Abstract
The last decade witnessed a significant progress in understanding the biology and immunology of colorectal cancer alongside with the technical innovations in radiotherapy. The stepwise implementation of intensity-modulated and image-guided radiation therapy by means of megavolt computed tomography and helical tomotherapy enabled us to anatomically sculpt dose delivery, reducing treatment related toxicity. In addition, the administration of a simultaneous integrated boost offers excellent local control rates. The novel challenge is the development of treatment strategies for medically inoperable patient and organ preserving approaches. However, distant control remains unsatisfactory and indicates an urgent need for biomarkers that predict the risk of tumor spread. The expected benefit of targeted therapies that exploit the tumor genome alone is so far hindered by high cost techniques and pharmaceuticals, hence hardly justifying rather modest improvements in patient outcomes. On the other hand, the immune landscape of colorectal cancer is now better clarified with regard to the immunosuppressive network that promotes immune escape. Both N2 neutrophils and myeloid-derived suppressor cells (MDSC) emerge as useful clinical biomarkers of poor prognosis, while the growing list of anti-MDSC agents shows promising ability to boost antitumor T-cell immunity in preclinical settings. Therefore, integration of genetic and immune biomarkers is the next logical step towards effective targeted therapies in the context of personalized cancer treatment.
Keywords: Rectal cancer, Image-guided radiotherapy, Intensity-modulated radiotherapy, Biomarkers, Targeted therapies, Myeloid-derived suppressor cells
Core tip: The stepwise implementation of intensity-modulated and image-guided radiation therapy enabled us to anatomically sculpt dose delivery and prescribe a simultaneous integrated boost, thus reducing treatment related toxicity. However, distant control remains unsatisfactory and indicates an urgent need for biomarkers of tumor spread. The immune landscape of colorectal cancer is now better clarified with regard to protumor N2 neutrophils and myeloid-derived suppressor cells (MDSC) that emerge as useful prognostic biomarkers. The growing list of anti-MDSC agents shows promising ability to boost antitumor T-cell immunity. Therefore, integration of genetic and immune biomarkers is the next logical step towards effective targeted therapies in the context of personalized cancer treatment.
INNOVATIONS IN RADIOTHERAPY
The addition of concomitant 5-fluorouracil (5-FU) or its prodrug capecitabine to preoperative radiotherapy is standard of care in patients with locally advanced rectal cancer. According to randomized trials, the combined treatment modality increases the pathologic complete remission rate and local control over radiotherapy alone, but has no impact on survival or the incidence of distant metastases[1,2]. However, this treatment is associated with significant acute and late digestive toxicity, when using 3D conformal radiotherapy. Current strategies mainly aim improving the outcome by addition of oxaliplatin and biologic agents such as cetuximab. The role of those agents in addition to 5-FU chemoradiotherapy is questionable as so far the results from phase III trials do not show improvement in local control or survival, nevertheless an increased toxicity[3-6]. Considering the excellent local control rates in rectal cancer in patients with a circumferential resection margin (CRM) > 1 mm, decreasing radiation enteritis should be an absolute priority in our opinion.
In an attempt to decrease treatment related toxicity we introduced the concept of intensity-modulated and image-guided RT (IMRT-IGRT) in the preoperative treatment of rectal cancer. The TomoTherapy Hi-Art II System is a linac that fully integrates IGRT by means of megavolt computed tomography and IMRT by means of helical tomotherapy. A pilot study explored the potential of the integrated megavolt computed tomography in decreasing the margin from the clinical target volume (CTV) to the planning target volume (PTV) compared to classic laser-skin marks, by measuring the setup error and internal organ motion. The CTV-PTV margin can be reduced from 15 mm isotropically to 8 mm in both lateral, 11 mm in the anterior and 7 mm in the posterior direction[7]. As a next step, we investigated to what extent the integration of IMRT and IGRT can reduce the irradiated volume of small bowel, which is the major predictor of radiation enteritis. To do so, 3D-conformal radiotherapy, IMRT (helical tomotherapy) and IMRT-IGRT (helical tomotherapy with reduced CTV-PTV margins) were compared in a dosimetric evaluation. This study demonstrated an additive effect between IMRT and IGRT in decreasing the probability for developing grade 2 + diarrhea to 18%, as opposed to a calculated risk of 27% and 40% for IMRT and 3D-CRT, respectively[8]. The clinical implementation of preoperative IMRT-IGRT in a phase II trial in 108 patients with locally advanced rectal cancer resulted in a favourable toxicity profile, with < 1% acute and < 10% late grade 3 + toxicity[9-10].
