Abstract
AIM: To analyze hepatitis C virus (HCV)-specific immune responses in chronically infected patients under triple therapy with interferon-α (IFN-α) plus ribavirin and CIGB-230.
METHODS: CIGB-230 was administered in different schedules with respect to IFN-α plus ribavirin therapy. Paired serum and peripheral blood mononuclear cells (PBMC) samples from baseline and end of treatment were analyzed. The HCV-specific humoral response was tested by enzyme-linked immunosorbent assay, neutralizing antibodies were evaluated by cell culture HCV neutralization assays, PBMC proliferation was assayed by carboxyfluorescein succinimidyl ester staining and IFN-γ secretion was assessed by enzyme-linked immunospot. Data on virological and histological response and their association with immune variables are also provided.
RESULTS: From week 12 to week 48, all groups of patients showed a significant reduction in mean leukocyte counts. Statistically significant reductions in antibody titers were frequent, but only individuals immunized with CIGB-230 as early add-on treatment sustained the core-IgG response, and the neutralizing antibody response was enhanced only in patients receiving CIGB-230. Cell-mediated immune responses also tended to decline, but significant reductions in IFN-γ secretion and total absence of core-specific lymphoproliferation were exclusive of the control group. Only CIGB-230-immunized individuals showed de novo induced lymphoproliferative responses against the structural antigens. Importantly, it was demonstrated that the quality of the CIGB-230-induced immune response depended on the number of doses and timing of administration in relation to the antiviral therapy. Specifically, the administration of 6 doses of CIGB-230 as late add-on to therapy increased the neutralizing antibody activity and the de novo core-specific IFN-γ secretion, both of which were associated with the sustained virological response.
CONCLUSION: CIGB-230, combined with IFN-α-based therapy, modifies the immune response in chronic patients. The study provides evidence for the design of more effective therapeutic vaccine interventions against HCV.
Keywords: Clinical trial, DNA vaccine, Enzyme-linked immunospot, Hepatitis C virus, Leukopenia
Core tip: We assayed, for the first time, the concomitant administration of CIGB-230, a DNA-based therapeutic vaccine candidate against hepatitis C virus (HCV), and non-peginterferon-α (IFN-α) plus ribavirin therapy to chronic, treatment-naive, HCV genotype 1b infected patients. We showed that CIGB-230 enhanced the neutralizing antibody response and induced de novo proliferative and IFN-γ secretion responses in the context of antiviral therapy. The quality of the induced immune response depended on both the number of doses and the timing of administration in relation to the antiviral therapy. In particular, the increases in neutralizing antibodies and IFN-γ were associated with the sustained virological response.
INTRODUCTION
Hepatitis C virus (HCV) poses a significant challenge for worldwide public health, since it infects approximately 3% of the world population[1], of whom 80% will develop a chronic infection[2] if not treated timely and appropriately. Recently, there have been rapid advances in the development of specific antivirals[3,4]. In the clinical setting, the combination of the most advanced antivirals, boceprevir and telaprevir, with the present standard of care, peginterferon-α (pegIFN-α) plus ribavirin, have been shown to induce a higher sustained viral response (SVR) and lower relapse rates than pegIFN-α plus ribavirin alone, in HCV genotype-1-infected patients, but this genotype remains persistent in 30% of treated patients[4,5]. Additionally, current therapies result in multiple adverse effects that lead to contraindications in many cases[4,5] and do not provide long-term protection against reinfection.
Given these elements, the development of vaccine strategies remains attractive although, so far, they have not demonstrated significant clinical impact[6]. In HCV chronic infection, a critical obstacle facing any vaccine candidate is the already established immune response, which is characterized by impairment of both the innate and adaptive responses[7-10]. Indeed, it is reasonable to consider that these defects may result in uncontrolled viral replication, which could be linked to the non-attainment of a SVR. In this respect, studies have given clues of the pervasive effects of high HCV viral load on virus specific T cells[11]. There exist evidence that HCV-specific T cell dysfunction can be reversed by viral clearance after antiviral therapy, at least in the early stages of the infection[12], although functional restoration may be incomplete[13]. Nevertheless, immune restoration seems more achievable in face of a moderate, instead of a high viral load. In this sense, the combination of therapeutic vaccine candidates with antiviral treatments, allowing the vaccine to function in a scenario of reduced viral load, seems a more promising strategy.
Previously, we demonstrated the capability of CIGB-230, a vaccine candidate based on the mixture of a plasmid for DNA immunization, expressing HCV structural proteins[14], with recombinant HCV core protein particles[15], to modify the HCV-specific neutralizing antibody response and to induce de novo cellular immune responses against the HCV core in chronically infected individuals, and non-responders to previous IFN-α plus ribavirin treatment[16]. In the present study, we assayed, for the first time, the impact of concomitant administration of CIGB-230 and non-pegIFN-α plus ribavirin antiviral therapy on the HCV-specific immune response in a cohort of chronic, treatment-naïve, HCV genotype 1b infected patients.
MATERIALS AND METHODS
Study population
The clinical trial (Protocol code: IG/VHI/HC/0701; Public Register Code: RPCEC00000074) was conducted at the National Institute of Gastroenterology (Havana, Cuba), and was approved by the institutional ethics committee and the National Regulatory Authority (CECMED, Havana, Cuba). Written informed consent was obtained from every patient. All procedures were conducted in accordance with the national ethics guidelines and the Helsinki Declaration of 1975, as revised in 1983. The study included 92 treatment-naïve patients, positive for plasma HCV RNA, genotype 1b, with diagnosed chronic hepatitis by liver biopsy and no other documented cause of liver disease. Exclusion criteria were pregnancy, nursing, co-infection with HIV or active HBV infection, liver cirrhosis or hepatocellular carcinoma, uncontrolled chronic diseases, blood disorders, immunosuppressive/immunomodulatory drug consumption in the previous 6 mo, autoimmune diseases, severe allergy, and suspected acute infection. Demographic and histological data of patients involved in the study are shown in Table 1. Histology was evaluated at baseline and on week 72, by liver biopsy analyzed according to the Ishak score[17].
Table 1.
Demographic, virological and histological data of patients included in the trial n (%)
| Control group | Group E6 | Group E9 | Group L6 | Group L9 | ||
| Demographics1 and virological response | ||||||
| n = 30 | n = 16 | n = 16 | n = 15 | n = 15 | ||
| Age (yr) | mean ± SD | 48.5 ± 7.4 | 47.4 ± 9.1 | 42.6 ± 7.9 | 47.3 ± 7.5 | 45.3 ± 7.3 |
| Sex | Feminine | 12 (40) | 11 (68.8) | 8 (50.0) | 11 (73.3) | 9 (60) |
| Masculine | 18 (60) | 5 (31.3) | 8 (50.0) | 4 (26.7) | 6 (40) | |
| Body Mass Index | mean ± SD | 26.7 ± 2.8 | 24.8 ± 3.5 | 28.6 ± 4.6 | 25.3 ± 5.0 | 24.9 ± 4.4 |
| Race | White | 16 (53.3) | 12 (75.0) | 11 (68.8) | 10 (66.7) | 8 (53.3) |
| Black | 4 (13.3) | 3 (18.8) | 3 (18.8) | 4 (26.7) | 3 (20.0) | |
| Mixed | 10 (33.3) | 1 (6.3) | 2 (12.5) | 1 (6.7) | 4 (26.7) | |
| Response to treatment2 | cEVR | 18 (60.0) | 10 (62.5) | 9 (56.3) | 7 (46.7) | 4 (26.7) |
| SVR | 14 (46.7) | 8 (50) | 8 (50) | 7 (46.7) | 2 (13.3) | |
| Histology3 | ||||||
| Week 0 | n | 30 | 16 | 16 | 15 | 15 |
| Necroinflammation | mean ± SD | 6.7 ± 3.6 | 6.4 ± 3.2 | 6.1 ± 3.3 | 5.1 ± 3.4 | 7.0 ± 4.1 |
| Fibrosis | 1.6 ± 1.4 | 1.9 ± 1.3 | 1.4 ± 1.4 | 1.6 ± 1.3 | 2.3 ± 1.8 | |
| Week 72 | n | 19 | 13 | 14 | 9 | 8 |
| Necroinflammation | mean ± SD | 3.3 ± 2.5 | 3.8 ± 2.2 | 3.2 ± 2.8 | 4.9 ± 3.0 | 5.6 ± 3.2 |
| Fibrosis | 1.3 ± 1.5 | 2.1 ± 1.3 | 1.1 ± 1.3 | 2.1 ± 1.6 | 2.5 ± 1.6 | |
Corresponding to baseline;
By intention to treat;
According to Ishak score[17]. cEVR: Complete early viral responders; SVR: Sustained viral responders.
Study design and interventions
The study was a Phase II, randomized, controlled, double-blind clinical trial. All patients received conventional IFN-α-2b (3 × 106 units, subcutaneously, three times a week) and ribavirin (1000 or 1200 mg daily, according to body weight) for 48 wk. Patients were randomized to five groups according to CIGB-230 treatment, as shown in Figure 1. The control group (n = 30) received 12 vaccine placebo inoculations. Two groups received 6 inoculations of CIGB-230, one (n = 16) starting simultaneously with the antiviral treatment as early add-on (E6), and the other (n = 15) starting on week 12 of therapy as late add-on (L6). The remaining two groups were inoculated 9 times with CIGB-230, one (n = 16) as early add-on (E9) and the other (n = 15) as late add-on (L9). All inoculations of the candidate vaccine or the placebo took place once every 4 wk. To maintain blinding of the study, placebo administrations took place in the immunization groups, once every 4 wk, on weeks not corresponding to vaccine candidate administration, according to the immunization schedule. CIGB-230 immunization consisted of intramuscular administration of 0.5 mg of pIDKE2 plasmid mixed with 0.05 mg of Co.120 recombinant protein in saline solution. Components of CIGB-230 were manufactured under sterile conditions, with at least 98% purity[16]. Blood samples were drawn on weeks 0 and 48 to evaluate the HCV-specific immune response. The virological response (presence of HCV RNA by RT-PCR UMELOSA, detection limit 101.7 IU/mL, Centro de Inmunoensayos, Havana, Cuba) was assessed on weeks 0, 12, 48 and 72. Complete early virological response (cEVR) and SVR were defined as undetectable HCV RNA on weeks 12 and 72, respectively.
