ejpb.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 115.Sortino S, Mazzaglia A, Scolaro LM, Merlo FM, Valveri V, Sciortino MT. Nanoparticles of cationic amphiphilic cyclodextrins entangling anionic porphyrins as carrier-sensitizer system in photodynamic cancer therapy. Biomaterials. 2006;27:4256–4265. doi: 10.1016/j.biomaterials.2006.03.035. [DOI] [PubMed] [Google Scholar]
- 116.Kandoth N, Vittorino E, Sciortino MT, Parisi T, Colao I, Mazzaglia A, Sortino S. A cyclodextrin-based nanoassembly with bimodal photodynamic action. Chem. Eur. J. 2012;18:1684–1690. doi: 10.1002/chem.201101635. [DOI] [PubMed] [Google Scholar]
- 117.Thiele C, Auerbach D, Jung G, Qiong L, Schneider M, Wenz G. Nanoparticles of anionic starch and cationic cyclodextrin derivatives for the targeted delivery of drugs. Polym. Chem. 2011;2:209–215. [Google Scholar]
- 118.Kang JC, Kumar V, Yang D, Chowdhury PR, Hohl RJ. Cyclodextrin complexation: influence on the solubility, stability, and cytotoxicity of camptothecin, an antineoplastic agent. Eur. J. Pharm. Sci. 2002;15:163–170. doi: 10.1016/s0928-0987(01)00214-7. [DOI] [PubMed] [Google Scholar]
- 119.Schluep T, Cheng JJ, Khin KT, Davis ME. Pharmacokinetics and biodistribution of the camptothecin–polymer conjugate IT-101 in rats and tumor-bearing mice. Cancer Chemther. Pharmacol. 2005;2006:654–662. doi: 10.1007/s00280-005-0091-7. [DOI] [PubMed] [Google Scholar]
- 120.Schluep T, Hwang JY, Cheng JJ, Heidel JD, Bartlett DW, Hollister B, Davis ME. Preclinical efficacy of the camptothecin-polymer conjugate IT-101 in multiple cancer models. Clin. Cancer Res. 2006;12:1606–1614. doi: 10.1158/1078-0432.CCR-05-1566. [DOI] [PubMed] [Google Scholar]
- 121.Svenson S, Wolfgang M, Hwang JY, Ryan J, Eliasof S. Preclinical to clinical development of the novel camptothecin nanopharmaceutical CRLX101. J. Control. Release. 2011;153:49–55. doi: 10.1016/j.jconrel.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 122.Schluep T, Hwang JY, Hildebrandt IJ, Czernin J, Choi CHJ, Alabi CA, Mack BC, Davis ME. Pharmacokinetics and tumor dynamics of the nanoparticle IT-101 from PET imaging and tumor histological measurements. Proc. Natl. Acad. Sci. U. S. A. 2009;106:11394–11399. doi: 10.1073/pnas.0905487106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schluep T, Gunawan P, Ma L, Jensen GS, Duringer J, Hinton S, Richter W, Hwang JY. Polymeric tubulysin-peptide nanoparticles with potent antitumor activity. Clin. Cancer Res. 2009;15:181–189. doi: 10.1158/1078-0432.CCR-08-1848. [DOI] [PubMed] [Google Scholar]
- 124.Zhang JX, Ellsworth K, Ma PX. Hydrophobic pharmaceuticals mediated self-assembly of beta-cyclodextrin containing hydrophilic copolymers: novel chemical responsive nano- vehicles for drug delivery. J. Control. Release. 2010;145:116–123. doi: 10.1016/j.jconrel.2010.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhang JX, Ma PX. Core-shell structured nanoassemblies based on β-cyclodextrin containing block copolymer and poly(β-benzyl L-aspartate) via host-guest complexation. Polymer. 2010;52:4928–4937. doi: 10.1016/j.polymer.2011.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rapoport N. Physical stimuli-responsive polymeric micelles for anti-cancer drug delivery. Prog. Polym. Sci. 2007;32:962–990. [Google Scholar]
