Abstract
AIM: To establish a reliable definition of postoperative liver failure (PLF) and allow the prediction of outcomes after hepatectomy.
METHODS: The clinical data of 478 consecutive patients who underwent hepatectomy were retrospectively analyzed. The examined prognostic factors included the ratio of total bilirubin (TBIL) on postoperative day (POD) X to TBIL on POD 1 (TBIL-r1) and the ratio of the international normalized ratio (INR) on POD X to the INR on POD 1 (INR-r1) for PODs 3, 5 and 7. Student’s t test, the χ2 test, logistic regression, survival analysis and receiver operating curve analysis were used to evaluate risk factors and establish the definition of postoperative liver failure (PLF).
RESULTS: Fourteen patients (2.9%) died of liver failure within 3 mo of surgery. Significant differences were found between patients who died of liver failure and the remaining patients in terms of TBIL-r1 and INR-r1 on PODs 3, 5 and 7. The combination of TBIL-r1 and INR-r1 on POD 5 showed strong predictive power for liver failure-related death (sensitivity 92.9% and specificity 90.1%). The hepatic damage score (HDs), which was derived from TBIL-r1 and INR-r1, was used to define the degree of metabolic functional impairment after resection as mild (HDs = 0), reversible hepatic “dysfunction” (HDs = 1) or fatal hepatic failure (HDs = 2). Furthermore, the indocyanine green retention rate at 15 min (ICG-R15) and the number of resected segments (RSs) were identified as independent predictors of the HDs. A linear relationship was found between ICG-R15 and RSs in the HDs = 2 group. The regression equation was: RSs = -0.168 × ICG-R15 + 5.625 (r2 = 0.613, F = 14.257, P = 0.004).
CONCLUSION: PLF can be defined by the HDs, which accurately predicts liver failure-related death after liver resection. Furthermore, the ICG-R15 and RSs can be used as selection criteria for hepatectomy.
Keywords: Liver failure, Hepatectomy, Mortality, Morbidity, Hepatic dysfunction
Core tip: We derived a new definition of postoperative liver failure (PLF) termed the hepatic damage score (HDs). The HDs was an ideal definition of PLF and reflected the degree of liver impairment after resection as mild (HDs = 0), reversible hepatic “dysfunction” (HDs = 1) to fatal hepatic failure (HDs = 2).
INTRODUCTION
Hepatic resection is the most effective treatment for selected patients with liver malignancies or a number of benign diseases, such as hepatocellular carcinoma and large hepatic cavernous hemangioma, among others[1-6]. With advances in surgical techniques and perioperative management and improvements in the criteria for patient selection, early postoperative mortality has been reduced from 13% for all resections and > 20% for major resections detected by Foster and Berman in 1977[7] to less than 8% in recent years[8,9]. However, the mortality caused by postoperative liver failure (PLF) has increased with increases of extended hepatectomy and complex liver resection[7,10-13].
In general, PLF is characterized as the failure of one or more of the synthetic and excretory functions of the liver, including hyperbilirubinemia, coagulopathy, hypoalbuminemia and different grades of hepatic encephalopathy[12-15]. Patients with PLF are often susceptible to complications, particularly septic events, and require long in-hospital stays to recover or ultimately succumb to death. Previous studies have reported some independent risk factors that affect PLF after liver resection[4,10-16]. However, the definitions of PLF are not uniform, which makes it difficult to compare outcome data across these studies and assess the efficacy of interventions designed to reduce the incidence of PLF. Early diagnosis of PLF is vital for appropriate postoperative treatments (such as optimization of drug therapy, surgical intervention, therapy using liver assist devices[17] or transplantation). Therefore, based on a large series of liver resections performed over a short period of time, the aim of the present study was to establish a reliable and standardized definition of PLF using an arbitrarily defined score combining the ratio of total bilirubin (TBIL) on postoperative day (POD) X to TBIL on POD 1 (TBIL-r1) and the ratio of the international normalized ratio (INR) on POD X to the INR on POD 1 (INR-r1), which can precisely predict early liver failure-related mortality and morbidity after liver resection in a timely fashion.
MATERIALS AND METHODS
Patients
From March 2009 to May 2012, 478 consecutive patients who had undergone liver resection at the Department of Liver Surgery, West China Hospital of Sichuan University, were enrolled in this study. All patients in this study underwent a preoperative indocyanine green (ICG) clearance test, which has been described in detail elsewhere[18,19].
