Abstract
A short and efficient synthesis of model spiroiminals that have the same stereochemistry but different conformations than marineosins A and B was carried out in 6-7 steps from 6-methyltetrahydropyran-2-one. These spiroiminals were also prepared biomimetically by reduction of an enol ether. A more highly substituted spiroiminal with the same stereochemistry and conformation as marineosin A was prepared in 11 steps from 6-methyl-4-propyl-3-phenyltetrahydropyran-2-one. A macrocyclic pyrrole lactone was prepared stereospecifically in 10 steps. A five step sequence converted the lactone to a late hemi-iminal intermediate that has resisted the methylation and spiroiminal formation that would lead to marineosin A.
Introduction
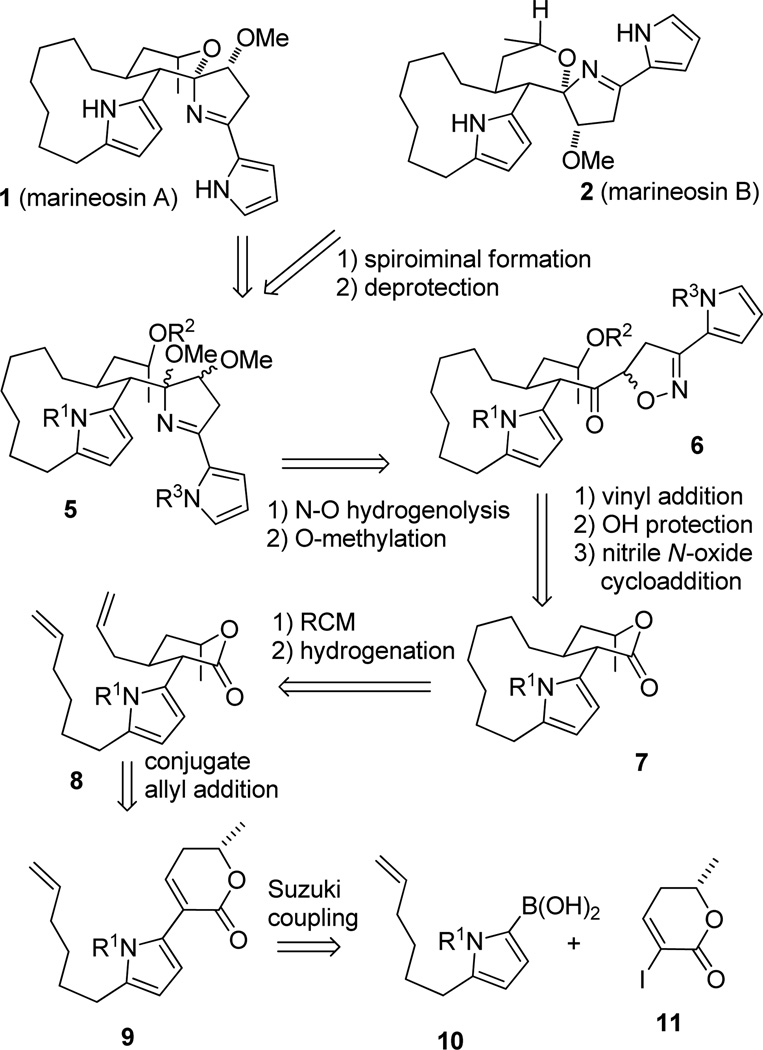
In 2008 Fenical and co-workers isolated the cytotoxic spiroiminals marineosins A (1) and B (2) from a marine-derived Streptomyces-related actinomycete (see Scheme 1).1 The structures were determined by analysis of the NMR spectra with the stereochemistry assigned by interpretation of the NOESY spectra. Marineosins A and B differ in stereochemistry at both C-7 and C-8. MMX calculations and examinations of models suggest that the marineosin isomers at the spiroiminal center (C-8) are much less stable than the two isolated isomers because of steric interactions between the methoxy group and the adjacent pyrrole in the macrocycle. The major isomer marineosin A (1) inhibited human colon carcinoma HCT-116 with an IC50 of 0.5 µM and testing in the NCI 60 cell line panel showed considerable selectivity against melanoma and leukemia cell lines. In contrast, marineosin B (2) showed considerably weaker cytotoxicity against human colon carcinoma HCT-116 with an IC50 of 46 µM.
SCHEME 1.
Structures and Biosynthesis of Marineosins A and B
Marineosins A (1) and B (2) are novel members of the prodigiosin family of bacterial pigments that appear to be derived from an undecylprodiginine (undecylprodigiosin).2 There are many examples of both spiroaminals and iminals, but the spiro-tetrahydropyran-dihydropyrrole (spiroiminal) moiety of the marineosins appears to be unprecedented. Fenical proposed that the biosynthesis of marineosins A and B involves an inverse electron demand intramolecular Diels-Alder reaction with a side chain enone as the diene to give a dihydropyran that undergoes a four-electron reduction to give the marineosins.1 Lindsley3 and Haran4 established that this Diels-Alder reaction could not be achieved in the laboratory suggesting that it is not the biosynthetic route.
Reynolds and Salem sequenced the gene cluster responsible for the biosynthesis of marineosins A and B in Streptomyces CNQ-617.5 The enzyme MarG, a RedG homolog from the mar gene cluster, oxidizes hydroxyundecylprodigiosine 3 at the asterisked carbon. Subsequent macrocyclization and spiroiminal formation affords dehydromarineosin A (4). The enzyme MarA, a putative dehydrogenase/reductase, catalyzes the reduction of 4 to afford marineosin A (1).
In 2010, we communicated the synthesis of spiroiminal models 18–21.6 Shi recently reported a very different approach to spiroiminals 18b–21b7 and Lindsley prepared analogous spiroiminals lacking the methyl group.8 Lindsley also reported the synthesis of the functionalized macrocyclic pyrrole core of marineosin A.9
We report here the full details of our spiroiminal model studies, including those with a fully substituted tetrahydropyran ring with the marineosin A stereochemistry and conformation. We also describe an approach to marineosin A that leads to a fully functionalized macrocyclic core lacking the spiroiminal ring. Our synthetic plan is shown in retrosynthetic form in Scheme 2. The synthesis of 1 and 2 will be completed by acid-catalyzed spiroiminal formation of methoxy iminal 5 and pyrrole deprotection. Hydrogenolysis of isoxazoline 6 over Raney nickel should give a hemi-iminal that will be methylated to give 5. Addition of a vinyl anion to lactone 7 and protection of the alcohol will give a vinyl ketone that will react with a protected pyrrole nitrile oxide to give isoxazoline 6, most likely as a mixture of diastereomers that will both be elaborated to both marineosins A or B. Ring-closing metathesis of diene 8 and hydrogenation will construct the macrocycle of 7. Conjugate addition of an allyl group to 9 should occur by axial attack from the face opposite the methyl group. Equilibration should give the desired stereoisomer of 8 with equatorial allyl and hexenylpyrrole groups. Pyrrole lactone 9 will be prepared by a Suzuki coupling of pyrrole boronic acid 10 and iodolactone 11.
SCHEME 2.
Retrosynthesis of Marineosins A and B
Results and Discussion
We started with a model study to prepare phenyl spiroiminals 18a–21a for two reasons (see Scheme 3). The unprecedented spiroiminal moiety is the most intriguing, but also most challenging, moiety of the marineosins (1 and 2). The phenyl group is more stable than the pyrrole group,10 and will allow us to first address the spiroiminal moiety without worrying about the instability of the pyrrole.
SCHEME 3.
Synthesis of Methoxy Iminal 16
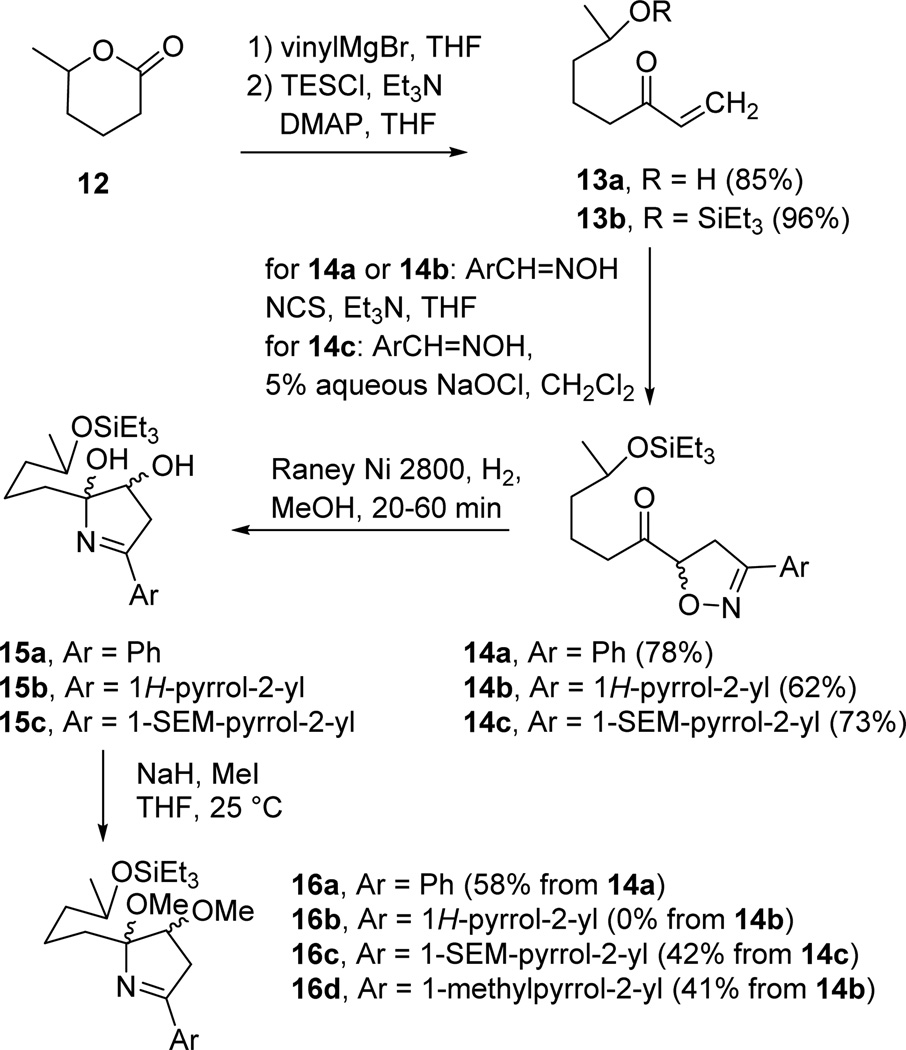
Treatment of readily available model lactone 12 with vinylmagnesium bromide afforded the known hydroxy ketone 13a in 85% yield (see Scheme 3).11 Benzaldehyde oxime was treated with NCS at room temperature to provide the chloro oxime, which was cooled to −78 °C and treated with Et3N to generate benzonitrile N-oxide, which was treated with enone 13a to provide the hemiketal form of the isoxazoline in 75% yield as a 1:1 mixture of two diastereomers (see eq 1). Torsell reported in 1983 that hydrogenolysis of the isoxazolinyl methyl ketone analogous to 14a gave a hemi-iminal.12 Vinyl ketone 13a exists preferentially in the open form because conjugation energy (~3 kcal/mol) is lost on cyclization to the hemiketal. However, once the isoxazoline is formed, the hemiketal dominates (>95%) in the equilibrium between the hemiketal and the corresponding saturated hydroxy ketone. Therefore it is not surprising that hydrogenolysis of the isoxazoline hemiketal formed from 13a over Raney nickel to reduce the isoxazoline failed to form a hemi-iminal by cyclization of the imine to the ketone. A variety of other reduction approaches were also unsuccessful.13

To prevent hemiketal formation we protected the hydroxy group of 13a with TESCl, Et3N, and DMAP to give 13b in 96% yield. Reaction of benzaldehyde oxime, NCS, and Et3N at 25 °C generated benzonitrile N-oxide which was cooled to −78 °C and treated with 13b to give the [3 + 2]-cycloadduct isoxazoline ketone 14a in 78% yield as a 1:1 mixture of diastereomers. As expected, treatment of isoxazoline ketone 14a with Raney Ni 2800 and H2 in MeOH afforded the desired hemi-iminal 15a as a mixture of diastereomers. Unfortunately, attempted deprotection of the silyl ether under a variety of acidic conditions resulted in decomposition, rather than formation of the desired hydroxy spiroiminal. Hemi-iminal 15a even decomposed in CDCl3 (containing adventitious HCl) in 10 h. We therefore treated 15a with sodium hydride and methyl iodide to afford methyl ether iminal 16a in 58% yield from 14a.
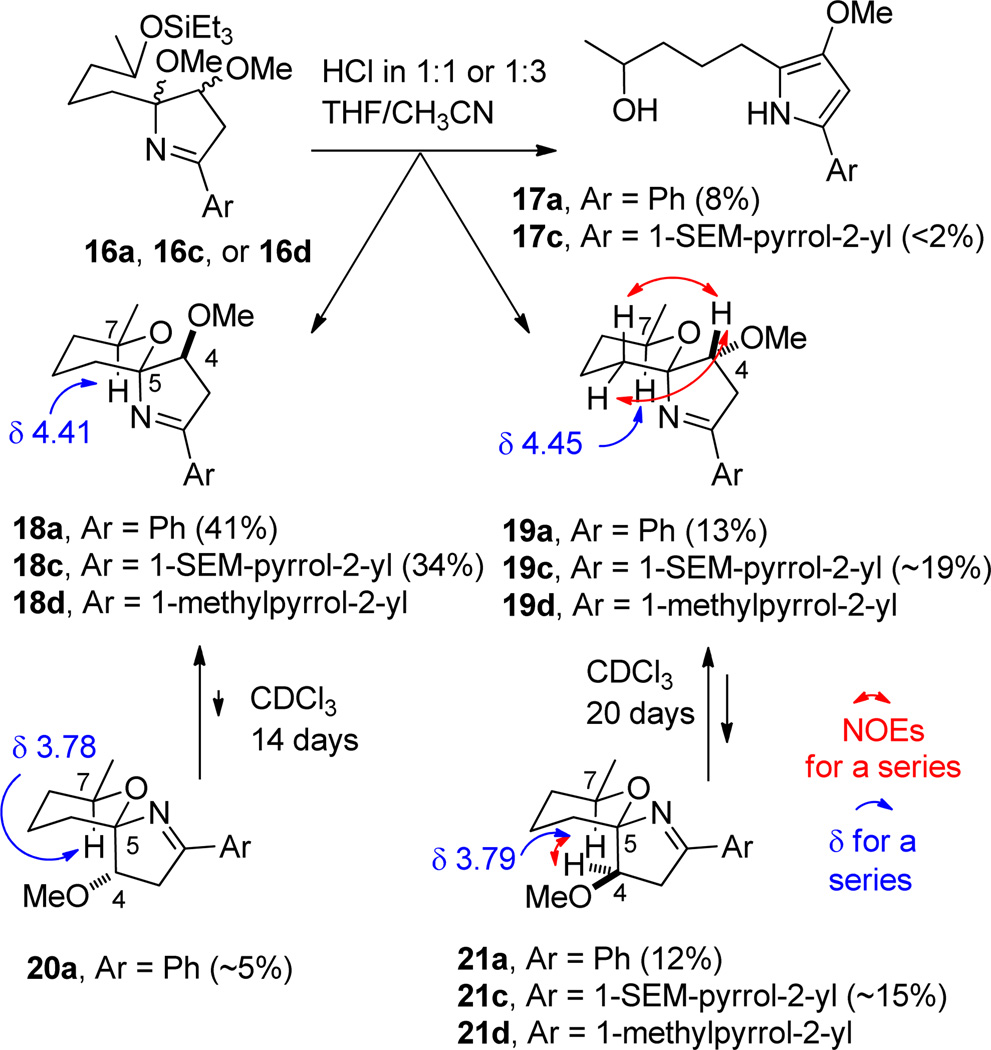
The conversion of 16a to spiroiminals 18a–21a was explored under a variety of acidic conditions (see Scheme 4). Treatment of 16a with 2% TFA in chloroform or PPTS in MeOH resulted in decomposition. Treatment of 16a with HF•Pyr and pyridine in THF gave the desired spiroiminals 18a–21a in only 15% yield and the undesired methoxypyrrole 17a in ~50% yield. Finally, we found that treatment of 16a with 2 M HCl in 1:1 THF/ CH3CN afforded three of the four desired spiroiminal diastereomers 18a (46%), 19a (13%) and 21a (12%) and only 8% of the undesired methoxypyrrole 17a. Over a 2–3 week period in CDCl3 (containing adventitious HCl), solutions of either pure 19a or 21a equilibrated to an identical 3:1 mixture of 19a and 21a. Therefore, these two spiroiminals differ only at the iminal center C-5 and have the identical relative stereochemistry at C-4 and C-7. Under the same conditions, the major isomer 18a equilibrated to give a 19:1 mixture of 18a and 20a. Therefore, these two compounds also differ only at the iminal center C-5. Attempts to accelerate the equilibration of 18a–21a by addition of 2% TFA to CDCl3 resulted in the formation of methoxypyrrole 17a.
SCHEME 4.
Preparation of Spiroiminals 18–21.
The structure of 21a was established by an NOE between the CHOMe proton H-4 and the CHMe proton H-7 as shown in Scheme 4. In the other three isomers these two protons are too far apart for an NOE to be observed. The structure of 19a follows from the structure of 21a because these compounds differ only in the stereochemistry at the spiroiminal center. The structure of 19a was confirmed by NOEs between the CHOMe proton H-4 and the adjacent tetrahydropyran methylene group as shown in Scheme 4. The CHMe proton H-7 in 18a (δ 4.41) and 19a (δ 4.45) is deshielded by the axial nitrogen and absorbs much further downfield than the CHMe proton H-7 in 20a (δ 3.78) and 21a (δ 3.79) with an axial carbon.14 The major isomer 18a has no NOEs as expected between the protons on the tetrahydropyran ring and those on the dihydropyrrole ring.
The presence of the imine double bond makes the formation of spiroiminals from 16a quite different from that of spiroketals and spiroaminals.15 Desilylation should occur easily to give the alcohol. Protonation of the resulting alcohol on the iminal methoxy group and loss of MeOH would give a stabilized allylic type cation C+-N=C that could cyclize to form the spiroiminal, but the nitrogen lone pair can't stabilize the cation by resonance because the five-membered ring precludes a linear cumulene C=N+=C. Formation and equilibration of the spiroiminals could also occur by initial isomerization of the imine to an enamine or by protonation on the imine nitrogen and ring opening of the dihydropyrrole ring to give an oxycarbenium ion.