Figure 1.
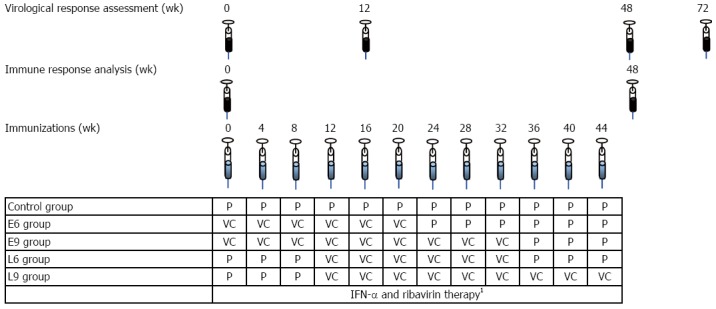
Study design. 148 wk of IFN-α-2b (3 × 106 units, subcutaneous, three times a week) plus ribavirin (1000 or 1200 mg daily, according to body weight)The control group (n = 30) received 12 vaccine placebo inoculations. Two groups received 6 inoculations of CIGB-230, one (n = 16) starting simultaneously with the antiviral treatment as early add-on (E6), and the other (n = 15) starting on week 12 of therapy as late add-on (L6). The remaining two groups were inoculated 9 times with CIGB-230, one (n = 16) as early add-on (E9) and the other (n = 15) as late add-on (L9). All inoculations relating the vaccine candidate or the placebo took place once every 4 wk. To maintain the blinding of the study, placebo administrations took place in the immunization groups, once every 4 wk, on weeks not corresponding to vaccine candidate administration, according to the immunization schedule. IFN: Interferon; VC: Vaccine candidate; P: Vaccine candidate’s placebo.
Antigens for analytical determinations
The recombinant protein NS3 was expressed in modified Escherichia coli and purified to 90%[18]. E2.680 recombinant protein was expressed in modified Picchia pastoris yeast and purified to 85%[19]. Recombinant Co.120 employed for immunization was also used for immunological evaluations. All the recombinant proteins corresponded to a genotype 1b strain.
White blood cells counts
White blood cells counts from whole blood were performed using an ABX Micros 60 hematology analyzer (ABX Diagnostics, Montpellier, France). These analyses were carried out at the clinical laboratory of the Cuban National Institute of Gastroenterology using routinely validated methods. Results are given as 109 cells/L in the case of leukocytes and as a percentage in the case of lymphocytes.
Evaluation of antibody response against HCV antigens
To detect human antibodies to the HCV capsid, E2 and NS3, an in-house enzyme-linked immunosorbent assay (ELISA) was performed[20]. For each individual evaluation, the cut-off value was established as at least 3 times the mean reactivity of a panel of 48 anti-HCV negative samples (UMELISA, Center for Immunoassay, Cuba). Titration was carried out by interpolation of a curve constructed by serially diluting a positive control sample of known reactivity.
Evaluation of neutralizing antibody response against HCV
For the evaluation of the neutralizing capacity of HCV-specific antibodies, total immunoglobulins were purified by Protein A-Sepharose 4 Fast flow (Amersham Biosciences, United Kingdom). Neutralization assays were performed essentially as described before[21]. Briefly, cell culture HCV (HCVcc, genotype 1a-2a chimera) and purified antibodies (50 μg/mL) were preincubated for 2 h at 37 °C before they were put in contact with Huh7 target cells; after 4 h, culture medium was replaced with fresh complete medium and cells were further incubated for 48 h. Finally, E1-specific immunostaining was carried out with E1-specific antibody and fluorophore-tagged antibody (anti-mouse Alexa 488). Foci forming units were counted in an Axiophot 2 microscope. Results are shown as percentage of HCVcc infectivity = (foci forming units in the presence of antibodies/foci forming units in the absence of antibodies) × 100. A sample was considered positive if at least a 50% reduction in the infectivity of HCVcc was observed in the presence of antibodies, compared to the absence of antibodies. Paired coded samples corresponding to each patient were evaluated simultaneously to avoid inter-experiment variability.
Peripheral blood mononuclear cell preparation
Peripheral blood mononuclear cell (PBMC) isolation and preservation was performed as previously described[20]. After quick thawing in a 37 °C water bath, cells were adjusted to 2 × 106 cells/mL in complete RPMI medium (Sigma-Aldrich, United States) with 10% FBS and then incubated at 37 °C for a resting period of 16 h.
Evaluation of PBMC proliferative response against HCV antigens
PBMC proliferation against Co.120, E2.680 and NS3 assays were performed essentially as previously described[20]. Cells were labeled with 6 μmol/L of CFSE (Fluka Biochemika, Switzerland), and stimulated in duplicate cultures of 0.25 × 106 cells, with protein antigens (8 μg/mL) in complete RPMI with 5% human AB serum (Sigma-Aldrich, United States) (RPMI/5) for 6 d at 37 °C. Cells incubated with medium alone and concanavalin A (ConA, Sigma-Aldrich, United States, 5 μg/mL) were considered the negative and positive controls, respectively. Cells were analyzed by flow cytometry (Partec Pass III, Germany). The stimulation index (SI) was calculated by dividing the proliferative frequency (%) in the presence of antigen by the proliferative frequency (%) without antigen. For each individual antigen, the cut-off value was established after the evaluation of samples from 10 HCV RNA negative donors: ≥ 2.5 (UMELOSA, Center for Immunoassay, Cuba). De novo responses were defined as those responses that were not detected on baseline evaluations and became detectable after treatment.
Evaluation of IFN-gamma secretory response against HCV antigens
The enzyme-linked immunospot (ELISPOT) assay was performed essentially as previously described[22] in nitrocellulose-backed microtiter plates (Millipore, France). Anti-IFN-γ (1-D1K clone, 5 μg/mL) and biotinylated anti-IFN-γ (7-B6-1clone, 0.5 μg/mL), both from Mabtech (Sweden), were used as capture and detection monoclonal antibodies, respectively. Previously, cells had undergone a 72-h stimulation period with proteins (8 μg/mL in RPMI/5) at 37 °C; 0.2 × 106 cells were transferred to the ELISPOT plate, in duplicate for each condition. Cells incubated with medium alone and concanavalin A (5 μg/mL) were considered the negative and positive controls, respectively. ELISPOT plates were incubated for 24 h at 37 °C. Spots were revealed with extravidin-peroxidase (1:800; Sigma-Aldrich, United States) followed by 0.5 mg/mL of AEC (Sigma-Aldrich, United States) solution. Spot counting was performed in a stereomicroscope (Carl Zeiss, Germany). For each individual antigen the cut-off value was established after the evaluation of samples from 10 HCV RNA negative donors (UMELOSA, Center for Immunoassay, Cuba), with the average spot number at least twice that obtained in the negative-control wells, and at least 50 spots/106 PBMC for E2.680 and NS3, and 180 spots/106 PBMC for Co.120. De novo responses were defined as those responses that were not detected on baseline evaluations and became detectable after treatment.
Statistical analysis
SPSS 15.0.0 Software for Windows was used to carry out statistical analysis. Normality was analyzed by the Shapiro-Wilk Test. The paired t test or Wilcoxon matched pairs test were used to compare the magnitude of a given response in a group between the two evaluated time points. For comparison of the number of positive samples at the two evaluation time points, in a given group, the McNemar test was used. Comparisons of the magnitude of the responses among all groups of patients in a given time point were performed using one way analysis of variance or the Kruskal-Wallis test. Frequency comparisons among the groups were performed by the Fisher exact test. Correlations between variables were analyzed by Spearman’s rank correlation coefficient. Relationships between nominal variables were analyzed by correspondence analysis. Significant differences were considered at P < 0.05.
RESULTS
Humoral response generally diminishes in IFN-α plus ribavirin treated patients, but the neutralizing activity may be enhanced by CIGB-230
Antibody responses against core, E2 and NS3 are summarized in Table 2. No significant differences in terms of response frequency or antibody titers were detected among the different groups at any evaluation time point. In all groups, more than 80% of patients showed a detectable IgG and IgM response against core and NS3 at both evaluated time points (Table 2). Regarding antibody titers against these antigens, statistically significant reductions were generally observed at week 48 in the majority of the groups (Table 2). In the case of E2, a tendency to a decrease in antibody titers was also often observed in the case of IgG (Table 2).
Table 2.
