Abstract
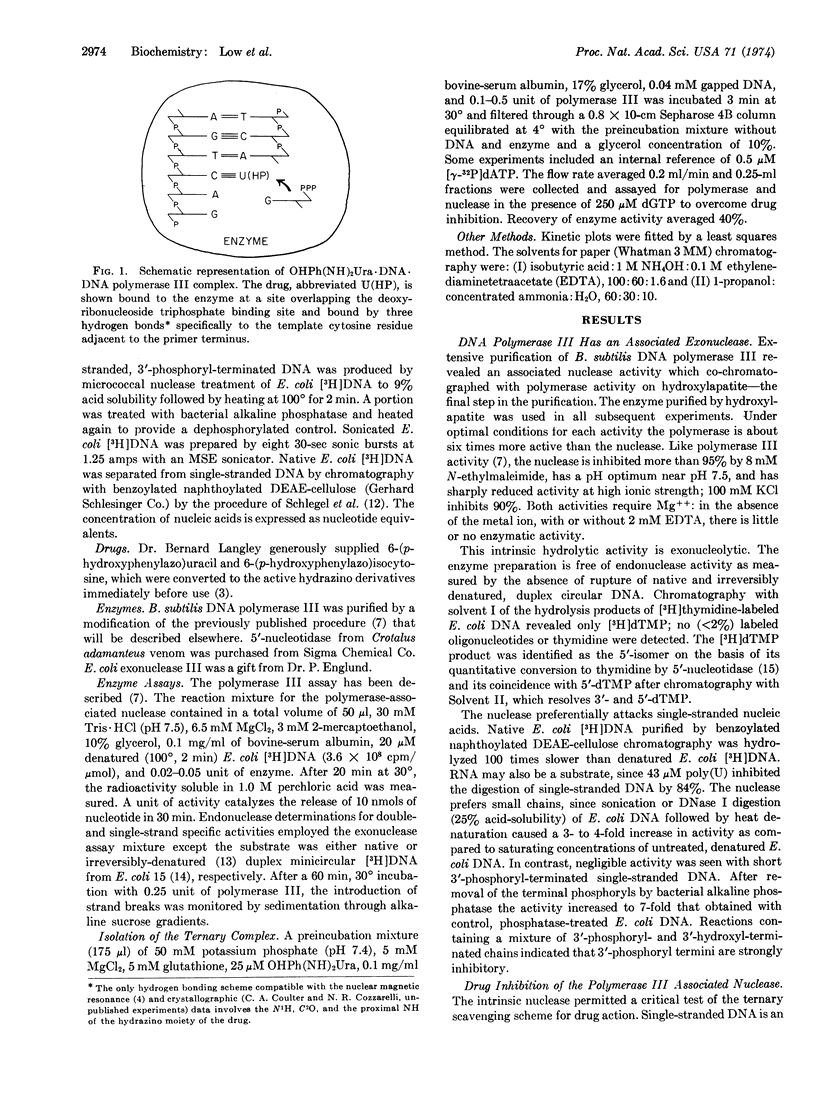
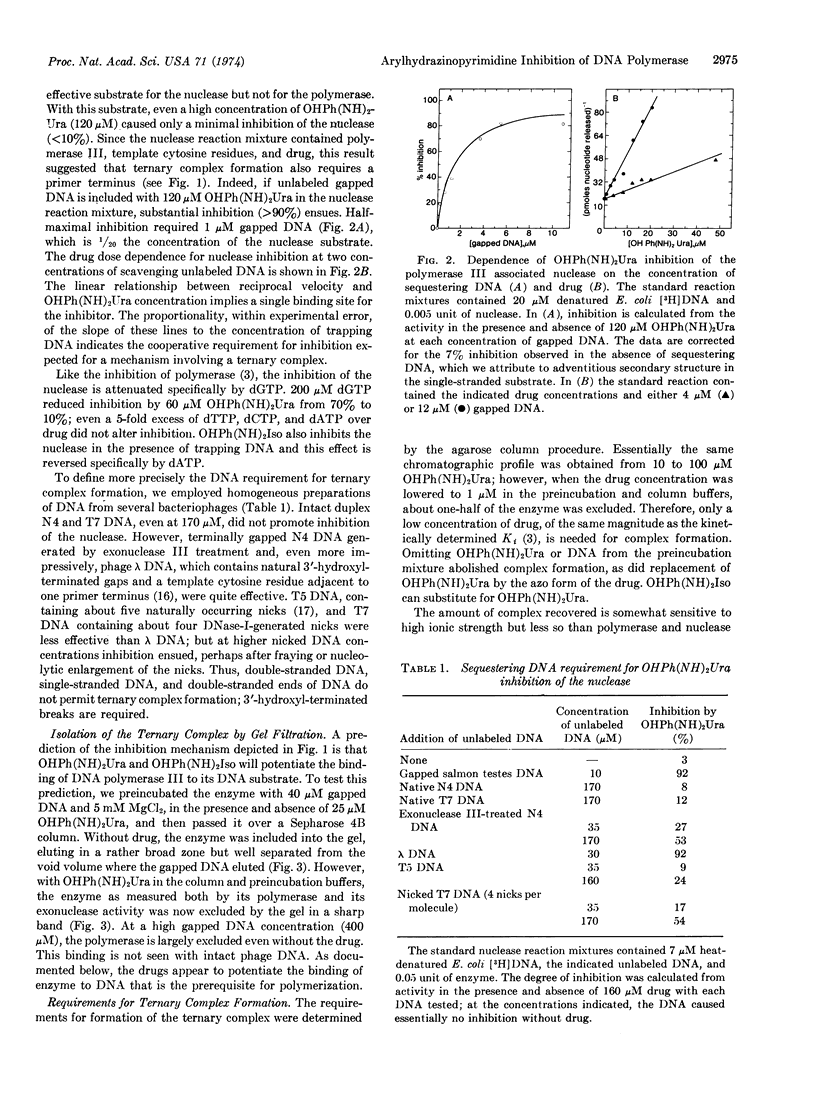
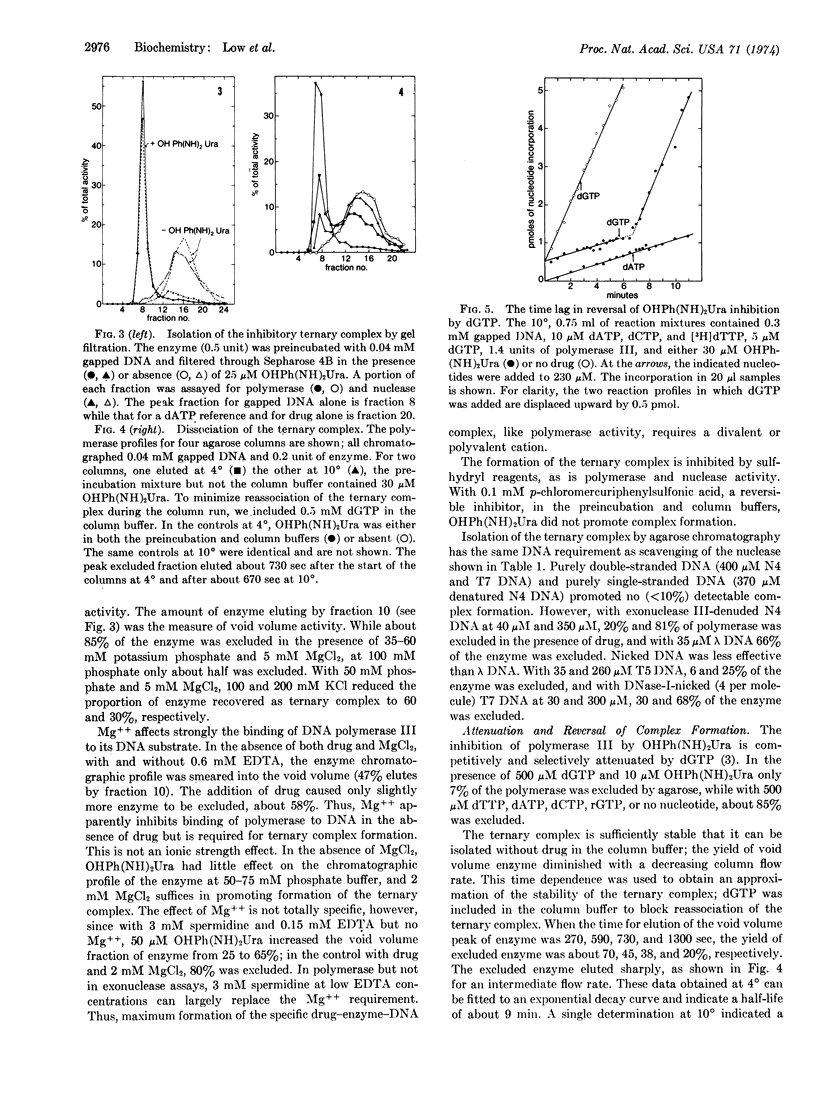
Arylhydrazinopyrimidines inhibit DNA synthesis in Bacillus subtilis by promoting formation of a specific, long-lived ternary complex with DNA polymerase III and the template-primer DNA. DNA polymerase III contains an associated, single-strand-selective exonuclease which generates 5′-mononucleotides. Drug inhibition of the nuclease similarly proceeds through formation of the ternary complex. The ternary complex was isolated by agarose chromatography. Like inhibition of the nuclease, optimum formation of the complex requires duplex DNA with single-stranded regions such as bacteriophage λ DNA (purely single- and double-stranded DNA are ineffective) and is antagonized by specific deoxyribonucleoside triphosphates. Formation of the ternary complex requires a di- or polyvalent cation and is inhibited by sulfhydryl reagents and high ionic strength. The complex dissociates with a half-life of the order of minutes at 4°.