Having developed a sequence to make phenyl-substituted spiroiminals 18a–21a, we turned our attention to making pyrrole-substituted spiroiminals 18b–21b, which have the same spiroiminal moieties as marineosins A and B (1 and 2). Treatment of 13b with 2-pyrrolecarboxaldehyde oxime in THF with NCS and Et3N in THF at −78 °C provided isoxazoline 14b in 62% yield. Hydrogenolysis of 14b over Raney nickel provided hemi-iminal 15b, but selective methylation of the hydroxyl groups without methylation of the pyrrole could not be achieved. The N-Me dimethyl ether 16d was obtained in 41% yield from 14b with NaH and MeI. Other methylation conditions were investigated unsuccessfully. Treatment of N-Me dimethyl ether 16d with 2 M HCl afforded N-Me pyrrole spiroiminals 18d, 19d, and 21d in 65% yield as a mixture of three diastereomers whose structures were assigned by analogy to 18a, 19a, and 21a.
We therefore needed to protect the pyrrole N-H to prevent N-methylation. Oxidation of N-Boc-2-pyrrolecarboxaldehyde oxime with PhI(OAc)216 generated N-Boc-2-pyrrole2-carbonitrile N-oxide, which added to enone 13b to produce the desired isoxazoline 14, Ar = N-Boc-pyrrol-2-yl, in 55% yield. However, treatment of the N-Boc pyrrole isoxazoline with Raney Ni and hydrogen not only cleaved the N-O bond of the isoxazoline but also hydrogenated the N-Boc pyrrole. The strong electron-withdrawing group on the pyrrole nitrogen reduces the aromaticity of the pyrrole ring making the pyrrole susceptible to hydrogenation.17
A SEM-protected pyrrole should be compatible with the hydrogenation step.18,19 N-SEM-pyrrole-2-carboxaldehyde18 was easily converted to the oxime with hydroxylamine hydrochloride and sodium acetate in aqueous MeOH. However, the oxidation conditions (NCS or iodobenzene diacetate) that were successful with other oximes gave 14c in <30% yield. Fortunately, reaction of N-SEM-pyrrole-2-carboxaldehyde oxime with 5% aqueous NaOCl20 in CH2Cl2 at 25 °C generated the nitrile N-oxide that reacted with enone 13b to give isoxazoline 14c in 73% yield. Hydrogenolysis over Raney Ni and methylation both now proceeded uneventfully to give 16c in 42% yield from 14c. Treatment of 16c with 2 M aqueous hydrochloric acid in 1:3 THF/CH3CN hydrolyzed the triethylsilyl ether and effected loss of methanol and cyclization to give SEM-protected spiroiminal 18c (34%), an inseparable 3:2 equilibrium mixture of SEM-protected spiroiminals 19c and 21c (34%), and <2% of methoxypyrrole 17c. The 1H and 13C NMR spectra of these spiroiminals in the aliphatic region spiroiminals are virtually identical to those of 18a–21a and their stereochemistry was assigned accordingly.
The initial model study was completed by deprotection of 18c with TBAF and molecular sieves in THF at 60 °C for 3 hours to provide spiroiminal 18b in 54% yield (see Scheme 5). Similarly, deprotection of the 3:2 mixture of 19c and 21c afforded a 7:3 mixture of 19b and 21b in 56% yield. The stereochemistry was again assigned from the NMR spectra, which are very similar to those of 18a–21a.
SCHEME 5.
Deprotection of 18c–21c
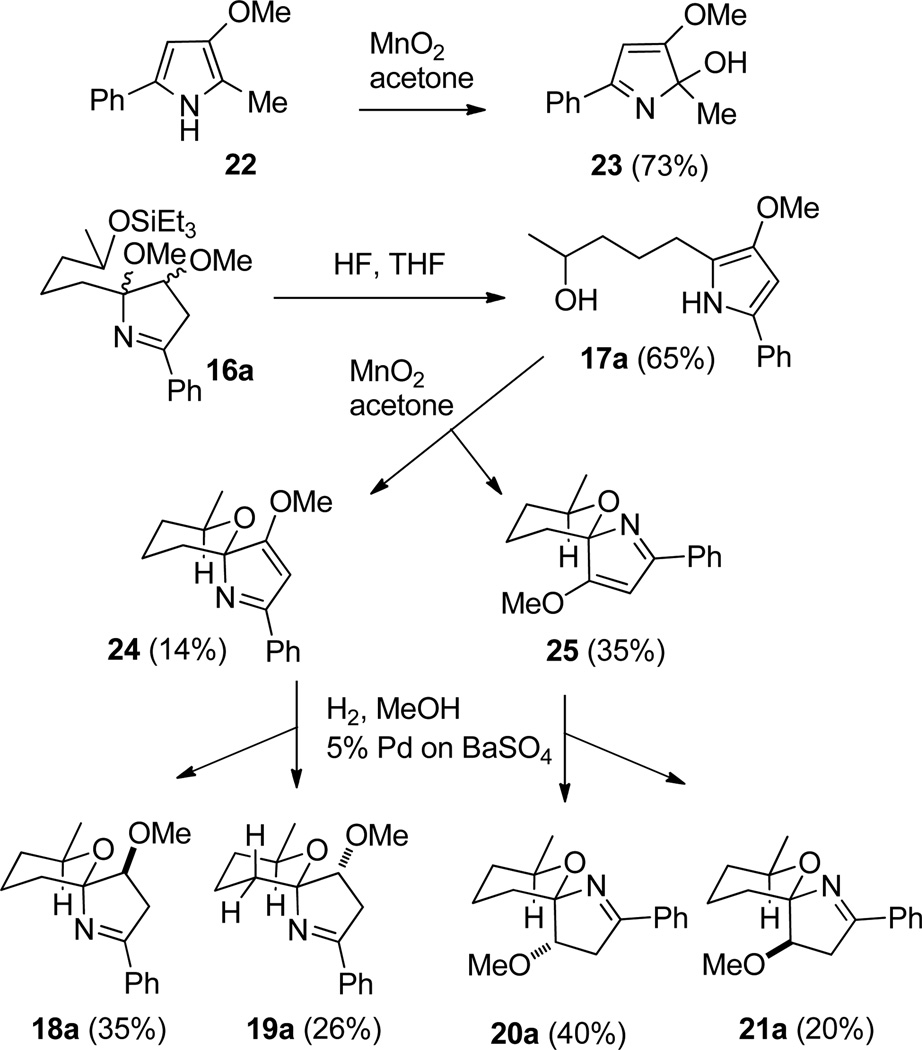
Salem and Reynolds’s biosynthetic studies established that the last step of the biosynthesis of marineosin A (1) is the reduction of the enol ether of 4 to give 1.5 We wanted to explore this approach for the preparation of model spiroiminals 18–21. Berner and co-workers reported that oxidation of 3-methoxypyrrole 22 with MnO2 in acetone afforded 23 in 73% yield (see Scheme 6).21 We thought that the analogous oxidation of 3-methoxypyrrole 17a would give 24 and 25, with the oxidized intermediate trapped intramolecularly by the hydroxy group in the side chain rather than intermolecularly by water. 3-Methoxy-5-phenylpyrrole 17a was a minor byproduct (8%) in the cyclization of 16a to 18a–21a with HCl in THF/CH3CN, but was formed in ~50% yield on treatment of 16a with HF•pyr and pyr. Further optimization led to the formation of 17a in 65% yield by treatment of 16a with HF in THF. As expected, treatment of 17a with MnO2 in acetone gave a mixture of easily separated spiroiminals 24 (14%) and 25 (35%), whose stereochemistry was assigned by hydrogenation to 18a–21a.
SCHEME 6.
Biomimetic Synthesis of 18a–21a
Hydrogenation of the major isomer 25 over Pd/C afforded a mixture of over-reduced spiroaminal diastereomers in ~70% yield in which both the imine and enol ether double bonds had been hydrogenated. Hydrogenation of 25 over Raney Ni gave a mixture of the previously prepared spiroiminals 20a (15%), and 21a (15%) in addition to 3-methoxy-5-phenylpyrrole 17a (45%) resulting from hydrogenolysis. The best results were obtained by hydrogenation of 25 over 5% Pd/BaSO4, which afforded the desired spiroiminals 20a (40%) and 21a (20%) and only 10% of 3-methoxypyrrole 17a. Similarly, hydrogenation of the minor isomer 24 over 5% Pd/BaSO4 provided 18a (35%), 19a (26%) and 17a (10%). We were pleased to find that spiroiminal 20a, which has the same stereochemistry as marineosin A (1) was obtained in 40% yield by hydrogenation of 25. This isomer was formed in trace amounts by the HCl-catalyzed spiroiminal formation from 16a and was formed in only ~5% yield during the equilibration of 18a in CDCl3 over two weeks.
3-Methoxy-5-phenylpyrrole 17a was obtained in 65% yield by treating 16a with HF in THF. Unfortunately treatment of N-SEM-pyrrole dimethyl ether 16c with HF•Pyr or HF in THF and a variety of other acidic conditions gave a complex mixture rather than the desired 2,2'-bi-1H–pyrrole 17c so this approach can't be used to prepare 18c–21c.22
Marineosin A (1) and model spiroiminal 20a have the same stereochemistry but very different conformations. As expected 20a adopts the conformation with an equatorial nitrogen and methyl group, whereas the macrocyclic ring of 1 locks the conformation of the tetrahydropyran ring so that both the nitrogen and methyl groups are axial. We therefore next turned to the preparation of a more highly substituted model lactone that would lead to a spiroiminal with the same conformation as marineosin A.
Both the second model study and the marineosin A synthesis start with (±)-parasorbic acid (26),23 but enantiomerically pure 26 can be easily prepared once the sequence is worked out (see Scheme 7).24 Parasorbic acid (26) was treated with iodine and pyridine to afford iodolactone 11 in 74% yield. Suzuki coupling of iodolactone 11 with phenylboronic acid and 10 mol% Pd(PPh3)4 afforded phenyl lactone 27 in 47% yield. The yield was increased to 85% by using Buchwald's Pd(OAc)2, SPhos and n-butanol conditions.25
SCHEME 7.
Preparation of Vinyl Ketone 33
All attempts to achieve 1,4-addition of an allylcuprate to 27 were unsuccessful as had previously been noted for a related unsaturated lactone by Waldmann and co-workers.26 Trauner and co-workers reported that treatment of a variety of enones with allyltributyltin and Tf2O afforded the corresponding vinyl triflates that can be used for intramolecular Heck reactions.27 Unsaturated lactone 27 reacted similarly with allyltributyltin, di-t-butyl peroxide and Tf2O to afford the vinyl triflate in 78% yield, which underwent Stille coupling with tributylvinyltin and Pd(PPh3)4 to afford 30 in 83% yield. Cycloaddition of 30 with N-SEM-pyrrolecarboxaldehyde oxime and NaOCl occurred selectively as desired on the vinyl group of 30 in 58% yield. However, we were not able to hydrolyze the dihydropyran enol ether to give the required hydroxy ketone precursor for the Raney nickel hydrogenation step.
Fortunately, treatment of unsaturated lactone 27 with allylmagnesium bromide, ZnBr2, and TMSCl as described by Waldmann afforded a 1:3 to 1:1 mixture of the desired conjugate addition products 28a and 29a in 63–72% yield. We expected that conjugate addition of the allyl group would occur by axial attack from the face opposite the methyl group. Treatment of the mixture with DMAP in CDCl3 provided a 6:1 equilibrium mixture favoring the desired trans isomer 28a. The equilibration of 28a and 29a establishes that they differ only in the stereochemistry at the phenyl substituted carbon. The vicinal coupling constants between the methine hydrogens establish that the phenyl and allyl groups are trans in 28a (J = 9.8 Hz) and cis in 29a (J = 5.6 Hz). The stereochemistry of 28a was confirmed by a strong NOE between the CHPh proton at δ 3.46 and CHMe at δ 4.66-4.56, which indicates that these two protons are mast protons in the expected boat conformer28 of 28a. The allyl double bond is needed for the ring-closing metathesis in the synthesis, but not for the model study, so the 6:1 mixture of 28a and 29a was hydrogenated over Raney nickel to afford a 6:1 mixture of trisubstituted lactones 28b and 29b.
Attempted addition of vinylmagnesium bromide to the 6:1 mixture of 28b and 29b resulted in enolization and the formation of a 1:1 mixture of 28b and 29b on acidification. The phenyl group makes the α-proton more acidic than those of lactone 12 and hinders the approach of the nucleophile to the carbonyl group. Addition of CeCl3 to the Grignard reagent helped, but the desired hydroxy vinyl ketone analogous to 13a was obtained in only 17% yield along with ~70% recovered 28b and 29b. Fortunately, treatment of the 6:1 mixture of lactones 28b and 29b with i-PrMgCl and N,O-dimethylhydroxylamine•HCl29 afforded Weinreb amides 31a (78%) and the diastereomer 31b (13%), which were easily separated. Reaction of 31a with TESCl, Et3N, and DMAP gave TES ether 32 in 85% yield, which was treated with vinylmagnesium bromide to give protected vinyl ketone 33 in 72% yield.
The sequence developed to elaborate 13b to 18c–21c worked efficiently to convert 33 to 36 and 37 (see Scheme 8). Cycloaddition of 33 with N-SEM-pyrrole oxime and NaOCl afforded a difficultly separable 7:1 mixture of 34 and 35 in 61% yield, whose stereochemistry was assigned from the stereochemistry of the final model spiroiminals 36 and 37. Hydrogenolysis of the isoxazoline over Raney nickel, methylation with NaH and MeI, and HCl-catalyzed hydrolysis of the silyl ether and cyclization afforded the spiroiminals 36 (33% from 34) and 37 (15% from a 1:1 mixture of 34 and 35).
SCHEME 8.
Preparation of Marineosin A Model 36
The stereochemistry of 36 and 37 was assigned by analysis of the 1H NMR spectral data. The CHPh proton H-10 absorbs as a doublet at δ 2.84 (J = 10.9 Hz) in 36 and at 8 3.01 (J = 11.6 Hz) in 37 thereby establishing that both the phenyl and propyl groups are equatorial in both 36 and 37. The axial methyl groups are deshielded by the 1,3-diaxial nitrogen and absorb at δ 1.47 in 36 and δ 1.57 in 37. These shifts are similar to that of the axial methyl group in marineosin A (1) at δ 1.51 and very different from those of the equatorial methyl groups of 18c, 19c and 21c at δ 1.13, 1.21 and 1.21, respectively and the methyl group of marineosin B (2) at δ 1.20. A large NOE between the axial CHPr proton H-9 and the axial methyl group confirmed this stereochemical assignment. The stereochemistry of the methoxy group was established by NOEs between the CHPh proton H-10 and the CHOMe proton H-7 in 36 and between the CHPh methine proton H-10 and the methoxy group in 37 as shown in Scheme 8.
The single stereocenter in 13b is too far from the vinyl ketone to affect the cycloaddition so that 14 was obtained as a 1:1 mixture of isomers. We were very encouraged by the observation that the two additional stereocenters in vinyl ketone 33 are close enough to the double bond to influence the stereochemistry of the cycloaddition leading to 7:1 selectivity favoring the isomer with the marineosin A stereochemistry. Furthermore spiroiminal 36 with a fully substituted tetrahydropyran ring adopts the same conformation as marineosin A, whereas model 20, which has the same stereochemistry as 36, adopts the other chair conformation with an equatorial nitrogen and methyl group. Minor spiroiminal 37 has the same stereochemistry as marineosin B (2) at the methoxy-substituted carbon, but the opposite stereochemistry at the spiroiminal carbon.
Having developed a practical route to 36 with the marineosin A stereochemistry and conformation, we turned our attention to preparing lactone 7 with the macrocyclic pyrrole tether. N-(Ts)-pyrrole-2-carboxaldehyde (38) was treated with 4-pentenylmagnesium bromide, NMO/TPAP, and then NaBH4 under Muchowski's conditions3,30 to generate 2-(5-hexenyl)pyrrole (39)31 in 47% overall yield (see Scheme 9). Protection of 39 with (Boc)2O, Et3N, and DMAP afforded N-Boc-pyrrole 40 in 74% yield. Boronic acid 41 was prepared using Fürstner's procedure for 4-pentenyl-N-Boc-pyrroleboronic acid.31 Deprotonation at the 5-position with lithium 2,2,6,6-tetramethylpiperidide followed by trapping of the resulting carbanion with trimethyl borate gave the unstable boronic acid 41 in about 60% yield. Great care was required during the workup. The organic phases from the extraction were slowly concentrated at room temperature until a solid started to precipitate. The mixture was then cooled to 0 °C and was filtered. Trituration of the solid with cold ether afforded 41 as a yellowish solid that was used immediately for the Suzuki coupling. To our delight, treatment of iodolactone 11 and boronic acid 41 with Pd(PPh3)4, Na2CO3, and LiCl in aqueous 1,2-dimethoxyethane at 80 °C afforded pyrrolyl lactone 42 in 55% yield.
SCHEME 9.
