Abstract
AIM: To investigate adjuvant chemotherapy, p53 and carcinoembryonic antigen (CEA) expression and prognosis after D2 gastrectomy for stage II/III gastric adenocarcinoma.
METHODS: A total of 286 patients with stage II or III gastric adenocarcinoma who underwent D2 radical gastrectomy between May 2007 and December 2010 were enrolled into this study. One hundred and sixty-nine of these patients received surgery plus adjuvant chemotherapy, and 117 patients received surgery alone. Tumor expression of p53 and CEA proteins in all patients was evaluated immunohistochemically and correlated with clinicopathological parameters. The Kaplan-Meier curves for overall survival (OS) and disease-free survival (DFS) with log-rank testing were used to compare the survival difference. A Cox proportional hazard regression model was used for multivariate analysis.
RESULTS: Patients with adjuvant chemotherapy had a significantly better median OS (50.87 mo vs 30.73 mo, P = 0.000) and median DFS (36.30 mo vs 25.60 mo, P = 0.001) than patients with surgery alone in the entire cohort. Consistent results with the entire cohort were found in stage II (P = 0.006 and P = 0.047), stage III (P = 0.005 and P = 0.030), and stage IIIB/IIIC patients (P = 0.000 and P = 0.001). The median OS and DFS advantages were confirmed by multivariate analysis (P = 0.000 and P = 0.008) and maintained when the analyses were restricted to fluoropyrimidine monotherapy (P = 0.003 and P = 0.001) and fluoropyrimidine plus platinum regimen (P = 0.001 and P = 0.007), however, not the fluoropyrimidine plus taxane (P = 0.198 and P = 0.777) or platinum plus taxane (P = 0.666 and P = 0.687) regimens. Median OS and median DFS did not differ significantly between the patients with p53(+) and p53(-) tumors (P = 0.608 and P = 0.064), or between patients with CEA(+) and CEA(-) tumors (P = 0.052 and P = 0.989), which were maintained when the analyses were restricted to surgery alone (p53: P = 0.864 and P = 0.431; CEA: P = 0.142 and P = 0.948), adjuvant chemotherapy (p53: P = 0.802 and P = 0.091; CEA: P = 0.223 and P = 0.946) and even different chemotherapy regimens (P > 0.05).
CONCLUSION: Patients after D2 gastrectomy for stage II/III gastric adenocarcinoma had significantly better survival after fluoropyrimidine monotherapy and fluoropyrimidine plus platinum. p53 and CEA were not prognostic.
Keywords: Gastric adenocarcinoma, Adjuvant chemotherapy, p53, Carcinoembryonic antigen, Immunohistochemistry
Core tip: Patients after D2 gastrectomy for stage II/III gastric adenocarcinoma had a significant survival benefit after adjuvant chemotherapy compared with surgery alone, which was maintained when restricted to stage II, III or IIIB/IIIC patients. The survival did not differ significantly between the patients with p53(+) and p53(-) tumors, or between patients with carcinoembryonic antigen (CEA)(+) and CEA(-) tumors, which were maintained when the analyses were restricted to surgery alone, adjuvant chemotherapy and even different chemotherapy regimens. p53 and CEA immunohistochemical expression is not prognostic for survival after D2 gastrectomy.
INTRODUCTION
Gastric cancer ranks second among the most common causes of cancer-related deaths worldwide, with a high incidence of recurrence and metastasis even after radical surgery, which is the main curative treatment for resectable gastric cancer[1]. Adjuvant chemotherapy is a standard component of treatment of resectable gastric cancer, however, the preferred treatment differs by geographical region and there is no extensive census on the regimens[2-6]. D2 gastrectomy is the standard treatment in Asia, and now recommended in Europe and the United States for resectable tumors[3,7]. So far, the National Comprehensive Cancer Network (NCCN) only recommends the capecitabine plus oxaliplatin (XELOX) and capecitabine plus cisplatin (XP) adjuvant regimens for D2 gastrectomy based on the Capecitabine and Oxaliplatin Adjuvant Study in Stomach Cancer (CLASSIC) in 2012 and the Adjuvant Chemoradiation Therapy in Stomach Cancer (ARTIST) trial in 2013, respectively[8,9]. Of note, according to the American Joint Committee on Cancer (AJCC, seventh edition), the CLASSIC study included stage II/III gastric cancers, but no T4bN+ or N3b (also meant as stage IV (M0) according to AJCC, sixth edition) was included. Thus, more evidence for stage IIIB and IIIC patients is warranted. The ARTIST trial compared postoperative XP regimen with XP plus radiotherapy, but did not compare postoperative XP regimen with surgery alone. Meanwhile, the Japanese recommendation after D2 gastrectomy is based on the Adjuvant Chemotherapy Trial of TS-1 for Gastric Cancer (ACTS-GC) study, which showed a survival benefit for stages II and IIIA patients[6]. Thus, more in-depth investigations are needed about the adjuvant chemotherapy including other regimens after D2 radical gastrectomy for stage II/III gastric adenocarcinoma.
Better understanding of the prognostic parameters is required. The tumor protein p53, which represents the product of the tumor suppressor gene p53, has been investigated in various cancers[10-13], and allelic loss of p53 occurs in > 60% of gastric carcinomas with tumor progression, however, there are still conflicting results about the clinical and prognostic significance of p53 mutations in gastric cancer[14-17]. Although carcinoembryonic antigen (CEA) immunoassay has found acceptance as a diagnostic adjunct in clinical diagnosis of gastrointestinal tumors, the prognostic value of CEA tissue status and its correlation with clinical parameters in gastric cancer remain to be explored[18-20]. Especially with the development of adjuvant chemotherapy, it is important to study the interaction of chemotherapy with p53 and CEA tumor status, which may help highlight biological behavior and potential individualized treatment[15,21].
This current study was deemed necessary to investigate the role of adjuvant chemotherapy, p53 and CEA immunohistochemical expression, their potential interactions, and prognosis after D2 radical gastrectomy for stage II/III gastric adenocarcinoma.
MATERIALS AND METHODS
Patients
We retrospectively analyzed the medical records of 286 patients who were pathologically proved and diagnosed with stage II or III gastric adenocarcinoma according to AJCC, seventh edition. All of the patients received radical gastrectomy (R0 or R1) with D2 nodal dissection by experienced surgeons in the Sun Yat-sen University Cancer Center between May 2007 and December 2010. Among them, 169 patients received adjuvant chemotherapy, while the remaining 117 patients did not. We excluded 174 patients with stage I gastric adenocarcinoma and patients with preoperative treatments. This study was approved by the Institutional Review Board of Sun Yat-sen University Cancer Center. Treatment, immunohistochemistry and retrospective analysis of medical records were performed after obtaining written informed consent from all patients and approval from the Research Ethics Committee at the Cancer Center of Sun Yat-sen University. All patients gave their consent for the two forms of treatment and were involved in the decision-making about receiving adjuvant chemotherapy. We conducted this retrospective research according to the principles of the Declaration of Helsinki.