Keywords: ternary complex, exonuclease, DNA replication, base pairing, polyamine
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bazill G. W., Gross J. D. Effect of 6-(p-hydroxyphenyl)-azouracil on B. subtilis DNA polymerases. Nat New Biol. 1972 Nov 15;240(98):82–83. doi: 10.1038/newbio240082a0. [DOI] [PubMed] [Google Scholar]
- Bujard H., Hendrickson H. E. Structure and function of the genome of coliphage T5. 1. The physical structure of the chromosome of T5 + . Eur J Biochem. 1973 Mar 15;33(3):517–528. doi: 10.1111/j.1432-1033.1973.tb02711.x. [DOI] [PubMed] [Google Scholar]
- Cozzarelli N. R., Kelly R. B., Kornberg A. A minute circular DNA from Escherichia coli 15. Proc Natl Acad Sci U S A. 1968 Jul;60(3):992–999. doi: 10.1073/pnas.60.3.992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzarelli N. R., Low R. L. Mutational alteration of Bacillus subtilis DNA polymerase 3 to hydroxyphenylazopyrimidine resistance: polymerase 3 is necessary for DNA replication. Biochem Biophys Res Commun. 1973 Mar 5;51(1):151–157. doi: 10.1016/0006-291x(73)90521-4. [DOI] [PubMed] [Google Scholar]
- Gass K. B., Cozzarelli N. R. Further genetic and enzymological characterization of the three Bacillus subtilis deoxyribonucleic acid polymerases. J Biol Chem. 1973 Nov 25;248(22):7688–7700. [PubMed] [Google Scholar]
- Gass K. B., Low R. L., Cozzarelli N. R. Inhibition of a DNA polymerase from Bacillus subtilis by hydroxyphenylazopyrimidines. Proc Natl Acad Sci U S A. 1973 Jan;70(1):103–107. doi: 10.1073/pnas.70.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie J. M., Neville M. M., Wright G. E., Brown N. C. Hydroxyphenylazopyrimidines: characterization of the active forms and their inhibitory action on a DNA polymerase from Bacillus subtilis. Proc Natl Acad Sci U S A. 1973 Feb;70(2):512–516. doi: 10.1073/pnas.70.2.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouwels P. H., Knijnenburg C. M., van Rotterdam J., Cohen J. A. Structure of the replicative form of bacteriphage phi X174. VI. Studies on alkali-denatured double-stranded phi X DNA. J Mol Biol. 1968 Mar 14;32(2):169–182. doi: 10.1016/0022-2836(68)90002-8. [DOI] [PubMed] [Google Scholar]
- RICHARDSON C. C., LEHMAN I. R., KORNBERG A. A DEOXYRIBONUCLEIC ACID PHOSPHATASE-EXONUCLEASE FROM ESCHERICHIA COLI. II. CHARACTERIZATION OF THE EXONUCLEASE ACTIVITY. J Biol Chem. 1964 Jan;239:251–258. [PubMed] [Google Scholar]
- Riva S., Silvestri L. G. Rifamycins: a general view. Annu Rev Microbiol. 1972;26:199–224. doi: 10.1146/annurev.mi.26.100172.001215. [DOI] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Schito G. C., Rialdi G., Pesce A. The physical properties of the deoxyribonucleic acid from N4 coliphage. Biochim Biophys Acta. 1966 Dec 21;129(3):491–501. doi: 10.1016/0005-2787(66)90064-5. [DOI] [PubMed] [Google Scholar]
- Schlegel R. A., Pyeritz R. E., Thomas C. A., Jr Analysis of DNA bearing single-chained terminals by BNC chromatography. Anal Biochem. 1972 Dec;50(2):558–568. doi: 10.1016/0003-2697(72)90066-8. [DOI] [PubMed] [Google Scholar]
- Wu R., Taylor E. Nucleotide sequence analysis of DNA. II. Complete nucleotide sequence of the cohesive ends of bacteriophage lambda DNA. J Mol Biol. 1971 May 14;57(3):491–511. doi: 10.1016/0022-2836(71)90105-7. [DOI] [PubMed] [Google Scholar]