To identify predictors of liver failure-related death, we investigated dynamic changes in TBIL and INR on postoperative days (PODs) 1, 3, 5 and 7. We found 2 abnormalities in the values of TBIL and INR: (1) stabilization at high levels in the first few days after liver resection, with no reduction afterward or only a very slight decrease; and (2) a gradual and stable increase after POD 1. These 2 trends were collectively classified into 2 parameters, namely, TBIL-r1 and INR-r1, which were calculated as the TBIL or INR value on POD X divided by their respective values on POD 1.
The first end point was liver failure-related death, which was defined as death in patients who had been classified as Child-Turcotte-Pugh (CTP) Class C on the day they died, including postoperative mortality related to liver failure or multisystem organ failure including liver failure. The second end point was postoperative major complications recorded during 90 d. According to the classification proposed by Clavien et al[20], postoperative major complications were defined as grades III, IV and V.
Surgical modalities
In total, 247 patients (51.7%) underwent minor hepatic resections, which predominantly included nonanatomical wedge resections (≤ 2 segments) or enucleation (n = 74, 15.5%) and left lateral segmentectomy (n = 66, 13.8%). The remaining minor hepatic resections included bisegmentectomy of segments VI and VII (n = 41), bisegmentectomy of segments V and VIII (n = 33) and segmentectomy (n = 33). Major hepatic resections were performed in 231 patients (48.3%), including right trisegmentectomy (n = 71), right hepatectomy (n = 54), left hepatectomy (n = 47), extended right hepatectomy (n = 21) and extended left hepatectomy (n = 16). Twenty-two (4.6%) patients underwent resection of 3 or more discontiguous segments, which was also considered as major hepatic resection. The average number of hepatic segments resected was 2.7 ± 1.3 (range 0-6). Written informed consent was obtained from each patient before treatment.
Follow-up
All patients were followed regularly in the outpatient clinic and monitored according to a standard protocol. The follow-up consisted of monthly blood tests to monitor remnant liver function and at least one ultrasonographic or contrast-enhanced computed tomography scan in the first 3 mo after surgery. Four patients died of PLF as outpatients and were included as liver failure-related deaths in this study. In addition, all other causes of deaths and major complications were all rectified within the 3 mo follow-up. Therefore, the numbers for liver failure-related deaths and major complications were not underestimated.
Statistical analysis
Descriptive statistics included means, ranges, standard deviations and proportions. Categorical data are presented as percentages, and differences between proportions were compared by the χ2 test or Fisher’s exact test. Continuous variables were compared using the unpaired Student’s t test. Multivariate analysis was performed to identify independent determinants of the hepatic damage score (HDs; logistic regression stepwise backward procedure). Survival curves were computed using the Kaplan-Meier method and compared using the log-rank test. All above statistical evaluations were performed using the SPSS for Windows package (SPSS 18.0 for Windows, Chicago, IL). For the receiver operating characteristic (ROC) curve analysis, we used MedCalc (version 12.0) to calculate the sensitivity, specificity and area under the curve and to select the optimal cut-off value for predicting liver failure-related death. The results with a P value < 0.05 were considered statistically significant.
RESULTS
Patient characteristics
There were 389 men (81.4%) and 89 women (18.6%), and their median age was 47 years (range, 18-77 years). In total, 432 patients were classified as CTP class A (90.4%) and 46 patients as CTP class B (9.6%). Of all patients, 340 (70.1%) were hepatitis B virus positive. The etiologies associated with liver disease in the included patients are shown in Table 1.
Table 1.
Liver disease etiologies among the enrolled patients (n = 478)
| Etiology | n (%) |
| Malignant disease | 371 (77.6) |
| HCC | 323 (67.6) |
| Colorectal metastasis | 25 (5.2) |
| Other metastatic disease | 12 (2.5) |
| Cholangiocarcinoma | 9 (1.9) |
| Gallbladder carcinoma | 2 (0.4) |
| Benign disease | 107 (22.4) |
| HCH | 72 (15.1) |
| FNH | 13 (2.7) |
| Hepatic echinococcosis | 7 (1.5) |
| Hepatic complex cysts | 2 (0.4) |
| Adenoma | 9 (1.9) |
| Cystadenoma | 3 (0.6) |
HCC: Hepatocellular carcinoma; HCH: Hepatic cavernous hemangioma; FNH: Focal nodular hyperplasia.