Humoral and neutralizing antibody response
| Antigen |
Control group |
E6 group |
E9 group |
L6 group |
L9 group |
||||||||||||
| Week 0 | Week 48 | P value | Week 0 | Week 48 | P value | Week 0 | Week 48 | P value | Week 0 | Week 48 | P value | Week 0 | Week 48 | P value | |||
| Humoral response | |||||||||||||||||
| Capsid | IgM | Seroc | 96.1 | 96.1 | - | 100.0 | 100.0 | - | 100.0 | 100.0 | - | 100.0 | 100.0 | - | 100.0 | 100.0 | - |
| Titer | 108.9 ± 96.4 | 57.3 ± 81.9 | 0.002 | 269.3 ± 313.5 | 107.4 ± 105.3 | 0.003 | 210.8 ± 223.0 | 98.3 ± 104.2 | 0.008 | 310.5 ± 338.4 | 273.8 ± 530.7 | ns | 174.9 ± 182.5 | 170.7 ± 221.6 | ns | ||
| IgA | Seroc | 84.6 | 73.1 | ns | 86.6 | 66.6 | ns | 86.6 | 86.6 | - | 92.8 | 85.7 | ns | 100.0 | 91.6 | ns | |
| Titer | 12.0 ± 20.8 | 10.0 ± 16.2 | 0.020 | 48.2 ± 76.9 | 23.6 ± 26.6 | 0.010 | 38.3 ± 49.2 | 29.3 ± 34.4 | ns | 34.1 ± 38.5 | 24.8 ± 41.3 | 0.006 | 30.3 ± 25.6 | 22.9 ± 26.9 | ns | ||
| IgG | Seroc | 96.1 | 96.1 | - | 100.0 | 100.0 | - | 100.0 | 100.0 | - | 100.0 | 100.0 | - | 91.6 | 91.6 | - | |
| Titer | 30056.0 ± 39875.0 | 17913.7 ± 24413.0 | < 0.001 | 49443.7 ± 40431.0 | 24135.7 ± 23793.0 | 0.002 | 30604.8 ± 22036.0 | 28932.5 ± 24813.0 | ns | 42400.4 ± 73634.0 | 31502.8 ± 62453.0 | 0.040 | 52700.6 ± 69336.1 | 33379.0 ± 52006.1 | 0.010 | ||
| E2 | IgM | Seroc | 34.6 | 19.2 | ns | 33.3 | 26.6 | ns | 13.3 | 13.3 | ns | 57.1 | 35.7 | ns | 25.0 | 33.3 | ns |
| Titer | 7.1 ± 3.5 | 9.7 ± 14.5 | ns | 7.8 ± 4.7 | 7.8 ± 2.4 | ns | 5.9 ± 2.0 | 5.9 ± 1.9 | ns | 8.7 ± 3.7 | 8.8 ± 5.5 | ns | 11.4 ± 19.1 | 9.4 ± 7.2 | ns | ||
| IgA | Seroc | 15.3 | 11.5 | ns | 6.6 | 6.6 | ns | 6.6 | 0.0 | - | 14.2 | 0.0 | - | 0.0 | 0.0 | - | |
| Titer | 5.8 ± 1.8 | 5.6 ± 1.6 | ns | 5.3 ± 1.3 | 5.3 ± 1.3 | ns | 5.3 ± 1.3 | 5.00 ± 0 | ns | 5.7 ± 1.8 | 5.0 ± 0 | ns | 5.0 ± 0.0 | 5.0 ± 0.0 | ns | ||
| IgG | Seroc | 61.5 | 80.7 | ns | 46.4 | 66.6 | ns | 86.6 | 86.6 | ns | 71.4 | 71.4 | ns | 66.6 | 83.3 | ns | |
| Titer | 325.9 ± 380.0 | 222.8 ± 221.0 | ns | 245.2 ± 355.0 | 176.5 ± 216 | ns | 611.8 ± 671.0 | 468.1 ± 707 | ns | 585.3 ± 1055.8 | 415.2 ± 644.8 | ns | 214.5 ± 306.4 | 159.4 ± 205.0 | ns | ||
| NS3 | IgM | Seroc | 92.3 | 84.6 | ns | 100.0 | 100.0 | - | 93.3 | 86.6 | ns | 100.0 | 92.8 | ns | 100.0 | 91.6 | ns |
| Titer | 171.0 ± 428.8 | 33.1 ± 43.9 | < 0.001 | 211.1 ± 300.6 | 62.18 ± 69.55 | 0.003 | 55.7 ± 85.7 | 23.6 ± 14.2 | 0.040 | 94.7 ± 146.0 | 132.3 ± 334.7 | ns | 214.3 ± 297.3 | 110.6 ± 192.2 | ns | ||
| IgA | Seroc | 76.9 | 50.0 | 0.008 | 66.6 | 60.0 | ns | 66.6 | 26.6 | 0.030 | 57.1 | 42.8 | ns | 83.3 | 75.0 | ns | |
| Titer | 30.2 ± 87.6 | 24.1 ± 75.9 | < 0.001 | 83.7 ± 240.4 | 16.3 ± 18.4 | 0.009 | 12.0 ± 7.7 | 8.2 ± 3.6 | 0.005 | 20.3 ± 31.8 | 19.8 ± 41.4 | ns | 47.8 ± 82.9 | 53.1 ± 100.2 | ns | ||
| IgG | Seroc | 96.1 | 96.1 | - | 100.0 | 100.0 | - | 100.0 | 100.0 | - | 100.0 | 100.0 | - | 100.0 | 100.0 | - | |
| Titer | 15907.0 ± 15723.0 | 9684.0 ± 12663.0 | 0.002 | 11881.0 ± 7195.0 | 6493.0 ± 5575.0 | 0.001 | 10695.0 ± 10046.4 | 5166.0 ± 5304.0 | 0.020 | 8135.0 ± 8076.2 | 4570.0 ± 5277.1 | 0.030 | 13893.1 ± 11677.0 | 8806.2 ± 8510.0 | ns | ||
| Neutralizing antibody response | |||||||||||||||||
| Positive (%) | 46.2 | 46.7 | ns | 66.7 | 58.3 | ns | 35.7 | 14.3 | ns | 23.1 | 30.8 | ns | 41.7 | 41.7 | ns | ||
| Infectivity (%) | 71.0 ± 77.7 | 58.7 ± 26.6 | ns | 52.6 ± 24.7 | 51.0 ± 20.0 | ns | 61.7 ± 25.1 | 76.1 ± 22.8 | ns | 73.5 ± 29.4 | 60.8 ± 23.8 | 0.040 | 53.2 ± 26.5 | 53.2 ± 26.5 | ns | ||
Seroc: Seroconversion; ns: Not significant. Seroconversion data are presented as percent of samples with detectable antibody responses. Titer and infectivity data are presented as mean ± SD, except for IgA response against the capsid in the control group, which is presented as median ± rank. Statistical values refer to time dependent (week 0 vs week 48) differences in one group (McNemar test and paired t test or Wilcoxon signed rank test). Significant differences were considered for P < 0.05. Positive (%) refers to the percent of samples with detectable neutralizing antibody response. Infectivity (%) = (foci forming units in the presence of antibodies from a patient/foci forming unit in the absence of antibodies) × 100.
The study of the associations between baseline humoral responses and the histological data showed that taking all the patients as one group, the IgG response specific to NS3 correlated positively with week 0 necroinflammation (R2 = 0.27; P = 0.01; Figure 2). It is worthwhile noting that both in the control group (Table 2), as well as in the immunized patients as the only group, a significant reduction in NS3-specific IgG was detected at week 48 when compared with week 0 (NS3-specific IgG titre for the immunized patients: 43335 vs 29243; P < 0.0001).
Figure 2.
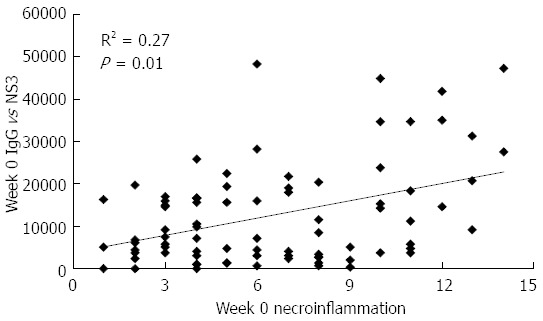
Baseline correlation between total IgG titer against NS3 and necroinflammation in patients of the study. Data refer to all the patients included in the study. Antibody titers are given in arbitrary units, necroinflammation was evaluated by liver biopsy according to the Ishak score[17]. Statistically significant differences were considered when P < 0.05 (Spearman’s rank correlation).
On the other hand, the analysis of the neutralizing antibody response (Table 2) showed no differences with respect to the percentage of individuals among groups with a positive response, or in each group between both time points. However, the L6 group showed a statistically significant enhancement in the neutralizing antibody response, between week 0 and week 48, while the control group did not (Table 2). In particular, the L6 group did not show a decline in the virological response from week 12 to week 72 (Table 1), and the significant enhancement in the neutralizing antibody response was observed in patients achieving the SVR (week 0 infectivity: 79.2 vs week 48 infectivity: 55.5; P = 0.024), in contrast to virological non-responders (week 0 infectivity: 66.9 vs week 48 infectivity: 66.9; P = 0.99).
CIGB-230 generates de novo HCV core-specific cell-mediated immune responses in an IFN-α plus ribavirin induced leukopenic state
As expected, lymphoproliferative responses were scarcely detected in treatment naïve patients (Table 3). A global tendency for disappearance of the immune response was observed towards week 48. At this time point the control group showed a complete absence of core-specific lymphoproliferative responses (Figure 3). Nevertheless, in the immunized groups all the responses against HCV structural antigens were generated de novo after CIGB-230 treatment. In fact, on week 48 a statistically significant difference was detected between CIGB-230 early add-on groups (E6 + E9) and the control group regarding the frequency of core-specific responses (P = 0.04). Additionally, a statistically significant difference was evidenced in core-specific lymphoproliferation between CIGB-230 early add-on groups (E6 + E9) and the control group on week 48 (1.04 vs 0.79, P = 0.018), as well as between virological responders from CIGB-230 early add-on groups (E6 + E9) and virological responders from the control on week 48 (1.13 vs 0.65, P = 0.008). We also observed that in the E6 group, one of the de novo core-responders was also a de novo E2-responder. This patient, although a late virological responder, achieved a SVR.
Table 3.