- 127.Bae Y, Kataoka K. Intelligent polymeric micelles from functional poly(ethylene glycol)-poly(amino acid) block copolymers. Adv. Drug Deliv. Rev. 2009;61:768–784. doi: 10.1016/j.addr.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 128.Cabral H, Nishiyama N, Kataoka K. Supramolecular nanodevices: from design validation to theranostic nanomedicine. Acc. Chem. Res. 2011;44:999–1008. doi: 10.1021/ar200094a. [DOI] [PubMed] [Google Scholar]
- 129.Layre AM, Wintgens V, Gosselet NM, Dalmas F, Amiel C. Tuning the interactions in cyclodextrin polymer nanoassemblies. Eur. Polym. J. 2009;45:3016–3026. [Google Scholar]
- 130.Dong HQ, Li YY, Cai SJ, Zhuo RX, Zhang XZ, Liu LJ. A facile one-pot construction of supramolecular polymer micelles from α-cyclodextrin and poly(e-caprolactone) Angew. Chem. Int. Ed. 2008;47:5573–5576. doi: 10.1002/anie.200800952. [DOI] [PubMed] [Google Scholar]
- 131.Li Q, Xia B, Branham M, Ha W, Wu H, Peng SL, Ding LS, Li BJ, Zhang S. Self-assembly of carboxymethyl konjac glucomannan-g-poly(ethylene glycol) and (a-cyclodextrin) to biocompatible hollow nanospheres for glucose oxidase encapsulation. Carbohydrate Polym. 2011;86:120–126. [Google Scholar]
- 132.Liu GY, Jin Q, Liu XS, Lv LP, Chen CJ, Ji J. Biocompatible vesicles based on PEO-b-PMPC/α-cyclodextrin inclusion complexes for drug delivery. Soft Matter. 2011;7:662–669. [Google Scholar]
- 133.Liu JH, Sondjaja HR, Tam KC. Alpha-cyclodextrin-induced self-assembly of a double-hydrophilic block copolymer in aqueous solution. Langmuir. 2007;23:5106–5109. doi: 10.1021/la063365r. [DOI] [PubMed] [Google Scholar]
- 134.Liu Y, Zhao DY, Ma RJ, Xiong DA, An YL, Shi LQ. Chaperone-like a-cyclodextrins assisted self-assembly of double hydrophilic block copolymers in aqueous medium. Polymer. 2009;50:855–859. [Google Scholar]
- 135.Croyle MA, Roessler BJ, Hsu CP, Sun R, Amidon GL. Beta-cyclodextrins enhance adenoviral-mediated gene delivery to the intestine. Pharm. Res. 1998;15:1348–1355. doi: 10.1023/a:1011985101580. [DOI] [PubMed] [Google Scholar]
- 136.Redenti E, Pietra C, Gerloczy A, Szente L. Cyclodextrins in oligonucleotide delivery. Adv. Drug Deliv. Rev. 2001;53:235–244. doi: 10.1016/s0169-409x(01)00230-7. [DOI] [PubMed] [Google Scholar]
- 137.Jessel N, Oulad-Abdelghani M, Meyer F, Lavalle P, Haikel Y, Schaaf P, Voegel JC. Multiple and time-scheduled in situ DNA delivery mediated by β-cyclodextrin embedded in a polyelectrolyte multilayer. PNAS. 2006;103:8618–8621. doi: 10.1073/pnas.0508246103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cryan SA, Holohan A, Donohue R, Darcy R, O'Driscoll CM. Cell transfection with polycationic cyclodextrin vectors. Eur. J. Pharm. Sci. 2004;21:625–633. doi: 10.1016/j.ejps.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 139.Arima H, Kihara F, Hirayama F, Uekama K. Enhancement of gene expression by polyamidoamine dendrimer conjugates with α-, β-, and γ-cyclodextrins. Bioconjugate Chem. 2001;12:476–484. doi: 10.1021/bc000111n. [DOI] [PubMed] [Google Scholar]