As alternative strategy to the administration of concomitant 5-FU, we decided exploring a simultaneous integrated boost in patients with a CRM < 2 mm on MRI, till 55.2 Gy or 120% of the prescription dose (46 Gy in daily fractions of 2 Gy, Figure 1). With a median follow-up of 60 mo a local recurrence rate of less than 3% was documented in this population at high risk for local failure[10]. The use of preoperative IMRT-IGRT with a simultaneous integrated boost is currently being compared to standard preoperative chemoradiotherapy in a multicenter phase III trial (NCT 01224392). The aim of this study is demonstrating non-inferiority of a higher radiation dose compared to concomitant chemotherapy, with tumor response as primary endpoint. An interim analysis after the first 80 patients, shows that patients receiving a higher radiation dose experience less acute grade 2 enteritis compared to the chemoradiotherapy arm (22% vs 44%). A comparable rate of major histomorphologic regression (Dworak grade 3-4) were recorded in both treatment arms.
Figure 1.
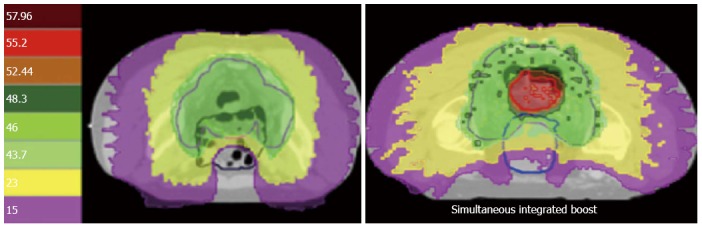
Dose distribution of helical tomotherapy. The left image shows a classic treatment of 46 Gy in daily fractions of 2 Gy. Note the horseshoe shaped distribution of the dose to spare the small bowel. On the right image a simultaneous integrated boost till 55.2 Gy is prescribed on the tumor.
With these excellent results for both local control and toxicity in the preoperative setting in mind, the research program of the UZ Brussel focuses on treatments for medically inoperable patients and organ preserving approaches. Besides improved radiation techniques and concomitant systemic treatments schemes, the identification of biomarkers for individualized-targeted therapies will become increasingly important.
PROSPECTS FOR TARGETED THERAPIES
For decades, the prognosis for patients with colorectal cancer was mainly determined by the timing of diagnosis and metastatic spread since the standard route of therapeutic practice had no tools to deal with the genetic landscape of individual tumors. Despite that the Human Genome Project was successfully accomplished in 2003, genetic testing remained to be limited keeping in mind that cancer mutations are rather unique (than heritable) and reflect an escalating heterogeneity along cancer progression. As a result, the American Society of Clinical Oncology considered only few prognostic biomarkers for gastrointestinal cancer, like, the chromosome arm 18q deletion, microsatellite instability, TP53 inactivation and EGFR/KRAS mutations (Figure 2). Thymidine synthase and other enzymes relevant to 5FU chemosensitivity were suggested as additional options in the context of predictive biomarkers[11].
Figure 2.