Synthesis of Macrocyclic Fused Lactone 47b
We were unable to remove the Boc protecting group at this point, so we treated unsaturated lactone 42 with allylmagnesium bromide, ZnBr2, and TMSCl. To our surprise, we obtained an inseparable 5:4 mixture of the undesired cis isomer 43 and ketene acylal 44 resulting from Boc migration in 56% yield. The CH-pyrrole proton of 43 absorbs as a doublet at δ 4.73 (J = 4.9 Hz) analogously to that of 29a, δ 3.94 (J = 5.6 Hz). There is no CH-pyrrole proton in 44, but LCMS analysis indicates that it has the same molecular weight as 43, suggesting that a precedented Boc migration occurred.32 Apparently, the enolate generated by conjugate addition of the allyl group undergoes kinetically controlled protonation from the face opposite the allyl group to give cis isomer 43 and Boc migration to give 44. Fortunately, treatment of the 5:4 mixture of 43 and 44 with TMSOTf and 2,6-lutidine32a removed the Boc groups from both compounds and epimerized the α position to afford a 19:1 mixture of the desired deprotected trans isomer 45 and the deprotected cis isomer in 91% yield. The CH-pyrrole proton of 45 absorbs as a doublet at δ 3.57 (J = 6.7 Hz) and shows a strong NOE to the CHMe proton establishing that lactone 45 also adopts a boat conformation. The coupling constant is smaller than those of the CHPh proton (J = 9.8 Hz) in 28a and the CH-N-SEM-pyrrole proton (J = 9.2 Hz) in the protected analogue of 45 that leads to 47a. This suggests that hydrogen bonding between the lactone carbonyl group and the pyrrole nitrogen perturbs the lactone conformation in 45
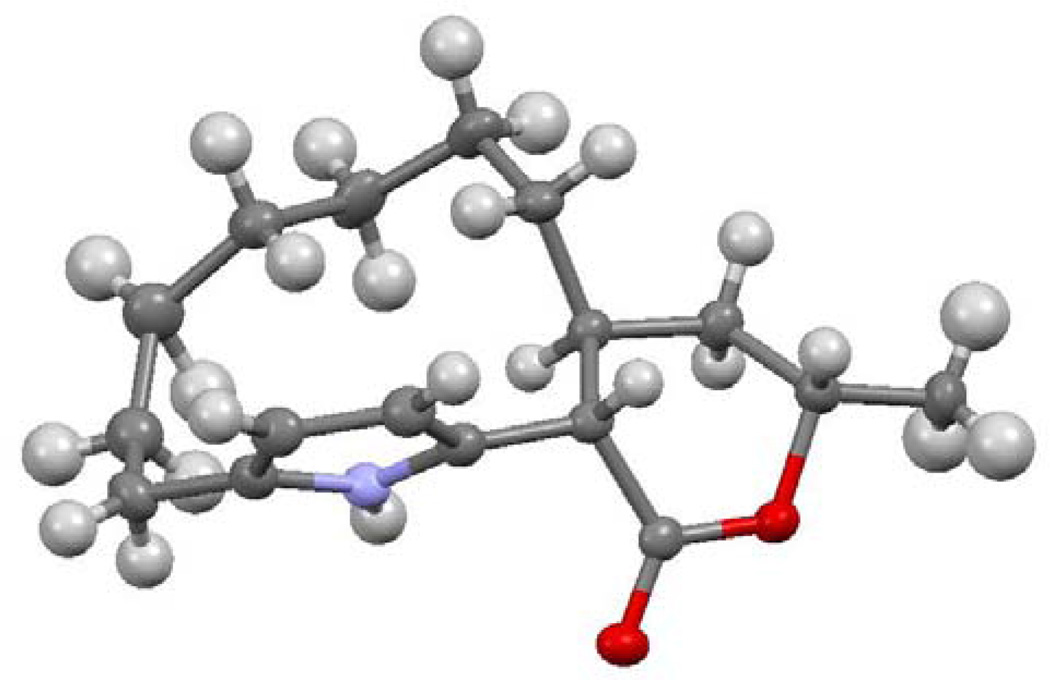
Treatment of a 10−4 molar solution of 45 with 15 mol% Grubbs II catalyst in CH2Cl2 at reflux for 12 hours gave 46 as a cis/trans mixture of isomers in 18% yield. The yield of 46 was increased to 41% by using more catalyst (2 × 15 mol%) and prolonging the reaction time to 16 h. Although this yield was not satisfactory, it provided sufficient material for further elaboration. Hydrogenation of 46 over Raney nickel reduced the double bond to afford 47b as a single compound in 90% yield. The 1H NMR spectrum of 47b shows the expected shielding of protons on the seven carbon methylene bridge by the pyrrole ring to δ 0.83 (m, 1) and δ 0.39 (m, 1) similar to that observed in marineosins A at δ 0.69 (m, 1) and δ 0.52 (m, 1). The large coupling constant for the CH-pyrrole proton at δ 3.47 (J = 12.1 Hz) indicates that the macrocycle is trans fused to the lactone. The structure of 47b was confirmed by X-ray crystal structure determination, which indicates that the lactone adopts the boat conformation as shown in Figure 1.
Figure 1.
Structure of 47b determined by X-ray crystallography. The tether is disordered with two positions for atom C10; only the major component (92.2%) is shown.
N-H pyrrole lactone 47b was converted to N-H pyrrole isoxazoline 51b by the sequence developed to prepare isoxazolines 34 and 35 (see Scheme 10). Treatment of 47b with i-PrMgCl and N,O-dimethylhydroxylamine•HCl afforded Weinreb amide 48b in 86% yield, which was protected to give triethylsilyl ether 49b in 77% yield. To our surprise, considerable reduction to the aldehyde occurred on treatment of Weinreb amide 49b with vinylmagnesium bromide. This problem has been previously noted, especially as the Grignard reagent ages.33,34 Fortunately, addition of a solution of vinyllithium34 freshly prepared from vinyl bromide and n-BuLi to 49b afforded enone 50b in 70% yield.
SCHEME 10.
Elaboration of 47b to Hemi-iminal 52b
The cycloaddition of 50b with N-SEM-pyrrole-2-carboxaldehyde oxime and NaOCl gave isoxazoline 51b in ~67% yield as a mixture of stereoisomers although the reaction was not as clean as those with enones 13b and 33. Hydrogenolysis of 51b over Raney nickel provided a crude mixture in ~67% yield that appeared to contain hemi-iminals 52b based on the similarity of the 1H NMR spectrum to those of 15 and the crude hydrogenation product from 34.
We were concerned that it might not be possible to methylate the two hydroxy groups of 52b without N-methylation of the pyrrole. However, to our disappointment, O-methylation of 52b, with or without concomitant N-methylation could not be accomplished. The use of NaH and MeI, which was successful in all the model studies, afforded a complex mixture. Other bases (KOH or KOtBu) and methylating reagents (Me2SO4) were also successful with 15a as was acid-catalyzed methylation with CH2N2 or TMSCHN2. Unfortunately, all of these conditions failed to methylate hemi-iminal 52b. Hemi-iminals are unstable and may decompose if the methylation doesn’t occur rapidly. Apparently the hydroxy groups of 52b are more hindered than those of the model hemi-iminals so that only decomposition occurs on attempted methylation.
We attempted to form the spiroiminal prior to methylation, but 52b decomposed on treatment with 2 M HCl or Dowex 50 WX ion exchange resin. Hemi-iminal 52b decomposed after 10 h in CDCl3 indicating that it is very acid sensitive.
We had previously prepared macrocyclic N-SEM pyrrole lactone 47a.13, 35 The four step sequence leading to isoxazoline 51a proceeded uneventfully, but all attempts to hydrogenolyze the isoxazoline and to form hemi-iminal 52a failed completely. We hypothesized that the protecting group on the pyrrole in the tether prevented formation of the hemi-iminal. We couldn't deprotect 47a to give 47b, so we developed the route to 47b described in detail above. We can form hemi-iminal 52b lacking the pyrrole protecting group, but the sequence fails one step later at the methylation stage.
In conclusion we have developed a short and efficient synthesis of model spiroiminals 18a–21a (six steps) and 18b–21b (seven steps) that have the same stereochemistry but different conformations than marineosins A and B. Phenyl substituted spiroiminals 18a–21a were also prepared biomimetically by reduction of an enol ether. More highly substituted spiroiminal 36 with the same stereochemistry and conformation as marineosin A was prepared in 11 steps. Macrocyclic pyrrole lactone 47b was prepared stereospecifically in 10 steps. A five step sequence converted the lactone to a late hemi-iminal intermediate 52b that has resisted the methylation and spiroiminal formation that would lead to marineosin A.
Experimental Section
General Experimental Methods
Reactions were conducted in flame- or oven-dried glassware under a nitrogen atmosphere and were stirred magnetically. The phrase "concentrated" refers to removal of solvents by means of a rotary evaporator attached to a diaphragm pump (15–60 Torr) followed by removal of residual solvents at < 1 Torr with a vacuum pump. Flash chromatography was performed on silica gel 60 (230–400 mesh). Analytical thin layer chromatography (TLC) was performed using silica gel 60 F-254 pre-coated glass plates (0.25 mm). TLC Plates were analyzed by short wave UV illumination, or by dipping in vanillin stain (27 g of vanillin in 380 mL of EtOH, 50 mL of water and 20 mL of concentrated sulfuric acid) and heating on a hot plate. THF and ether were dried and purified by distillation from sodium/benzophenone. Et3N was distilled from CaH2. 1H and 13C NMR spectra were obtained on a 400 MHz spectrometer in CDCl3 with CHCl3 as an internal standard (δ 7.26, CDCl3 at δ 77.00) unless otherwise indicated. Chemical shifts are reported in δ (ppm downfield from tetramethylsilane). Coupling constants are reported in Hz with multiplicities denoted as s (singlet), d (doublet), t (triplet), q (quartet), p (pentet), m (multiplet) and br (broad). IR spectra were acquired on an FT-IR spectrometer and are reported in wave numbers (cm−1). High resolution mass spectra were obtained using the following ionization techniques: chemical ionization (CI), electron impact (EI), electrospray ionization analyzed by quadrupole time of flight (QTof).
Benzaldehyde Oxime
A solution of benzaldehyde (530 mg, 5.0 mmol) in 20 mL of EtOH was treated with a mixture of NaOH (300 mg, 7.50 mmol) and NH2OH•HCl (783 mg, 11.4 mmol) in 10 mL of H2O. The reaction mixture was stirred at 25 °C for 6 h, concentrated to remove EtOH, diluted with CH2Cl2, washed with brine, and dried (Na2SO4). Flash chromatography on silica gel (8:1 hexanes/EtOAc) gave 482 mg (84%) of the oxime as a brown solid with data identical to those previously reported.41
1-[[2-(Trimethylsilyl)ethoxy]methyl]-1H-pyrrole-2-carboxaldehyde was prepared by the literature procedure.18 A solution of pyrrole-2-carboxaldehyde (245 mg, 2.57 mmol) in anhydrous THF (2 mL) was added dropwise to a suspension of NaH (60% in mineral oil, 124 mg, 3.09 mmol) in THF (10 mL) at 0 °C. The mixture was stirred at 0 °C for 30 min and SEMCl (0.50 mL, 2.83 mmol) was added by syringe over 3 min. The reaction was warmed to 25 °C and stirred for 2 h. The mixture was quenched with saturated aqueous NH4Cl (3 mL). The aqueous layer was extracted with EtOAc and the combined organic layers were dried (Na2SO4) and concentrated. Flash chromatography on silica gel (8:1 hexanes/EtOAc) gave 538 mg (93%) of N-SEM-pyrrole-2-carboxaldehyde as a pale yellow gum: 1H NMR 9.58 (s, 1), 7.15-7.13 (m, 1), 6.96 (dd, 1, J = 1.5, 3), 6.30 (dd, 1, J = 3, 4), 5.70 (s, 2), 3.54 (t, 2, J = 8.1), 0.89 (t, 2, J = 8.1), −0.04 (s, 9); 13C NMR 179.3, 131.6, 130.8, 125.0, 110.2, 76.2, 65.8, 17.5, −1.7 (3 C); IR (neat) 1671.
1-[[2-(Trimethylsilyl)ethoxy]methyl]-1H-pyrrole-2-carboxaldehyde Oxime
A solution of N-SEM-pyrrole-2-carboxaldehyde (538 mg, 2.39 mmol) in 11 mL of 10:1 MeOH/H2O was treated with NH2OH•HCl (183 mg, 2.63 mmol) and NaOAc (295 mg, 3.59 mmol). The resulting mixture was stirred at 25 °C for 2.5 h, concentrated to remove MeOH, diluted with CH2Cl2, washed with brine, dried (Na2SO4), and concentrated. Flash chromatography on silica gel (8:1 hexanes/EtOAc) gave 482 mg (84%) of the oxime as a brown gum: 1H NMR 8.92 (s, 1, OH), 8.20 (s, 1), 6.87-6.85 (m, 1), 6.52 (dd, 1, J = 1.2, 2.5), 6.19 (dd, 1, J = 3, 4), 5.46 (s, 2), 3.50 (t, 2, J = 8.2), 0.91 (t, 2, J = 8.2), −0.03 (s, 9); 13C NMR 142.3, 126.2, 125.2, 115.0, 109.1, 76.9, 65.5, 17.5, −1.6 (3 C); IR (neat) 3376, 1624; HRMS (EI) calcd for C11H20N2O2Si (M+) 240.1294, found 240.1298.
7-Hydroxy-1-octen-3-one (13a) was prepared by the literature procedure.11 A solution of 6-Methyltetrahydropyran-2-one (12) (0.92 g, 8.76 mmol) in anhydrous THF (15 mL) was treated with vinylmagnesium bromide (1 M in THF, 10.51 mL, 10.51 mmol) by syringe over 15 min under nitrogen at −78 °C. The resulting solution was stirred at −78 °C for 4 h. The mixture was quenched with saturated aqueous NH4Cl, diluted with EtOAc, washed with brine, dried (Na2SO4), and concentrated to give 1.11 g of crude 13a. Flash chromatography on MeOH-deactivated silica gel (4:1 hexanes/EtOAc) gave 1.06 g (85%) of 13a as a colorless liquid: 1H NMR 6.36 (dd, 1, J = 10.4, 17.4), 6.24 (d, 1, J = 17.4), 5.85 (d, 1, J = 10.4), 3.81-3.76 (m, 1), 2.64 (t, 2, J = 6.7), 2.37 (s, 1, OH), 1.75-1.65 (m, 2), 1.50-1.43 (m, 2), 1.19 (d, 3, J = 6.7); 13C NMR 201.0, 136.3, 128.2, 67.3, 39.2, 38.4, 23.3, 19.8; IR (neat) 3452 (br), 1729.
7-Triethylsilyloxy-1-octen-3-one (13b)
A solution of alcohol 13a (836 mg, 5.88 mmol) in 15 mL of THF was treated with Et3N (1.36 mL, 9.41 mmol), DMAP (69 mg, 0.59 mmol), and TESCl (1.58 mL, 9.41 mmol). The mixture was stirred at 25 °C for 3 h. The reaction was then diluted with Et2O (10 mL) and washed with brine (3 × 5 mL). The organic layer was dried (MgSO4) and concentrated to give 1.78 g of crude 13b. Flash chromatography on silica gel (18:1 hexanes/EtOAc) gave 1.45 g (96%) of 13b as a sticky liquid: 1H NMR 6.34 (dd, 1, J = 10.6, 17.6), 6.21 (d, 1, J = 17.6), 5.81 (d, 1, J = 10.6), 3.82-3.78 (m, 1), 2.59 (t, 2, J = 6.4), 1.72-1.58 (m, 2), 1.46-1.39 (m, 2), 1.14 (d, 3, J = 6.4), 0.95 (t, 9, J = 7.6), 0.58 (q, 6, J = 7.6); 13C NMR 200.8, 136.5, 127.9, 68.2, 39.6, 39.1, 23.8, 20.3, 6.9 (3 C), 4.9 (3 C); IR (neat) 1682; HRMS (EI) calcd for C14H27O2Si (M-H+) 255.1780, found 255.1787.
1-(4,5-Dihydro-3-phenyl-5-isoxazolyl)-5-triethylsilyloxy-1-hexanone (14a)
A solution of N-chlorosuccinimide (220 mg, 1.65 mmol) in anhydrous THF (3 mL) was added dropwise by syringe over 20 min to a solution of benzaldehyde oxime (170 mg, 1.40 mmol) in THF (6 mL). The mixture was stirred at 25 °C for 5 h, cooled to −78 °C, and treated with a solution of enone 13b (300 mg, 1.17 mmol) in THF (2 mL) and then Et3N (240 µL, 1.65 mmol). The mixture was gradually warmed to 25 °C and stirred for 3 h. The reaction mixture was diluted with EtOAc, washed with brine, dried (Na2SO4), and concentrated. Flash chromatography on MeOH-deactivated silica gel (12:1 hexanes/EtOAc) gave 341 mg (78%) of 14a as a 1:1 mixture of diastereomers as a colorless gum: 1H NMR 7.67 (d, 2, J = 6.1), 7.43-7.39 (m, 3), 5.03 (dd, 1, J = 6.1, 12.1), 3.79 (tq, 1, J = 6.1, 6.1), 3.64 (dd, 1, J = 6.1, 16.8), 3.48 (dd, 1, J = 12.1, 16.8), 2.73 (t, 2, J = 7.3), 1.73-1.52 (m, 2), 1.50-1.34 (m, 2), 1.12 (d, 3, J = 5.5), 0.94 (t, 9, J = 6.6), 0.57 (q, 6, J = 6.6); 13C NMR 209.5, 156.6, 130.5, 128.8 (2 C), 128.5, 126.8 (2 C), 84.1, 68.1, (38.96, 38.94), (38.82, 38.81), (37.28, 37.25), 23.7, (19.30, 19.27), 6.8 (3 C), 4.9 (3 C); IR (neat) 1721, 1595; HRMS (EI) calcd for C19H28O3NSi (M+-CH2CH3) 346.1838, found 346.1837.
1-[4,5-Dihydro-3-[1-[[2-(trimethylsilyl)ethoxy]methyl]-1H-pyrrol-2-yl]-5-isoxazolyl]-5-triethylsilyloxy-1-hexanone (14c)
A mixture of N-SEM-pyrrole-2-carboxaldehyde oxime (440 mg, 1.83 mmol) and enone 13b (610 mg, 2.28 mmol) in CH2Cl2 (15 mL) was treated with bleach (5.25% aqueous NaOCl, 5.15 mL, 271 mg of NaOCl, 3.66 mmol) and Et3N (40 µL, 0.28 mmol) at 0 °C. The resulting mixture was warmed to 25 °C and stirred for 3 h. The reaction was then diluted with CH2Cl2, washed with brine, dried (Na2SO4), and concentrated. Flash chromatography on MeOH-deactivated silica gel (12:1 hexanes/EtOAc) gave 661 mg (73%) of 14c as a mixture of diastereomers as a pale yellow gum: 1H NMR 7.00–6.98 (m, 1), 6.46-6.44 (m, 1), 6.23-6.12 (m, 1), 5.67 (d, 1, J = 10.4), 5.60 (d, 1, J = 10.4), 4.87 (dd, 1, J = 6.2, 11.3), 3.79 (tq, 1, J = 6.1, 6.1), 3.60 (dd, 1, J = 6.2, 16.3), 3.53-3.46 (m, 3), 2.78-2.63 (m, 2), 1.72-1.50 (m, 2), 1.48-1.34 (m, 2), 1.12 (d, 3, J = 6.1), 0.94 (t, 9, J = 7,8), 0.89 (t, 2, J = 7.9), 0.57 (q, 6, J = 7.8), −0.04 (s, 9); 13C NMR 209.7, 150.1, 127.5, 121.3, 116.0, 109.3, 82.4, 77.4, 68.1, 65.7, 39.4, 39.0, 38.8, (23.72, 23.70), 19.3, 17.7, 6.9 (3 C), 4.9 (3 C), −1.5 (3 C); IR (neat) 1721, 1598; HRMS (EI) calcd for C25H46O4N2Si2 (M+) 494.2996, found 494.2989.