Cell-mediated immune responses
| Antigen |
Control group |
E6 group |
E9 group |
L6 group |
L9 group |
|||||||||||
| Week 0 | Week 48 | P value | Week 0 | Week 48 | P value | Week 0 | Week 48 | P value | Week 0 | Week 48 | P value | Week 0 | Week 48 | P value | ||
| Lymphoproliferative response | ||||||||||||||||
| Capsid | Total n | 25 | 25 | 14 | 13 | 15 | 14 | 13 | 14 | 12 | 11 | |||||
| Positive (%) | 4 | 0 | ns | 7.1 | 15.4 | ns | 20 | 14.3 | ns | 38.5 | 7.1 | ns | 8.3 | 18.2 | ns | |
| SI1 | 1.2 ± 0.8 | 0.8 ± 0.5 | ns | 1.1 ± 0.6 | 1.2 ± 0.9 | ns | 1.8 ± 1.2 | 2.5 ± 3.4 | ns | 2.3 ± 2.0 | 1.5 ± 1.2 | ns | 1.5 ± 0.8 | 1.7 ± 1.8 | ns | |
| E2 | Total n | 25 | 23 | 13 | 9 | 14 | 10 | 13 | 10 | 12 | 9 | |||||
| Positive (%) | 16 | 8.7 | ns | 7.7 | 11.1 | ns | 21.4 | 0 | ns | 23.1 | 0 | ns | 13.3 | 0 | ns | |
| SI | 1.5 ± 1.8 | 1.6 ± 3.1 | ns | 0.9 ± 0.7 | 1.2 ± 1.2 | ns | 2.2 ± 2.2 | 0.6 ± 0.3 | 0.04 | 2.3 ± 2.4 | 1.1 ± 0.6 | ns | 1.4 ± 1.1 | 1.2 ± 0.8 | ns | |
| NS3 | Total n | 24 | 21 | 13 | 9 | 13 | 10 | 13 | 10 | 12 | 8 | |||||
| Positive (%) | 8.3 | 0 | ns | 7.7 | 11.1 | ns | 7.7 | 0 | ns | 30.8 | 0 | ns | 8.3 | 0 | ns | |
| SI | 1.2 ± 0.9 | 1.0 ± 0.6 | ns | 1.2 ± 0.8 | 2.0 ± 3.5 | ns | 1.7 ± 1.4 | 1.1 ± 0.4 | ns | 2.0 ± 1.3 | 0.9 ± 0.4 | 0.03 | 1.3 ± 0.8 | 1.1 ± 0.5 | ns | |
| IFN-γ ELISPOT response | ||||||||||||||||
| Capsid | Total n | 24 | 22 | 13 | 9 | 11 | 7 | 12 | 9 | 9 | 7 | |||||
| Positive (%) | 16.7 | 9.1 | ns | 23.1 | 0 | ns | 27.3 | 12.5 | ns | 8.3 | 27.3 | ns | 33.3 | 0 | - | |
| SFC | 151.4 ± 228.4 | 38.2 ± 67.3 | 0.003 | 232.9 ± 466.2 | 49.8 ± 94.1 | ns | 422.4 ± 731.7 | 50.5 ± 80.1 | ns | 120.9 ± 112.4 | 101.0 ± 89.5 | ns | 330.0 ± 214.7 | 116.0 ± 125.3 | ns | |
| E2 | Total n | 15 | 16 | 10 | 7 | 5 | 4 | 6 | 4 | 2 | 4 | |||||
| Positive (%) | 20 | 12.5 | ns | 10 | 0 | - | 0 | 0 | - | 0 | 20 | ns | 50 | 0 | - | |
| SFC | 84.6 ± 117.9 | 42.0 ± 82.4 | 0.040 | 96.8 ± 144.1 | 31.2 ± 54.8 | ns | 245.7 ± 409.4 | 3.9 ± 1.7 | ns | 19.9 ± 14.4 | 30.0 ± 33.6 | ns | 120.6 ± 73.9 | 23.3 ± 24.8 | ns | |
| NS3 | Total n | 17 | 12 | 9 | 4 | 1 | 4 | 7 | 3 | 4 | 3 | |||||
| Positive (%) | 15.4 | 8.3 | ns | 11.1 | 0 | - | 100 | 0 | ns | 100 | 0 | - | 25 | 0 | - | |
| SFC | 132.1 ± 259.7 | 52.0 ± 97.4 | 0.030 | 329.1 ± 452.3 | 2.5 ± 3.5 | ns | 110.8 ± 0.0 | 7.92 ± 10.7 | - | 31.3 ± 30.8 | 26.9 ± 23.5 | ns | 260.7 ± 315.7 | 15.2 ± 8.4 | ns | |
Data on number of positive samples are presented as percent of total evaluated samples. Stimulation index and spot forming cells data are presented as mean ± SD. Statistical values refer to time dependent differences in one group (week 0 vs week 48; P < 0.05, Wilcoxon signed rank test).
A statistically significant difference regarding core-specific lymphoproliferation was observed among the groups on week 0 (P = 0.04, Kruskal-Wallis test), but the multiple comparison test was not potent enough to indicate which of the groups accounted for the difference. SI: Stimulation index; ns: Not significant; SFC: Sport forming cells/106 cells.
Figure 3.
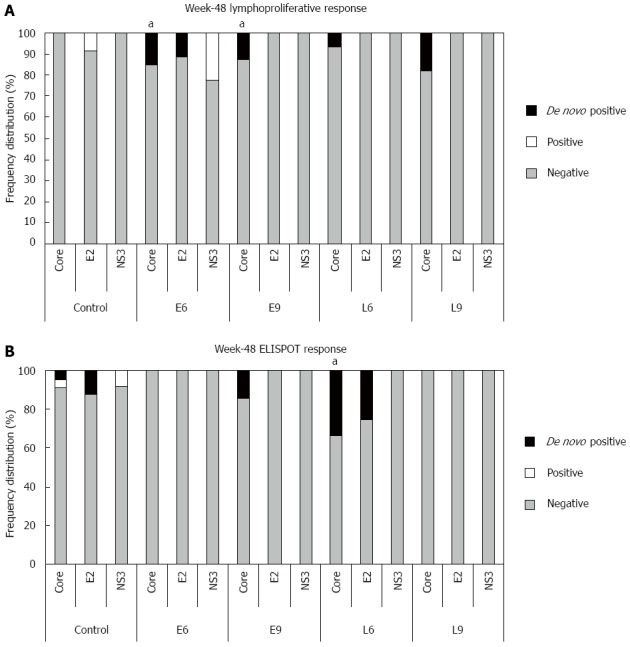
Frequency of hepatitis C virus-specific cell-mediated immune responses induced by CIGB-230 in combination with interferon-α plus ribavirin on week 48. A: Frequency of lymphoproliferative responses against hepatitis C virus (HCV) antigens. aDenotes a statistically significant difference between the CIGB-230 early add-on groups (E6 + E9) and the control group regarding the frequency of de novo core-specific responses (P = 0.04); B: Frequency of interferon-γ secretion responses against HCV antigens. aDenotes a statistically significant difference between L6 group and the control group with respect to the frequency of de novo core specific responses (P = 0.03). Significant differences were considered for P < 0.05; Fisher’s exact test. In the Figure “De novo positive” refers to responses that were undetectable on week 0 and became detectable on week 48; “Positive” refers to detectable responses on week 48 that were also detectable on week 0, and “Negative” refers to undetectable responses on week 48.
The correspondence analysis showed that in the control group, the E2-specific proliferative response, at both evaluation time points, was associated with the presence of serum HCV RNA on week 48 (P < 0.05). Moreover, the study of the baseline associations between lymphoproliferative responses and the histological data showed that taking all the patients as one group, the E2-specific response correlated positively with fibrosis (R2 = 0.24; P = 0.03; Figure 4). Remarkably, this immune response, which was associated with a bad prognosis, regarding both virological and histological responses, was significantly reduced in immunized patients (week 0: 1.49 vs week 48: 1.01; P = 0.02), while the control group did not show a significant change (week 0: 1.53 vs week 48: 1.65; P > 0.05; Table 3).
Figure 4.
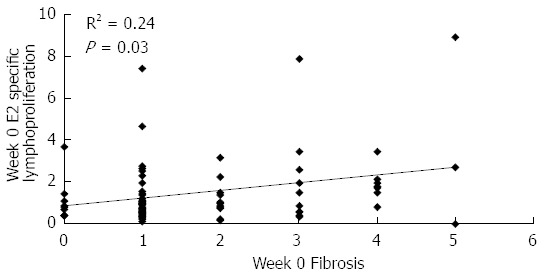
Baseline correlation between E2-specific lymphoproliferation and fibrosis in patients of the study. Data refer to all patients included in the study. E2-specific lymphoproliferation is given as stimulation index, fibrosis was evaluated by liver biopsy according to the Ishak score[17]. Statistically significant differences were considered when P < 0.05 (Spearman’s rank correlation).
According to PBMC availability, IFN-γ secretion against HCV antigens was also assessed. A trend for the disappearance of the responses was observed on week 48 (Table 3). However, significant decreases against all evaluated antigens were only observed in the control group; however, in the rest of the groups the small number of assayed samples could be masking possible differences.
Notably, on week 48, the L6 group showed a greater frequency of de novo IFN-γ responders against core than the control group (P = 0.03; Figure 3). The correspondence analysis showed that in this group, the positive association between an individual increase by at least a factor of 1.5 in this effector mechanism and the SVR was near statistical significance (P = 0.06). Moreover, taking into account patients showing an increase in the neutralizing antibody response and/or in the IFN-γ secretion in the L6 group, a statistically significant correspondence was detected with the SVR (P = 0.02). In this group, none of the week 72 RNA positive patients showed any increase in these effector mechanisms.
It is important to note that these changes in HCV-specific cell-mediated immune responses took place in the context of a significant leukopenia induced by IFN-α plus ribavirin treatment. The leukopenic state was already significant on week 12 of treatment in all groups of patients. Regarding lymphocyte counts, only the control group showed a significant decline at week 48 (Table 4).
Table 4.
Leukocyte and lymphocyte counts in hepatitis C virus chronically infected patients treated with interferon-α plus ribavirin
| Groups | Control group | Group E6 | Group E9 | Group L6 | Group L9 | |
| Total number of patients | 30 | 16 | 16 | 15 | 15 | |
| Baseline leukocyte counts | mean ± SD | 6.7 ± 2.1 | 7.0 ± 1.5 | 7.7 ± 2.3 | 6.4 ± 2.0 | 7.0 ± 2.2 |
| (Min; Max) | (3.5; 12.1) | (4.2; 9.0) | (3.8; 12.2) | (4.0; 11.1) | (2.0; 10.5) | |
| Week 12 leukocyte counts | mean ± SD | 4.5 ± 1.5 | 4.4 ± 1.8 | 5.0 ± 1.5 | 3.7 ± 0.9 | 4.7 ± 0.9 |
| (Min; Max) | (2.3; 9.0) | (2.3; 8.4) | (3.1; 7.3) | (2.7; 5.1) | (3.4; 6.8) | |
| Week 48 leukocyte counts | mean ± SD | 4.3 ± 1.3 | 3.6 ± 1.2 | 4.4 ± 1.5 | 3.7 ± 1.3 | 4.9 ± 0.9 |
| (Min; Max) | (2.5; 7.6) | (1.6; 6.7) | (2.3; 7.6) | (2.5; 6.6) | (2.9; 5.8) | |
| Statistics (P value)1 | < 0.001 | 0.001 | < 0.001 | 0.001 | 0.013 | |
| Statistics (P value)2 | < 0.010 | 0.001 | 0.001 | 0.001 | 0.023 | |
| Baseline lymphocyte counts | mean ± SD | 37.8 ± 8.6 | 35.7 ± 7.6 | 33.7 ± 7.5 | 36.7 ± 10.7 | 37.6 ± 8.7 |
| (Min; Max) | (22.8; 58.8) | (21.8; 52.5) | (19.8; 53.8) | (18.3; 51.4) | (25.4; 50.7) | |
| Week 12 lymphocyte counts | mean ± SD | 38.2 ± 9.7 | 34.6 ± 6.9 | 30.5 ± 8.0 | 36.1 ± 9.5 | 36.5 ± 7.5 |
| (Min; Max) | (20.3; 52.9) | (22.0; 44.1) | (15.8; 47.4) | (22.5; 55.2) | (25.3; 52.0) | |
| Week 48 lymphocyte counts | mean ± SD | 32.0 ± 8.9 | 37.1 ± 8.3 | 31.2 ± 6.9 | 34.9 ± 11.3 | 33.1 ± 6.2 |
| (Min; Max) | (13.3; 57.8) | (19.5; 50.9) | (21.3; 45.0) | (19.8; 54.0) | (23.5; 42.3) | |
| Statistics (P value)1 | ns | ns | ns | ns | ns | |
| Statistics (P value)2 | 0.004 | ns | ns | ns | ns | |
Week 12 vs baseline in one group;
Week 48 vs baseline in one group (Differences were considered significant when P < 0.05; Wilcoxon signed ranks test for leukocyte counts, Paired t test for lymphocyte counts). Results are given as 109 cells/L in the case of leukocytes and as % in the case of lymphocytes. ns: Not significant.