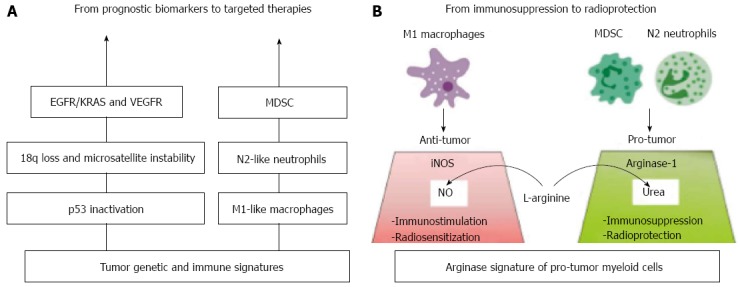
The genetic/immune landscape of colorectal cancer and therapeutic implications. The concept of personalized treatments in colorectal cancer should be based on integral knowledge of both tumor and immune cell signatures that would ideally provide therapeutic targets as well. A: As a result of genome profiling, epidermal growth factor receptor (EGFR) and vascular endothelial growth factor (VEGF) have been identified as promising targets for personalized therapies, while other biomarkers, like p53 mutations, 18q loss and microsatellite instability, lack prognostic/predictive value. The immune profile of colorectal cancer is rather unique with regard to macrophages (and Tregs), which unexpectedly point to favourable prognosis, yet being immunosuppressive in the most tumor types. Hence, the monocyte lineage of myeloid cells may reveal antitumor M1-like polarization within the compartment of tumor-associated macrophages. The granulocyte lineage of myeloid cells, comprising undifferentiated myeloid-derived suppressor cells (MDSC), feature clear protumor N2-like polarization and contribute to poor prognosis. Those cells overexpress Arg that causes L-arginine depletion and thereby suppresses antitumor T-cell immunity; B: We hypothesize that Arg+ neutrophils and MDSC may also display radioprotective properties, as L-arginine deficiency would neutralize the radiosensitizing potential of M1 macrophages. Indeed, classically activated M1 macrophages are known to produce the radiosensitizing molecule nitric oxide (NO) through the iNOS/L-arginine pathway. Therefore, Arg+ neutrofils and MDSC emerge as promising biomarkers and candidates for future targeted therapies, aiming at reversing impaired immune and radiation responses.
With the recent advance of next-generation DNA sequencing, the concept of personalized targeted therapy is one step closer to prospectively tailor medical care based on tumor profiling. Since 2009, the major role of KRAS mutations in colon carcinogenesis has been acknowledged in standard practice[12-14]. These developments emphasized the use of cetuximab and panitumumab predominantly in KRAS wild-type cases, and explained the importance of specific codon 12 mutations in downstream signalling through the RAF/MEK/ERK and PI3K/Akt pathways. Despite this evident progress toward personalized anti-EGFR treatments, the increase in survival of relapsed patients remains modest due to the outgrowth of resistant clones. Conversely, biomarkers that predict sensitivity to anti-angiogenic agents, like bevacizumab, are still lacking. Finally, the current difficulties to extract meaningful clinical results for the majority of patients, while facing adverse effects, provide a strong motivation to further look into the tumor genomic signatures.
In this context, the cyclooxygenase-2 (COX-2)/PGE2 pathway has been extensively revisited since a seminal observation that the anti-inflammatory drug aspirin significantly reduces the risk of colorectal cancer[15]. In addition, the selective COX-2 inhibitor celecoxib was shown to inhibit adenomatous polyposis, pointing to a key role of PGE2 in the pathogenesis of colorectal cancer. Recently, the multifaceted effects of PGE2 on cell proliferation and motility have been linked to G-protein coupled receptors (GPCR) that initiate or/and modulate a cascade of intracellular events including the transactivation of EGFR[16]. These findings led to an explosion of cancer-relevant research on GPCR and their therapeutic targeting, while the PGE2 receptor EP4 was established as a critical link to EGFR (and downstream P13K/Akt and RAS/MAPK/ERK pathways) through the beta-arrestin 1/c-Src signalling complex. Thus, next to their known GPCR-desensitizing functions, arrestins may act as adaptors that facilitate signalling events responsible for metastases. Indeed, the PGE2-induced transactivation of EGFR and metastatic spread to the liver was accelerated in the case of arrestin-expressing colorectal cancer cells, as compared with mutant counterparts[17]. Since existing COX-2 inhibitors cause cardiovascular toxicity, a further downstream targeting of EP4 by specific ligand antagonists may be more beneficial for cancer treatment, and could be combined with anti-EGFR agents.