3,4-Dihydro-2,3-dimethoxy-2-(4-triethylsilyloxypentyl)-4-phenyl-2H-pyrrole (16a)
A solution of isoxazoline 14a (178 mg, 0.47 mmol) in 10 mL of MeOH was treated with a wet slurry of Raney nickel 2800 (~50 mg) and the suspension was stirred at 25 °C under H2 (1 atm) for 35 min. The mixture was then diluted with EtOAc and filtered. The filtrate was washed with brine (3 × 5 mL), dried (MgSO4), and concentrated to give 174 mg of crude hydroxy hemi-iminal 15a as a mixture of four diastereomers that was used for the next step.
A solution of crude 15a in anhydrous THF (2 mL) was added dropwise to a suspension of NaH (60% in mineral oil, 152 mg, 3.80 mmol) in THF (5 mL) at 0 °C. The mixture was stirred at 0 °C for 30 min and MeI (237 µL, 3.80 mmol) was then added by syringe over 3 min. The resulting mixture was warmed to 25 °C and stirred for 4 h. The reaction was quenched with saturated aqueous NH4Cl (3 mL). The aqueous layer was extracted with EtOAc (3 × 5 mL). The combined organic layers were dried (Na2SO4) and concentrated to give 163 mg of crude 16a. Flash chromatography on silica gel (18:1 hexanes/EtOAc) gave 110 mg (58% for two steps) of 16a as a mixture of four diastereomers as a colorless gum: 1H NMR (major (75–80%) pair of diastereomers with either cis or trans methoxy groups) 7.87 (d, 2, J = 7.3), 7.46-7.39 (m, 3), 3.96-3.91 (m, 1), 3.84-3.75 (m, 1), 3.48 (s, 6), 3.20 (dd, 1, J = 7.3, 17.4), 3.02 (dd, 1, J = 3.0, 17.4), 1.96-1.82 (m, 1), 1.67-1.35 (m, 5), 1.14 (d, 3, J = 6.1), 0.93 (t, 9, J = 7.8), 0.58 (q, 6, J = 7.8); 1H NMR (minor (20–25%) pair of diastereomers with either trans or cis methoxy groups) 3.48-3.24 (m, 2 or 3); IR (neat) 2955, 1619, 1449; HRMS (EI) calcd for C23H39O3NSi (M+) 405.2699, found 405.2710.
3,4-Dihydro-2,3-dimethoxy-2-(4-triethylsilyloxypentyl)-4-(1-[[2-(trimethylsilyl)-ethoxy]methyl]-1H-pyrrol-2-yl)-2H-pyrrole (16c)
A solution of isoxazoline 14c (203 mg, 0.41 mmol) in 12 mL of 5:1 MeOH/H2O was treated with a wet slurry of Raney nickel 2800 (~50 mg) and the suspension was stirred at 25 °C under H2 (1 atm) for about 50 min. The mixture was then diluted with EtOAc and filtered. The filtrate was washed with brine (3 × 5 mL), dried (Na2SO4), and concentrated to give 191 mg of crude hydroxy hemi-iminal 15c.
A solution of crude 15c in THF (2 mL) was added dropwise to a suspension of NaH (60% in mineral oil, 130 mg, 3.24 mmol) in THF (5 mL) at 0 °C. The mixture was stirred at 0 °C for 30 min and MeI (203 µL, 3.24 mmol) was added dropwise by syringe over 3 min. The resulting mixture was warmed to 25 °C and stirred for 4 h. The reaction was quenched with saturated aqueous NH4Cl (3 mL) and the aqueous layer was extracted with EtOAc. The combined organic layers were dried (Na2SO4), and concentrated to give 151 mg of crude 16c. Flash chromatography on silica gel (15:1 hexanes/EtOAc) gave 91 mg (42% for two steps) of 16c (pale yellow gum) as a mixture of four diastereomers in which two predominate: 1H NMR 7.03–7.01 (m, 1), 6.58-6.56 (m, 1), 6.21-6.19 (m, 1), 5.93 (d, 1, J = 10.4), 5.90 (d, 1, J = 10.4), 3.80-3.77 (m, 2), 3.54 (t, 2, J = 7.9), 3.44 (s, 3), 3.43 (s, 3), 3.12 (dd, 1, J = 6.7, 17.1), 2.96 (dd, 1, J = 2.4, 17.1), 1.83-1.77 (m, 1), 1.55-1.37 (m, 5), 1.13 (d, 3, J = 6.1), 0.94 (t, 9, J = 7.8), 0.87 (t, 2, J = 7.9), 0.57 (q, 6, J = 7.8), −0.05 (s, 9); IR (neat) 2954, 1617; HRMS (EI) calcd for C27H52O4N2Si2 (M+) 524.3466, found 524.3475.
3-Methoxy-α-methyl-5-phenyl-1H-pyrrole-2-butanol (17a) and (±)-(4S,5R,7R)-, (±)-(4R,5R,7R)-, and (±)-(4R,5S,7R)-4-Methoxy-7-methyl-2-phenyl-6-oxa-1-azaspiro[4.5]dec-1-ene (18a, 19a, and 21a)
A solution of 16a (101 mg, 243 µmol) in 6 mL of 1:1 CH3CN/THF was treated with 2 M HCl (2.49 mL, 4.98 mmol) at 0 °C. The resulting mixture was stirred at 0 °C for 40 min. Saturated NaHCO3 (5 mL) was added to bring the pH to 7. The reaction was extracted with EtOAc and the organic layer was washed with brine, dried (Na2SO4), and concentrated. Flash chromatography on MeOH-deactivated silica gel (7:1 to 2:1 hexanes/EtOAc) gave 26 mg (41%) of 18a as a colorless gum, followed by 8.1 mg (13%) of 19a as a colorless gum, 7.4 mg (12%) of 21a as a colorless gum, and then 5.2 mg (8%) of 17a as a pale yellow gum.
The data for 17a: 1H NMR (recorded in C6D6 because the compound is unstable in CDCl321) 7.83 (br, 1, NH), 7.30 (d, 2, J = 7.3), 7.20 (t, 2, J = 7.3), 7.04 (t, 1, J = 7.3), 6.30 (d, 1, J = 2.5), 3.60 (s, 3), 3.58-3.48 (m, 1), 2.61 (t, 2, J = 7.4), 1.69-1.51 (m, 2), 1.38-1.25 (m, 2), 0.92 (d, 3, J = 6.1); 13C NMR (C6D6) 146.0, 133.9, 129.0 (2 C), 126.9, 125.5, 123.4 (2 C), 117.5, 95.0, 67.8, 58.5, 38.3, 26.3, 24.5, 24.0; IR (neat) 3316, 2934, 1630; HRMS (EI) calcd for C16H19O2N (M+-2H) 257.1416, found 257.1407. This compound is unstable and oxidizes easily to 24 and 25.
The data for 18a: 1H NMR 7.84 (d, 2, J = 6.7), 7.44-7.38 (m, 3), 4.43-4.39 (m, 1, H-7), 3.88 (dd, 1, J = 7.0, 7.0, H-4), 3.46 (s, 3), 3.30 (dd, 1, J = 17.1, 7.0, H-3), 2.77 (dd, 1, J = 16.4, 7.0, H-3), 2.07 (br ddd, 1, J = 11, 11, 11, H-9ax), 1.79 (ddd, 1, J = 11, 11, 3, H-10ax), 1.77-1.69 (m, 2, H-8eq, H-9eq), 1.51 (br d, 1, J = 11, H-10eq), 1.36 (br ddd, 1, J = 11, 11, 11, H-8ax), 1.16 (d, 3, J = 6.1, H-7 Me); 13C NMR 169.6, 134.8, 130.8, 128.4 (2 C), 127.6 (2 C), 103.8, 87.2, 68.7, 58.2, 39.1, 33.6, 28.7, 22.4, 19.8; IR (neat) 2932, 1615, 1448; HRMS (EI) calcd for C16H21O2N (M+) 259.1572, found 259.1560. A 1D NOESY experiment with irradiation of H-4 at δ 3.88 showed NOEs to OMe at δ 3.46 (OMe) and H-3s at δ 3.30 and 2.77. A 1D NOESY experiment with irradiation of H-7 at δ 4.43-4.39 showed NOEs to H-9ax at δ 2.07, H-8eq at δ 1.77-1.69 and 7-Me at δ 1.16.
The data for 19a: 1H NMR 7.86 (d, 2, J = 7.3), 7.46-7.36 (m, 3), 4.49-4.42 (m, 1, H-7), 3.77 (dd, 1, J = 6.1, 4.0, H-4), 3.50 (s, 3), 3.13 (dd, 1, J = 17.1, 6.1, H-3), 3.05 (dd, 1, J = 17.1, 4.0, H-3), 2.12 (br ddd, 1, J = 11, 11, 11, H-9ax), 1.77 (ddd, 1, J = 11, 11, 3, H-10ax), 1.76-1.68 (m, 2, H-8eq, H-9eq), 1.48 (br d, 1, J = 11, H-10eq), 1.40 (br ddd, 1, J = 11, 11, 11, H-8ax), 1.23 (d, 3, J = 6.7, H-7 Me); 13C NMR 170.3, 134.8, 130.7, 128.3 (2 C), 127.7 (2 C), 101.7, 85.4, 68.6, 58.8, 39.6, 34.7, 33.3, 22.3, 20.4; IR (neat) 2930, 1616, 1448; HRMS (EI) calcd for C16H21O2N (M+) 259.1572, found 259.1570. A 1D NOESY experiment with irradiation of H-4 at δ 3.77 showed NOEs to OMe at δ 3.50 (OMe), H-3s at δ 3.13 and 3.05, and H-10ax at δ 1.77 and H-10eq at δ 1.48.
The data for 21a: 1H NMR 7.90 (d, 2, J = 7.4), 7.44-7.36 (m, 3), 4.11 (dd, 1, J = 6.7, 3.6, H-4), 3.83-3.76 (m, 1, H-7), 3.40 (s, 3), 3.36 (dd, 1, J = 17.4, 6.7, H-3), 2.94 (dd, 1, J = 17.4, 3.6, H-3), 2.06-2.01 (m, 1), 1.88-1.75 (m, 3), 1.62 (br d, 1, J = 11), 1.46-1.38 (m, 1), 1.23 (d, 3, J = 6.1); 13C NMR 171.9, 134.0, 131.1, 128.2 (2 C), 128.1 (2 C), 105.4, 83.8, 69.9, 57.7, 40.1, 32.2, 28.9, 22.3, 20.6; IR (neat) 2930, 1627, 1448; HRMS (EI) calcd for C16H21O2N (M+) 259.1572, found 259.1556. A 1D NOESY experiment with irradiation of H-4 at δ 4.11 showed NOEs to H-7 at δ 3.83-3.76, OMe at δ 3.40, and H-3s at δ 3.36 and 2.94.
Equilibration of 19a and 21a
A solution of 19a in 0.6 mL of CDCl3 (containing HCl/DCl from decomposition of CDCl3) equilibrated to a 3:1 mixture of 19a and 21a. The percentage of 19a in the mixture was determined as a function of time by 1H NMR spectroscopy: initial, 100%; 7 days, 90%; 14 days, 80%; 20 days, 75%. The spectrum did not change at longer times. A solution of 21a in 0.6 mL of CDCl3 (containing HCl/DCl from decomposition of CDCl3) equilibrated to a 3:1 mixture of 19a and 21a. The percentage of 19a in the mixture was determined as a function of time by 1H NMR spectroscopy: initial, <2%; 5 days, 25%; 10 days, 60%, 15 days, 75%. The spectrum did not change at longer times.
Equilibration of 18a and 20a
A solution of 18a in 0.6 mL of CDCl3 (containing HCl/DCl from decomposition of CDCl3) was monitored by 1H NMR for 14 days, at which time a 19:1 mixture of 18a and 20a was present. Partial data for 20a were determined from the mixture: 1H NMR 7.91 (d, 2, J = 7.6), 7.40-7.20 (m, 3), 4.13 (d, 1, J = 4.9, H-4), 3.80-3.74 (m, 1, H-7), 3.36 (s, 3, OMe), 3.20 (d, 1, J = 17.4, H-3), 2.99 (dd, 1, J = 17.4, 4.9, H-3), 1.30 (d, 3, J = 6.4).
(±)-(4S,5R,7R)-, (±)-(4R,5R,7R)-, and (±)-(4R,5S,7R)-4-Methoxy-7-methyl-2-(1-[[2-(trimethylsilyl)ethoxy]methyl]-1H-pyrrol-2-yl)-6-oxa-1-azaspiro[4.5]dec-1-ene (18c, 19c, and 21c)
A solution of 16c (78 mg, 149 µmol) in 8 mL of 3:1 CH3CN/THF was treated with aqueous 2 M HCl (1.49 mL, 2.98 µmol) at 25 °C. The resulting mixture was stirred at 25 °C for 11 h. Saturated NaHCO3 (3 mL) was added to bring the pH to 7. The reaction was extracted with EtOAc and the organic layer was washed with brine, dried (Na2SO4), and concentrated to give 77 mg of a mixture of spiroiminals. Flash chromatography on MeOH-deactivated silica gel (18:1 to 2:1 hexanes/EtOAc) gave 19 mg (34%) of isomer 18c as a colorless gum followed by 19 mg (34%) of an inseparable 3:2 mixture of isomers 19c and 21c as a colorless gum.
The data for 18c: 1H NMR 7.02–7.00 (m, 1), 6.57-6.55 (m, 1), 6.21-6.19 (m, 1), 6.01 (d, 1, J = 10.1), 5.88 (d, 1, J = 10.1), 4.29-4.22 (m, 1, H-7), 3.77 (dd, 1, J = 6.9, 6.9, H-4), 3.55 (t, 2, J = 8.2), 3.43 (s, 3), 3.23 (dd, 1, J = 6.9, 16.3, H-3), 2.76 (dd, 1, J = 6.9, 16.3, H-3), 1.98 (br ddd, 1, J = 11, 11, 11, H-9ax), 1.76 (ddd, 1, J = 11, 11, 3, H-10ax), 1.76-1.64 (m, 2, H-8eq, H-9eq), 1.49 (br d, 1, J = 11, H-10eq), 1.34 (br ddd, 1, J = 11, 11, 11, H-8ax), 1.13 (d, 3, J = 6.1), 0.88 (t, 2, J = 8.2), −0.05 (s, 9); 13C NMR 162.6, 127.6, 127.5, 116.6, 108.9, 104.3, 86.2, 76.8, 68.6, 65.5, 58.2, 40.5, 33.6, 29.0, 22.4, 20.0, 18.0, −1.5 (3 C); IR (neat) 1610; HRMS (EI) calcd for C20H34O3N2Si (M+) 378.2339, found 378.2325.
The data for 19c and 21c: IR (CDCl3) 1613; HRMS (EI) calcd for C20H34O3N2Si (M+) 378.2339, found 378.2350.
NMR data for 19c were determined from the mixture: 1H NMR 7.03–7.01 (m, 1), 6.57-6.55 (m, 1), 6.20-6.17 (m, 1), 6.16 (d, 1, J = 10.1), 5.74 (d, 1, J = 10.1), 4.35-4.29 (m, 1), 3.67 (dd, 1, J = 6.0, 4.8), 3.56 (t, 2, J = 8.5), 3.47 (s, 3), 3.06 (dd, 1, J = 17.2, 6.0), 3.01 (dd, 1, J = 17.2, 4.8), 2.06-1.99 (m, 1), 1.78-1.35 (m, 5), 1.21 (d, 3, J = 6.1), 0.90-0.85 (m, 2), −0.05 (s, 9); 13C NMR 162.9, 127.6, 127.4, 116.7, 108.9, 102.0, 84.3, 68.6, 65.6, 58.6, 40.8, 34.8, 33.4, 22.3, 20.6, 18.0, −1.5 (3 C), (one peak is obscured by the CDCl3 triplet at δ 77.0).
NMR data for 21c were determined from the mixture: 1H NMR 7.01–6.99 (m, 1), 6.57-6.55 (m, 1), 6.38 (d, 1, J = 10.4), 6.20-6.17 (m, 1), 5.54 (d, 1, J = 10.4), 3.97 (dd, 1, J = 6.0, 3.1), 3.81-3.75 (m, 1), 3.51 (t, 2, J = 8.5), 3.36 (s, 3), 3.28 (dd, 1, J = 16.8, 6.0), 2.86 (dd, 1, J = 16.8, 3.1), 2.06-1.99 (m, 1), 1.78-1.35 (m, 5), 1.21 (d, 3, J = 6.1), 0.90-0.85 (m, 2), −0.04 (s, 9) 13C NMR 164.8, 127.6, 127.4, 117.0, 108.9, 106.2, 82.0, 70.1, 65.6, 57.4, 41.2, 32.2, 29.4, 22.4, 20.5, 17.9, −1.5 (3 C), (one peak is obscured by the CDCl3 triplet at δ 77.0).
(±)-(4S,5R,7R)-4-Methoxy-7-methyl-2-(1H-pyrrol-2-yl)-6-oxa-1-azaspiro[4.5]dec-1-ene (18b)
A mixture of 18c (19 mg, 50.2 µmol) and molecular sieves (4 Å, 100 mg) in freshly distilled THF (3 mL) was treated with TBAF (1 M in THF, 1.01 mL, 1.01 mmol) dropwise at 50 °C. The resulting mixture was stirred at 60 °C for 3 h. The reaction was cooled, diluted with Et2O (15 mL), washed with brine (2 × 5 mL) and H2O (3 × 5 mL). The organic layer was dried (MgSO4) and concentrated to give 59 mg crude of 18b. Flash chromatography on MeOH-deactivated silica gel (4:1 hexanes/EtOAc) gave 5.7 mg (54%) of 18b as a pale yellow gum: 1H NMR 6.94-6.91 (m, 1), 6.57-6.54 (m, 1), 6.25-6.23 (m, 1), 4.26-4.20 (m, 1), 3.82 (dd, 1, J = 6.7, 6.1), 3.43 (s, 3), 3.19 (dd, 1, J = 16.4, 6.7), 2.73 (dd, 1, J = 16.4, 6.1), 1.97 (br ddd, 1, J = 11, 11, 11, H-9ax), 1.81-1.66 (m, 3, H-10ax, H-8eq, H-9eq), 1.54 (br d, 1, J = 11, H-10eq), 1.32 (br ddd, 1, J = 11, 11, 11, H-8ax), 1.12 (d, 3, J = 6.1, H-7 Me), the pyrrole NH was not observed; 13C NMR 162.8, 127.7, 122.1, 113.7, 109.8, 103.4, 86.9, 68.5, 58.1, 38.5, 33.4, 28.7, 22.4, 19.7; IR (CDCl3) 2930, 1607, 1432, 743; HRMS (EI) calcd C14H20N2O2 (M+) 248.1525, found 248.1532.