- 140.Menuel S, Fontanay S, Clarot I, Duval RE, Diez L, Marsura A. Synthesis and complexation ability of a novel bis-(guanidinium)-tetrakis-(β-cyclodextrin) dendrimeric tetrapod as a potential gene delivery (DNA and siRNA) system. study of cellular siRNA transfection. Bioconjugate Chem. 2008;19:2357–2362. doi: 10.1021/bc800193p. [DOI] [PubMed] [Google Scholar]
- 141.Tsutsumi T, Hirayama F, Uekama K, Arima H. Evaluation of polyamidoamine dendrimer/α-cyclodextrin conjugate (generation 3, G3) as a novel carrier for small interfering RNA (siRNA) J. Control. Release. 2007;119:349–359. doi: 10.1016/j.jconrel.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 142.Forrest ML, Gabrielson N, Pack DW. Cyclodextrin-polyethylenimine conjugates for targeted in vitro gene delivery. Biotechnol. Bioeng. 2005;89:416–423. doi: 10.1002/bit.20356. [DOI] [PubMed] [Google Scholar]
- 143.Choi HS, Yamashita A, Ooya T, Yui N, Akita H, Kogure K, Ito R, Harashima H. Sunflower-shaped cyclodextrin-conjugated poly(ε-lysine) polyplex as a controlled intracellular trafficking device. ChemBioChem. 2005;6:1986–1990. doi: 10.1002/cbic.200500242. [DOI] [PubMed] [Google Scholar]
- 144.Harada A, Takashima Y, Yamaguchi H. Cyclodextrin-based supramolecular polymers. Chem. Soc. Rev. 2009;38:875–882. doi: 10.1039/b705458k. [DOI] [PubMed] [Google Scholar]
- 145.Choi HS, Ooya T, Lee SC, Sasaki S, Kurisawa M, Uyama H, Yui N. pH Dependence of polypseudorotaxane formation between cationic linear polyethylenimine and cyclodextrins. Macromolecules. 2004;37:6705–6710. [Google Scholar]
- 146.Yamashita A, Choi HS, Ooya T, Yui N, Akita H, Kogure K, Harashima H. Improved cell viability of linear polyethylenimine through γ-cyclodextrin inclusion for effective gene delivery. ChemBioChem. 2006;7:297–302. doi: 10.1002/cbic.200500348. [DOI] [PubMed] [Google Scholar]
- 147.Ooya T, Choi HS, Yamashita A, Yui N, Sugaya Y, Kano A, Maruyama A, Akita H, Ito R, Kogure K, Harashima H. Biocleavable polyrotaxane-plasmid DNA polyplex for enhanced gene delivery. J. Am. Chem. Soc. 2006;128:3852–3853. doi: 10.1021/ja055868+. [DOI] [PubMed] [Google Scholar]
- 148.Li J, Yang C, Li HZ, Wang X, Goh SH, Ding JL, Wang DY, Leong KW. Cationic supramolecules composed of multiple oligoethylenimine-grafted b-cyclodextrins threaded on a polymer chain for efficient gene delivery. Adv. Mater. 2006;18:2969–2974. [Google Scholar]
- 149.Liu Y, Yu L, Chen Y, Zhao YL, Yang H. Construction and DNA condensation of cyclodextrin-based polypseudorotaxanes with anthryl grafts. J. Am. Chem. Soc. 2007;129:10656–10657. doi: 10.1021/ja073882b. [DOI] [PubMed] [Google Scholar]
- 150.Burckbuchler V, Wintgens V, Leborgne C, Lecomte S, Leygue N, Scherman D, Kichler A, Amiel C. Development and characterization of new cyclodextrin polymer-based DNA delivery systems. Bioconjugate Chem. 2008;19:2311–2320. doi: 10.1021/bc800070f. [DOI] [PubMed] [Google Scholar]
- 151.Diaz-Moscoso A, Balbuena P, Gomez-Garcia M, Mellet CO, Benito JM, Gourrierec LL, Giorgio CD, Vierling P, Mazzaglia A, Micali N, Defaye J, Fernandez JMG. Rational design of cationic cyclooligosaccharides as efficient gene delivery systems. Chem. Commun. 2008:2001–2003. doi: 10.1039/b718672j. [DOI] [PubMed] [Google Scholar]