Another on-going area of biomedical cancer research, with a clear focus on targeted therapies, addresses tumor immunotolerance at the level of myeloid-derived suppressor cells (MDSC). MDSC were originally identified in tumor-bearing mice as the CD11b+GR1+ set of poorly differentiated myeloid cells, both monocytes and granulocytes, which reveal a strong potency to suppress T-cell immunity through the production of reactive oxygen and nitrogen species[18]. In addition, the dominant polymorphonuclear subset of MDSC appeared to inhibit Th1 lymphocytes through profound L-arginine depletion due to overexpressed arginase-1 (Arg). This enzyme is currently considered as a surrogate marker of tumor immunosuppression, while MDSC emerge as a biomarker of tumor progression and as a novel therapeutic target. The list of screened MDSC inhibitors has recently extended from cytotoxic anticancer drugs (5FU, gemcitabine) to other classes that suppress MDSC functions (PDE and COX2 inhibitors) and sustain their differentiation (vitamin A, CpG ODNs), or disrupt their signalling pathways [JAK2/STAT3 and vascular endothelial growth factor (VEGF)], thereby illuminating a possibility to restore antitumor immunity[18,19].
In many human malignancies, a similar though much more heterogeneous type of undifferentiated granulocytic MDSC (Lin-HLA-DR-CD33+) has been documented as well, and elevated levels of MDSC seem to compromise both prognosis and therapy outcomes[19,20]. In renal cell carcinoma, the significance of Arg (in plasma) and immunosuppressive MDSC (in blood) is already established[21], and their sensitivity to the VEGF inhibitor sunitinib was explained by targeting the STAT3 signalling[22]. The immunosuppressive signature of colorectal cancer remains to be the matter of debate. First, patient prognosis is favoured by an intensive pro-inflammatory infiltrate suggesting a possible antitumor (M1-like) polarization of tumor-associated macrophages[23]. Unlike, other malignancies mostly feature a protumor (M2) phenotype, which thought to promote tumor growth and contribute to poor prognosis[24]. Next, the immunosuppressive FoxP3+ Tregs generally point to good prognosis in colorectal cancer, contrasting to other tumor types[25]. However, the neutrophil lineage of myeloid cells in colorectal cancer reveals a clear protumor (N2) rather than antitumor (N1) polarization[26], as both increased neutrophil-to-lymphocyte ratios and granulocytic MDSC contribute to poor prognosis [20,27,28].
In line, our pilot clinical study suggests that the levels of circulating Arg+ (N2-like) neutrophils and their MDSC subset (Lin-HLA-DR-CD33+CD15+CD16low) are significantly higher in colorectal cancer patients, as compared with healthy donors (unpublished data). Of note, immunosuppressive MDSC comprise only a minor part of abundant neutrophils that overexpress Arg and hence are capable of L-arginine depletion. This paradigm of N1-to-N2 shift in colorectal cancer suggests not only a rationale for interrogating the tumor immune landscape by FACS analysis of blood neutrophils but illuminates also a possible mechanistic link between immunosuppression and radioprotection. Indeed, our recent studies demonstrated that classically activated M1 macrophages produce a high output of nitric oxide (NO) by inducible nitric oxide synthase (iNOS), sufficient to block oxygen consumption and reverse impaired radioresponse of hypoxic tumor cells[29,30]. The radiosensitizing effect through the iNOS pathway is however critically dependent on the bioavailability of L-arginine, an essential substrate for NO synthesis[31].