(±)-(4R,5R,7R)- and (±)-(4R,5S,7R)-4-Methoxy-7-methyl-2-(1H-pyrrol-2-yl)-6-oxa-1-azaspiro[4.5]dec-1-ene (19b and 21b)
A 3:2 mixture of 19c and 21c (19 mg, 50.2 µmol), and molecular sieves (4 Å, 100 mg) in freshly distilled THF (3 mL) was treated with TBAF (1 M in THF, 1.01 mL, 1.01 mmol) dropwise at 50 °C. The resulting mixture was then stirred at 60 °C for 3 h. The reaction was cooled, diluted with Et2O (15 mL), washed with brine (2 × 5 mL) and H2O (3 × 5 mL). The organic layer was dried (MgSO4) and concentrated to give 65 mg crude of 19b and 21b. Flash chromatography on MeOH-deactivated silica gel (4:1 to 2:1 hexanes/EtOAc) gave 5.9 mg (56%) of an inseparable 7:3 mixture of 19b and 21b as a pale yellow gum: IR (CDCl3) 2933, 1612, 1434, 744; HRMS (EI) calcd C14H20N2O2 (M+) 248.1525, found 248.1533.
NMR data for 19b were determined from the mixture: 1H NMR 6.94-6.92 (m, 1), 6.56-6.54 (m, 1), 6.25-6.21 (m, 1), 4.31-4.23 (m, 1), 3.70 (dd, 1, J = 6.1, 4.3), 3.48 (s, 3), 3.03 (dd, 1, J = 16.4, 6.1), 2.97 (dd, 1, J = 16.4, 4.3), 2.08-1.98 (m, 1), 1.81-1.32 (m, 5), 1.19 (d, 3, J = 6.1), the pyrrole NH was not observed; 13C NMR 163.1, (127.8 or 127.1), (122.5 or 122.2), (114.2 or 113.6), (109.9 or 109.8), 100.7, 85.3, 68.4, 58.8, 38.6, 34.3, 33.3, 22.3, 20.3.
NMR data for 21b were determined from the mixture: 1H NMR 6.92-6.90 (m, 1), 6.56-6.54 (m, 1), 6.25-6.21 (m, 1), 4.07 (dd, 1, J = 6.1, 3.0), 3.81-3.73 (m, 1), 3.38 (s, 3), 3.24 (dd, 1, J = 17.0, 6.1), 2.85 (dd, 1, J = 17.0, 3.0), 2.08-1.98 (m, 1), 1.81-1.32 (m, 5), 1.21 (d, 3, J = 6.1), the pyrrole NH was not observed; 13C NMR 164.4, (127.8 or 127.1), (122.5 or 122.2), (114.2 or 113.6), (109.9 or 109.8), 105.0, 83.1, 70.1, 57.5, 39.4, 32.3, 29.1, 22.4, 20.7.
3-Methoxy-α-methyl-5-phenyl-1H-pyrrole-2-butanol (17a)
A solution of dimethyl ether 16a (64 mg, 0.25 mmol) in 10 mL of THF was treated with HF (1.35 M in THF, 3.7 mL, prepared by addition of 1 mL of 48% aqueous HF to 15 mL of THF). The mixture was stirred at 0 °C for 1.5 h. The mixture was diluted with EtOAc, washed with NaHCO3 (25 mL), brine (10 mL), dried (Na2SO4), and concentrated. Flash chromatography of the residue on MeOH-deactivated silica gel (3:1 hexanes/EtOAc) gave 26 mg (65%) of 17a as a pale yellow gum.
(±)-(5R,7R)- and (±)-(5S,7R)-4-methoxy-7-methyl-2-phenyl-6-oxa-1-azaspiro[4,5]deca-1,3-diene (24 and 25)
A solution of pyrrole 17a (260 mg, 1 mmol) in 12 mL of acetone was treated with activated MnO2 (435 mg, 5 mmol) at 25 °C. The mixture was stirred for 20 min and filtered through a pad of Celite. The filtrate was concentrated to give 247 mg of crude product. Flash chromatography of the residue on silica gel (10:1 to 4:1 hexanes/EtOAc) gave 56 mg (14%) of 24 as a yellow gum followed by 90 mg (35%) of 25 as a yellow gum.
The data for 24: 1H NMR 7.91 (d, 2, J = 7.1), 7.45-7.39 (m, 3), 5.64 (s, 1), 4.45 (dqd, 1, J = 3.0, 6.4, 12.8, H-7), 3.89 (s, 3), 2.22 (ddddd, 1,J = 4.0, 4.0, 12.8, 12.8, 12.8, H-9ax), 1.92 (ddd, 1, J= 3.0, 12.8, 12.8, H-10ax), 1.85 (br d, 1,J = 12.8, H-9eq), 1.76 (br d, 1,J = 12.8, H-8eq), 1.44 (dddd, 1,J = 3.0, 12.8, 12.8, 12.8, H-8ax), 1.35 (br d, 1,J = 12.8, H-10eq), 1.21 (d, 3,J = 6.1); 13C NMR 182.0, 171.4, 134.9, 130.6, 128.4 (2 C), 127.5 (2 C), 99.7, 92.9, 70.0, 59.0, 32.6, 31.2, 22.1, 21.2; IR (neat) 2934, 1631; HRMS (QTof) calcd for C16H20NO2 (MH+) 258.1494, found 258.1485.
The data for 25: 1H NMR 7.94 (d, 2, J = 7.1), 7.44-7.38 (m, 3), 5.60 (s, 1), 4.43 (dqd, 1, J = 3.0, 6.4, 12.8, H-7), 3.87 (s, 3), 2.15 (br dd, 1, J = 12.8. 12.8, H-10ax), 2.10 (ddddd, 1, J = 3.0, 3.0, 12.8, 12.8, 12.8, H-9ax), 1.84 (ddd, 1, J = 3.0, 3.0, 12.8, H-10eq), 1.72-1.64 (m, 2, H-8eq, H-9eq), 1.44 (dddd, J = 3.0, 12.8, 12.8, 12.8, H-8ax), 1.25 (d, 3, J = 6.1);13C NMR 185.6, 171.5, 134.1, 130.8, 128.3 (2 C), 127.7 (2 C), 98.9, 92.2, 69.0, 58.9, 32.0, 30.6, 22.3, 18.5; IR (neat) 2954, 1616; HRMS (QTof) calcd for C16H20NO2 (MH+) 258.1494, found 258.1496.
(4S,5R,7R)- and (4R,5R,7R)-rel-4-Methoxy-7-methyl-2-phenyl-6-oxa-1-azaspiro[4.5]dec-1-ene (18a and 19a)
A solution of 24 (13 mg, 0.19 mmol) in 5 mL of MeOH was treated with 5% Pd/BaSO4 (20 mg) and the suspension was stirred at 25 °C under H2 (1 atm) for 3 h. The mixture was then diluted with EtOAc and filtered through a pad of Celite. The filtrate was concentrated to afford 11 mg of crude product. Flash chromatography of the residue on MeOH-deactivated silica gel (5:1 to 2:1 hexanes/EtOAc) gave 5 mg (35%) of 18a as a colorless gum, followed by 3 mg (26%) of 19a as a colorless gum, and then 2 mg (15%) 17a as a pale yellow gum.
(±)-(4S,5S,7R)- and (±)-(4R,5S,7R)-4-Methoxy-7-methyl-2-phenyl-6-oxa-1-azaspiro[4.5]dec-1-ene (20a and 21a)
A solution of 25 (21 mg, 0.19 mmol) in 6 mL of MeOH was treated with 5% Pd on BaSO4 (20 mg) and the suspension was stirred at 25 °C under H2 (1 atm) for 3 h. The mixture was then diluted with EtOAc and filtered through a pad of Celite. The filtrate was concentrated to afford 46 mg crude product. Flash chromatography of the residue on MeOH-deactivated silica gel (5:1 to 2:1 hexanes/EtOAc) gave 4 mg (20%) of 21a as a colorless gum, followed by 8 mg (40%) of 20a as a colorless gum, and then 3 mg (15%) 17a as a pale yellow gum.
3-Iodo-6-methyl-5,6-dihydropyan-2-one (11)
A solution of parascorbic acid (26)23 (740 mg, 6.59 mmol) in 12 mL of 1:1 ether/pyridine was treated with iodine (5.02 g, 19.8 mmol) and the mixture was stirred for 8 h. The reaction was diluted with ether, washed with saturated aqueous Na2SO3 (30 mL), saturated aqueous CuSO4 (3 × 20 mL), and brine (20 mL). The organic layer was dried (Na2SO4) and concentrated. Flash chromatography of the residue on silica gel (6:1 hexanes/ EtOAc) gave 1.19 g (76%) of 11 as a pale yellow solid: mp 61–64 °C; 1H NMR 7.53 (dd, 1, J = 3.0, 6.1), 4.72-4.63 (m, 1), 2.47-2.33 (m, 2), 1.44 (d, 3, J = 6.1); 13C NMR 160.4, 153.7, 89.3, 75.1, 35.0, 20.4; HRMS (QTof) calcd for C6H8O2I (MH+) 238.9569, found 238.9560. The 1H NMR and 13C NMR data are identical to those previously reported for the (R) enantiomer.42
3-Phenyl-6-methyl-5,6-dihydro-2H-pyran-2-one (27)
A sealed tube was filled with iodolactone 26 (480 mg, 2.0 mmol), phenylboronic acid (726 mg, 6.0 mmol), Pd(OAc)2 (22 mg, 0.1 mmol), SPhos (41 mg, 0.1mmol) and K3PO4 (1.5 g, 7.0 mmol). Degassed n-butanol (5 mL) was added and the mixture was stirred at 80 °C for 6 h. The mixture was diluted with EtOAc and filtered. The filtrate was washed with brine (15 mL), dried (Na2SO4) and concentrated. Flash chromatography of the residue on silica gel (8:1 hexanes/EtOAc) gave 322 mg (85%) of 27 as a yellow solid: mp 89–90 °C; 1H NMR 7.45 (d, 2, J = 7.0), 7.40-7.30 (m, 3), 6.94 (dd, 1, J = 3.0, 5.9), 4.71-4.61 (m, 1), 2.58-2.43 (m, 2), 1.49 (d, 3, J = 6.3); 13C NMR 164.5, 140.7, 135.5, 133.1, 128.3 (2 C), 128.2 (3 C), 74.3, 31.8, 20.7; IR (neat) 2976, 1706; HRMS (QTof) calcd for C12H13O2 (MH+) 189.0916, found 189.0915.
(±)-(2S,3S,5S)-5-Hydroxy-2-phenyl-4-propyl-N-methoxy-N-methyl-hexanamide (31a)
A solution of ZnBr2 (430 mg, 1.83 mmol) in 12 mL THF was treated with allylmagnesium bromide (1.1 M in THF, 3.33 mL, 3.66 mmol) at 0 °C under nitrogen. The mixture was stirred for 30 min at 0 °C and cooled to −78 °C. A mixture of unsaturated lactone 27 (115 mg, 0.61 mmol) and TMSCl (0.47 mL, 3.66 mmol) in 4 mL THF was added dropwise. The reaction was stirred at −78 °C for 3 h. Aqueous NH4Cl solution was added and the mixture was extracted with EtOAc (3 × 15 mL). The combined organic layers were washed with brine (15 mL), dried (Na2SO4) and concentrated. Flash chromatography of the residue on silica gel (10:1 hexanes/EtOAc) gave 377 mg (72%) of a 1:3 mixture of 28a and 29a as a pale yellow gum. A 1:3 mixture of 28a and 29a in CDCl3 was treated with DMAP. The percentage of 28a in the mixture was determined as a function of time by 1H NMR spectroscopy: initial, 25%; 3 h, 50%; 6 h, 75%; 12 h, 85%. The spectrum did not change at longer times. A similar reaction on a 6:5 mixture also gave a 6:1 mixture of 28a and 29a.
Data of 28a were determined from a 6:1 mixture of 28a and 29a: 1H NMR 7.36 (t, 2, J = 7.6), 7.29 (t, 1, J = 7.6), 7.19 (d, 2, J = 7.6), 5.72-5.62 (m, 1), 5.09 (d, 1, J = 10.1), 5.03 (d, 1, J = 17.1), 4.66-4.56 (m, 1), 3.46 (d, 1, J = 9.8), 2.34-2.24 (m, 1), 2.16 (ddd, 1,J = 4.8, 4.8, 14.0), 1.93 (ddd, 1,J = 8.4, 8.4, 14.0), 1.90-1.83 (m, 2), 1.43 (d, 3, J = 6.3). A 1D NOESY experiment with irradiation of CHPh at δ 3.46 showed NOEs to the protons at δ 7.19 (ortho phenyl), δ 4.66-4.56 (CHMe), δ 2.34-2.24, and δ 1.93.
Data of 29a were determined from a 1:3 mixture of 28a and 29a: 1H NMR 7.36-7.24 (m, 3), 7.18 (d, 2, J = 7.6), 5.62-5.50 (m, 1), 5.10-4.98 (m, 2), 4.82-4.73 (m, 1), 3.94 (d, 1, J = 5.6), 2.31-2.22 (m, 1), 2.10-2.02 (m, 2), 1.89-1.80 (m, 1), 1.73 (ddd, 1,J = 3.9, 9.2, 14.0). 1.42 (d, 3, J = 6.1).
A 6:1 mixture of 28a and 29a (375 mg, 1.6 mmol) was dissolved in 6 mL MeOH and treated with a wet slurry of Raney Ni (~30 mg). The suspension was stirred at 25 °C under H2 (1 atm) for 0.5 h and filtered through a pad of Celite. The filtrate was concentrated to give 341 mg (91%) of a 6:1 mixture of 28b and 29b that was used without further purification.
Data of 28b were determined from the mixture: 1H NMR 7.37-7.16 (m, 5), 4.66-4.58 (m, 1), 3.40 (d, 1,J = 9.4), 2.20-1.90 (m, 1), 1.89(ddd, 1,J = 8.0, 10.0, 14.4), 1.76(ddd, 1,J = 4.0, 4.0, 14.4), 1.42 (d, 3, J = 6.3), 1.40-1.14 (m, 4), 0.81 (t, 3, J = 7.0); IR (neat) 2929, 1735, 1188.
Partial data of 29b were determined from the mixture: 1H NMR 7.37-7.16 (m, 5), 4.83-4.62 (m, 1), 3.89 (d, 1,J = 3.9), 2.05 (ddd, 1,J = 4.8, 6.8, 11.6).
A 6:1 mixture of lactones 28b and 29b (175 mg, 0.75 mmol) and NH(OMe)Me•HCl (300 mg, 3.05 mmol) in 8 mL THF was treated with i-PrMgCl (1.3 M in THF, 6.40 mL) at −20 °C. The reaction was warmed to 0 °C in 30 min and stirred at 0 °C for 2.5 h. Aqueous NH4Cl solution was added and the mixture was extracted with EtOAc (3 × 15 mL). The organic layers were washed with brine (10 mL), dried (Na2SO4) and concentrated. Flash chromatography of the residue on silica gel (3:1 hexanes/EtOAc) gave 29 mg (13%) of 31b as a colorless gum, followed by 173 mg (78%) of 31a as a colorless gum.
Data for 31a: 1H NMR 7.34-7.21 (m, 5), 3.91 (1, d,J = 10.5), 3.78-3.68 (m, 1), 3.56 (s, 3), 3.15 (s, 3), 2.86 (s, 1, OH), 2.50-2.41 (m, 1), 1.66 (ddd, 1,J = 5.2, 8.8, 14.0), 1.37 (ddd, 1,J = 4.4, 7.6, 14.0), 1.30-1.18 (m, 1), 1.20 (d, 3, J = 6.2), 1.20-1.02 (m, 2), 0.98-0.88 (m, 1), 0.69 (t, 3, J = 7.2); 13C NMR 175.5, 138.7, 128.9 (2 C), 128.4 (2 C), 127.0, 64.5, 61.3, 51.8, 43.5, 36.3, 33.9, 32.4, 23.4, 18.7, 14.2; IR (neat) 2931, 1649; HRMS (QTof) calcd for C17H28NO3 (MH+) 294.2069, found 294.2065.
Data for 31b: 1H NMR 7.34 (d, 2, J = 7.2), 7.28 (t, 2, J = 7.2), 7.22 (t, 1, J = 7.2), 4.03 (br d, 1, J = 8.2), 3.60-3.53 (m, 1), 3.53 (s, 3), 3.15 (s, 3), 2.36-2.26 (m, 1), 1.62 (s, 1, OH), 1.42-1.34 (m, 5), 1.32 (ddd, 1,J = 6.8, 6.8, 14.0), 1.21 (ddd, 1,J = 6.0, 6.0, 14.0), 0.97 (d, 3, J = 5.9), 0.91 (t, 3, J = 6.8); IR (neat) 2959, 1642, 1377.