CIGB-230 induces differential courses of cell-mediated immunity in virological responder and non-responder patients under therapy with IFN-alpha plus ribavirin
We also determined whether there were any differences in HCV-specific immune responses associated with differing virological responses in immunized and non-immunized individuals. In fact, no differences in SVR rates were detected among the different groups. Reductions in the magnitude of several of the immune variables were detected (Table 5). Week 48 virological non-responders in the control group and CIGB-230-immunized week 48 virological responders showed a significant decline in E2-specific IgG; however, in the latter, the decline was exclusive of relapsers, as verified in a separate analysis (relapsers: P = 0.02; SVR: P = 0.10).
Table 5.
CIGB-230 administration induces differential courses of cell-mediated immunity in virological responder and non-responder patients under therapy with interferon-α plus ribavirin
| Immune response |
HCV RNA (-) on week 48 |
HCV RNA (+) on week 48 |
HCV RNA (-) on week 72 |
HCV RNA (+) on week 72 |
|||||||||
| Week 0 | Week 48 | P value | Week 0 | Week 48 | P value | Week 0 | Week 48 | P value | Week 0 | Week 48 | P value | ||
| CIGB-230 immunized patients | |||||||||||||
| IgG | Core | 32193.3 | 15486.7 | 0.0001 | 22068.7 | 15130.5 | ns | 32193.3 | 15486.7 | 0.003 | 22920.0 | 15130.5 | 0.0041 |
| E2 | 272.3 | 103.5 | 0.0061 | 50 | 295.3 | ns | 138.8 | 103.1 | ns | 254.3 | 138.7 | ns | |
| NS3 | 8277.2 | 2969.1 | < 0.0001 | 7177.4 | 5555.5 | 0.006 | 8013.1 | 2900.1 | 0.002 | 7263.8 | 5555.5 | 0.0002 | |
| IFN-γ | Core | 69.2 | 15 | ns | 88.8 | 57.5 | ns | 68.8 | 18.3 | ns | 76.3 | 43.5 | 0.041 |
| E2 | 17.4 | 5.6 | ns | 10.8 | 5.8 | ns | 22.5 | 5.2 | ns | 10.8 | 5.8 | ns | |
| NS3 | 14.6 | 2.5 | ns | 38.8 | 16.3 | ns | 15 | 0 | ns | 26.3 | 15.1 | ns | |
| SI | Core | 1.3 | 1 | ns | 1.4 | 1 | ns | 1.3 | 1 | 0.04 | 1.4 | 1 | ns |
| E2 | 1 | 0.9 | 0.0061 | 0.9 | 1.2 | ns | 1 | 0.8 | ns | 0.9 | 1.2 | ns | |
| NS3 | 1.2 | 1 | ns | 0.9 | 0.9 | ns | 1.2 | 1.1 | ns | 1.1 | 0.9 | ns | |
| Control patients | |||||||||||||
| IgG | Core | 26820.1 | 9096.3 | 0.0010 | 14778.9 | 10761.0 | 0.008 | 28066.9 | 12800.0 | 0.002 | 14778.9 | 8873.2 | 0.0005 |
| E2 | 85.7 | 233.1 | ns | 339.3 | 94.5 | 0.040 | 85.7 | 185 | ns | 339.3 | 155 | ns | |
| NS3 | 5150.3 | 2856.7 | 0.0010 | 15003.7 | 10833.1 | ns | 5150.3 | 2856.7 | 0.003 | 15003.8 | 8577.9 | ns | |
| IFN-γ | Core | 78.7 | 19.1 | 0.0410 | 60.3 | 15.6 | ns | 80.4 | 35 | ns | 60.3 | 14 | 0.0200 |
| E2 | 39.5 | 6.6 | ns | 5 | 0 | ns | 36.2 | 7.5 | ns | 32.5 | 2.5 | ns | |
| NS3 | 35.9 | 4.1 | 0.0310 | 11.6 | ne | - | 47.7 | 13 | ns | 12.9 | 4.2 | ns | |
| SI | Core | 0.9 | 0.8 | ns | 1.2 | 0.8 | ns | 1 | 0.7 | ns | 1.1 | 1 | ns |
| E2 | 0.8a | 0.8 | ns | 1.8a | 1.4 | ns | 0.8c | 0.7 | ns | 1.4c | 1.4 | ns | |
| NS3 | 0.8 | 0.8 | ns | 1.5 | 1.1 | ns | 0.8 | 0.7 | ns | 1.2 | 1.1 | ns | |
Median values are shown. SI: Stimulation index; ns: Not significant; ne: Not evaluated. Differences were considered statistically significant when P < 0.05 (Wilcoxon signed rank test).
Not significant when virological relapsers and non-responders were separately analyzed. a,cIndicate differences between virological responders and non responders, on both evaluation time points (week 48 and week 72) (P < 0.05, Unpaired t test). HCV: Hepatitis C virus.
Regarding cell-mediated immune responses, a statistically significant reduction in IFN-γ secretion was only noted in the week 72 virological non-responders against core (Table 5). On the other hand, in the non-immunized patients no significant changes were detected in the lymphoproliferative response. However, in CIGB-230-treated week 48 virological responders a significant decline was verified against E2 (Table 5).
In the control group, no significant correlations were found between cell-mediated immune responses and the histological findings, when virological responders and non responders were analyzed separately. In contrast, in the CIGB-230-immunized virological non-responders, the baseline proliferative response against all tested antigens showed a positive correlation with week 72 necroinflammation or fibrosis (Figure 5A-D), and week 48 core-specific IFN-γ secretion showed a positive correlation with both histological parameters at week 72 (Figure 5E and F).
Figure 5.
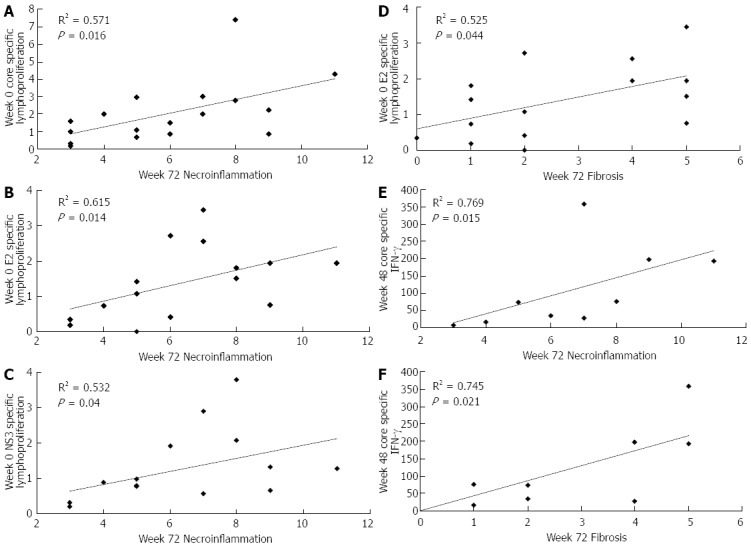
Correlations between hepatitis C virus-specific cell-mediated immune responses and histological parameters in patients of the study. Data refer to CIGB-230 immunized patients who were not able to clear viral RNA after treatment (virological non-responders). Lymphoproliferation is given as stimulation index; interferon-γ (IFN-γ) secretion is given as spot forming cells/106 cells; histological parameters were evaluated by liver biopsy according to the Ishak score[17]. Statistically significant differences were considered when P < 0.05 (Spearman’s rank correlation).
DISCUSSION
IFN-α has long been a cornerstone in the standard of care for HCV chronic infection. Nevertheless, its administration at therapeutic doses leads to several adverse events[23] which, together with those related to ribavirin, provoke frequent dose adjustments, treatment interruptions or contraindication in certain patients[24,25]. Hematologic toxicity is one of IFN-α plus ribavirin’s common adverse events[26,27]. Correspondingly, in our study from week 12 to week 48, all groups of patients showed a significant reduction in mean leukocyte counts. Nonetheless, mean lymphocyte counts were not significantly reduced in any of the immunized groups, unlike the control. This difference could support a possible immune stimulating effect of the CIGB-230 vaccine candidate.