Therefore, we hypothesize that Arg+ neutrophils/MDSC may neutralize the radiosensitizing potential of M1 macrophages through the same mechanism of accelerated L-arginine depletion that suppresses T-cell immunity on the first place. As such, the balance of Arg+ myeloid cells vs iNOS+ macrophages within the tumor microenvironment may determine radiotherapy responses through competitive L-arginine turnover. The myeloid signature and a possible cross-talk between macrophages and Arg+ neutrophils with regard to the L-arginine metabolism in colorectal cancer are schematically summarized in Figure 2. Future studies will clarify whether scoring of N2 neutrophils and MDSC is predictive for identifying patients at increased risk of tumor relapse/spread following radiotherapy. Those patients would need MDSC-targeted therapies to reverse the functional deficiency of Th1 lymphocytes and M1 macrophages, and to fully benefit from radiotherapy. Remarkably, 5FU-based chemotherapy at reduced doses may be an option to eliminate MDSC while sparing T cells, as recently reported in experimental studies[19].
Footnotes
Supported by Grants from the Vlaamse Liga tegen Kanker
P- Reviewers: Ionov Y, Roesler R S- Editor: Song XX L- Editor: A E- Editor: Liu XM
References
- 1.Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, Daban A, Bardet E, Beny A, Ollier JC. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355:1114–1123. doi: 10.1056/NEJMoa060829. [DOI] [PubMed] [Google Scholar]
- 2.Gérard JP, Conroy T, Bonnetain F, Bouché O, Chapet O, Closon-Dejardin MT, Untereiner M, Leduc B, Francois E, Maurel J, et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: results of FFCD 9203. J Clin Oncol. 2006;24:4620–4625. doi: 10.1200/JCO.2006.06.7629. [DOI] [PubMed] [Google Scholar]
- 3.Aschele C, Cionini L, Lonardi S, Pinto C, Cordio S, Rosati G, Artale S, Tagliagambe A, Ambrosini G, Rosetti P, et al. Primary tumor response to preoperative chemoradiation with or without oxaliplatin in locally advanced rectal cancer: pathologic results of the STAR-01 randomized phase III trial. J Clin Oncol. 2011;29:2773–2780. doi: 10.1200/JCO.2010.34.4911. [DOI] [PubMed] [Google Scholar]
- 4.Dewdney A, Cunningham D, Tabernero J, Capdevila J, Glimelius B, Cervantes A, Tait D, Brown G, Wotherspoon A, Gonzalez de Castro D, et al. Multicenter randomized phase II clinical trial comparing neoadjuvant oxaliplatin, capecitabine, and preoperative radiotherapy with or without cetuximab followed by total mesorectal excision in patients with high-risk rectal cancer (EXPERT-C) J Clin Oncol. 2012;30:1620–1627. doi: 10.1200/JCO.2011.39.6036. [DOI] [PubMed] [Google Scholar]
- 5.Gérard JP, Azria D, Gourgou-Bourgade S, Martel-Laffay I, Hennequin C, Etienne PL, Vendrely V, François E, de La Roche G, Bouché O, et al. Comparison of two neoadjuvant chemoradiotherapy regimens for locally advanced rectal cancer: results of the phase III trial ACCORD 12/0405-Prodige 2. J Clin Oncol. 2010;28:1638–1644. doi: 10.1200/JCO.2009.25.8376. [DOI] [PubMed] [Google Scholar]
- 6.Roh MS, Colangelo LH, O’Connell MJ, Yothers G, Deutsch M, Allegra CJ, Kahlenberg MS, Baez-Diaz L, Ursiny CS, Petrelli NJ, et al. Preoperative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum: NSABP R-03. J Clin Oncol. 2009;27:5124–5130. doi: 10.1200/JCO.2009.22.0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tournel K, De Ridder M, Engels B, Bijdekerke P, Fierens Y, Duchateau M, Linthout N, Reynders T, Verellen D, Storme G. Assessment of intrafractional movement and internal motion in radiotherapy of rectal cancer using megavoltage computed tomography. Int J Radiat Oncol Biol Phys. 2008;71:934–939. doi: 10.1016/j.ijrobp.2008.02.032. [DOI] [PubMed] [Google Scholar]