(±)-(2S,3S,5S)-5-Triethylsilyloxy-2-phenyl-4-propyl-N-methoxy-N-methyl-hexanamide (32)
A solution of alcohol 31a (87 mg, 0.29 mmol) in THF was treated with TESCl (162 µL, 0.43 mmol), Et3N (181 µL 0.58 mmol) and DMAP (4 mg, 0.03 mmol). The mixture was stirred at 25 °C for 3 h. The reaction was then diluted with Et2O (10 mL) and washed with brine. The organic layers were dried (MgSO4) and concentrated. Flash chromatography of the residue on silica gel (10:1 hexanes/EtOAc) gave 102 mg (85%) of 32 as a colorless gum: 1H NMR 7.35 (d, 2, J = 7.2), 7.28 (t, 2, J = 7.2), 7.21 (t, 1,J = 7.2), 3.97 (br d, 1, J = 9.4), 3.91-3.83 (m, 1), 3.55 (s, 3), 3.12 (s, 3), 2.32-2.22 (m, 1), 1.56 (ddd, 1, J = 5.2, 9.2, 13.6), 1.43 (ddd, 1,J = 3.4, 8.0, 13.6), 1.28-1.10 (m, 4), 1.18 (d, 3, J = 5.9), 0.97 (t, 9, J = 8.0), 0.71 (t, 3, J = 7.1), 0.61 (q, 6, J = 8.0); 13C NMR 174.4, 138.5, 129.0 (2 C), 128.2 (2 C), 126.8, 67.2, 61.3, 51.0, 42.0, 37.8, 32.1 (2 C), 23.3, 17.9, 14.4, 6.8 (3 C), 4.8 (3 C); IR (neat) 2956, 1661, 1264; HRMS (QTof) calcd for C23H41NO3NaSi (MNa+) 430.2753, found 430.2756.
(±)-(4S,5S,7S)-4-Phenyl-5-propyl-7-triethylsilyloxy-1-octen-3-one (33)
A solution of Weinreb amide 32 (81 mg, 0.2 mmol) in 6 mL of THF was treated with vinylmagnesium bromide (0.7 M, 0.43 mL) slowly at 25 °C. The reaction was stirred for 1.5 h and aqueous NH4Cl (5 mL) solution was added. The mixture was extracted with EtOAc (3 × 10 mL). The organic layers were washed with brine (10 mL), dried (Na2SO4) and concentrated. Flash chromatography of the residue on silica gel (10:1 hexanes/EtOAc) gave 53 mg (72%) of 33 as a colorless gum: 1H NMR 7.32-7.09 (m, 5), 6.34 (dd, 1, J = 10.1, 17.3), 6.24 (d, 1, J = 17.3), 5.64 (d, 1, J = 10.1), 3.94 (d, 1, J = 9.4), 3.94-3.86 (m, 1), 2.41-2.31 (m, 1), 1.55 (ddd, 1, J = 6.4, 6.4, 13.6), 1.43 (ddd, 1, J = 4.4, 6.8, 13.6), 1.30-1.17 (m, 4), 1.17 (d, 3, J = 6.3), 0.96 (t, 9, J = 8.0), 0.73 (t, 3, J = 7.1), 0.61 (q, 6, J = 8.0); 13C NMR 199.8, 137.2, 136.2, 129.3 (2 C), 128.6 (2 C), 127.9, 127.1, 67.0, 60.3, 41.6, 36.5, 32.4, 23.6, 18.3, 14.4, 6.9 (3 C), 5.0 (3 C); IR (neat) 2955, 1699, 1676; HRMS (QTof) calcd for C23H38O2NaSi (MNa+) 397.2539, found 397.2545.
(±)-(2S,3S,5S)-1-[(5R)-4,5-Dihydro-3-[[2-(trimethylsilyl)ethoxy]methyl]-1H-pyrrole-5-isoxazolyl]-2-phenyl-3-propyl-5-triethylsilyloxy-1-hexanone (34) and (±)-(2S,3S,5S)-1-[(5S)-4,5-Dihydro-3-[[2-(trimethylsilyl)ethoxy]methyl]-1H-pyrrole-5-isoxazolyl]-2-phenyl-3-propyl-5-triethylsilyloxy-1-hexanone (35)
A mixture of N-SEM-pyrrole-2-carboxaldehyde oxime (122 mg, 0.51 mmol) and enone 33 (96 mg, 0.26 mmol) in CH2Cl2 (15 mL) was treated with bleach (5.25% aqueous NaOCl, 1.12 mL, 58 mg of NaOCl, 0.77 mmol) and Et3N (13 µL, 0.3 mmol) at 0 °C. The resulting mixture was warmed to 25 °C and stirred for 3 h. The reaction was then diluted with CH2Cl2, washed with brine, dried (Na2SO4), and concentrated. Flash chromatography of the residue on MeOH-deactivated silica gel (18:1 hexanes/EtOAc) gave 30 mg (20%) of pure 34 as a colorless gum, followed by a 4:1 mixture of 34 and 35 (64 mg, 41% yield) as a colorless gum. Further chromatography of this fraction gave a 1:1 mixture of 34 and 35 (24 mg).
Data for 34: 1H NMR 7.32-7.22 (m, 5), 6.97-6.94 (m, 1), 6.40-6.37 (m, 1), 6.20-6.17 (m, 1), 5.72 (d, 1, J = 10.2), 5.49 (d, 1, J = 10.2), 4.77 (dd, 1, J = 6.7, 11.5), 4.28 (d, 1, J = 10.2), 3.81 (tq, 1, J = 5.8, 5.8), 3.63 (dd, 1, J = 6.7, 16.8), 3.52 (t, 2, J = 7.8), 3.23 (dd, 1, J = 11.5, 16.8), 2.34-2.25 (m, 1), 1.51 (ddd, 1, J = 5.6, 8.4, 14.0), 1.25-1.12 (m, 4), 1.10 (d, 3, J = 5.8), 0.98-0.90 (m, 1), 0.93 (t, 9, J = 7.8), 0.93 (t, 2, J = 7.8), 0.67 (t, 3, J = 7.0), 0.55 (q, 6, J = 7.8), −0.06 (s, 9); 13C NMR 207.2, 150.3, 136.5, 129.5 (2 C), 128.8 (2 C), 127.4, 127.2, 121.4, 115.9, 109.3, 91.5, 81.0, 77.4, 67.2, 65.7, 58.5, 42.0, 37.8, 36.1, 32.2, 23.5, 17.8, 14.5, 6.9 (3 C), 4.9 (3 C), −1.4 (3 C); IR (neat) 2956, 1720, 1358; HRMS (QTof) calcd for C34H57N2O4Si2 (MH+) 613.3857, found 613.3860.
Partial data for 35 were obtained from a 1:1 mixture of 34 and 35: 1H NMR 7.34-7.12 (m, 5), 6.93-6.92 (m, 1), 6.17-6.15 (m, 1), 6.14-6.12 (m, 1), 5.57 (d, 1, J = 10.2), 5.41 (d, 1, J = 10.2), 4.86 (dd, 1, J = 6.4, 12.0), 4.33 (d, 1, J = 10.2), 3.92-3.84 (m, 1), 3.48 (t, 2, J = 7.8), 3.40 (dd, 1, J = 12.0, 16.8), 3.16 (dd, 1, J = 6.4, 16.8), 2.34-2.25 (m, 1), 1.64-1.55 (m, 1), 1.35-1.12 (m, 4), 1.17 (d, 3, J = 5.8), 0.90 (t, 9, J = 7.8), 0.63 (q, 6, J = 7.8), −0.06 (s, 9).
(±)-(4R,5R,7S,9S,10S)-4-Methoxy-7-methyl-9-propyl-10-phenyl-2-(1-[[2-(trimethylsilyl)ethoxy]methyl]-1H-pyrrol-2-yl)-6-oxa-1-azaspiro[4.5]dec-1-ene (36)
A solution of pure isoxazoline 34 (29 mg, 47 µmol) in 6 mL of MeOH was treated with a wet slurry of Raney nickel 2800 (~20 mg) and the suspension was stirred at 25 °C under H2 (1 atm) for about 30 min. The mixture was then diluted with EtOAc and filtered. The filtrate was washed with brine (3 × 5 mL), dried (Na2SO4), and concentrated to give 27 mg of crude hydroxy hemi-iminal.
A solution of crude hydroxy hemi-iminal in THF (1 mL) was added dropwise to a suspension of NaH (60% in mineral oil, 15 mg, 0.37 mmol) in THF (5 mL) at 0 °C. The mixture was stirred at 0 °C for 10 min and MeI (23 µL, 0.37 mmol) was added dropwise by syringe over 2 min. The resulting mixture was warmed to 25 °C and stirred for 4 h. The reaction was quenched with saturated aqueous NH4Cl (3 mL) and the aqueous layer was extracted with EtOAc. The combined organic layers were dried (Na2SO4), and concentrated to give 23 mg of crude dimethyl ether. Flash chromatography on silica gel (25:1 hexanes/EtOAc) gave 13 mg (45% for two steps) of a single isomer of the dimethyl ether as a pale yellow gum: 1H NMR 7.16-7.10 (m, 5), 7.06–7.03 (m, 1), 6.50-6.46 (m, 1), 6.21-6.18 (m, 1), 6.01 (d, 1, J = 9.8), 5.77 (d, 1, J = 9.8), 3.98 (tq, 1, J = 5.9, 5.9), 3.64 (dd, 1, J = 3.1, 7.9), 3.50 (t, 2, J = 8.2), 3.43 (s, 3), 3.34 (s, 3), 3.03 (d, 1, J = 4.3), 2.64 (dd, 1, J = 3.1, 17.4), 2.58-2.48 (m, 1), 2.40 (dd, 1, J = 7.9, 17.4), 2.15-2.05 (m, 1), 1.46-1.14 (m, 4), 1.19 (d, 3, J = 5.9), 0.98 (t, 9, J = 7.8), 0.94-0.80 (m, 6), 0.64 (q, 6, J = 7.8), −0.03 (s, 9); IR (neat) 2955, 1615.
A solution of the dimethyl ether (13 mg, 21 µmol) in 8 mL of 3:1 CH3CN/THF was treated with aqueous 2 M HCl (210 µL, 420 nmol) at 25 °C. The resulting mixture was stirred at 25 °C for 20 h. Saturated NaHCO3 (4 mL) was added to bring the pH to 7. The reaction was extracted with EtOAc and the organic layer was washed with brine, dried (Na2SO4), and concentrated to give 12.5 mg of crude 36. Flash chromatography of the residue on MeOH-deactivated silica gel (6:1 hexanes/EtOAc) gave 7.2 mg (70%) of 36 as a colorless gum: 1H NMR (CDCl3) 7.25-7.18 (m, 2), 7.16-7.11 (m, 3), 7.02–6.98 (m, 1), 6.37-6.33 (m, 1), 6.27 (d, 1, J = 9.8), 6.15-6.11 (m, 1), 5.71 (d, 1, J = 9.8), 4.50-4.41 (m, 1), 3.64-3.53 (m, 3), 3.36 (s, 3), 2.84 (d, 1, J = 10.9), 2.66 (dd, 1, J = 5.2, 16.2), 2.62-2.51 (m, 1), 2.06 (dd, 1, J = 7.0, 16.2), 1.99 (ddd, 1, J = 4.0, 4.0, 12.8), 1.73 (ddd, 1, J = 5.6, 9.6, 12.8), 1.47 (d, 3, J = 6.6), 1.40-1.27 (m, 1), 1.18-1.02 (m, 2), 1.02-0.88 (m, 3), 0.74 (t, 3, J = 7.1), 0.00 (s, 9); 1H NMR (acetone-d6) 7.36-7.30 (m, 2), 7.18-7.10 (m, 4), 6.42-6.38 (m, 1), 6.32 (d, 1, J = 9.8), 6.11-6.08 (m, 1), 5.73 (d, 1, J = 9.8), 4.40-4.30 (m, 1), 3.66 (t, 2, J = 7.1), 3.56 (dd, 1, J = 4.2, 7.0), 3.39 (s, 3), 2.90 (d, 1, J = 11.3), 2.62-2.51 (m, 1), 2.50 (dd, 1, J = 4.2, 16.2), 2.12-2.03 (m, 1), 1.90 (dd, 1, J = 7.0, 16.2), 1.73 (ddd, 1, J = 4.8, 7.6, 12.8), 1.40 (d, 3, J = 6.6), 1.42-1.33 (m, 1), 1.20-1.07 (m, 2), 1.07-0.82 (m, 3), 0.74 (t, 3, J = 7.1), 0.00 (s, 9);13C NMR (CDCl3) 164.3, 140.1, 130.4 (br, 2 C), 127.7 (2 C), 127.3, 127.0, 126.3, 116.9, 108.4, 103.4, 82.1, 77.4, 69.4, 65.7, 58.4, 56.0, 39.6, 36.1, 35.8, 31.8, 23.4, 19.2, 18.1, 14.2, −1.4 (3 C); 13C NMR (acetone-d6) 164.8, 141.3, 131.5 (br, 2 C), 129.1 (br, 2 C), 128.6, 128.2, 127.2, 117.9, 109.1, 105.1, 83.2, 78.0, 68.9, 66.0, 58.7, 56.9, 41.5, 37.1, 36.9, 33.3, 23.9, 20.1, 18.7, 14.6, −1.2 (3 C); IR (neat) 2925, 1616, 1084. HRMS (QTof) calcd for C29H45N2O3Si (MH+) 497.3199, found 497.3192.
A 2D NOESY experiment in CDCl3 showed NOEs between CHOMe H-4 at δ 3.64-3.53 and both CHPh H-10 at δ 2.84 and the phenyl protons at δ 7.25-7.18 and between CHPr H-9 at δ 2.62-2.51 and 7-Me at δ 1.47.
(±)-(4S,5R,7S,9S,10S)-4-Methoxy-7-methyl-9-propyl-10-phenyl-2-(1-[[2-(trimethylsilyl)ethoxy]methyl]-1H-pyrrol-2-yl)-6-oxa-1-azaspiro[4.5]dec-1-ene (37)
A 1:1 mixture of isoxazolines 34 and 35 (24 mg, 39 µmol) in 5 mL of MeOH was treated with a wet slurry of Raney nickel 2800 (~20 mg) and the suspension was stirred at 25 °C under H2 (1 atm) for about 30 min. The mixture was then diluted with EtOAc and filtered. The filtrate was washed with brine (3 × 5 mL), dried (Na2SO4), and concentrated to give 22 mg of crude hydroxy hemi-iminals.
A solution of crude hydroxy hemi-iminals in THF (1 mL) was added dropwise to a suspension of NaH (60% in mineral oil, 15 mg, 0.37 mmol) in THF (5 mL) at 0 °C. The mixture was stirred at 0 °C for 10 min and MeI (23 µL, 0.37 mmol) was added dropwise by syringe over 2 min. The resulting mixture was warmed to 25 °C and stirred for 4 h. The reaction was quenched with saturated aqueous NH4Cl (3 mL) and the aqueous layer was extracted with EtOAc. The combined organic layers were dried (Na2SO4), and concentrated to give 17 mg of crude mixture of dimethyl ethers. Flash chromatography on silica gel (25:1 hexanes/EtOAc) gave 11 mg (45% for two steps) of a 1:1 mixture of two diastereomeric dimethyl ethers as a colorless gum.
Data for the isomer not obtained from 34 were determined from the mixture: 1H NMR 7.46-7.41 (d, 2, J = 7.0), 7.28-7.19 (m, 3), 7.06–7.03 (m, 1), 6.59-6.56 (m, 1), 6.46 (d, 1, J = 9.8), 6.21-6.18 (m, 1), 5.54 (d, 1, J = 9.8), 3.85 (tq, 1, J = 5.9, 5.9), 3.66 (d, 1, J = 3.0, 7.2), 3.61-3.54 (m, 2), 3.36 (s, 3), 3.08 (dd, 1, J = 7.2, 17.6), 2.96 (s, 3), 2.82 (dd, 1, J = 3.0, 17.6), 2.55 (d, 1, J = 4.8), 2.29-2.20 (m, 1), 2.15-2.05 (m, 1), 1.46-1.14 (m, 4), 1.06 (d, 3, J = 5.9), 0.92-0.80 (m, 6), 0.87 (t, 9, J = 7.8), 0.49 (m, 6, J = 7.8), −0.03 (s, 9).
A 1:1 mixture of dimethyl ethers (22 mg, 36 µmol) in 8 mL of 3:1 CH3CN/THF was treated with aqueous 2 M HCl (360 µL, 720 µmol) at 25 °C. The resulting mixture was stirred at 25 °C for 20 h. Saturated NaHCO3 (10 mL) was added to bring the pH to 7. The reaction was extracted with EtOAc and the organic layer was washed with brine, dried (Na2SO4), and concentrated to give 24 mg of a crude mixture of 36 and 37. Flash chromatography of the residue on MeOH-deactivated silica gel (10:1 hexanes/EtOAc) gave 5.6 mg (32%) of 37 as a colorless gum, followed by 5.0 mg (29%) of 36 as a colorless gum.
Data for 37: 1H NMR (CDCl3) 7.23 (d, 2, J = 7.2), 7.07-7.6.98 (m, 3), 6.98-6.96 (m, 1), 6.19 (d, 1, J = 10.0), 6.18-6.15 (m, 1), 6.11-6.08 (m, 1), 5.70 (d, 1, J = 10.0), 4.44-4.36 (m, 1), 3.80 (dd, 1, J = 8.8, 8.8), 3.66-3.55 (m, 2), 3.42 (s, 3), 3.01 (d, 1, J = 11.6), 2.61 (dd, 1, J = 8.8, 15.6), 2.47-2.36 (m, 1), 1.96 (ddd, 1, J = 4.0, 4.0, 13.6), 1.70 (ddd, 1, J = 5.2, 10.0, 13.6), 1.57 (d, 3, J = 6.8), 1.38-1.26 (m, 1), 1.11 (dd, 1, J = 8.8, 15.6), 1.16-0.88 (m, 5), 0.72 (t, 3, J = 7.1), 0.00 (s, 9); 1H NMR (acetone-d6) 7.32 (d, 2, J = 7.2), 7.12-7.09 (m, 1), 7.09-6.92 (m, 3), 6.29-6.23 (m, 3), 6.08-6.04 (m, 1), 5.71 (d, 1, J = 10.0), 4.38-4.29 (m, 1), 3.78 (dd, 1, J = 8.8, 8.8), 3.66 (t, 2, J = 7.1), 3.41 (s, 3), 3.00 (d, 1, J = 11.4), 2.69 (dd, 1, J = 8.8, 15.6), 2.55-2.45 (m, 1), 1.97 (ddd, 1, J = 3.6, 3.6, 13.6), 1.67 (ddd, 1, J = 6.4, 10.0, 13.6), 1.56 (d, 3, J = 7.1), 1.41-1.28 (m, 1), 1.10 (dd, 1, J = 8.8, 15.6), 1.20-0.85 (m, 5), 0.72 (t, 3, J = 7.1), 0.00 (s, 9); 13C NMR (CDCl3) 163.0, 140.0, 131.5 (br, 2 C), 127.24, 127.19, 126.7 (2 C), 125.7, 116.8, 108.4, 105.4, 88.3, 69.5, 65.5, 58.3, 52.1, 39.2, 35.7 (2 C), 31.2, 22.9, 19.2, 18.0, 14.1, −1.4 (3 C), one peak is obscured by the CDCl3 triplet at δ 77.0; 13C NMR (acetone-d6) 163.7, 141.3, 132.6 (br, 2 C), 129.1 (br, 2 C), 128.0, 127.5, 126.5, 117.8, 109.0, 106.3, 89.4, 77.9, 69.9, 65.9, 58.5, 53.2, 40.1, 36.8, 36.7, 32.2, 23.5, 20.0, 18.6, 14.6, −1.2 (3 C); IR (neat) 2928, 1619; HRMS (QTof) calcd for C29H45N2O3Si (MH+) 497.3199, found 497.3199.