Fourteen patients (2.9%) died of irreversible postoperative liver failure or multisystem organ failure associated with liver failure within 3 mo after the operation. The median time to liver failure-related death was 31 d (range 14-63 d). Seven additional perioperative deaths were caused by uncontrolled hemorrhage in 1 patient, intra-abdominal hemorrhage later in the postoperative period in 1 patient, severe sepsis in 2 patients and multisystem organ failure without associated liver failure in 3 patients.
The median hospital stay after hepatectomy was 7 d (range 4-87 d). Major postoperative complications occurred in 142 (29.7%) patients and were graded as follows: grade IIIa (n = 79; 16.5%), grade IIIb (n = 28; 5.9%), grade IVa (n = 11; 2.3%), grade IVb (n = 3; 0.6%) and grade V (n = 21; 4.4%). Table 2 lists the relevant complications and their frequencies. Most patients suffered at least two complications within 3 mo after the operation. Hyperbilirubinemia was not included as a postoperative major complication in the calculation of the composite end point.
Table 2.
Postoperative complications in the entire patient cohort (n = 478)
| Complication | n (%) |
| Cardiopulmonary | 90 (18.8) |
| Myocardial infarction | 4 (0.8) |
| Cardiac arrest | 15 (3.1) |
| Congestive heart failure | 6 (1.3) |
| Respiratory insufficiency/failure1 | 12 (2.5) |
| Pneumonia | 34 (7.1) |
| Pleural effusion (symptomatic) | 19 (4.0) |
| Liver/biliary | 70 (14.6) |
| Increased bilirubin2 | 59 (12.3) |
| Bile leak/biloma | 5 (1.0) |
| Cholangitis | 6 (1.3) |
| Gastrointestinal | 12 (2.5) |
| Ileus | 5 (1.0) |
| Gastrointestinal hemorrhage | 7 (1.5) |
| Infection | 34 (7.1) |
| Deep wound infection | 23 (4.8) |
| Sepsis/bacteremia | 11 (2.3) |
| Renal and genitourinary | 59 (12.3) |
| Renal insufficiency/failure | 17 (3.6) |
| Urinary tract infection | 23 (4.8) |
| Urinary retention | 19 (4.0) |
| Miscellaneous | 23 (4.8) |
| Deep venous thrombosis | 5 (1.0) |
| Need for reoperation | 7 (1.5) |
| Perioperative hemorrhage | 11 (2.3) |
| Others3 | 10 (2.1) |
Prolonged ventilatory requirement or reintubation, hypoxia with prolonged supplemental oxygen requirement;
Not included in calculation of composite endpoint as a major postoperative complication;
Stroke/TIA (n = 1), pericarditis/pericardial effusion (n = 2), perihepatic abscess (n = 2), portal vein thrombosis (n = 1), biliary stricture (n = 3), perforated duodenal ulcer (n = 1).
Kinetics of postoperative liver function tests
The postoperative TBIL, INR, TBIL-r1 and INR-r1 in cases of liver failure-related death (group I) and the remaining patients (group II) are shown in Figure 1. At baseline, the TBIL level in group I was 16.2 ± 4.9 μmol/L, compared to 17.8 ± 6.2 μmol/L in group II. After liver resection, the TBIL value in group I increased during the first 5 d and was then maintained at a high level. In contrast, the TBIL value in group II exhibited a slight increase in the first 3 d and thereafter exhibited a stable decline until it reached the baseline value. The two groups significantly differed on POD 5 and POD 7, with P values of 0.001 and 6.0e-5, respectively (Figure 1A). The postoperative INR level was the highest on POD 1 in both groups. Thereafter, it remained high in group I and steadily decreased to the baseline level in group II. INR levels also showed significant differences between groups I and II on PODs 5 and 7, with P values of 0.015 and 4.8e-5, respectively (Figure 1B). The TBIL-r1 in group I significantly increased, whereas it exhibited a stable decrease in group II. The TBIL-r1 values significantly differed between the two groups on PODs 3, 5 and 7 (P < 0.05; Figure 1C). The INR-r1 in group II demonstrated a slight and continuous decrease compared with the INR-r1 in group I, which remained stable from POD 3 to POD 7. The differences between the groups remained significant at all time points (P < 0.05; Figure 1D).