The adjuvant chemotherapy was mainly based on fluoropyrimidine (either injection or oral fluoropyrimidines such as S-1 or capecitabine), with or without a combination of platinum (oxaliplatin, cisplatin, lobaplatin, or nedaplatin), taxane (paclitaxel or docetaxal), or both. There was also a small subset of patients with a regimen of platinum and taxane.
Clinicopathological evaluation included medical history, physical examination, complete blood count, serum chemistry, serum tumor markers, electrocardiography before treatment, and surgical-pathological evaluation. All regular follow-up assessments were completed by June 30, 2013. The median follow-up was 46.0 mo (range, 0.6-62.2 mo).
Specimens and immunohistochemistry
Paraffin-embedded tumor specimens from 286 patients were cut into 5-μm-thick sections and subjected to immunohistochemical analyses. The primary antibodies were mouse anti-human p53 protein (1:50; Santa Cruz Biotechnology, Santa Cruz, CA, United States) and rabbit anti-human CEA protein (1:100; IBL, Tokyo, Japan). After incubation with the primary antibody, the sections were incubated with the secondary antibody and avidin-biotin-peroxidase complex. The slides were counterstained with hematoxylin and eosin. Positivity for p53 was defined as nuclear staining in > 10% of the tumor cell nuclei. Positivity for CEA was > 10% cytoplasmic staining. Immunohistochemical reactivity was interpreted blindly by two independent investigators[15,19].
Statistical analysis
The χ2 test was used to compare categorical variables. Nonparametric tests were used to compare continuous variables. The Kaplan-Meier method with log-rank testing was used to estimate the distribution of time to events. Overall survival (OS) was calculated from the date of surgery to death from any cause. Disease-free survival (DFS) was determined from the date of surgery to recurrence or death from any cause. Prognostic factors were analyzed by searching clinicopathological factors in univariate analysis. All variables with P < 0.05 in the univariate analysis entered into multivariate analysis using Cox proportional hazard regression models. A two-sided P value < 0.05 was considered significant, and HR and 95%CI were calculated. All statistical analyses were performed using SPSS version 19.0 software (Chicago, IL, United States).
RESULTS
Patient characteristics
All the clinicopathological characteristics for the surgery plus adjuvant chemotherapy group and surgery alone group were compared (Table 1). Among the 169 patients with surgery plus adjuvant chemotherapy, 43 received fluoropyrimidine monotherapy, while 72 received fluoropyrimidine plus platinum, 32 fluoropyrimidine plus taxane, two fluoropyrimidine plus both platinum and taxane, and 19 platinum and taxane.
Table 1.
Clinicopathological characteristics of patients with stage II/III gastric cancer n (%)
| Characteristic | Surgery alone | Surgery + chemotherapy | P value |
| No. of patients | 117 | 169 | |
| Age (yr) | |||
| Median (range) | 62 (31-80) | 58 (25-80) | 0.063 |
| < 70 | 89 (76.1) | 142 (84.0) | 0.093 |
| ≥ 70 | 28 (23.9) | 27 (16.0) | |
| Sex, n | 0.924 | ||
| Men | 83 (70.9) | 119 (70.4) | |
| Women | 34 (29.1) | 50 (20.6) | |
| Karnofsky performance status | 0.515 | ||
| ≥ 90 | 116 (99.1) | 166 ( 98.2) | |
| ≥ 80 | 1(0.9) | 3 (1.8) | |
| Tumor location | 0.054 | ||
| Proximal | 77 (65.8) | 92 (54.4) | |
| Distal | 40 (34.2) | 77 (45.6) | |
| Histological grade | 0.120 | ||
| High differentiation | 0 (0.0) | 4 (2.4) | |
| Moderate differentiation | 32 (27.4) | 35 (20.7) | |
| Low differentiation | 85 (72.6) | 130 (76.9) | |
| Gloss type | 0.429 | ||
| Protrusion | 41 (35.0) | 64 (37.9) | |
| Ulcer | 71 (60.7) | 102 (60.4) | |
| Infiltration | 5 (4.3) | 3 (1.8) | |
| Tumor size | 0.359 | ||
| < 5 cm | 41 (35.3) | 51 (30.2) | |
| ≥ 5 cm | 75 (64.7) | 118 (60.8) | |
| Preoperative serum CEA | 0.207 | ||
| Median (range) | 2.42 (0.2–326.8) | 2.235 (0.2–265.5) | |
| Preoperative serum CA19-9 | 0.729 | ||
| Median (range) | 11.33 (0.6–206.9) | 10.83 (0.6–3098.0) | |
| CEA tumor status | 0.792 | ||
| – | 12 (10.3) | 19 (11.2) | |
| + | 105 (89.7) | 150 (88.8) | |
| P53 tumor status | 0.813 | ||
| – | 27 (23.1) | 37 (21.9) | |
| + | 90 (76.9) | 132 (78.1) | |
| T category | 0.182 | ||
| T1 | 0 (0.0) | 2 (1.2) | |
| T2 | 7 (6.0) | 20 (11.8) | |
| T3 | 91 (77.8) | 116 (68.6) | |
| T4 | 19 (12.6) | 31 (18.3) | |
| N category | 0.069 | ||
| N0 | 31 (26.5) | 37 (21.9) | |
| N1 | 19 (16.2) | 29 (17.2) | |
| N2 | 33 (28.2) | 31 (18.3) | |
| N3 | 34 (29.1) | 72 (42.6) | |
| AJCC stage (7th) | 0.066 | ||
| IIA | 29 (24.8) | 38 (22.5) | |
| IIB | 11 (9.4) | 26 (15.4) | |
| IIIA | 34 (29.1) | 34 (20.1) | |
| IIIB | 40 (34.2) | 56 (33.1) | |
| IIIC | 3 (2.6) | 15 (8.9) | |
CEA: Carcinoembryonic antigen.
In the 286 tumor specimens, immunohistochemical expression of p53 and CEA proteins was observed in 222 (77.6%) and 255 (89.2%), respectively. The p53 tumor status was not correlated with CEA tumor status (P = 0.669). We found no correlation of p53 and CEA tumor status with clinicopathological parameters, except that the proximal gastric cancer had a more intense immunoreactivity of CEA (P = 0.040) (Table 2).