- 152.Hwang SJ, Bellocq NC, Davis ME. Effects of structure of α-cyclodextrin-containing polymers on gene delivery. Bioconjugate Chem. 2001;12:280–290. doi: 10.1021/bc0001084. [DOI] [PubMed] [Google Scholar]
- 153.Reineke TM, Davis ME. Structural effects of carbohydrate-containing polycations on gene delivery. 1. carbohydrate size and its distance from charge centers. Bioconjugate Chem. 2003;14:247–254. doi: 10.1021/bc025592k. [DOI] [PubMed] [Google Scholar]
- 154.Reineke TM, Davis ME. Structural effects of carbohydrate-containing polycations on gene delivery. 2. charge center type. Bioconjugate Chem. 2003;14:255–261. doi: 10.1021/bc025593c. [DOI] [PubMed] [Google Scholar]
- 155.Bartlett DW, Davis ME. Physicochemical and biological characterization of targeted, nucleic acid-containing nanoparticles. Bioconjugate Chem. 2007;18:456–468. doi: 10.1021/bc0603539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Pun SH, Tack F, Bellocq NC, Cheng JJ, Grubbs BH, Jensen GS, Davis ME, Brewster M, Janicot M, Janssens B, Floren W, Bakker A. Targeted delivery of RNA-cleaving DNA enzyme (DNAzyme) to tumor tissue by transferrin-modified, cyclodextrin-based particles. Cancer Biol. Ther. 2004;3:641–650. doi: 10.4161/cbt.3.7.918. [DOI] [PubMed] [Google Scholar]
- 157.Hu-Lieskovan S, Heidel JD, Bartlett DW, Davis ME, Triche TJ. Sequence-specific knockdown of EWS-FLI1 by targeted, nonviral delivery of small interfering RNA inhibits tumor growth in a murine model of metastatic ewing's sarcoma. Cancer Res. 2005;65:8984–8992. doi: 10.1158/0008-5472.CAN-05-0565. [DOI] [PubMed] [Google Scholar]
- 158.Heidel JD, Yu ZP, Liu JYC, Rele SM, Liang YC, Zeidan RK, Kornbrust DJ, Davis ME. Administration in non-human primates of escalating intravenous doses of targeted nanoparticles containing ribonucleotide reductase subunit M2 siRNA. Proc. Natl. Acad. Sci. U. S. A. 2007;104:5715–5721. doi: 10.1073/pnas.0701458104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Davis ME, Zuckerman JE, Choi CHJ, Seligson D, Tolcher A, Alabi CA, Yen Y, Heidel JD, Ribas A. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature. 2010;464:1067–1070. doi: 10.1038/nature08956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Greco WR, Bravo G, Parsons JC. The search for synergy: a critical review from a response surface perspective. Pharmacol. Rev. 1995;47:331–385. [PubMed] [Google Scholar]
- 161.Hu CMJ, Aryal S, Zhang LF. Nanoparticle-assisted combination therapies for effective cancer treatment. Ther. Deliv. 2010;1:323–334. doi: 10.4155/tde.10.13. [DOI] [PubMed] [Google Scholar]
- 162.Rebuffat A, Bernasconi A, Ceppi M, Wehrli H, Verca SB, Ibrahim M, Frey BM, Frey FJ, Rusconi S. Selective enhancement of gene transfer by steroid-mediated gene delivery. Nat. Biotechnol. 2001;19:1155–1161. doi: 10.1038/nbt1201-1155. [DOI] [PubMed] [Google Scholar]
- 163.Liu F, Shollenberger LM, Huang L. Nonimmunostimulatory nonviral vectors. FASEB J. 2004;18:1779–1781. doi: 10.1096/fj.04-2187fje. [DOI] [PubMed] [Google Scholar]
- 164.Wang Y, Gao SJ, Ye WH, Yoon HS, Yang YY. Codelivery of drugs and DNA from cationic core-shell nanoparticles self-assembled from a biodegradable copolymer. Nat. Mater. 2006;5:791–796. doi: 10.1038/nmat1737. [DOI] [PubMed] [Google Scholar]