In this context, despite being diverse and numerous from baseline, the humoral responses against HCV antigens tended to decline with treatment in all groups. Significant drops in NS3-specific IgG observed here have also been reported in IFN-α or IFN-α plus ribavirin responders[28]. In our work, only individuals from the E9 group were able to sustain the IgG response to the capsid. Further analyses revealed a significant decline in E2-specific IgG in control non-responders and immunized relapsers, which could indicate that these antibodies are pivotal for viral control. In fact, several E2 epitopes have been identified as targets of neutralizing antibodies[29]. Interestingly, in this study we observed that the neutralizing antibody response was only enhanced in a CIGB-230-treated group, while no significant reduction in this effector mechanism was observed in any of the groups. Modification of the neutralizing antibody response by CIGB-230 was also detected in a previous clinical trial[16]. In the present study, it should be noted that in the L6 group, which showed a significant enhancement of the neutralizing antibody response, the typical fall in the percentage virological response that has been described for pegIFN plus ribavirin therapy from week 12 to week 72[30] was not observed. Additionally, in this group we detected significantly greater induction of de novo core-specific IFN-γ ELISPOT responders with respect to the control. HCV-specific IFN-γ secretion has been previously associated with the clearance of HCV infection[31] and our results support this fact, since patients with detectable viral RNA on week 72, either immunized or not with CIGB-230, showed a significant decrease in the core-specific IFN-γ secretion.
The observed effect in the virological response in the L6 group might be correlated with the enhancement of both effector mechanisms, neutralizing antibodies and core-specific IFN-γ ELISPOT response, because a positive association was found between the increase in at least one of them and the achievement of a SVR. It is likely that, in this case, the moment of vaccine administration in relation to both the stage of antiviral therapy and the typically associated viral kinetics might play a pivotal role in the generation of effector and memory cells in a time, quality and quantity able to favorably impact viral control rates.
The HCV-specific cell-mediated immune response, in accord with previous studies, was scarce before the initiation of therapy[32,33] and showed a trend to disappear during treatment. Similar results have been reported by several[34-36], but not all studies[12,37]. This contradiction makes it difficult to draw definitive conclusions regarding the actual impact of IFN-based therapy on immune-mediated HCV clearance; however, the immune modulatory and antiproliferative properties of IFN-α and ribavirin have been clearly described[38-42]. Nonetheless, our results indicate that the inclusion of CIGB-230 is capable of changing the immune context of patients under IFN-α plus ribavirin treatment.
In addition to the above-mentioned findings, despite IFN-α-induced leukopenia, CIGB-230 administration was able to induce significantly greater numbers of de novo core-specific lymphoproliferative and IFN-γ responses than antiviral therapy alone. In a previous clinical trial, CIGB-230 had already demonstrated its capacity to induce de novo cell-mediated immune responses against the structural antigens in chronic patients, non-responders to therapy[16]. Certainly, in the present study conducted in treatment naïve patients, the percentage of de novo responses was inferior to that expected according to previous results, which might be most probably due to the immune modulatory effects of the combined therapy. In contrast, Wedemeyer and colleagues showed that the immunogenicity of the IC41 peptide vaccine candidate was not affected when administered as late add-on to the standard treatment[33]. This discrepancy may be attributable to the differences in the mechanisms of action of both vaccine candidates: peptides, not requiring extensive processing for presentation vs DNA, requiring full expression and processing. Additionally, the samples of selected patients also differ: treatment naïve vs early virological responders. In particular, the characteristics of the population sample may be critical determinants of treatment outcome, since several host factors have been found to be associated with therapy response[43-45]. In fact, in our exploratory study, in which randomization by single nucleotide polymorphisms, HLA, viral load or histological damage was not performed; differential predisposition to an IFN-α plus ribavirin therapy response was observed in the different groups, the impact being evident in the cEVR rate. In this sense, comparison among different groups of patients may be misleading and the effect of therapeutic interventions should be analyzed in an intra-group basis, comparing baseline with end of treatment outcome.
The study of HCV-specific cell-mediated immune responses in chronic patients has frequently shown that specific proliferation and cytokine secretion are not commonly associated[33,46], and may be differentially affected by IFN-α based therapy[47]. Our results suggest that the early and late add-on schedules differ in their capacity to induce de novo HCV-specific proliferative and IFN-γ responses. This phenomenon may be directly linked to the timing of vaccine administration in relation to the antiviral therapy. In fact, it has been observed that a leukopenic environment promotes changes in lymphocyte physiology and induces T-cell proliferation through homeostatic peripheral expansion[48]. It has been suggested that the immediate period of lymphopenia after cytoreduction provides a unique therapeutic window for immunotherapy[49], but the intensity of this proliferation and the characteristics of the resulting cells, in terms of proliferative and cytokine secretion capacity, will depend on the degree and duration of lymphopenia[50-52]. In our study, in the early add-on schedule, the specific antigen was administered at the initiation of the antiviral therapy, before the stabilization of lymphopenia at its nadir. These conditions might be favorable to stimulate the development of proliferating uncommitted cells, while in late add-on, the more stable, chronic-like lymphopenic environment could be suitable for the generation of proinflammatory IFN-γ secreting cells.
Additionally, it cannot be ruled out that the profile of the immune variables tested in this trial depends also on the characteristics of each of the different immunization schedules, regarding the number of inoculations, as well as the moment in which they are evaluated. Further understanding of the mechanisms governing the differential induction of HCV-specific proliferation and cytokine secretion in the context of triple therapy with CIGB-230 and IFN-α plus ribavirin is needed to finally achieve the ultimate goal of generation of multifunctional cells, which have been found to be associated with resolution of the disease, while monofunctional cells have not[53].
Several data support the idea that the hepatic damage caused by HCV infection is mainly immune-mediated[54-56]. Our quest to find a relationship between immune response and therapy outcome showed an association between the E2-specific lymphoproliferative response and hepatic injury. This result, together with the association between this type of immune response against E2 with the inability to clear the viral RNA in the control group, might implicate the E2-specific lymphoproliferative response in the generation and/or maintenance of the histological damage, and a poorer treatment outcome. The causality of this relationship demands a more detailed study regarding epitopic specificity as well as the characteristics of the cell populations involved.
In conclusion, our results are evidence of the depressive effects of IFN-α plus ribavirin therapy on the HCV-specific immune response. They show that CIGB-230, in this unfavorable context, is capable of modifying the immune response established in chronic patients, and that the quality of the induced response will depend on both the number of doses and the timing in which they are administered in relation to the antiviral therapy. In particular, the administration of six CIGB-230 doses as late add-on to therapy increased the neutralizing antibody activity and the de novo core-specific IFN-γ secretion, with a positive impact on the virological response. Nevertheless, SVR rates observed with the triple therapy including CIGB-230 were lower than those reported for the recently licensed direct acting antivirals[57]. This fact obviously calls for more research regarding the optimization of CIGB-230 administration. The information obtained in this work sheds light on the design of more effective therapeutic vaccine interventions against HCV, given the fact that any strategy must change the already established immune response, which is usually the result of decades of infection and failed attempts to control it. Future studies must explore other immunization schedules with CIGB-230, aiming at the generation of polyfunctional cells, rather than monofunctional ones. Therefore, regardless of the potential of IFN-based therapy to reduce the HCV viral load, the success of its combination with therapeutic vaccination cannot be taken for granted, given its antiproliferative properties; hence the optimal immunization schedule is still to be defined.
ACKNOWLEDGMENTS
The authors would like to acknowledge Elena Ferrer, Julia Vancol, Yelaimne Tamayo, Aina Méndez, Ricardo Pérez, for technical assistance in collection and processing of blood samples, as well as isolation and conservation of lymphocytes. Authors also recognize Marbelis Linares, Miladys Limonta, Odalys Ruiz, Dinorah Torres, Martha Pupo, Gabriel Marquez, Edel Torres and Eduardo Martínez for recombinant proteins used in this work. We are equally indebted to Yahíma Chacón and Freya Freire for excellent technical assistance with flow cytometry data acquisition. We also acknowledge Dr Boris Acevedo, Dr. Luis Herrera, Dr. Gerardo Guillén, Dr. Verena Muzio, Dr. Pedro Velbes, Dr. Carlos Domínguez, Dr. Susana Sainz, Dr. Bienvenido Grá, Dr. Dayamí González, Dr. Zaylí Dorta, Ivón Lidice, Osvaldo Seijas, Lisset González, Sergio Ojeda, Ivón Jiménez, Sara Nápoles, Sacha Lazo, Susana Mir, Isabel González, Alejandrina Piña, Georgina Zambrano, Rafael Ibargollín, Dubel Bueno, Dr. Lourdes Pérez, Ania Inés Cuellar, Dr. Rafael López, Dr. Pedro López, and Dr. Enrique Arús for contribution to patient recruitment and follow-up, as well as for constructive revision of the manuscript.
COMMENTS
Background
There are rapid advances in the development of specific antivirals against hepatitis C virus (HCV). Nevertheless, approximately 30% of genotype-1-infected patients remain persistently infected after therapy. Additionally, current therapies comprise multiple adverse effects and do not provide long-term protection against HCV reinfection. Therefore, the development of vaccine strategies remains attractive although, so far, they have not demonstrated significant clinical impacts.
Research frontiers
A critical obstacle facing any therapeutic vaccine candidate against HCV is the already established immune response, which is characterized by impairment of both the innate and adaptive responses. The combination of therapeutic vaccine candidates with antiviral treatments, allowing the vaccine to function in a scenario of reduced viral load, seems a promising strategy.
Innovations and breakthroughs
In the present study we assayed, for the first time, the impact of the concomitant administration of CIGB-230, a DNA based vaccine candidate, and non-peginterferon (pegIFN)-α plus ribavirin antiviral therapy on HCV-specific immune response in a cohort of chronic, treatment naïve, HCV genotype 1b infected patients. The administration of six doses of CIGB-230 as late add-on to therapy increased the neutralizing antibody activity and the de novo core-specific IFN-γ secretion, both of which are associated with the sustained virological response (SVR).
Applications
The demonstration that the quality of CIGB-230-induced immune response depends on the number of doses and timing of administration in relation to the antiviral therapy will allow design of more effective therapeutic vaccine interventions against HCV.
Terminology
SVR is the reduction in HCV viral RNA to undetectable levels up to 6 mo after the end of therapy.
Peer review
The manuscript titled “HCV-specific immune responses induced by CIGB-230 in combination with IFN-α plus ribavirin “is an interesting phase II clinical study evaluating the effects of triple therapy INF-α plus ribavirin and CIGB-230 in treatment naive, HCV genotype 1b infected patients and found that CIGB-230 modifies immune response in chronic patients and immune response depends on number of doses and time of administration in relation to antiviral therapy. Overall, it is a well-structured and organized study, which provides new potential option for future treatment of HCV patients.