Table 2.
p53 tumor status, carcinoembryonic antigen tumor status and clinicopathological parameters n (%)
| Characteristic | p53(+) (n = 222) | CEA(+) (n = 255) |
| Age (yr) | P = 0.406 | P = 0.056 |
| < 70 | 177 (76.6) | 202 (87.4) |
| ≥ 70 | 45 (81.8) | 53 (96.4) |
| Sex, n | P = 0.804 | P = 0.227 |
| Men | 156 (77.2) | 183 (90.6) |
| Women | 66 (78.6) | 72 (85.7) |
| Tumor location | P = 0.271 | P = 0.040 |
| Proximal | 135 (79.9) | 156 (92.3) |
| Distal | 87 (74.4) | 99 (84.6) |
| Histological grade | P = 0.248 | P = 0.248 |
| High differentiation | 4 (100) | 4 (100) |
| Moderate differentiation | 48 (71.6) | 63 (94.0) |
| Low differentiation | 170 (79.1) | 188 (87.4) |
| Gloss type | P = 0.041 | P = 0.954 |
| Protrusion | 73 (69.5) | 93 (88.6) |
| Ulcer | 142 (82.1) | 155 (89.6) |
| Infiltration | 7 (87.5) | 7 (87.5) |
| Tumor size | P = 0.684 | P = 0.103 |
| < 5 cm | 70 (76.1) | 86 (93.5) |
| ≥ 5 cm | 151 (78.2) | 168 (87.0) |
| T category | P = 0.420 | P = 0.883 |
| T1 | 2 (100) | 2 (100) |
| T2 | 19 (70.4) | 25 (92.6) |
| T3 | 165 (79.7) | 184 (88.9) |
| T4 | 36 (72.0) | 44 (88.0) |
| N category | P = 0.923 | P = 0.198 |
| N0 | 54 (79.4) | 57 (83.8) |
| N1 | 38 (79.2) | 45 (93.8) |
| N2 | 50 (78.1) | 60 (93.8) |
| N3 | 80 (75.5) | 90 (87.7) |
| AJCC stage (7th) | P = 0.211 | P = 0.741 |
| IIA | 51 (76.1) | 58 (86.6) |
| IIB | 30 (81.1) | 33 (89.2) |
| IIIA | 58 (85.3) | 63 (92.6) |
| IIIB | 72 (75.0) | 86 (89.6) |
| IIIC | 11 (61.1) | 15 (83.3) |
CEA: Carcinoembryonic antigen.
Overall survival
The median OS was significantly higher in the surgery plus adjuvant chemotherapy group than in the surgery alone group (50.87, 95%CI: 44.14-57.60 vs 30.73, 95%CI: 22.99-38.47 mo, P = 0.000) (Figure 1A). The median OS advantage of surgery plus adjuvant chemotherapy over surgery alone was maintained when the analyses were restricted to patients with stage II disease (55.70 mo vs 41.93 mo; P = 0.006), stage III (39.90 mo vs 25.60 mo, P = 0.005), and even stage IIIB/IIIC (39.90 mo vs 18.20 mo, P = 0.000) (Figure 1B).
Figure 1.
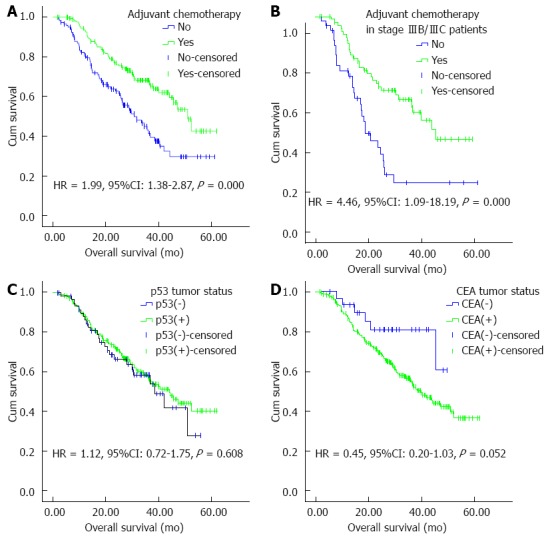
Kaplan-Meier curves for overall survival of patients after D2 gastrectomy for stage II/III gastric adenocarcinoma. A: Adjuvant chemotherapy or not in the entire cohort; B: Adjuvant chemotherapy or not in stage IIIB/IIIC patients; C: p53 tumor status as measured by immunohistochemistry; D: CEA tumor status as measured by immunohistochemistry. HR and 95%CI were calculated with green curves as references. CEA: Carcinoembryonic antigen.
When we compared the two groups stratified by regimens in detail, we still found that median OS favored surgery plus fluoropyrimidine monotherapy over surgery alone (P = 0.003), or fluoropyrimidine plus platinum over surgery alone (P = 0.001), however, it did not favor fluoropyrimidine combined with taxane (P = 0.198), or platinum plus taxane (P = 0.666) over surgery alone.
The median OS of patients with p53(+) and p53(-) tumors was 38.47 (26.69-50.25) and 44.0 (35.45-52.55) mo, respectively, and the difference was not significant (P = 0.608) (Figure 1C). There was no significant difference in median OS between patients with p53(+) and p53(-) tumors in the surgery alone group (P = 0.864) and surgery plus adjuvant chemotherapy group (P = 0.802). There was no significant difference in median OS in the fluoropyrimidine monotherapy subgroup (P = 0.234), fluoropyrimidine plus platinum subgroup (P = 0.082), fluoropyrimidine plus taxane subgroup (P = 0.144), and platinum plus taxane subgroup (P = 0.288).
The median OS of patients with CEA(+) and CEA(-) tumors was 44.24 (38.94-49.53) and 39.90 (34.30-45.50) mo, with a borderline significant difference (P = 0.052) (Figure 1D). No significant difference was found in the median OS between CEA(+) and CEA(-) tumors in the surgery alone group (P = 0.142) and surgery plus adjuvant chemotherapy group (P = 0.223). There was no significant difference in median OS in the fluoropyrimidine monotherapy subgroup (P = 0.665), fluoropyrimidine plus platinum subgroup (P = 0.453), fluoropyrimidine plus taxane subgroup (P = 0.229), and platinum plus taxane subgroup (P = 0.738).
Disease-free survival
The median DFS also significantly favored surgery plus adjuvant chemotherapy over surgery alone (36.30, 28.35-44.25 mo vs 25.60, 20.13-31.07 mo; P = 0.001) (Figure 2A). The median DFS advantage was maintained when the analyses were restricted to patients with stage II disease (55.50 mo vs 40.47 mo; P = 0.047), stage III (26.80 mo vs 18.93 mo; P = 0.030), and even stage IIIB/IIIC (26.80 mo vs 15.80 mo; P = 0.001) (Figure 2B).
Figure 2.
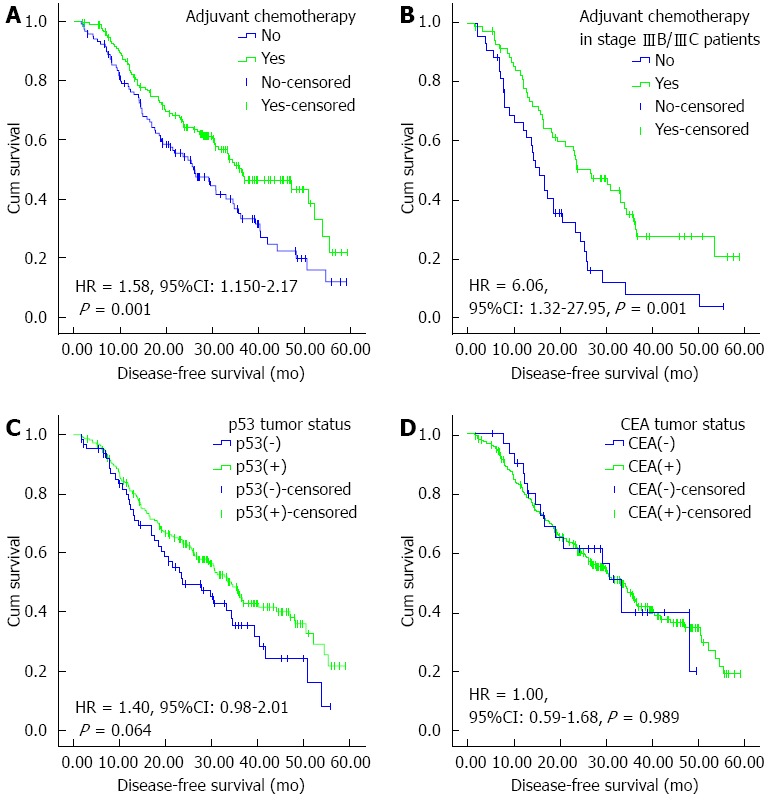
Kaplan-Meier curves for disease-free survival of patients after D2 gastrectomy for stage II/III gastric adenocarcinoma. A: Adjuvant chemotherapy or not in the entire cohort; B: Adjuvant chemotherapy or not in stage IIIB/IIIC patients; C: p53 tumor status as measured by immunohistochemistry; D: CEA tumor status as measured by immunohistochemistry. HR and 95%CI were calculated with green curves as references. CEA: Carcinoembryonic antigen.