- 8.Engels B, De Ridder M, Tournel K, Sermeus A, De Coninck P, Verellen D, Storme GA. Preoperative helical tomotherapy and megavoltage computed tomography for rectal cancer: impact on the irradiated volume of small bowel. Int J Radiat Oncol Biol Phys. 2009;74:1476–1480. doi: 10.1016/j.ijrobp.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 9.De Ridder M, Tournel K, Van Nieuwenhove Y, Engels B, Hoorens A, Everaert H, Op de Beeck B, Vinh-Hung V, De Grève J, Delvaux G, et al. Phase II study of preoperative helical tomotherapy for rectal cancer. Int J Radiat Oncol Biol Phys. 2008;70:728–734. doi: 10.1016/j.ijrobp.2007.07.2332. [DOI] [PubMed] [Google Scholar]
- 10.Engels B, Tournel K, Everaert H, Hoorens A, Sermeus A, Christian N, Storme G, Verellen D, De Ridder M. Phase II study of preoperative helical tomotherapy with a simultaneous integrated boost for rectal cancer. Int J Radiat Oncol Biol Phys. 2012;83:142–148. doi: 10.1016/j.ijrobp.2011.05.068. [DOI] [PubMed] [Google Scholar]
- 11.Locker GY, Hamilton S, Harris J, Jessup JM, Kemeny N, Macdonald JS, Somerfield MR, Hayes DF, Bast RC. ASCO 2006 update of recommendations for the use of tumor markers in gastrointestinal cancer. J Clin Oncol. 2006;24:5313–5327. doi: 10.1200/JCO.2006.08.2644. [DOI] [PubMed] [Google Scholar]
- 12.Siena S, Sartore-Bianchi A, Di Nicolantonio F, Balfour J, Bardelli A. Biomarkers predicting clinical outcome of epidermal growth factor receptor-targeted therapy in metastatic colorectal cancer. J Natl Cancer Inst. 2009;101:1308–1324. doi: 10.1093/jnci/djp280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parsons BL, Myers MB. Personalized cancer treatment and the myth of KRAS wild-type colon tumors. Discov Med. 2013;15:259–267. [PubMed] [Google Scholar]
- 14.Rodriguez J, Zarate R, Bandres E, Viudez A, Chopitea A, García-Foncillas J, Gil-Bazo I. Combining chemotherapy and targeted therapies in metastatic colorectal cancer. World J Gastroenterol. 2007;13:5867–5876. doi: 10.3748/wjg.v13.i44.5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flossmann E, Rothwell PM, British Doctors Aspirin Trial and the UK-TIA Aspirin Trial. Effect of aspirin on long-term risk of colorectal cancer: consistent evidence from randomised and observational studies. Lancet. 2007;369:1603–1613. doi: 10.1016/S0140-6736(07)60747-8. [DOI] [PubMed] [Google Scholar]
- 16.Lappano R, Maggiolini M. G protein-coupled receptors: novel targets for drug discovery in cancer. Nat Rev Drug Discov. 2011;10:47–60. doi: 10.1038/nrd3320. [DOI] [PubMed] [Google Scholar]
- 17.Buchanan FG, Gorden DL, Matta P, Shi Q, Matrisian LM, DuBois RN. Role of beta-arrestin 1 in the metastatic progression of colorectal cancer. Proc Natl Acad Sci USA. 2006;103:1492–1497. doi: 10.1073/pnas.0510562103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poschke I, Kiessling R. On the armament and appearances of human myeloid-derived suppressor cells. Clin Immunol. 2012;144:250–268. doi: 10.1016/j.clim.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 20.Solito S, Falisi E, Diaz-Montero CM, Doni A, Pinton L, Rosato A, Francescato S, Basso G, Zanovello P, Onicescu G, et al. A human promyelocytic-like population is responsible for the immune suppression mediated by myeloid-derived suppressor cells. Blood. 2011;118:2254–2265. doi: 10.1182/blood-2010-12-325753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ochoa AC, Zea AH, Hernandez C, Rodriguez PC. Arginase, prostaglandins, and myeloid-derived suppressor cells in renal cell carcinoma. Clin Cancer Res. 2007;13:721s–726s. doi: 10.1158/1078-0432.CCR-06-2197. [DOI] [PubMed] [Google Scholar]