- 165.Zhang JX, Sun HL, Ma PX. Host-guest interactions mediated polymeric assemblies: multifunctional nanoparticles for drug and gene delivery. ACS Nano. 2010;4:1049–1059. doi: 10.1021/nn901213a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wu J, Lu Y, Lee A, Pan X, Yang X, Zhao X, Lee RJ. Reversal of multidrug resistance by transferrin-conjugated liposomes co-encapsulating doxorubicin and verapamil. J. Pharm. Pharm. Sci. 2007;10:350–357. [PubMed] [Google Scholar]
- 167.Saad M, Garbuzenko OB, Minko T. Co-delivery of siRNA and an anticancer drug for treatment of multidrug-resistant cancer. Nanomedicine. 2008;3:761–776. doi: 10.2217/17435889.3.6.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Fan H, Hu QD, Xu FJ, Liang WQ, Tang GP, Yang WT. In vivo treatment of tumors using host-guest conjugated nanoparticles functionalized with doxorubicin and therapeutic gene pTRAIL. Biomaterials. 2012;33:1428–1436. doi: 10.1016/j.biomaterials.2011.10.043. [DOI] [PubMed] [Google Scholar]
- 169.Takae S, Miyata K, Oba M, Ishii T, Nishiyama N, Itaka K, Yamasaki Y, Koyama H, Kataoka K. PEG-detachable polyplex micelles based on disulfide-linked block catiomers as bioresponsive nonviral gene vectors. J. Am. Chem. Soc. 2008;130:6001–6009. doi: 10.1021/ja800336v. [DOI] [PubMed] [Google Scholar]
- 170.Tang LY, Wang YC, Li Y, Du JZ, Wang J. Shell-detachable micelles based on disulfide-linked block copolymer as potential carrier for intracellular drug delivery. Bioconjugate Chem. 2009;20:1095–1099. doi: 10.1021/bc900144m. [DOI] [PubMed] [Google Scholar]
- 171.Zhang JX, Feng K, Cuddihy M, Kotov NA, Ma PX. Spontaneous formation of temperature-responsive assemblies by molecular recognition of a β-cyclodextrin containing block copolymer and poly(N-isopropylacrylamide) Soft Matter. 2010;6:610–617. doi: 10.1039/c000898b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ravoo BJ, Darcy R. Cyclodextrin bilayer vesicles. Angew. Chem. Int. Ed. 2000;39:4324–4326. doi: 10.1002/1521-3773(20001201)39:23<4324::AID-ANIE4324>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 173.Falvey P, Lim CW, Darcy R, Revermann T, Karst U, Giesbers M, Marcelis ATM, Lazar A, Coleman AW, Reinhoudt DN, Ravoo BJ. Bilayer vesicles of amphiphilic cyclodextrins: Host membranes that recognize guest molecules. Chem. Eur. J. 2005;11:1171–1180. doi: 10.1002/chem.200400905. [DOI] [PubMed] [Google Scholar]
- 174.Versluis F, Tomatsu I, Kehr S, Fregonese C, Tepper AWJW, Stuart MCA, Ravoo BJ, Koning RI, Kros A. Shape and release control of a peptide decorated vesicle through pH sensitive orthogonal supramolecular interactions. J. Am. Chem. Soc. 2009;131:13186–13187. doi: 10.1021/ja9026264. [DOI] [PubMed] [Google Scholar]
- 175.Zhang JX, Li XD, Li XH. Stimuli-triggered structural engineering of synthetic and biological polymeric assemblies. Prog. Polym. Sci. 2012;37:1130–1176. [Google Scholar]
- 176.Deng W, Yamaguchi H, Takashima Y, Harada A. Construction of chemical-responsive supramolecular hydrogels from guest-modified cyclodextrins. Chem. Asian J. 2008;3:687–695. doi: 10.1002/asia.200700378. [DOI] [PubMed] [Google Scholar]