The median DFS still favored surgery plus fluoropyrimidine monotherapy over surgery alone (P = 0.001), and fluoropyrimidine plus platinum over surgery alone (P = 0.007). However, it did not favor fluoropyrimidine combined with taxane (P = 0.777) and platinum plus taxane (P = 0.687) over surgery alone.
The median DFS of patients with p53(+) and p53(-) tumors was 34.33 (30.25-38.41) and 23.73 (14.05-33.41) mo, and the difference was not significant (P = 0.064) (Figure 2C). There was no significant difference in median DFS between patients with p53(+) and p53(-) tumors in the surgery alone group (P = 0.431) and surgery plus adjuvant chemotherapy group (P = 0.091). There was no significant difference in median DFS in the fluoropyrimidine monotherapy subgroup (P = 0.431), fluoropyrimidine plus platinum subgroup (P = 0.665), fluoropyrimidine plus taxane subgroup (P = 0.924), and platinum plus taxane subgroup (P = 0.055).
The median DFS of patients with CEA(+) and CEA(-) tumors was 32.93 (27.48-38.38) and 33.43 (28.12-38.75) mo, and the difference was not significant (P = 0.989) (Figure 2D). There was no significant difference in median OS between the CEA(+) and CEA(-) tumors in the surgery alone group (P = 0.948) and surgery plus adjuvant chemotherapy group (P = 0.946). There was no significant difference in median DFS in the fluoropyrimidine monotherapy subgroup (P = 0.419), fluoropyrimidine plus platinum subgroup (P = 0.889), fluoropyrimidine plus taxane subgroup (P = 0.177), and platinum plus taxane subgroup (P = 0.923).
Prognostic factors
All clinicopathological factors were searched in univariate analysis. Table 3 shows the results of univariate and multivariate analyses of factors prognostic for patient survival. Multivariate analysis showed that adjuvant chemotherapy, AJCC stage II, and R0 radical surgery were independently prognostic for prolonged DFS, while adjuvant chemotherapy, AJCC stage II, and first-line chemotherapy were independently prognostic for prolonged OS.
Table 3.
Univariate and multivariate analyses of survival
| Clinicopathological factor |
DFS |
OS |
|||||
| Univariate | Multivariate | Multivariate P value | Univariate | Multivariate | Multivariate P value | ||
| HR (95%CI) | HR (95%CI) | HR (95%CI) | HR (95%CI) | ||||
| Sex | Women | Reference | Reference | ||||
| Men | 0.89 (0.63-1.26) | 1.00 (0.67-1.51) | |||||
| Age (yr) | ≥ 70 | Reference | Reference | ||||
| < 70 | 0.87 (0.59-1.30) | 0.65 (0.42-1.0) | |||||
| Performance status | ≥ 90 | Reference | Reference | ||||
| ≥ 80 | 2.64 (0.84-8.34) | 1.84 (0.45-7.47) | |||||
| Tumor location | Distal | Reference | Reference | 0.099 | |||
| Proximal | 1.22 (0.88-1.70) | 1.51 (1.02-2.24) | 1.40 (0.94-2.09) | ||||
| Differentiation | Low | Reference | Reference | ||||
| Moderate | 0.81 (0.56-1.19) | 0.78 (0.50-1.21) | |||||
| High | 0.20 (0.03-1.47) | 0.31 (0.04-2.27) | |||||
| Gloss type | Protrusion | Reference | 0.081 | Reference | 0.008 | ||
| Ulcer | 0.81 (0.58-1.14) | 0.83 (0.59-1.18) | 0.301 | 0.70 (0.47-1.03) | 0.69 (0.46-1.03) | 0.072 | |
| infiltration | 2.31 (1.05-5.07) | 1.94 (0.86-4.37) | 0.108 | 2.56 (1.15-5.69) | 2.19 (0.95-5.05) | 0.066 | |
| Tumor size | < 5 cm | Reference | 0.363 | Reference | 0.099 | ||
| ≥ 5 cm | 1.45 (1.04-2.01) | 1.17 (0.84-1.63) | 1.67 (1.15-2.43) | 1.40 (0.94-2.09) | |||
| Preoperative serum CEA | ≥ median | Reference | Reference | ||||
| < median | 0.94 (0.68-1.31) | 0.84 (0.58-1.23) | |||||
| Preoperative serum CA19-9 | ≥ median | Reference | Reference | ||||
| < median | 0.84 (0.575-1.24) | 0.85 (0.54-1.34) | |||||
| CEA tumor status | + | Reference | Reference | ||||
| - | 1.00 (0.59-1.68) | 0.45 (0.20-1.03) | |||||
| P53 tumor status | + | Reference | Reference | ||||
| - | 1.40 (0.98-2.01) | 1.12 (0.72-1.75) | |||||
| AJCC stage (7th) | III | Reference | 0 | Reference | 0.000 | ||
| II | 0.36 (0.24-0.53) | 0.63 (0.51-0.77) | 0.32 (0.20-0.51) | 0.59 (0.46-0.75) | |||
| Radical surgery | R1 | Reference | 0.006 | Reference | 0.214 | ||
| R0 | 0.34 (0.19-0.60) | 0.43 (0.23-0.78) | 0.45 (0.23-0.86) | 0.65 (0.33-1.28) | |||
| Adjuvant chemotherapy | Yes | Reference | 0.008 | Reference | 0.000 | ||
| No | 1.58 (1.15-2.17) | 1.55 (1.12-2.14) | 1.99 (1.38-2.87) | 2.01 (1.37-2.94) | |||
| First-line chemotherapy | Yes | Reference | 0.013 | ||||
| No | 1.87 (1.14-3.07) | 1.95 (1.15-3.30) | |||||
CEA: Carcinoembryonic antigen; OS: Overall survival; DFS: Disease-free survival.