Footnotes
P- Reviewer: Jun DW S- Editor: Wen LL L- Editor: Cant MR E- Editor: Ma S
References
- 1.Williams R. Global challenges in liver disease. Hepatology. 2006;44:521–526. doi: 10.1002/hep.21347. [DOI] [PubMed] [Google Scholar]
- 2.Amoroso P, Rapicetta M, Tosti ME, Mele A, Spada E, Buonocore S, Lettieri G, Pierri P, Chionne P, Ciccaglione AR, et al. Correlation between virus genotype and chronicity rate in acute hepatitis C. J Hepatol. 1998;28:939–944. doi: 10.1016/s0168-8278(98)80340-1. [DOI] [PubMed] [Google Scholar]
- 3.Sarrazin C, Rouzier R, Wagner F, Forestier N, Larrey D, Gupta SK, Hussain M, Shah A, Cutler D, Zhang J, et al. SCH 503034, a novel hepatitis C virus protease inhibitor, plus pegylated interferon alpha-2b for genotype 1 nonresponders. Gastroenterology. 2007;132:1270–1278. doi: 10.1053/j.gastro.2007.01.041. [DOI] [PubMed] [Google Scholar]
- 4.Hézode C, Forestier N, Dusheiko G, Ferenci P, Pol S, Goeser T, Bronowicki JP, Bourlière M, Gharakhanian S, Bengtsson L, et al. Telaprevir and peginterferon with or without ribavirin for chronic HCV infection. N Engl J Med. 2009;360:1839–1850. doi: 10.1056/NEJMoa0807650. [DOI] [PubMed] [Google Scholar]
- 5.Kwo PY, Lawitz EJ, McCone J, Schiff ER, Vierling JM, Pound D, Davis MN, Galati JS, Gordon SC, Ravendhran N, et al. Efficacy of boceprevir, an NS3 protease inhibitor, in combination with peginterferon alfa-2b and ribavirin in treatment-naive patients with genotype 1 hepatitis C infection (SPRINT-1): an open-label, randomised, multicentre phase 2 trial. Lancet. 2010;376:705–716. doi: 10.1016/S0140-6736(10)60934-8. [DOI] [PubMed] [Google Scholar]
- 6.Inchauspé G, Michel ML. Vaccines and immunotherapies against hepatitis B and hepatitis C viruses. J Viral Hepat. 2007;14 Suppl 1:97–103. doi: 10.1111/j.1365-2893.2007.00922.x. [DOI] [PubMed] [Google Scholar]
- 7.Ryan EJ, Stevenson NJ, Hegarty JE, O’Farrelly C. Chronic hepatitis C infection blocks the ability of dendritic cells to secrete IFN-α and stimulate T-cell proliferation. J Viral Hepat. 2011;18:840–851. doi: 10.1111/j.1365-2893.2010.01384.x. [DOI] [PubMed] [Google Scholar]
- 8.Ma CJ, Ni L, Zhang Y, Zhang CL, Wu XY, Atia AN, Thayer P, Moorman JP, Yao ZQ. PD-1 negatively regulates interleukin-12 expression by limiting STAT-1 phosphorylation in monocytes/macrophages during chronic hepatitis C virus infection. Immunology. 2011;132:421–431. doi: 10.1111/j.1365-2567.2010.03382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bode JG, Brenndörfer ED, Häussinger D. Subversion of innate host antiviral strategies by the hepatitis C virus. Arch Biochem Biophys. 2007;462:254–265. doi: 10.1016/j.abb.2007.03.033. [DOI] [PubMed] [Google Scholar]
- 10.Radziewicz H, Ibegbu CC, Hon H, Osborn MK, Obideen K, Wehbi M, Freeman GJ, Lennox JL, Workowski KA, Hanson HL, et al. Impaired hepatitis C virus (HCV)-specific effector CD8+ T cells undergo massive apoptosis in the peripheral blood during acute HCV infection and in the liver during the chronic phase of infection. J Virol. 2008;82:9808–9822. doi: 10.1128/JVI.01075-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pilli M, Zerbini A, Penna A, Orlandini A, Lukasiewicz E, Pawlotsky JM, Zeuzem S, Schalm SW, von Wagner M, Germanidis G, et al. HCV-specific T-cell response in relation to viral kinetics and treatment outcome (DITTO-HCV project) Gastroenterology. 2007;133:1132–1143. doi: 10.1053/j.gastro.2007.06.059. [DOI] [PubMed] [Google Scholar]
- 12.Abdel-Hakeem MS, Bédard N, Badr G, Ostrowski M, Sékaly RP, Bruneau J, Willems B, Heathcote EJ, Shoukry NH. Comparison of immune restoration in early versus late alpha interferon therapy against hepatitis C virus. J Virol. 2010;84:10429–10435. doi: 10.1128/JVI.01094-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Missale G, Pilli M, Zerbini A, Penna A, Ravanetti L, Barili V, Orlandini A, Molinari A, Fasano M, Santantonio T, et al. Lack of full CD8 functional restoration after antiviral treatment for acute and chronic hepatitis C virus infection. Gut. 2012;61:1076–1084. doi: 10.1136/gutjnl-2011-300515. [DOI] [PubMed] [Google Scholar]
- 14.Dueñas-Carrera S, Alvarez-Lajonchere L, César Alvarez-Obregón J, Pérez A, Acosta-Rivero N, Vázquez DM, Martínez G, Viña A, Pichardo D, Morales J. Enhancement of the immune response generated against hepatitis C virus envelope proteins after DNA vaccination with polyprotein-encoding plasmids. Biotechnol Appl Biochem. 2002;35:205–212. doi: 10.1042/ba20010089. [DOI] [PubMed] [Google Scholar]
- 15.Acosta-Rivero N, Rodriguez A, Mussachio A, Poutou J, Falcon V, Torres D, Aguilar JC, Linares M, Alonso M, Perez A, et al. A C-terminal truncated hepatitis C virus core protein variant assembles in vitro into virus-like particles in the absence of structured nucleic acids. Biochem Biophys Res Commun. 2005;334:901–906. doi: 10.1016/j.bbrc.2005.06.185. [DOI] [PubMed] [Google Scholar]
- 16.Alvarez-Lajonchere L, Shoukry NH, Grá B, Amador-Cañizares Y, Helle F, Bédard N, Guerra I, Drouin C, Dubuisson J, González-Horta EE, et al. Immunogenicity of CIGB-230, a therapeutic DNA vaccine preparation, in HCV-chronically infected individuals in a Phase I clinical trial. J Viral Hepat. 2009;16:156–167. doi: 10.1111/j.1365-2893.2008.01058.x. [DOI] [PubMed] [Google Scholar]
- 17.Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween RN. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–699. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 18.Palenzuela DO, Pedroso S, Roca J, Villareal A, Lemos G, Reyes O, Novoa LI. A new NS3 recombinant protein shows improved antigenic properties for HCV diagnosis. Biotecnologia Aplicada. 2006;23:94–98. [Google Scholar]
- 19.Martinez-Donato G, Acosta-Rivero N, Morales-Grillo J, Musacchio A, Vina A, Alvarez C, Figueroa N, Guerra I, Garcia J, Varas L, et al. Expression and processing of hepatitis C virus structural proteins in Pichia pastoris yeast. Biochem Biophys Res Commun. 2006;342:625–631. doi: 10.1016/j.bbrc.2006.01.157. [DOI] [PubMed] [Google Scholar]
- 20.Amador-Cañizares Y, Alvarez-Lajonchere L, Guerra I, Rodríguez-Alonso I, Martínez-Donato G, Triana J, González-Horta EE, Pérez A, Dueñas-Carrera S. Induction of IgA and sustained deficiency of cell proliferative response in chronic hepatitis C. World J Gastroenterol. 2008;14:6844–6852. doi: 10.3748/wjg.14.6844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Helle F, Vieyres G, Elkrief L, Popescu CI, Wychowski C, Descamps V, Castelain S, Roingeard P, Duverlie G, Dubuisson J. Role of N-linked glycans in the functions of hepatitis C virus envelope proteins incorporated into infectious virions. J Virol. 2010;84:11905–11915. doi: 10.1128/JVI.01548-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmittel A, Keilholz U, Bauer S, Kuhne U, Stevanovic S, Thiel E, Scheibenbogen C. Application of the IFN-gamma ELISPOT assay to quantify T cell responses against proteins. J Immunol Methods. 2001;247:17–24. doi: 10.1016/s0022-1759(00)00305-7. [DOI] [PubMed] [Google Scholar]
- 23.Bergman SJ, Ferguson MC, Santanello C. Interferons as therapeutic agents for infectious diseases. Infect Dis Clin North Am. 2011;25:819–834. doi: 10.1016/j.idc.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wada M, Marusawa H, Yamada R, Nasu A, Osaki Y, Kudo M, Nabeshima M, Fukuda Y, Chiba T, Matsuda F. Association of genetic polymorphisms with interferon-induced haematologic adverse effects in chronic hepatitis C patients. J Viral Hepat. 2009;16:388–396. doi: 10.1111/j.1365-2893.2009.01095.x. [DOI] [PubMed] [Google Scholar]
- 25.Manns MP, Wedemeyer H, Cornberg M. Treating viral hepatitis C: efficacy, side effects, and complications. Gut. 2006;55:1350–1359. doi: 10.1136/gut.2005.076646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dormann H, Krebs S, Muth-Selbach U, Brune K, Schuppan D, Hahn EG, Schneider HT. Rapid onset of hematotoxic effects after interferon alpha in hepatitis C. J Hepatol. 2000;32:1041–1042. doi: 10.1016/s0168-8278(00)80113-0. [DOI] [PubMed] [Google Scholar]
- 27.Pawlowska M, Pilarczyk M, Foksinska A, Smukalska E, Halota W. Hematological Adverse events and Sustained Viral Response in Children Undergoing Therapy for Chronic Hepatitis C Infection. Hepat Mon. 2011;11:968–974. doi: 10.5812/kowsar.1735143X.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang ZX, Milich DR, Peterson DL, Birkett A, Schvarcz R, Weiland O, Sällberg M. Interferon-alpha treatment induces delayed CD4 proliferative responses to the hepatitis C virus nonstructural protein 3 regardless of the outcome of therapy. J Infect Dis. 1997;175:1294–1301. doi: 10.1086/516459. [DOI] [PubMed] [Google Scholar]