- 177.Li J. Self-assembled supramolecular hydrogels based on polymer-cyclodextrin inclusion complexes for drug delivery. NPG Asia Mater. 2010;2:112–118. [Google Scholar]
- 178.Harada A, Kobayashi R, Takashima Y, Hashidzume A, Yamaguchi H. Macroscopic self-assembly through molecular recognition. Nat. Chem. 2011;3:34–37. doi: 10.1038/nchem.893. [DOI] [PubMed] [Google Scholar]
- 179.Yamaguchi H, Kobayashi Y, Kobayashi R, Takashima Y, Hashidzume A, Harada A. Photoswitchable gel assembly based on molecular recognition. Nat. Commun. 2012;3:1–5. doi: 10.1038/ncomms1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Nakahata M, Takashima Y, Yamaguchi H, Harada A. Redox-responsive self-healing materials formed from host−guest polymers. Nat. Commun. 2011;2:1–6. doi: 10.1038/ncomms1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Li J, Harada A, Kamachi M. Sol-gel transition during inclusion complex formation between a-cyclodextrin and high molecular weight poly(ethylene glycol)s in aqeuous solution. Polym. J. 1994;26:1019–1026. [Google Scholar]
- 182.Nogueiras-Nieto L, Sobarzo-Sanchez E, Gomez-Amoza JL, Otero-Espinar FJ. Competitive displacement of drugs from cyclodextrin inclusion complex by polypseudorotaxane formation with poloxamer: Implications in drug solubilization and delivery. Eur. J. Pharm. Biopharm. 2012;80:585–595. doi: 10.1016/j.ejpb.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 183.Chen Y, Pang XH, Dong CM. Dual stimuli-responsive supramolecular polypeptide-based hydrogel and reverse micellar hydrogel mediated by host-guest chemistry. Adv. Funct. Mater. 2010;20:579–586. [Google Scholar]
- 184.Yu JH, Fan HL, Huang J, Chen JH. Fabrication and evaluation of reduction-sensitive supramolecular hydrogel based on cyclodextrin/polymer inclusion for injectable drug-carrier application. Soft Matter. 2011;7:7386–7394. [Google Scholar]
- 185.Liao XJ, Chen GS, Liu XX, Chen WX, Chen F, Jiang M. Photoresponsive pseudopolyrotaxane hydrogels based on competition of host-guest interactions. Angew. Chem. Int. Ed. 2010;49:4409–4413. doi: 10.1002/anie.201000141. [DOI] [PubMed] [Google Scholar]
- 186.Li J, Ni XP, Leong KW. Injectable drug-delivery systems based on supramolecular hydrogels formed by poly(ethylene oxide)s and α-cyclodextrin. J. Biomed. Mater. Res. 2003;65A:196–202. doi: 10.1002/jbm.a.10444. [DOI] [PubMed] [Google Scholar]
- 187.Hashim IIA, Higashi T, Anno T, Motoyama K, Abd-ElGawad AEH, El-Shabouri MH, Borg TM, Arima H. Potential use of γ-cyclodextrin polypseudorotaxane hydrogels as an injectable sustained release system for insulin. Int. J. Pharm. 2010;392:83–91. doi: 10.1016/j.ijpharm.2010.03.026. [DOI] [PubMed] [Google Scholar]
- 188.Ma D, Zhang LM, Xie X, Liu T, Xie MQ. Tunable supramolecular hydrogel for in situ encapsulation and sustained release of bioactive lysozyme. J. Colloid Interface Sci. 2011;359:399–406. doi: 10.1016/j.jcis.2011.04.032. [DOI] [PubMed] [Google Scholar]
- 189.Simoes SMN, Veiga F, Torres-Labandeira JJ, Ribeiro ACF, Sandez-Macho MI, Concheiro A, Alvarez-Lorenzo C. Syringeable Pluronic-α-cyclodextrin supramolecular gels for sustained delivery of vancomycin. Eur. J. Pharm. Biopharm. 2012;89:103–112. doi: 10.1016/j.ejpb.2011.09.017. [DOI] [PubMed] [Google Scholar]