A 2D NOESY experiment showed NOEs between the OMe at δ 3.42 and CHPh H-10 at δ 3.01 and the phenyl protons at δ 7.23 and between CHPr H-9 at δ 2.47-2.36 and 7-Me at δ 1.57.
2-(Hex-5-enyl)-1H-pyrrole (39) was prepared by Muchowski's procedure.3,30 Magnesium ribbon (400 mg, 16.67 mmol) and a small crystal of iodine (~20 mg) were placed in a 100 mL flask. The flask was flushed with nitrogen and was treated with 30 mL of THF. The suspension of magnesium in THF was slowly treated with 4-bromo-1-pentene (1.64 g, 11.0 mmol) and was heated to reflux gently. The resulting solution was refluxed for 2 h, cooled to 25 °C and cannulated to a solution of 1-(phenylsulfonyl)-2-pyrrolecarboxaldehyde43 (1.95 g, 8.25 mmol) in THF (15 mL) at 0 °C. The mixture was then stirred at 25 °C for 3 h and was quenched with 0.5 M HCl (8 mL). The aqueous layer was extracted with EtOAc and the combined organic layers were dried (Na2SO4) and concentrated. Flash chromatography on silica gel (5:1 hexanes/EtOAc) gave 1.62 g (64%) of α-5-hexen-1-yl-1-(phenylsulfonyl)-1H–pyrrole-2-methanol as a pale yellow gum: 1H NMR 7.78 (d, 2, J = 7.4), 7.62 (t, 1, J = 7.4), 7.51 (t, 2, J = 7.4), 7.30 (dd, 1, J = 1.5, 3.3), 6.32-6.25 (m, 2), 5.79-5.68 (m, 1), 4.98-4.90 (m, 2), 4.81 (t, 1, J = 6.0), 2.74 (br s, 1, OH), 2.06-1.92 (m, 2), 1.89-1.73 (m, 2), 1.54-1.42 (m, 1), 1.42-1.31 (m, 1); 13C NMR 139.2, 138.4, 138.2, 133.9, 129.4 (2 C), 126.4 (2 C), 123.5, 114.7, 112.4, 111.7, 65.0, 34.4, 33.2, 25.2; IR (neat) 3554, 1364, 1175; HRMS (Qtof) calcd for C16H19NO3NaS (MNa+) 328.0983, found 328.0980.
A solution of the above alcohol (1.62 g, 5.26 mmol) in CH2Cl2 (20 mL) was treated with 4A molecular sieves (2.5 g), N-methylmorpholine-N-oxide (1.23 g, 10.52 mmol) and tetrapropylammonium perruthenate (0.19 g, 0.53 mmol) at 0 °C. The reaction was stirred at 25 °C for 4 h and filtered through a pad of Celite. The filtrate was concentrated to afford 2.78 g reaction crude as a black oil. Flash chromatography on silica gel (8:1 hexanes/EtOAc) gave 1.44 g (89%) of 1-(1-(phenylsulfonyl)-1H–pyrrol-2-yl)hex-5-en-1-one as a pale yellow gum: 1H NMR 7.99 (d, 2, J = 7.2), 7.80 (dd, 1, J = 3.2, 1.6), 7.59 (t, 1, J = 7.2), 7.52 (t, 2, J = 7.2), 7.03 (dd, 1, J = 3.2, 1.6), 7.34 (t, 1, J = 1.6), 5.78-5.66 (m, 1), 5.01–4.92 (m, 2), 2.67 (t, 2, J = 7.6), 2.00 (dt, 2, J = 7.6, 7.6), 1.69 (tt, 2, J = 7.6, 7.6); 13C NMR (rotamer) 188.7, 138.9, 137.8, 133.5, 133.4, 130.10 (130.06), 128.7 (2 C), 128.1 (2 C), 123.3, 115.3 (br), 110.4 (110.3), 38.4, 32.9, 23.7; IR (neat) 1672, 1438, 1141; HRMS (Qtof) calcd for C16H18NO3S (MH+) 304.1007, found 304.0999.
A solution of the ketone (1.44 g, 4.75 mmol) in i-PrOH (50 mL) was treated with NaBH4 (1.26 g, 33.3 mmol). The mixture was refluxed for 16 h, cooled to 25 °C, and slowly quenched with water (50 mL) and saturated aqueous NH4Cl (30 mL). The mixture was extracted with EtOAc (3 × 20 mL). The combined organic layers were dried (MgSO4) and concentrated to give 1.06 g of crude 39. Flash chromatography on silica gel (12:1 hexanes/EtOAc) gave 0.65 g (84%) of 39 as a pale colorless gum with data identical to those previously reported.31
2-(Hex-5-enyl)-pyrrole-1-carboxylic Acid tert-Butyl Ester (40)
A solution of pyrrole 3931 (654 mg, 4.08 mmol) in 8 mL of CH2Cl2 was treated with (Boc)2O (1.24 g, 5.69 mmol), Et3N (0.82 mL, 5.69 mmol) and DMAP (30 mg, 0.43 mmol). The resulting solution was stirred for 5 h and concentrated. Flash chromatography of the residue on silica gel (25:1 hexanes/EtOAc) gave 811 mg (74%) of 40 as a colorless gum: 1H NMR 7.19 (dd, 1 J = 1.8, 3.3), 6.08 (t, 1, J = 3.3), 5.96 (dd, 1, J = 1.8, 3.3), 5.82 (ddt, 1, J = 10.2, 17.2, 6.7), 5.01 (ddt, 1, J = 1.4, 17.2, 1.4), 4.95 (ddt, 1, J =1.4, 10.2, 1.0), 2.85 (t, 2, J = 7.6), 2.10 (dt, 2, J = 6.7, 7.6), 1.64 (tt, 2, J = 7.6, 7.6), 1.59 (s, 9), 1.48 (tt, 2, J = 7.6, 7.6); 13C NMR (rotamer) 149.5, 138.9 (138.8), 136.3, 120.8 (120.7), 114.3, 110.8 (110.6), 109.9 (109.7), 83.1, 33.6 (br), 28.7, 28.6, 28.3, 28.0 (3 C); IR (neat) 2935, 1742, 1330; HRMS (EI) calcd for C15H23NO2 (M+) 249.1729, found 249.1733.
3-[5-(Hex-5-enyl)-1-tert-butoxycarbonylpyrrol-2-yl]-6-methyl-5,6-dihydro-2H-pyran-2-one (42)
A solution of 2,2,6,6-tetramethylpiperidine (1.37 mL, 8.13 mmol) in 12 mL of THF was treated with n-BuLi (1.6 M in THF, 5.08 mL) dropwise at −78 °C under nitrogen. The solution was stirred for 15 min, warmed to 0 °C for 30 min, and recooled to −78 °C. A solution of pyrrole 40 (1.35 g, 5.40 mmol) in 2 mL of THF was added dropwise and the reaction mixture was stirred at −78 °C for 2 h. Trimethyl borate (6.3 mL, 27.0 mmol) was added at −78 °C and the solution was warmed to 25 °C over 2 h and stirred overnight. Aqueous HCl (0.2 N, 45 mL) was added and the resulted mixture was extracted with EtOAc (3× 20 mL). The combined organic layers were washed with brine (25 mL) and dried (Na2SO4). The solution was slowly concentrated until a white solid started to precipitate. 5 mL of dry 1,2-dimethoxyethane was added and the solution was slowly concentrated to 2 mL to remove the remaining EtOAc. The solution was kept at 0 °C and degassed. Boronic acid 41 was unstable and was used immediately after being degassed.
A resealable tube was filled with iodolactone 26 (400 mg, 1.67 mmol), LiCl (214 mg, 5.00 mmol), Na2CO3 (1.06 g, 10.0 mmol), and Pd(PPh3)4 (155 mg, 0.13 mmol) and was purged with nitrogen. 2 mL of the above 1,2-dimethoxyethane solution of boronic acid 41 was added to the mixture, and then 0.3 mL of degassed H2O was added to the mixture. The tube was sealed and stirred at 80 °C for 8 h. The mixture was diluted with EtOAc and filtered. The filtrate was washed with brine (15 mL), dried (Na2SO4) and concentrated. Flash chromatography of the residue on silica gel (8:1 hexanes/EtOAc) gave 330 mg (55%) of 42 as a yellow gum: 1H NMR 6.74 (dd, 1, J = 3.1, 5.5), 6.06 (d, 1, J = 2.7), 5.88 (d, 1, J = 2.7), 5.81 (ddd, 1, J = 6.6, 10.2, 17.2), 5.00 (br d, 1, J = 17.2), 4.94 (br d, 1, J = 10.2), 4.76-4.67 (m, 1), 2.85-2.72 (m, 2), 2.48-2.35 (m, 2), 2.08 (m, 2), 1.65-1.40 (m, 4), 1.55 (s, 9), 1.47 (d, 3, J = 6.1); 13C NMR 164.3, 150.1, 138.8, 137.4, 136.9, 129.3, 128.6, 114.4, 112.6, 109.3, 84.1, 74.2, 33.6, 31.4, 29.3, 28.7, 28.5, 27.9 (3 C), 20.7; IR (neat) 1730 (br), 1367, 1216; HRMS (QTof) calcd for C21H30NO4 (MH+) 360.2175, found 360.2177.
(±)-(3S,4S,6S)-3-[5-(Hex-5-enyl)-1H-pyrrol-2-yl]-4-(2-propen-1-yl)-6-methyl-3,4,5,6-tetrahydro-2H-pyran-2-one (45)
A solution of ZnBr2 (1.00 g, 4.44 mmol) in 20 mL THF was treated with allylmagnesium bromide (1.7 M in THF, 5.22 mL) at 0 °C under nitrogen. The mixture was stirred for 30 min at 0 °C and cooled down to −78 °C. A mixture of unsaturated lactone 42 (400 mg, 1.11 mmol) and TMSCl (1.13 mL, 4.44 mmol) in 4 mL THF was added dropwise. The reaction was stirred at −78 °C for 3 h. Aqueous NH4Cl solution was added and the mixture was extracted with EtOAc (3 × 15 mL). The combined organic layers were washed with brine (15 mL), dried (Na2SO4) and concentrated. Flash chromatography of the residue on silica gel (10:1 hexanes/EtOAc) gave 249 mg (56%) of a 5:4 mixture of cis-addition product 43 and Boc-migration product 44 as a pale yellow gum
Partial data of 43 were determined from the mixture: 1H NMR 6.04–6.02 (m, 1), 5.90-5.86 (m, 1), 5.86-5,72 (m, 1), 5.65-5.52 (m, 1), 5.20-4.82 (m, 4), 4.72 (d, 1, J = 4.9), 4.72-4.64 (m, 1), 2.80-2.70 (m, 2), 2.48-2.30 (m, 2), 2.20-1.70 (m, 5), 1.59 (s, 9), 1.62-1.40 (m, 4), 1.39 (d, 3, J = 6.1).
Partial data of 44 were determined from the mixture: 1H NMR 5.98-5.95 (m, 1), 5.90-5.86 (m, 1), 5.86-5.65 (m, 2), 5.20-4.82 (m, 4), 4,60-4.52 (m, 1), 2.80-2.70 (m, 2), 2.48-2.30 (m, 1), 2.20-1.70 (m, 6), 1.59 (s, 9), 1.62-1.40 (m, 4), 1.41 (d, 3, J = 6.1).
A solution of the mixture of 43 and 44 (270 mg, 0.67 mmol) in CH2Cl2 was treated with 2,6-lutidine (0.43 mL, 4.05 mmol) and TMSOTf (0.6 mL, 2.7 mmol) at 0 °C. The reaction was stirred at 25 °C for 8 h, diluted with CH2Cl2, and treated with AcOH (0.5 mL). The mixture was washed with water (10 mL), NaHCO3 (5 mL), and brine (3 × 10 mL). The organic phase was dried (Na2SO4) and concentrated. Flash chromatography of the residue on silica gel (6:1 hexanes/EtOAc) gave 182 mg (91%) of a 19:1 equilibrium mixture of 45 and the cis isomer 45 as a pale yellow gum.
Data of 45 were determined from the mixture: 1H NMR 8.39 (br s, 1, NH), 5.92 (t, 1, J = 2.7), 5.79 (t, 1, J = 2.7), 5.86-5.69 (m, 2), 5.11 (d, 1, J = 10.0), 5.09 (d, 1, J = 17.2), 5.00 (d, 1, J = 17.2), 4.94 (d, 1, J = 10.4), 4.64-4.58 (m, 1), 3.57 (d, 1, J = 6.7), 2.56 (t, 2, J = 7.6), 2.44-2.30 (m, 2), 2.17-2.10 (m, 1), 2.07 (dt, 2, J = 7.0, 7.0), 1.89 (ddd, 1, J = 6.4, 10.0, 14.4), 1.80 (ddd, 1, J = 3.6, 3.6, 14.4), 1.62 (tt, 2, J = 7.6, 7.6), 1.45 (tt, 2, J = 7.6, 7.6), 1.37 (d, 3, J = 6.4); 13C NMR 173.1, 138.8, 134.9, 133.4, 124.2, 118.0, 114.5, 106.5, 104.6, 73.7, 43.6, 38.4, 33.6, 33.5, 32.9, 28.9, 28.6, 27.6, 21.3; IR (neat) 3365, 1704; HRMS (QTof) calcd for C19H28NO2 (MH+) 302.2120, found 302.2114. A 2D NOESY experiment showed NOEs between H-3 at δ 3.57 and H-6 at δ 4.64-4.58.
Partial data for the cis isomer of 45 were determined from the mixture: 1H NMR 4.09 (d, 1, J = 6.8).
(±)-(3S,4aS,15aS)-3-Methyl-3,4,4a,5,6,7,8,9,10,11-decahydro-12,15-epiminocycloundeca[c]pyran-1(15aH)-one (47b)
A solution of diene 45 (40 mg, 0.13 mmol) in 250 mL of degassed CH2Cl2 was slowly treated with a solution of Grubbs II catalyst (8 mg, 72 µmol) in 5 mL degassed CH2Cl2 at reflux under nitrogen. The solution was stirred at reflux for 8 h, and another solution of Grubbs II catalyst (8 mg, 72 µmol) in 5 mL CH2Cl2 was slowly added. The solution was stirred at reflux for another 8 h. The reaction was cooled to 25 °C, and five drops of DMSO were added. The solution was stirred overnight and concentrated. Flash chromatography of the residue on silica gel (6:1 hexanes/EtOAc) gave 16 mg (41%) of 46 as a brown solid, which was used directly for the next step.
A solution of alkene 46 (48 mg, 0.18 mmol, from three runs of the previous reaction) in 5 mL MeOH was treated with a wet slurry of Raney nickel 2800 (~15 mg) and the suspension was stirred at 25 °C under H2 (1 atm) for 25 min. The mixture was then diluted with EtOAc and filtered through a pad of Celite. The filtrate was concentrated and filtered again through a pad of silica gel to give 43 mg (90%) of 47b as a white solid: mp 165 °C (decomposed); 1H NMR 8.23 (br s, 1, NH), 5.92 (m, 1), 5.77 (m, 1), 4.64-4.54 (m, 1), 3.47 (d, 1, J = 12.1), 2.55 (ddd, 1, J = 4.4, 4.4, 14.4), 2.44 (ddd, 1, J = 3.8, 10.8, 14.4), 2.20-2.09 (m, 1), 2.04 (ddd, 1, J = 10.0, 10.0, 14.0), 1.67 (ddd, 1, J = 4.0, 4.0, 14.0), 1.64-1.44 (m, 4), 1.39 (d, 3, J = 6.4), 1.37-1.02 (m, 5), 0.91-0.72 (m, 2), 0.48-0.35 (m, 1); 13C NMR 175.0, 134.0, 124.4, 110.2, 105.7, 73.1, 45.5, 38.3, 33.1, 32.5, 28.1, 26.8, 25.5, 24.6, 24.2, 24.1, 20.9; IR (neat) 3343, 2924, 1725, 1211; HRMS (QTof) calcd for C17H26NO2 (MH+) 276.1964, found 276.1969.
(±)-(2S,3S)-3-((S)-2-hydroxy)propyl)-N-methoxy-N-methyl-14-azabicyclo[9.2.1]tetradeca-1(13),11-diene-2-carboxamide (48b)
A mixture of lactone 47b (43 mg, 0.16 mmol) and NH(OMe)Me•HCl (64 mg, 0.65 mmol) in 8 mL THF was treated with i-PrMgCl (1.3 M in THF, 1.37 mL) at −20 °C. The reaction was warmed to 0 °C in 30 min and stirred at 0 °C for 2.5 h. Aqueous NH4Cl solution was added and the mixture was extracted with EtOAc (3 × 15 mL). The combined organic layers were washed with brine (10 mL), dried (Na2SO4) and concentrated. Flash chromatography of the residue on silica gel (3:1 hexanes/EtOAc) gave 43 mg (86%) of 48b as a colorless gum: 1H NMR 8.43 (br s, 1, NH), 5.86 (m, 1), 5.74 (m, 1), 3.91 (d, 1, J = 11.0), 3.75 (s, 3), 3.74-3.65 (m, 1), 3.19 (s, 3), 2.72 (br s, 1, OH), 2.65 (ddd, 1, J = 4.0, 4.0, 14.4), 2.49 (ddd, 1, J = 3.2, 11.2, 14.4), 2.28-2.17 (m, 1), 1.75-1.20 (m, 9), 1.18 (d, 3, J = 6.1), 1.18-1.04 (m, 2), 1.04-0.74 (m, 3), 0.47-0.35 (m, 1); 13C NMR 175.5, 133.7, 127.2, 108.5, 105.3, 64.8, 61.6, 46.4, 45.0, 35.1, 32.2, 28.8, 28.2, 27.8, 25.2, 25.0, 24.8, 24.5, 23.3; IR (neat) 2958, 1667; HRMS (QTof) calcd for C19H33N2O3 (MH+) 337.2491, found 337.2485.