DISCUSSION
As the incidence of recurrence and metastasis is high after radical gastrectomy, it is important to investigate adjuvant chemotherapy and prognostic parameters as well as their potential interactions. Although the CLASSIC study and ACTS-GC study confirmed the improved 3-year DFS rate of XELOX and 5-year OS rate of S-1 in adjuvant chemotherapy after D2 gastrectomy, there are limited published data concerning stage IIIB and IIIC patients according to AJCC stage (7th)[6,8]. The ARTIST trial demonstrated a good 3-year DFS rate with the postoperative XP regimen, without comparison of postoperative XP regimen and surgery alone[9]. In addition, investigations of other regimens after D2 gastrectomy for stage II/III disease are still warranted.
In the present single-center study, we observed that patients had a significant OS and DFS benefit after postoperative chemotherapy in the entire stage II/III cohort, as well as for stage II, III and IIIB/IIIC individually. This provided an insight into current treatment for stage II/III disease, especially Stage IIIB and IIIC, in our center, which adds to current knowledge about the population benefit of adjuvant chemotherapy. Three-year DFS and OS reported in the CLASSIC study and ARTIST trial have not yet been formally validated as surrogate measures, therefore, we chose traditional median OS and median DFS as the primary outcomes. Furthermore, we confirmed the significant difference in 3-year OS rate between patients with adjuvant chemotherapy and patients with surgery alone (72.8% vs 53.8%, P = 0.001), and similarly for the 3-year DFS rate (57.4% vs 45.3%, P = 0.044). Although the results of different studies could not be compared directly, the 3-year OS and 3-year DFS rates of the two groups were lower than those reported in the CLASSIC study (83% vs 78%, P = 0.0493, 74% vs 59%, P < 0.0001), in the ACTS-GC study (80.1% vs 70.1%, P = 0.003, 72.2% vs 59.6%, P < 0.001) and the 3-year DFS rate of XP chemotherapy in the ARTIST trial (74.2%). However, the conclusions were the same. We excluded the patients with preoperative treatment that could otherwise have improved the R0 resection rates and confused the effects of adjuvant chemotherapy. The R1 radical gastrectomy existed reasonably and inclusion of R1 radical gastrectomy reflected the overall treatment situation of resectable gastric cancer in our center. The above three phase-3 trials only focused on R0 radical gastrectomy. Thus, the inclusion of R1 radical gastrectomy resulted in reduced OS and DFS in the present study. R1 gastrectomy was a poor prognostic factor for DFS vs R0 surgery, as revealed by multivariate analysis. This was consistent with the results for the entire cohort in the R0 subgroup (OS: 52.27 mo vs 31.67 mo, P = 0.000; DFS: 36.93 mo vs 26.20 mo, P = 0.004; 3-year OS rate: 74.2% vs 55.0%, P = 0.001; 3-year DFS rate: 59.7% vs 45.4%, P = 0.036) after excluding the R1 subgroup. Furthermore, we focused on patients after R0 gastrectomy excluding patients with T4bN+ or N3b disease (3-year OS rate: 76.1% vs 56.4%, P = 0.000; 3-year DFS rate: 61.0% vs 48.2%, P = 0.006), for whom survival came nearer to results of the CLASSIC study. Of note, 13.6% patients with T4bN+ or N3b disease were included in our entire population, higher than 6.9% in the ACTS-GC study. The subgroup results excluding T4bN+ or N3b disease came nearer to those in the ACTS-GC study (3-year OS rate: 82.63% vs 75.2%, 3-year DFS rate: no subgroup analysis), too. Our data on R1 gastrectomy are an important complement to the current studies of R0 gastrectomy.
Although the XELOX and XP regimens were recommended by NCCN in 2012 and 2013, respectively, there is no consensus on the regimens for adjuvant chemotherapy. Some have suggested that patients would benefit from adjuvant chemotherapy and should not be influenced by different schemes, including monotherapy, double therapy and triple therapy[22]. Some have also reported that significant benefits can be detected from a fluoropyrimidine-based monotherapy regimen and a fluoropyrimidine-based polychemotherapy regimen, but not from chemotherapy regimens without fluoropyrimidines[2]. In our study, we examined the fluoropyrimidine-based regimens and regimens without fluoropyrimidines vs surgery alone and only detected a significant survival benefit for fluoropyrimidine-based regimens over surgery alone [OS: 52.25 mo vs 31.67 mo, P = 0.000, HR = 0.46 (95%CI: 0.31-0.68); DFS: 36.93 mo vs 26.20 mo, P = 0.033, HR = 0.70 (0.50-0.97)]. However, there was no benefit for the regimens without fluoropyrimidines vs surgery alone [OS: 29.93 vs 31.67 mo, P = 0.666, HR = 0.85 (95%CI: 0.41-1.78); DFS: 23.90 mo vs 26.20 mo, P = 0.687, HR = 1.13 (95%CI: 0.62-2.09)]. These results were in accordance with the optimization of fluoropyrimidines in current gastric cancer treatment regimens established by previous research. We investigated four categories of regimens but only found survival benefits over surgery alone for surgery plus fluoropyrimidine monotherapy, and fluoropyrimidine combined with platinum. There was no benefit for fluoropyrimidine plus taxane, or platinum and taxane over surgery alone. The benefit of fluoropyrimidine monotherapy or fluoropyrimidine combined with platinum was consistent with the outcomes of the ACTS-GC study, CLASSIC study and ARTIST trial[6,8,9]. Previous studies revealed the genetic mechanism of cellular cytotoxicity to 5-fluorouracil (5-FU), and platinum may act synergistically to enhance the antitumor effects of 5-FU in terms of DNA synthesis inhibition and could act as a modulator of 5-FU in gastric cancer[23-25]. The platinum plus taxane regimen that we used here, which lacked fluoropyrimidines, yielded no survival benefit over surgery alone. Moreover, only two patients received fluoropyrimidine-based triple therapy, which was too small a size to draw any conclusions in comparison of triple therapy with surgery alone. Although much work has demonstrated improved efficacy for triple therapy, in 2013 the NCCN guidelines withdrew the recommendation of triple therapy because of obvious toxicity. A recent phase II clinical trial found that adjuvant therapy with S-1 plus docetaxel yielded promising OS and DFS in patients with stage IIIA but not stage IIIB gastric cancer after D2 gastrectomy[26]. Our fluoropyrimidine plus taxane regimen included injection or oral fluoropyrimidines, such as S-1, capecitabine, and paclitaxel or docetaxal. The sample size restricted the subgroup analysis stratified by the disease stage. Therefore, the role of different combinations remains to be investigated in detail with regard to disease stage. We suggested that stage II/III patients after D2 gastrectomy may gain a survival benefit from fluoropyrimidine monotherapy and fluoropyrimidine combined with platinum.