- 190.Ma D, Tu K, Zhang LM. Bioactive supramolecular hydrogel with controlled dual drug release characteristics. Biomacromolecules. 2010;11:2204–2212. doi: 10.1021/bm100676a. [DOI] [PubMed] [Google Scholar]
- 191.Ma D, Zhang LM. Supramolecular gelation of a polymeric prodrug for its encapsulation and sustained release. Biomacromolecules. 2011;12:3124–3130. doi: 10.1021/bm101566r. [DOI] [PubMed] [Google Scholar]
- 192.Harris JM, Chess RB. Effect of pegylation on pharmaceuticals. Nat. Rev. Drug Discov. 2003;2:214–221. doi: 10.1038/nrd1033. [DOI] [PubMed] [Google Scholar]
- 193.Auzely-Velty R, Rinaudo M. New supramolecular assemblies of a cyclodextrin-grafted chitosan through specific complexation. Macromolecules. 2002;35:7955–7962. [Google Scholar]
- 194.van de Manakker F, van de Pot M, Vermonden T, van Nostrum CF, Hennink WE. Self-assembling hydrogels based on β-cyclodextrin/cholesterol inclusion complexes. Macromolecules. 2008;41:1766–1773. [Google Scholar]
- 195.van de Manakker F, Braeckmans K, el Morabit N, Smedt SCD, van Nostrum CF, Hennink WE. Protein-release behavior of self-assembled PEG-β-cyclodextrin/PEG-cholesterol hydrogels. Adv. Funct. Mater. 2009;19:2992–3001. [Google Scholar]
- 196.van de Manakker F, Kroon-Batenburg LMJ, Vermonden T, van Nostrum CF, Hennink WE. Supramolecular hydrogels formed by β-cyclodextrin self-association and host-guest inclusion complexes. Soft Matter. 2010;6:187–194. [Google Scholar]
- 197.Peng K, Tomatsu I, Kros A. Light controlled protein release from a supramolecular hydrogel. Chem. Commun. 2010;46:4094–4096. doi: 10.1039/c002565h. [DOI] [PubMed] [Google Scholar]
- 198.Tamesue S, Takashima Y, Yamaguchi H, Shinkai S, Harada A. Photoswitchable supramolecular hydrogels formed by cyclodextrins and azobenzene polymers. Angew. Chem. Int. Ed. 2010;49:7461–7464. doi: 10.1002/anie.201003567. [DOI] [PubMed] [Google Scholar]
- 199.Li C, Luo GF, Wang HY, Zhang J, Gong YH, Cheng SX, Zhuo RX, Zhang XZ. Host-guest assembly of pH-responsive degradable microcapsules with controlled drug release behavior. J. Phys. Chem. 2011;C(115):17651–17659. [Google Scholar]
- 200.Xiao W, Chen WH, Zhang J, Li C, Zhuo RX, Zhang XZ. Design of a photoswitchable hollow microcapsular drug delivery system by using a supramolecular drug-loading approach. J. Phys. Chem. B. 2011;115:13796–13802. doi: 10.1021/jp208692c. [DOI] [PubMed] [Google Scholar]
- 201.Wintgens V, Nielsen TT, Larsen KL, Amiel C. Size-controlled nanoassemblies based on cyclodextrin-modified dextrans. Macromol. Biosci. 2011;11:1254–1263. doi: 10.1002/mabi.201100097. [DOI] [PubMed] [Google Scholar]
- 202.Bohm I, Ritter H. Stimuli responsive size control of hyperbranched polymers. Macromol. Chem. Phys. 2011;212:1080–1085. [Google Scholar]
- 203.Bohm I, Isenbugel K, Ritter H, Branscheid R, Kolb U. Cyclodextrin and adamantane host-guest interactions of modified hyperbranched poly(ethylene imine) as mimetics for biological membranes. Angew. Chem. Int. Ed. 2011;50:7896–7899. doi: 10.1002/anie.201101604. [DOI] [PubMed] [Google Scholar]