- 29.Zeisel MB, Fafi-Kremer S, Fofana I, Barth H, Stoll-Keller F, Doffoel M, Baumert TF. Neutralizing antibodies in hepatitis C virus infection. World J Gastroenterol. 2007;13:4824–4830. doi: 10.3748/wjg.v13.i36.4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davis GL, Wong JB, McHutchison JG, Manns MP, Harvey J, Albrecht J. Early virologic response to treatment with peginterferon alfa-2b plus ribavirin in patients with chronic hepatitis C. Hepatology. 2003;38:645–652. doi: 10.1053/jhep.2003.50364. [DOI] [PubMed] [Google Scholar]
- 31.Smyk-Pearson S, Tester IA, Lezotte D, Sasaki AW, Lewinsohn DM, Rosen HR. Differential antigenic hierarchy associated with spontaneous recovery from hepatitis C virus infection: implications for vaccine design. J Infect Dis. 2006;194:454–463. doi: 10.1086/505714. [DOI] [PubMed] [Google Scholar]
- 32.Tatsumi T, Takehara T, Miyagi T, Nakazuru S, Mita E, Kanto T, Hiramatsu N, Hayashi N. Hepatitis C virus-specific CD8+ T cell frequencies are associated with the responses of pegylated interferon-α and ribavirin combination therapy in patients with chronic hepatitis C virus infection. Hepatol Res. 2011;41:30–38. doi: 10.1111/j.1872-034X.2010.00734.x. [DOI] [PubMed] [Google Scholar]
- 33.Wedemeyer H, Schuller E, Schlaphoff V, Stauber RE, Wiegand J, Schiefke I, Firbas C, Jilma B, Thursz M, Zeuzem S, et al. Therapeutic vaccine IC41 as late add-on to standard treatment in patients with chronic hepatitis C. Vaccine. 2009;27:5142–5151. doi: 10.1016/j.vaccine.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 34.Barnes E, Gelderblom HC, Humphreys I, Semmo N, Reesink HW, Beld MG, van Lier RA, Klenerman P. Cellular immune responses during high-dose interferon-alpha induction therapy for hepatitis C virus infection. J Infect Dis. 2009;199:819–828. doi: 10.1086/597072. [DOI] [PubMed] [Google Scholar]
- 35.Burton JR, Klarquist J, Im K, Smyk-Pearson S, Golden-Mason L, Castelblanco N, Terrault N, Rosen HR. Prospective analysis of effector and regulatory CD4+ T cells in chronic HCV patients undergoing combination antiviral therapy. J Hepatol. 2008;49:329–338. doi: 10.1016/j.jhep.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 36.Hammond T, Lee S, Watson MW, Flexman JP, Cheng W, Price P. Decreased IFNγ production correlates with diminished production of cytokines by dendritic cells in patients infected with hepatitis C virus and receiving therapy. J Viral Hepat. 2011;18:482–492. doi: 10.1111/j.1365-2893.2010.01331.x. [DOI] [PubMed] [Google Scholar]
- 37.Badr G, Bédard N, Abdel-Hakeem MS, Trautmann L, Willems B, Villeneuve JP, Haddad EK, Sékaly RP, Bruneau J, Shoukry NH. Early interferon therapy for hepatitis C virus infection rescues polyfunctional, long-lived CD8+ memory T cells. J Virol. 2008;82:10017–10031. doi: 10.1128/JVI.01083-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bergamini A, Bolacchi F, Cepparulo M, Demin F, Uccella I, Bongiovanni B, Ombres D, Angelico F, Liuti A, Hurtova M, et al. Treatment with ribavirin and interferon-alpha reduces interferon-gamma expression in patients with chronic hepatitis C. Clin Exp Immunol. 2001;123:459–464. doi: 10.1046/j.1365-2249.2001.01468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Furusyo N, Kubo N, Toyoda K, Takeoka H, Nabeshima S, Murata M, Nakamuta M, Hayashi J. Helper T cell cytokine response to ribavirin priming before combined treatment with interferon alpha and ribavirin for patients with chronic hepatitis C. Antiviral Res. 2005;67:46–54. doi: 10.1016/j.antiviral.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 40.Jain MK, Zoellner C. Role of ribavirin in HCV treatment response: now and in the future. Expert Opin Pharmacother. 2010;11:673–683. doi: 10.1517/14656560903580001. [DOI] [PubMed] [Google Scholar]
- 41.Martín J, Navas S, Quiroga JA, Pardo M, Carreño V. Effects of the ribavirin-interferon alpha combination on cultured peripheral blood mononuclear cells from chronic hepatitis C patients. Cytokine. 1998;10:635–644. doi: 10.1006/cyto.1997.0333. [DOI] [PubMed] [Google Scholar]
- 42.Rigopoulou EI, Abbott WG, Williams R, Naoumov NV. Direct evidence for immunomodulatory properties of ribavirin on T-cell reactivity to hepatitis C virus. Antiviral Res. 2007;75:36–42. doi: 10.1016/j.antiviral.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 43.Poordad F, Bronowicki JP, Gordon SC, Zeuzem S, Jacobson IM, Sulkowski MS, Poynard T, Morgan TR, Molony C, Pedicone LD, et al. Factors that predict response of patients with hepatitis C virus infection to boceprevir. Gastroenterology. 2012;143:608–18.e1-5. doi: 10.1053/j.gastro.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 44.Swiątek BJ. Is interleukin-10 gene polymorphism a predictive marker in HCV infection? Cytokine Growth Factor Rev. 2012;23:47–59. doi: 10.1016/j.cytogfr.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 45.Tsubota A, Shimada N, Yoshizawa K, Furihata T, Agata R, Yumoto Y, Abe H, Ika M, Namiki Y, Chiba K, et al. Contribution of ribavirin transporter gene polymorphism to treatment response in peginterferon plus ribavirin therapy for HCV genotype 1b patients. Liver Int. 2012;32:826–836. doi: 10.1111/j.1478-3231.2011.02727.x. [DOI] [PubMed] [Google Scholar]
- 46.Barnes E, Harcourt G, Brown D, Lucas M, Phillips R, Dusheiko G, Klenerman P. The dynamics of T-lymphocyte responses during combination therapy for chronic hepatitis C virus infection. Hepatology. 2002;36:743–754. doi: 10.1053/jhep.2002.35344. [DOI] [PubMed] [Google Scholar]
- 47.Alvarado Esquivel C, Elewaut A, Philippé J, Elewaut AE, Desombere I, Maertens G, Leroux-Roels G. Evolution of hepatitis C virus-specific T cell responses and cytokine production in chronic hepatitis C patients treated with high doses of interferon-alpha. Rev Invest Clin. 2002;54:41–50. [PubMed] [Google Scholar]
- 48.Tchao NK, Turka LA. Lymphodepletion and homeostatic proliferation: implications for transplantation. Am J Transplant. 2012;12:1079–1090. doi: 10.1111/j.1600-6143.2012.04008.x. [DOI] [PubMed] [Google Scholar]
- 49.Gattinoni L, Finkelstein SE, Klebanoff CA, Antony PA, Palmer DC, Spiess PJ, Hwang LN, Yu Z, Wrzesinski C, Heimann DM, et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med. 2005;202:907–912. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Neujahr DC, Chen C, Huang X, Markmann JF, Cobbold S, Waldmann H, Sayegh MH, Hancock WW, Turka LA. Accelerated memory cell homeostasis during T cell depletion and approaches to overcome it. J Immunol. 2006;176:4632–4639. doi: 10.4049/jimmunol.176.8.4632. [DOI] [PubMed] [Google Scholar]
- 51.Min B, Yamane H, Hu-Li J, Paul WE. Spontaneous and homeostatic proliferation of CD4 T cells are regulated by different mechanisms. J Immunol. 2005;174:6039–6044. doi: 10.4049/jimmunol.174.10.6039. [DOI] [PubMed] [Google Scholar]
- 52.Onoe T, Kalscheuer H, Chittenden M, Zhao G, Yang YG, Sykes M. Homeostatic expansion and phenotypic conversion of human T cells depend on peripheral interactions with APCs. J Immunol. 2010;184:6756–6765. doi: 10.4049/jimmunol.0901711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaplan DE, Sugimoto K, Newton K, Valiga ME, Ikeda F, Aytaman A, Nunes FA, Lucey MR, Vance BA, Vonderheide RH, et al. Discordant role of CD4 T-cell response relative to neutralizing antibody and CD8 T-cell responses in acute hepatitis C. Gastroenterology. 2007;132:654–666. doi: 10.1053/j.gastro.2006.11.044. [DOI] [PubMed] [Google Scholar]
- 54.Accapezzato D, Francavilla V, Paroli M, Casciaro M, Chircu LV, Cividini A, Abrignani S, Mondelli MU, Barnaba V. Hepatic expansion of a virus-specific regulatory CD8(+) T cell population in chronic hepatitis C virus infection. J Clin Invest. 2004;113:963–972. doi: 10.1172/JCI20515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bolacchi F, Sinistro A, Ciaprini C, Demin F, Capozzi M, Carducci FC, Drapeau CM, Rocchi G, Bergamini A. Increased hepatitis C virus (HCV)-specific CD4+CD25+ regulatory T lymphocytes and reduced HCV-specific CD4+ T cell response in HCV-infected patients with normal versus abnormal alanine aminotransferase levels. Clin Exp Immunol. 2006;144:188–196. doi: 10.1111/j.1365-2249.2006.03048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kaplan DE, Ikeda F, Li Y, Nakamoto N, Ganesan S, Valiga ME, Nunes FA, Rajender Reddy K, Chang KM. Peripheral virus-specific T-cell interleukin-10 responses develop early in acute hepatitis C infection and become dominant in chronic hepatitis. J Hepatol. 2008;48:903–913. doi: 10.1016/j.jhep.2008.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Poordad F, Dieterich D. Treating hepatitis C: current standard of care and emerging direct-acting antiviral agents. J Viral Hepat. 2012;19:449–464. doi: 10.1111/j.1365-2893.2012.01617.x. [DOI] [PubMed] [Google Scholar]