Figure 1.
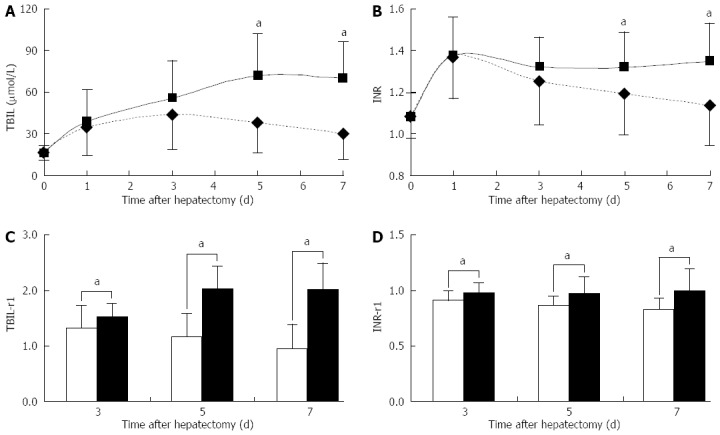
Dynamic changes in postoperative liver function indexes in cases of liver failure-related death and the remaining patients. Values are expressed as mean SD, aP < 0.05 between groups. A: Total bilirubin (TBIL); B: International normalized ratio (INR); C: The ratio of total bilirubin on postoperative day (POD) X to the value on POD 1(TBIL-r1); D: The ratio of international normalized ratio on POD X to the value on POD 1 (INR-r1).
Predictors of liver failure-related death
ROC curve analysis of TBIL, INR, TBIL-r1 and INR-r1 to predict liver failure-related death was performed to define PLF. A cut-off value was determined by seeking the largest sum of the sensitivity and specificity values. The diagnostic characteristics of TBIL, INR, TBIL-r1 and INR-r1 are summarized in Table 3. The results suggested that at each time point after hepatectomy, the predictive powers of TBIL-r1 and INR-r1 were stronger than those of TBIL and INR. In the clinical setting, a predictor of PLF must have strong predictive power and should be able to predict PLF as early as possible. Thus, we chose the combination of TBIL-r1 and INR-r1 on POD 5 as a strong and early predictor of PLF. Although the combination of TBIL-r1 and INR-r1 on POD 7 was slightly superior to the combination of TBIL-r1 and INR-r1 on POD 5 regarding predictive power, 2 additional days were required to obtain the values. Moreover, on POD 3, the predictive power of these values was not sufficiently strong.
Table 3.
Diagnostic characteristics of total bilirubin, international normalized ratio and combined criteria on postoperative day (3, 5, 7) in the prediction of liver failure-related death
| Criteria | AUC | 95%CI | Sensitivity | Specificity | P value | Cut-off value |
| POD 3 | ||||||
| TBIL-r1 | 0.686 | 0.642-0.727 | 92.90% | 51.70% | 0.018 | 1.24 |
| INR-r1 | 0.658 | 0.614-0.701 | 64.30% | 64.90% | 0.046 | 0.94 |
| Combination1 | 0.719 | 0.676-0.759 | 64.30% | 74.80% | 0.005 | 0.0337 |
| POD 5 | ||||||
| TBIL-r1 | 0.918 | 0.890-0.941 | 85.70% | 87.50% | 1.0e-7 | 1.68 |
| INR-r1 | 0.719 | 0.677-0.759 | 50.00% | 94.80% | 0.006 | 1.02 |
| Combination1 | 0.951 | 0.927-0.968 | 92.90% | 90.10% | 9.0e-9 | 0.0379 |
| TBIL | 0.810 | 0.772-0.844 | 78.60% | 81.50% | 7.7e-5 | 51.0 |
| INR | 0.708 | 0.665-0.748 | 85.70% | 64.40% | 0.008 | 1.23 |
| Combination2 | 0.829 | 0.792-0.861 | 99.80% | 57.50% | 2.8e-5 | 0.0164 |
| POD 7 | ||||||
| TBIL-r1 | 0.937 | 0.912-0.957 | 85.70% | 91.60% | 2.4e-8 | 1.61 |
| INR-r1 | 0.796 | 0.757-0.831 | 71.40% | 80.00% | 1.5e-4 | 0.91 |
| Combination1 | 0.975 | 0.956-0.987 | 92.90% | 94.40% | 1.4e-9 | 0.0566 |
| TBIL | 0.886 | 0.854-0.913 | 92.90% | 79.10% | 1.0e-6 | 49.5 |
| INR | 0.798 | 0.760-0.833 | 85.70% | 73.30% | 1.4e-4 | 1.22 |
| Combination2 | 0.908 | 0.879-0.933 | 92.90% | 79.70% | 8.7e-7 | 0.0277 |
The combination of TBIL-r1 and INR-r1 used the predicted probabilities, which were calculated using logistic regression analysis;
The combination of TBIL and INR used the predicted probabilities, which were calculated using logistic regression analysis. AUC: Area under the receiver operating curve; TBIL: Total bilirubin; INR: International normalized ratio; TBIL-r1: The ratio of total bilirubin on postoperative day (POD) X to the value on POD 1; INR-r1: The ratio of international normalized ratio on POD X to the value on POD 1.