- 22.Ko JS, Rayman P, Ireland J, Swaidani S, Li G, Bunting KD, Rini B, Finke JH, Cohen PA. Direct and differential suppression of myeloid-derived suppressor cell subsets by sunitinib is compartmentally constrained. Cancer Res. 2010;70:3526–3536. doi: 10.1158/0008-5472.CAN-09-3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forssell J, Oberg A, Henriksson ML, Stenling R, Jung A, Palmqvist R. High macrophage infiltration along the tumor front correlates with improved survival in colon cancer. Clin Cancer Res. 2007;13:1472–1479. doi: 10.1158/1078-0432.CCR-06-2073. [DOI] [PubMed] [Google Scholar]
- 24.Biswas SK, Allavena P, Mantovani A. Tumor-associated macrophages: functional diversity, clinical significance, and open questions. Semin Immunopathol. 2013;35:585–600. doi: 10.1007/s00281-013-0367-7. [DOI] [PubMed] [Google Scholar]
- 25.deLeeuw RJ, Kost SE, Kakal JA, Nelson BH. The prognostic value of FoxP3+ tumor-infiltrating lymphocytes in cancer: a critical review of the literature. Clin Cancer Res. 2012;18:3022–3029. doi: 10.1158/1078-0432.CCR-11-3216. [DOI] [PubMed] [Google Scholar]
- 26.Piccard H, Muschel RJ, Opdenakker G. On the dual roles and polarized phenotypes of neutrophils in tumor development and progression. Crit Rev Oncol Hematol. 2012;82:296–309. doi: 10.1016/j.critrevonc.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 27.Sun HL, Zhou X, Xue YF, Wang K, Shen YF, Mao JJ, Guo HF, Miao ZN. Increased frequency and clinical significance of myeloid-derived suppressor cells in human colorectal carcinoma. World J Gastroenterol. 2012;18:3303–3309. doi: 10.3748/wjg.v18.i25.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mallappa S, Sinha A, Gupta S, Chadwick SJ. Preoperative neutro phil to lymphocyte ratio > 5 is a prognostic factor for recurrent colorectal cancer. Colorectal Dis. 2013;15:323–328. doi: 10.1111/codi.12008. [DOI] [PubMed] [Google Scholar]
- 29.Jiang H, De Ridder M, Verovski VN, Sonveaux P, Jordan BF, Law K, Monsaert C, Van den Berge DL, Verellen D, Feron O, et al. Activated macrophages as a novel determinant of tumor cell radioresponse: the role of nitric oxide-mediated inhibition of cellular respiration and oxygen sparing. Int J Radiat Oncol Biol Phys. 2010;76:1520–1527. doi: 10.1016/j.ijrobp.2009.10.047. [DOI] [PubMed] [Google Scholar]
- 30.Jiang H, Verovski VN, Leonard W, Law KL, Vermeersch M, Storme G, Van den Berge D, Gevaert T, Sermeus A, De Ridder M. Hepatocytes determine the hypoxic microenvironment and radiosensitivity of colorectal cancer cells through production of nitric oxide that targets mitochondrial respiration. Int J Radiat Oncol Biol Phys. 2013;85:820–827. doi: 10.1016/j.ijrobp.2012.07.2359. [DOI] [PubMed] [Google Scholar]
- 31.De Ridder M, Verellen D, Verovski V, Storme G. Hypoxic tumor cell radiosensitization through nitric oxide. Nitric Oxide. 2008;19:164–169. doi: 10.1016/j.niox.2008.04.015. [DOI] [PubMed] [Google Scholar]