(±)-(2S,3S)-N-methoxy-N-methyl-3-((S)-2-((triethylsilyl)oxy)propyl)-14-azabicyclo[9.2.1]tetradeca-1(13),11-diene-2-carboxamide (49b)
A solution of alcohol 48b (43 mg, 0.16 mmol) in 3 mL of THF was treated with TESCl (90 µL, 0.24 mmol), Et3N (100 µL, 0.32 mmol) and DMAP (2 mg, 0.02 mmol). The mixture was stirred at 25 °C for 3 h. The reaction was then diluted with Et2O (10 mL) and washed with brine (10 mL). The organic layer was dried (MgSO4) and concentrated. Flash chromatography of the residue on silica gel (10:1 hexanes/EtOAc) gave 52 mg (77%) of 49b as a pale yellow gum: 1H NMR 8.49 (br s, 1, NH), 5.83 (t, 1, J = 2.7), 5.74 (t, 1, J = 2.7), 3.88 (tq, 1, J = 6.1, 6.1), 3.85 (d, 1, J = 11.0), 3.71 (s, 3), 3.17 (s, 3), 2.65 (ddd, 1, J = 4.6, 4.6, 14.6), 2.46 (ddd, 1, J = 3.7, 11.2, 14.6), 2.10-1.98 (m, 1), 1.79-1.44 (m, 3), 1.46-1.20 (m, 7), 1.18 (d, 3, J = 6.1), 1.18-1.02 (m, 2), 0.96 (t, 9, J = 8.0), 0.92-0.72 (m, 2), 0.59 (q, 6, J = 8.0), 0.45-0.35 (m, 1); 13C NMR 174.5, 133.2, 127.4, 108.1, 105.6, 66.7, 61.5, 46.9, 45.8, 35.9, 31.9, 29.1, 28.1, 26.6, 25.9, 25.2, 24.9, 24.6, 23.0, 6.9 (3 C), 4.8 (3 C); IR (neat) 2935, 1679, 1268; HRMS (QTof) calcd for C25H46N2O3NaSi (MNa+) 473.3175, found 473.3173.
(±)-1-((2S,3S)-3-((S)-2-((triethylsilyl)oxy)propyl)-14-azabicyclo[9.2.1]tetradeca-1(13),11-dien-2-yl)prop-2-en-1-one (50b)
A solution of vinyl bromide (24 µL, 76 µmol) in 4 mL of ether was treated with n-BuLi (1.6 M in THF, 48 µL) dropwise at −78 °C. Then the solution was transferred to a solution of Weinreb amide 49b (17 mg, 38 µmol) in 2 mL of ether by cannula at 0 °C. The mixture was stirred at 0 °C for 2 h. Aqueous NH4Cl solution was added and extracted with EtOAc (3 × 5 mL). The organic solution was washed with brine (5 mL), dried (Na2SO4) and concentrated. Flash chromatography of the residue on silica gel (16:1 hexanes/EtOAc) gave 11 mg (70%) of 50b as a pale yellow gum (containing 5% of bisvinyl tertiary alcohol): 1H NMR 8.13 (br s, 1, NH), 6.44 (dd, 1, J = 10.4, 17.6), 6.22 (d, 1, J = 17.6), 5.88 (t, 1, J = 2.8), 5.80 (d, 1, J = 10.4), 5.75 (t, 1, J = 2.8), 3.90 (tq, 1, J = 6.1, 6.1), 3.86 (d, 1, J = 10.2), 2.63 (ddd, 1, J = 4.6, 4.6, 14.6), 2.47 (ddd, 1, J = 3.7, 11.2, 14.6), 2.18-2.10 (m, 1), 1.62-1.50 (m, 2), 1.44-1.24 (m, 7), 1.18 (d, 3, J = 6.1), 1.18-1.02 (m, 2), 0.96 (t, 9, J = 8.0), 0.95-0.70 (m, 2), 0.60 (q, 6, J = 8.0), 0.55-0.43 (m, 1); 13C NMR 201.2, 136.2, 133.6, 129.0, 125.9, 109.0, 106.2, 66.6, 53.9, 46.3, 35.3, 29.2, 28.0, 26.2, 25.3, 25.2, 25.0, 24.9, 23.1, 6.9 (3 C), 4.9 (3 C); HRMS (QTof) calcd for C25H44NO2Si (MH+) 418.3136. found 418.3142.
X-Ray data collection, solution, and refinement for 47b
All operations were performed on a modern, kappa-geometry X-ray diffractometer equipped with a CCD detector and graphite-monochromated MoKα radiation. All diffractometer manipulations, including data collection, integration, scaling, and absorption corrections were carried out using standard software.44 Preliminary cell constants were obtained from three sets of 12 frames. Data collection was carried out at 120K, using a frame time of 30 sec and a detector distance of 60 mm. The optimized strategy used for data collection consisted of three phi and one omega scan sets, with 0.5° steps in phi or omega; completeness was 99.7 %. A total of 2133 frames were collected. Final cell constants were obtained from the xyz centroids of 7440 reflections after integration.
From the systematic absences, the observed metric constants and intensity statistics, space group C2/c was chosen initially; subsequent solution and refinement confirmed the correctness of this choice. The structure was solved using direct methods,45 and refined by using full-matrix-least squares methods.46 The asymmetric unit contains one complex (Z = 8; Z’ = 1). All ordered non-hydrogen atoms were refined using anisotropic displacement parameters. After location of H atoms on electron-density difference maps, the H atoms were initially refined with soft restraints on the bond lengths and angles to regularize their geometry (C---H in the range 0.93–0.98 Å and Uiso (H) in the range 1.2–1.5 times Ueq of the parent atom), after which the positions were refined with riding constraints.47 Minor ring disorder was observed at atom C(10); the two orientations were modeled as C(10) and C(101), and the occupancies were constrained to sum to 1.0. The major component occupancy (atom C(10), refined using an anisotropic displacement parameters) was 0.922(5); atom C(101) was refined using an isotropic displacement parameter. Atom H(1), attached to N(1) was refined using an isotropic displacement parameter. The final least-squares refinement converged to R1 = 0.0434 (I > 2σ(I), 3018 data) and wR2 = 0.1117 (F2, 3333 data, 190 parameters). The final CIF is available as supporting material.
Supplementary Material
Acknowledgment
We are grateful to the National Institutes of Health (GM-50151) for partial support of this work. We thank Mark W. Bezpalko, Brandeis University, for carrying out the X-ray crystallographic structure determination.
Footnotes
Supporting Information Available: Copies of 1H, 13C, and 2D NOESY NMR spectral data. Crystallographic data for compound 47b. This material is available free of charge via the Internet at http://pubs.acs.org.
References and Notes
- 1.Boonlarppradab C, Kauffman CA, Jensen PR, Fenical W. Org. Lett. 2008;10:5508–5508. doi: 10.1021/ol8020644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.(a) Fürstner A. Angew. Chem., Int. 2003;42:3582–3603. doi: 10.1002/anie.200300582. [DOI] [PubMed] [Google Scholar]; (b) Williamson NR, Fineran PC, Leeper FJ, Salmond GPC. Nat. Rev. Microbiol. 2006;4:887–899. doi: 10.1038/nrmicro1531. [DOI] [PubMed] [Google Scholar]; (c) Williamson NR, Fineran PC, Gristwood T, Chawrai SR, Leeper FJ, Salmond GPC. Future Microbiol. 2007;2:605–618. doi: 10.2217/17460913.2.6.605. [DOI] [PubMed] [Google Scholar]; (d) Mo S, Sydor PK, Corre C, Alhamadsheh MM, Stanley AE, Haynes SW, Song L, Reynolds KA, Challis GL. Chem. Biol. 2008;15:137–148. doi: 10.1016/j.chembiol.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 3.Aldrich LN, Dawson EW, Lindsley CW. Org. Lett. 2010;12:2231–2234. doi: 10.1021/ol100034p. [DOI] [PubMed] [Google Scholar]
- 4.Ross DJ, Harran P. ORGN 657; 239th ACS National Meeting; March 21-25, 2010; San Francisco, CA. [Google Scholar]
- 5.(a) Reynolds KA. NORM 132; 68th Northwest Regional Meeting of the American Chemical Society; July 21-24, 2013; Corvallis, OR. [Google Scholar]; (b) Salem SM. Ph.D. Thesis. Portland State University; 2012. [Google Scholar]
- 6.For a preliminary communication of a portion of this work, see: Cai X-C, Wu X, Snider BB. Org. Lett. 2010;12:1600–1603. doi: 10.1021/ol100333d.
- 7.Li G, Zhang X, Li Q, Feng P, Shi Y. Org. Biomol. Chem. 2013;11:2936–2938. doi: 10.1039/c3ob40208h. [DOI] [PubMed] [Google Scholar]
- 8.Panarese JD, Konkol LC, Berry CB, Bates BS, Aldrich LN, Lindsley CW. Tetrahedron Lett. 2013;54:2231–2234. doi: 10.1016/j.tetlet.2013.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aldrich LN, Berry CB, Bates BS, Konkol LC, So M, Lindsley CW. Eur. J. Org. Chem. 2013:4215–4218. doi: 10.1002/ejoc.201300643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cycloadditions with the nitrile N-oxide derived from benzaldehyde oxime can be carried out at room temperature, whereas those with the nitrile N-oxide of pyrrole-2-carboxaldehyde must be carried out at −78 °C Ghabrial SS, Thomsen I, Torssell KBG. Acta Chem. Scand. B. 1987;B41:426–434.
- 11.Cohen N, Banner BL, Blount JF, Weber G, Tsai M, Saucy G. J. Org. Chem. 1974;39:1824–1833. [Google Scholar]
- 12.Andersen SH, Sharma KK, Torssell KBG. Tetrahedron. 1983;39:2241–2245. [Google Scholar]
- 13.For full details Cai X. Ph.D. Thesis. Brandeis University; 2013.
- 14.For a similar effect by an axial oxygen in spiroketals, see: Doubský J, Šaman D, Zedník J, Vašíčková S, Koutek B. Tetrahedron Lett. 2005;46:7923–7926.
- 15.For the formation and equilibration of spiroketals, see: Zhou X-T, Lu L, Furkert DP, Wells CE, Carter RG. Angew. Chem. Int. Ed. 2006;45:7622–7626. doi: 10.1002/anie.200603353. Taber DF, Joerger J-M. J. Org. Chem. 2007;72:3454–3457. doi: 10.1021/jo0626461. Philips ST, Shair MD. J. Am. Chem. Soc. 2007;129:6589–6598. doi: 10.1021/ja0705487. Sinibaldi M-E, Canet I. Eur. J. Org. Chem. 2008:4391–4399. Wurst JM, Verano AL, Tan DS. Org. Lett. 2012;14:4442–4445. doi: 10.1021/ol3019456.
- 16.(a) Das B, Holla H, Mahender G, Banerjee J, Reddy MR. Tetrahedron Lett. 2004;45:7347–7350. [Google Scholar]; (b) Mendelsohn BA, Lee S, Kim S, Teyssier F, Aulakh VS, Ciufolini MA. Org. Lett. 2009;11:1539–1542. doi: 10.1021/ol900194v. [DOI] [PubMed] [Google Scholar]
- 17.Kaiser H-P, Muchowski JM. J. Org. Chem. 1984;49:4203–4209. [Google Scholar]
- 18.Muchowski JM, Solas DR. J. Org. Chem. 1984;49:203–205. [Google Scholar]
- 19.For a comprehensive review of pyrrole protection and deprotection Jolicoeur B, Chapman EE, Thompson A, Lubell WD. Tetrahedron. 2006;62:11531–11563.
- 20.Lee GA. Synthesis. 1982:508–509. [Google Scholar]
- 21.Berner H, Schulz G, Reinshagen H. Monatsh. Chem. 1978;109:137–145. [Google Scholar]
- 22.Hydrogenolysis of 14a over Raney nickel containing boric acid afforded the expected 2-hydroxy-1,4-diketone (65%). Methylation of the hydroxy group with CH2N2 (65%), formation of the pyrrole with ammonium acetate (57%), and hydrolysis of the silyl ether with HCl provided 17a. However, hydrogenolysis of 14c under these conditions gave a complex mixture rather than the analogous desired 2-hydroxy-1,4-diketone
- 23.Stevenson R, Weber JV. J. Nat. Prod. 1988;51:1215–1219. [Google Scholar]
- 24.Sperry J, Yuen TY, Brimble MA. Synthesis. 2009:2561–2569. [Google Scholar]
- 25.Billingsley KL, Anderson KW, Buchwald SL. Angew. Chem. Int. Ed. 2006;45:3484–3488. doi: 10.1002/anie.200600493. [DOI] [PubMed] [Google Scholar]
- 26.Brohm D, Philippe N, Metzger S, Bhargava A, Müller O, Folker L, Waldmann H. J. Am. Chem. Soc. 2002;124:13171–13178. doi: 10.1021/ja027609f. [DOI] [PubMed] [Google Scholar]
- 27.Beaulieu ED, Voss L, Trauner D. Org. Lett. 2008;10:869–872. doi: 10.1021/ol702996t. [DOI] [PubMed] [Google Scholar]
- 28.The analogous triphenyl substituted lactone also adopts the boat conformation see: Fuller-Stanley JA, Loehlin JH, Bolin KA, Fairbrother G, Nazaire F. J. Org. Chem. 2002;67:27–31. doi: 10.1021/jo0155963.
- 29.Hartmann E, Oestreich M. Angew. Chem. Int. Ed. 2010;49:6195–6198. doi: 10.1002/anie.201002916. [DOI] [PubMed] [Google Scholar]
- 30.Greenhouse R, Ramierz C, Muchowski JM. J. Org. Chem. 1985;50:2961–2964. [Google Scholar]
- 31.Fürstner A, Grabowski J, Lehmann CW. J. Org. Chem. 1999;64:8275–8280. doi: 10.1021/jo991021i. [DOI] [PubMed] [Google Scholar]
- 32.(a) Xue F, Silverman RB. Tetrahedron Lett. 2010;51:2536–2538. doi: 10.1016/j.tetlet.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Albanese D, Landini D, Penso M, Tagliabue A, Carlini E. Org. Proc. Res. Dev. 2010;14:705–711. [Google Scholar]
- 33.For differences in the reactivity of freshly prepared and commercially available vinylMgBr, see: Ushakov DB, Navickas V, Ströbele M, Maichle-Mössmer C, Sasse F, Maier ME. Org. Lett. 2011;13:2090–2093. doi: 10.1021/ol200499t. Han X-J, Yao M, Lu C-D. Org. Lett. 2012;14:2906–2909. doi: 10.1021/ol301194e.
- 34.(a) Reich HJ, Holtan RC, Bolm C. J. Am. Chem. Soc. 1990;112:5609–5617. [Google Scholar]; (b) Reich HJ, Olson RE, Clark MC. J. Am. Chem. Soc. 1980;102:1423–1424. [Google Scholar]
- 35.Halogen-metal exchange of 2-bromo-N-SEM-pyrrole36 with n-BuLi and alkylation with 6-iodo-1-hexene37 afforded 2-(5-hexenyl)-N-SEM pyrrole in 67% yield. The preparation of 2-pyrroleboronic acids requires an electron withdrawing protecting group on the nitrogen,38,39 so we treated the pyrrole with NBS to prepare 5-bromo-2-(5-hexenyl)-N-SEM pyrrole in 91% yield. Metal-halide exchange of iodolactone 11 with isopropylmagnesium chloride and treatment with PinBOMe afforded the unstable pinacol boronic ester in 74% yield.40 Treatment of the bromopyrrole and lactone pinacol boronic ester with Pd(PPh3)4 and K3PO4 afforded the compound analogous to 42 with an N-SEM, rather than N-Boc protecting group in 55% yield. Further elaboration to 47a was analogous to that described above for 47b
- 36.Garg NK, Caspi DD, Stoltz BM. J. Am. Chem. Soc. 2004;126:9552–9553. doi: 10.1021/ja046695b. [DOI] [PubMed] [Google Scholar]
- 37.Panarello AP, Khinast JG. Tetrahedron Lett. 2003;44:4095–4098. [Google Scholar]
- 38.Billingsley K, Buchwald SL. J. Am. Chem. Soc. 2007;129:3358–3366. doi: 10.1021/ja068577p. [DOI] [PubMed] [Google Scholar]
- 39.Primas N, Bouillon A, Rault S. Tetrahedron. 2010;66:8121–8136. and references cited therein. [Google Scholar]
- 40.(a) Ashburn BO, Rathbone LK, Camp EH, Carter RG. Tetrahedron. 2008;64:856–865. [Google Scholar]; (b) Knochel P, Dohle W, Gommermann N, Kneisel FF, Kopp F, Korn T, Sapountzis I, Vu VA. Angew. Chem. Int. Ed. 2003;42:4302–4320. doi: 10.1002/anie.200300579. and references therein. [DOI] [PubMed] [Google Scholar]
- 41.Jain N, Kumar A, Chauhan SMS. Tetrahedron Lett. 2005;46:2599–2602. [Google Scholar]
- 42.Moreau X, Campagne J-M. J. Org. Chem. 2003;68:5346–5350. doi: 10.1021/jo034195f. [DOI] [PubMed] [Google Scholar]
- 43.Hinz W, Jones RA, Anderson T. Synthesis. 1986:620–623. [Google Scholar]
- 44.Apex2, Version 2 User Manual, M86-E01078, Bruker Analytical X-ray Systems. Madison, WI: 2006. Jun, [Google Scholar]
- 45.Altomare A, Cascarano G, Giacovazzo G, Guagliardi A, Burla MC, Polidori G, Camalli M. J. Appl. Cryst. 1994;27:435–436. [Google Scholar]
- 46.Betteridge PW, Carruthers JR, Cooper RI, Prout K, Watkin DJ. In: J. Appl. Cryst. Prout CK, Pearce LJ, editors. Vol. 36. Oxford, UK: CAMERON, Chemical Crystallography Laboratory; 2003. p. 1487. 1996. [Google Scholar]
- 47.Cooper RI, Thompson AL, Watkin DJ. J. Appl. Cryst. 2010;43:1100–1107. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.