- 204.Bohm I, Kreth SK, Ritter H, Branscheid R, Kolb U. Switchable supramolecular crosslinking of cyclodextrin-modified hyperbranched polyethylenimine via anthraquinone dyes. Macromol. Chem. Phys. 2012;213:243–248. [Google Scholar]
- 205.Zhang XJ, Zhang XG, Wu ZM, Gao XJ, Cheng C, Wang Z, Li CX. A hydrotropic β-cyclodextrin grafted hyperbranched polyglycerol co-polymer for hydrophobic drug delivery. Acta Biomater. 2011;7:585–592. doi: 10.1016/j.actbio.2010.08.029. [DOI] [PubMed] [Google Scholar]
- 206.Zhang XJ, Zhang XG, Wu ZM, Gao XJ, Shu SJ, Wang Z, Li CX. β-Cyclodextrin grafting hyperbranched polyglycerols as carriers for nasal insulin delivery. Carbohydrate Polym. 2011;84:1419–1425. [Google Scholar]
- 207.Yhaya F, Lim J, Kim Y, Liang M, Gregory AM, Stenzel MH. Development of micellar novel drug carrier utilizing temperature-sensitive block copolymers containing cyclodextrin moieties. Macromolecules. 2011;44:8433–8445. [Google Scholar]
- 208.Chen SP, Wang YT. Study on β-cyclodextrin grafting with chitosan and slow release of its inclusion complex with radioactive iodine. J. Appl. Polym. Sci. 2001;82:2414–2421. [Google Scholar]
- 209.Prabaharan M, Mano JF. Hydroxypropyl chitosan bearing β-cyclodextrin cavities: Synthesis and slow release of its inclusion complex with a model hydrophobic drug. Macromol. Biosci. 2005;5:965–973. doi: 10.1002/mabi.200500087. [DOI] [PubMed] [Google Scholar]
- 210.Prabaharan M, Gong SQ. Novel thiolated carboxymethyl chitosan-g-β-cyclodextrin as mucoadhesive hydrophobic drug delivery carriers. Carbohydrate Polym. 2008;73:117–125. [Google Scholar]
- 211.Liu Y, Yu ZL, Zhang YM, Guo DS, Liu YP. Supramolecular architectures of β-cyclodextrin-modified chitosan and pyrene derivatives mediated by carbon nanotubes and their DNA condensation. J. Am. Chem. Soc. 2008;130:10431–10439. doi: 10.1021/ja802465g. [DOI] [PubMed] [Google Scholar]
- 212.Huang Y, Kang Q. Synthesis of conjugates of β-cyclodextrin with polyamidoamine dendrimers and their molecular inclusion interaction with levofloxacin lactate. J. Incl. Phenom. Macrocycl. Chem. 2012;72:55–61. [Google Scholar]
- 213.Zhu YX, Che L, He HM, Jia Y, Zhang JX, Li XH. Highly efficient nanomedicines assembled via polymer-drug multiple interactions: tissue-selective delivery carriers. J. Control. Release. 2011;152:317–324. doi: 10.1016/j.jconrel.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 214.Pun SH, Bellocq NC, Liu AJ, Jensen G, Machemer T, Quijano E, Schluep T, Wen SF, Engler H, Heidel JD, Davis ME. Cyclodextrin-modified polyethylenimine polymers for gene delivery. Bioconjugate Chem. 2004;15:831–840. doi: 10.1021/bc049891g. [DOI] [PubMed] [Google Scholar]
- 215.Wang H, Wang ST, Su HL, Chen KJ, Armijo AL, Lin WY, Wang YJ, Sun J, Kamei KI, Czernin J, Radu CG, Tseng HR. A supramolecular approach for preparation of size-controlled nanoparticles. Angew. Chem. Int. Ed. 2009;48:4344–4348. doi: 10.1002/anie.200900063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Chen Y, Zhou LZ, Pang W, Qiu F, Jiang XL, Zhu XY, Yan DY, Chen Q. Photoluminescent hyperbranched poly(amido amine) containing β-cyclodextrin as a nonviral gene delivery vector. Bioconjugate Chem. 2011;22:1162–1170. doi: 10.1021/bc200010w. [DOI] [PubMed] [Google Scholar]