The level of wild-type p53 protein is low, and because of its short half-life, it is undetectable by standard immunohistochemical staining in normal tissues. The mutated p53 protein accumulates by binding to other oncogenic proteins or by prolonging its half-life[27], which can be detected with immunohistochemistry. Previous studies have shown a significant association between P53 mutations and immunohistochemical p53 reactivity[28]. There are conflicting results in studies on the prognostic significance of p53 mutations after gastrectomy. Some studies have shown that p53 overexpression leads to poor DFS and OS after radical gastrectomy, either by univariate analysis[16,29,30] or by multivariate analysis[31,32]. However, other studies have found that p53 overexpression is not related to prognosis[15,33,34]. With fewer than 200 patients included in most studies, we thus conducted a larger study with balanced characteristics and took into account other prognostic parameters, especially the potential interaction of adjuvant chemotherapy. Our univariate analysis found that p53 tumor status was not prognostic for OS or DFS after radical gastrectomy. The consistently nonsignificant prognostic effects of p53, stratified by surgery or different adjuvant regimens, are compatible with previous studies that showed no significant influence of p53 expression upon tumor response[35]. Here, p53(+) tumor status tended to yield prolonged OS or DFS compared with p53(-) tumor status, although the difference was not significant. This is consistent with the study by Potrc et al[36] that median survival was higher in patients with p53 positivity, although not significantly. One study showed that patients with p53(-) gastric cancer had a poor prognosis by univariate analysis[37]. One possible reason is that the increase in p53 staining is not always due to a mutated gene, and overexpression of the wild-type protein might be detected in a subset of patients[38]. Another is that p53 plays a complicated role as both a tumor suppressor and oncogene. In addition, we observed no correlation between p53 expression and clinicopathological variables, as in previous studies[15,32,39]. These results all suggest that p53 immunohistochemical expression should not be judged as a prognostic biomarker.
In this study, immunoreactivity of CEA was observed in 89.2% of patients, similar to that observed by Kim et al[19]. A high percentage (100%) of CEA positivity was seen in four highly differentiated gastric cancer specimens, compared to 94% in 63 moderately differentiated and 87.4% in 188 lowly differentiated cancer specimens, in accordance with the relationship between CEA intensity and differentiation reported previously[40,41]. However, the difference was not significant, which may be due to the small number of highly differentiated gastric cancer specimens. As compared to the distal localization of gastric cancer, proximal cancer had more intense immunoreactivity of CEA (92.3% vs 84.6%, P = 0.040). The difference between proximal and distal localization was also investigated by Mărgăritescu et al[42]. Despite the decline in gastric cancer incidence, adenocarcinomas from the proximal stomach have tended to be more frequent during the past three decades. These differences in CEA tumor status indicate that proximal adenocarcinomas may be a different, specific subtype of gastric carcinoma. Although CEA immunoassay as a diagnostic adjunct and a potential prognostic factor in gastrointestinal tumors has been discussed[43,44], the value of CEA tumor status as a prognostic indicator for gastric carcinoma remains unclear. Kim et al[19] demonstrated that the CEA(-) group (78.2%) had a significantly better 3-year survival rate than the CEA(+) group (60.2%) in univariate analysis, however, not in multivariate analysis. We found that the CEA(-) group had better median OS than the CEA(+) group, although only with borderline significance. We also observed that the 3-year survival rate in the CEA(-) group was higher than that in the CEA(+) group, although still without significance (80.9% vs 55.7%, P = 0.083). Whether this was due to the small size of the CEA(-) group or a true clinicopathological principle is conjectural. In addition, the nonsignificant effect of CEA tumor status was maintained when it was restricted to surgery alone and different regimens. The present data suggest that CEA immunohistochemistry in tumor tissues is of limited value in obtaining precise prognostic information.
Besides adjuvant chemotherapy, the positive prognostic values of AJCC stage II for DFS and OS, as well as first-line chemotherapy for OS have been reported previously[45].
In conclusion, we suggest that patients after D2 gastrectomy for stage II/III gastric adenocarcinoma can gain a significant survival benefit from fluoropyrimidine monotherapy and fluoropyrimidine combined with platinum adjuvant regimens. p53 and CEA immunohistochemical expression is not prognostic for survival after D2 gastrectomy.
COMMENTS
Background
Gastric cancer ranks second among the most common causes of cancer deaths worldwide. A high incidence of recurrence and metastasis after radical gastrectomy indicates that investigation of adjuvant chemotherapy and prognostic parameters is important.
Research frontiers
Although the CLASSIC study and ACTS-GC study confirmed the improved 3-year disease-free survival (DFS) rate of the XELOX and 5-year overall survival rate of S-1 in adjuvant chemotherapy after D2 gastrectomy, there are limited published data concerning stage IIIB and IIIC patients. The ARTIST trial demonstrated a good 3-year DFS rate after the postoperative XP regimen, without comparison of postoperative XP regimen and surgery alone. In addition, investigations of other regimens after D2 gastrectomy for stage II/III disease are still warranted. Better understanding of the prognostic parameters is required. There are still conflicting results on the clinical and prognostic significance of p53 tissue status and carcinoembryonic antigen (CEA) tissue status in gastric cancer.
Innovations and breakthroughs
The authors found that patients after D2 gastrectomy for stage II/III gastric adenocarcinoma gained a significant survival benefit from adjuvant chemotherapy compared with surgery alone. This advantage was maintained when the analyses were restricted to patients with stage II, III and IIIB/IIIC disease, and patients treated with fluoropyrimidine monotherapy or fluoropyrimidine plus platinum. The survival did not differ significantly between the patients with p53(+) and p53(-) tumors, or between patients with CEA(+) and CEA(-) tumors. This was maintained when the analyses were restricted to surgery alone, adjuvant chemotherapy, and even different chemotherapy regimens. p53 and CEA immunohistochemical expression is not prognostic for survival after D2 gastrectomy.
Applications
The findings in this study demonstrated that fluoropyrimidine monotherapy and fluoropyrimidine combined with platinum adjuvant regimens should be considered in patients after D2 gastrectomy for stage II/III gastric adenocarcinoma. Meanwhile, more investigations of prognostic parameters after D2 gastrectomy are warranted on the basis of the nonprognostic value of p53 and CEA immunohistochemical expression.
Terminology
CLASSIC study, the Capecitabine and Oxaliplatin Adjuvant Study in Stomach Cancer study; ARTIST trial, Adjuvant Chemoradiation Therapy in Stomach Cancer trial; ACTS-GC, Adjuvant Chemotherapy Trial of TS-1 for Gastric Cancer study; XELOX, capecitabine plus oxaliplatin; XP, capecitabine plus cisplatin.
Peer review
This study concluded that patients after D2 gastrectomy for stage II/III gastric adenocarcinoma can gain a significant survival benefit from fluoropyrimidine monotherapy and fluoropyrimidine combined with platinum adjuvant regimens. p53 and CEA immunohistochemical expression is not prognostic for survival after D2 gastrectomy. This study had some indications of how gastric cancer patients at stage II/III could be treated with better outcome.
Footnotes
Supported by National High Technology Research and Development Program of China (863 Program), China, No. 2012AA02A506; Science and Technology Department of Guangdong Province, China, No. 2012B031800088; and Medical Scientific Research Foundation of Guangdong Province, China, No. C2011019
P- Reviewers: Abdel-Salam OME, Cho CH, Shinohara T S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Ma S
References
- 1.Yoo CH, Noh SH, Shin DW, Choi SH, Min JS. Recurrence following curative resection for gastric carcinoma. Br J Surg. 2000;87:236–242. doi: 10.1046/j.1365-2168.2000.01360.x. [DOI] [PubMed] [Google Scholar]
- 2.Paoletti X, Oba K, Burzykowski T, Michiels S, Ohashi Y, Pignon JP, Rougier P, Sakamoto J, Sargent D, Sasako M, et al. Benefit of adjuvant chemotherapy for resectable gastric cancer: a meta-analysis. JAMA. 2010;303:1729–1737. doi: 10.1001/jama.2010.534. [DOI] [PubMed] [Google Scholar]
- 3.Okines A, Verheij M, Allum W, Cunningham D, Cervantes A. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21 Suppl 5:v50–v54. doi: 10.1093/annonc/mdq164. [DOI] [PubMed] [Google Scholar]
- 4.Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, Haller DG, Ajani JA, Gunderson LL, Jessup JM, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725–730. doi: 10.1056/NEJMoa010187. [DOI] [PubMed] [Google Scholar]
- 5.Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 6.Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, Yamaguchi T, Nashimoto A, Fujii M, Nakajima T, Ohashi Y. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011;29:4387–4393. doi: 10.1200/JCO.2011.36.5908. [DOI] [PubMed] [Google Scholar]
- 7.Nakajima T. Gastric cancer treatment guidelines in Japan. Gastric Cancer. 2002;5:1–5. doi: 10.1007/s101200200000. [DOI] [PubMed] [Google Scholar]
- 8.Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, Lee KH, Lee KW, Kim YH, Noh SI, Cho JY, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012;379:315–321. doi: 10.1016/S0140-6736(11)61873-4. [DOI] [PubMed] [Google Scholar]
- 9.Lee J, Lim do H, Kim S, Park SH, Park JO, Park YS, Lim HY, Choi MG, Sohn TS, Noh JH, et al. Phase III trial comparing capecitabine plus cisplatin versus capecitabine plus cisplatin with concurrent capecitabine radiotherapy in completely resected gastric cancer with D2 lymph node dissection: the ARTIST trial. J Clin Oncol. 2012;30:268–273. doi: 10.1200/JCO.2011.39.1953. [DOI] [PubMed] [Google Scholar]
- 10.Murata A, Baba Y, Watanabe M, Shigaki H, Miyake K, Karashima R, Imamura Y, Ida S, Ishimoto T, Iwagami S, et al. p53 immunohistochemical expression and patient prognosis in esophageal squamous cell carcinoma. Med Oncol. 2013;30:728. doi: 10.1007/s12032-013-0728-z. [DOI] [PubMed] [Google Scholar]
- 11.Malats N, Bustos A, Nascimento CM, Fernandez F, Rivas M, Puente D, Kogevinas M, Real FX. P53 as a prognostic marker for bladder cancer: a meta-analysis and review. Lancet Oncol. 2005;6:678–686. doi: 10.1016/S1470-2045(05)70315-6. [DOI] [PubMed] [Google Scholar]
- 12.Tada M, Matsumoto R, Iggo RD, Onimaru R, Shirato H, Sawamura Y, Shinohe Y. Selective sensitivity to radiation of cerebral glioblastomas harboring p53 mutations. Cancer Res. 1998;58:1793–1797. [PubMed] [Google Scholar]
- 13.Yu SJ, Yu JK, Ge WT, Hu HG, Yuan Y, Zheng S. SPARCL1, Shp2, MSH2, E-cadherin, p53, ADCY-2 and MAPK are prognosis-related in colorectal cancer. World J Gastroenterol. 2011;17:2028–2036. doi: 10.3748/wjg.v17.i15.2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lowe SW, Ruley HE, Jacks T, Housman DE. p53-dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell. 1993;74:957–967. doi: 10.1016/0092-8674(93)90719-7. [DOI] [PubMed] [Google Scholar]
- 15.Tsujitani S, Saito H, Wakatsuki T, Ikeguchi M, Shirabe K, Morita M, Kakeji Y, Yano T, Maehara Y. Relationship between expression of apoptosis-related proteins and the efficacy of postoperative chemotherapy in patients with T3 gastric cancer. Surg Today. 2012;42:225–232. doi: 10.1007/s00595-011-0062-z. [DOI] [PubMed] [Google Scholar]
- 16.Drebber U, Baldus SE, Nolden B, Grass G, Bollschweiler E, Dienes HP, Hölscher AH, Mönig SP. The overexpression of c-met as a prognostic indicator for gastric carcinoma compared to p53 and p21 nuclear accumulation. Oncol Rep. 2008;19:1477–1483. [PubMed] [Google Scholar]
- 17.Oh SY, Kwon HC, Kim SH, Jang JS, Kim MC, Kim KH, Han JY, Kim CO, Kim SJ, Jeong JS, et al. Clinicopathologic significance of HIF-1alpha, p53, and VEGF expression and preoperative serum VEGF level in gastric cancer. BMC Cancer. 2008;8:123. doi: 10.1186/1471-2407-8-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kojima O, Ikeda E, Uehara Y, Majima T, Fujita Y, Majima S. Correlation between carcinoembryonic antigen in gastric cancer tissue and survival of patients with gastric cancer. Gann. 1984;75:230–236. [PubMed] [Google Scholar]
- 19.Kim DY, Kim HR, Shim JH, Park CS, Kim SK, Kim YJ. Significance of serum and tissue carcinoembryonic antigen for the prognosis of gastric carcinoma patients. J Surg Oncol. 2000;74:185–192. doi: 10.1002/1096-9098(200007)74:3<185::aid-jso4>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 20.Zhou J, Zhang L, Gu Y, Li K, Nie Y, Fan D, Feng Y. Dynamic expression of CEACAM7 in precursor lesions of gastric carcinoma and its prognostic value in combination with CEA. World J Surg Oncol. 2011;9:172. doi: 10.1186/1477-7819-9-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kojima N, Kunieda K, Matsui K, Kato H, Saji S. Evaluation of carcinoembryonic antigen mRNA in living, necrotic, and apoptotic gastric cancer cells by reverse transcriptase-polymerase chain reaction. Surg Today. 2003;33:839–846. doi: 10.1007/s00595-003-2617-0. [DOI] [PubMed] [Google Scholar]
- 22.Liu TS, Wang Y, Chen SY, Sun YH. An updated meta-analysis of adjuvant chemotherapy after curative resection for gastric cancer. Eur J Surg Oncol. 2008;34:1208–1216. doi: 10.1016/j.ejso.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 23.Matsusaka S, Nagareda T, Yamasaki H. Does cisplatin (CDDP) function as a modulator of 5-fluorouracil (5-FU) antitumor action? A study based on a clinical trial. Cancer Chemother Pharmacol. 2005;55:387–392. doi: 10.1007/s00280-004-0860-8. [DOI] [PubMed] [Google Scholar]
- 24.Park JS, Young Yoon S, Kim JM, Yeom YI, Kim YS, Kim NS. Identification of novel genes associated with the response to 5-FU treatment in gastric cancer cell lines using a cDNA microarray. Cancer Lett. 2004;214:19–33. doi: 10.1016/j.canlet.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 25.Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama W, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9:215–221. doi: 10.1016/S1470-2045(08)70035-4. [DOI] [PubMed] [Google Scholar]
- 26.Fujitani K, Tamura S, Kimura Y, Tsuji T, Matsuyama J, Iijima S, Imamura H, Inoue K, Kobayashi K, Kurokawa Y, et al. Three-year outcomes of a phase II study of adjuvant chemotherapy with S-1 plus docetaxel for stage III gastric cancer after curative D2 gastrectomy. Gastric Cancer. 2013:Jun 5; Epub ahead of print. doi: 10.1007/s10120-013-0273-7. [DOI] [PubMed] [Google Scholar]
- 27.Lane DP, Benchimol S. p53: oncogene or anti-oncogene? Genes Dev. 1990;4:1–8. doi: 10.1101/gad.4.1.1. [DOI] [PubMed] [Google Scholar]
- 28.Wynford-Thomas D. P53 in tumour pathology: can we trust immunocytochemistry? J Pathol. 1992;166:329–330. doi: 10.1002/path.1711660402. [DOI] [PubMed] [Google Scholar]
- 29.Saito H, Osaki T, Murakami D, Sakamoto T, Kanaji S, Ohro S, Tatebe S, Tsujitani S, Ikeguchi M. Prediction of sites of recurrence in gastric carcinoma using immunohistochemical parameters. J Surg Oncol. 2007;95:123–128. doi: 10.1002/jso.20612. [DOI] [PubMed] [Google Scholar]
- 30.Lee KE, Lee HJ, Kim YH, Yu HJ, Yang HK, Kim WH, Lee KU, Choe KJ, Kim JP. Prognostic significance of p53, nm23, PCNA and c-erbB-2 in gastric cancer. Jpn J Clin Oncol. 2003;33:173–179. doi: 10.1093/jjco/hyg039. [DOI] [PubMed] [Google Scholar]
- 31.Díez M, Medrano MJ, Gutierrez A, López A, Mugüerza JM, Hernández P, Lozano O, Noguerales F, Ruíz A, Granell J. P53 protein expression in gastric adenocarcinoma. Negative predictor of survival after postoperative adjuvant chemotherapy. Anticancer Res. 2000;20:3929–3933. [PubMed] [Google Scholar]
- 32.Büyükçelik A, Onur H, Akbulut H, Bülent Y, Ensari A, Utkan G, Onal BS, Içli F. Expression of p53 protein and DNA flow cytometry in gastric adenocarcinoma: implications in patients treated with adjuvant etoposide, adriamycin and cisplatin. Tumori. 2005;91:302–308. doi: 10.1177/030089160509100403. [DOI] [PubMed] [Google Scholar]
- 33.Ismail HM, Moneer M, El-Baradie M, Khorshid O, Touny A. Clinicopathologic and prognostic significance of overexpression of her-2/neu and p53 oncoproteins in gastric carcinoma using tissue microarray. J Egypt Natl Canc Inst. 2007;19:147–157. [PubMed] [Google Scholar]
- 34.Tsujinaka T, Shiozaki H, Yano M, Kikkawa N, Takami M, Monden M. Prognostic factors for recurrence in stage II and III gastric cancer patients receiving a curative resection and postoperative adjuvant chemotherapy. Oncol Rep. 2001;8:33–38. doi: 10.3892/or.8.1.33. [DOI] [PubMed] [Google Scholar]
- 35.Sirak I, Petera J, Hatlova J, Vosmik M, Melichar B, Dvorak J, Tycova V, Zoul Z, Lesko M. Expression of p53, p21 and p16 does not correlate with response to preoperative chemoradiation in gastric carcinoma. Hepatogastroenterology. 2001;56:1213–1218. [PubMed] [Google Scholar]
- 36.Potrc S, Gadiijev E, Hajdinjak T, Kavalar R. Clinicopathological and immunohistochemical markers after radical gastrectomy for gastric cancer. Hepatogastroenterology. 2007;54:308–314. [PubMed] [Google Scholar]
- 37.Deveci MS, Deveci G. Prognostic value of p53 protein and MK-1 (a tumor-associated antigen) expression in gastric carcinoma. Gastric Cancer. 2007;10:112–116. doi: 10.1007/s10120-007-0418-7. [DOI] [PubMed] [Google Scholar]
- 38.Bataille F, Rümmele P, Dietmaier W, Gaag D, Klebl F, Reichle A, Wild P, Hofstädter F, Hartmann A. Alterations in p53 predict response to preoperative high dose chemotherapy in patients with gastric cancer. Mol Pathol. 2003;56:286–292. doi: 10.1136/mp.56.5.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCulloch P, Taggart T, Ochiai A, O’Dowd G, Nash J, Sasako M. c-erbB2 and p53 expression are not associated with stage progression of gastric cancer in Britain or Japan. Eur J Surg Oncol. 1997;23:304–309. doi: 10.1016/s0748-7983(97)90669-7. [DOI] [PubMed] [Google Scholar]
- 40.Santeusanio G, Peronace L, Castagna G, De Muro G, Santi D, D’Orazio A, Amanti C, Midiri G, Campisi C, D’Ambra G. Immunohistochemical study of carcinoembryonic antigen (CEA) in gastric tumors: correlation with preoperative serum levels, histologic type, and grade of anaplasia of the tumor. J Surg Oncol. 1988;37:13–19. doi: 10.1002/jso.2930370105. [DOI] [PubMed] [Google Scholar]
- 41.Bhatnagar J, Heroman W, Murphy M, Austin GE. Immunohistochemical detection of carcinoembryonic antigen in esophageal carcinomas: a comparison with other gastrointestinal neoplasms. Anticancer Res. 2002;22:1849–1857. [PubMed] [Google Scholar]
- 42.Mărgăritescu C, Mogoantă L, Mănescu P, Simionescu C, Pirici D, Streba L, Mercuţ D. The immunohistochemical profile of the adenocarcinoma of upper gastric pole. Rom J Morphol Embryol. 2007;48:215–235. [PubMed] [Google Scholar]
- 43.Chen S, Chen YB, Li YF, Feng XY, Zhou ZW, Yuan XH, Qian CN. Normal carcinoembryonic antigen indicates benefit from perioperative chemotherapy to gastric carcinoma patients. World J Gastroenterol. 2012;18:3910–3916. doi: 10.3748/wjg.v18.i29.3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu YB, Ge SH, Zhang LH, Wang XH, Xing XF, DU H, Hu Y, Li YA, Jia YN, Lin Y, et al. [Clinical value of serum CEA, CA19-9, CA72-4 and CA242 in the diagnosis and prognosis of gastric cancer] Zhonghua Weichang Waike Zazhi. 2012;15:161–164. [PubMed] [Google Scholar]
- 45.Kim DH, Kim SM, Hyun JK, Choi MG, Noh JH, Sohn TS, Bae JM, Kim S. Changes in postoperative recurrence and prognostic risk factors for patients with gastric cancer who underwent curative gastric resection during different time periods. Ann Surg Oncol. 2013;20:2317–2327. doi: 10.1245/s10434-012-2700-0. [DOI] [PubMed] [Google Scholar]