The HDs was assigned values of 0, 1 and 2 when neither, either or both TBIL-r1 and INR-r1 exceeded the cut-off values of 1.68 and 1.02, respectively, on POD 5. Patients were categorized into groups A, B and C according to HDss of 0, 1 and 2, respectively. There were 381 patients (79.7%) in group A (HDs = 0) with a liver failure-related mortality rate of only 0.3% (n = 1) and a major complication morbidity rate of 22.8% (n = 87). Group B (HDs = 1) consisted of 86 patients (18.0%), of whom 7 (8.1%) suffered liver failure-related deaths; the major complication morbidity rate was 54.7% (n = 47), which was much higher than that in group A. There were only 11 cases (2.3%) in group C (HDs = 2), which had the highest liver failure-related mortality rate and the highest major complication morbidity rate, at 54.5% (n = 6) and 72.7% (n = 8), respectively. Any two groups exhibited a significant difference in liver failure-related mortality (Figure 2A). If the patients who died of any causes not related to liver failure was calculated as censored data, the cumulative three-month survival rates were 99.7%, 91.9% and 45.5% in groups A, B and C, respectively (Figure 2B).
Figure 2.

Liver failure-related mortality and major complication morbidity rates (A) and cumulative survival curves (B) of the three groups. A: Any two groups had significant differences in liver failure-related mortality and major complications morbidity (P < 0.001), except the major complication morbidity between groups B and C (P = 0.414); B: Patients who died of any causes not related to liver failure were calculated as censored data.
Univariate and multivariate analyses of the HDs
Since the HDs was strongly correlated with liver failure-related death, we believed that the HDs could be a reliable and standardized definition of PLF. Thus, we next investigated predictors of the HDs. Group C did not contain a sufficient number of cases; therefore, we combined groups B and C for comparison with group A. In univariate analyses, the following variables were significantly associated with the HDs: ICG-R15, CTP grade, intraoperative blood loss (mL), intraoperative blood transfusion volume (mL) and RSs (all with P < 0.05) (Table 4). These variables were included in a multivariate logistic regression model to identify independent predictors of the HDs. As shown in Table 5, ICG-R15 and RSs were independent predictors of the HDs. Comparing the ICG-R15 and RSs values of the patients with an HDs of 2 after surgery using regression analysis revealed a liner relationship (r2 = 0.613, F = 14.257, P = 0.004). The regression equation was as follows: RSs = - 0.168 × ICG-R15 + 5.625 (Figure 3).
Table 4.
Univariate analysis of factors associated with the hepatic damage score (n = 478)
| Variable | Group 1 (n = 380) | Group 2 (n = 98) | t | χ2 | P value |
| Age (yr) | 47.5 ± 12.4 | 46.2 ± 11.4 | 0.946 | 0.345 | |
| Gender | 0.639 | 0.424 | |||
| Male | 306 | 83 | |||
| Female | 72 | 15 | |||
| TBIL (µmol/L)1 | 17.5 ± 6.2 | 18.6 ± 5.7 | -1.516 | 0.130 | |
| ALB (g/L)1 | 40.0 ± 5.0 | 39.6 ± 5.0 | 0.737 | 0.462 | |
| ALT (IU/L)1 | 55 ± 47 | 54 ± 30 | 0.117 | 0.907 | |
| AST (IU/L)1 | 60 ± 54 | 64 ± 41 | -0.557 | 0.578 | |
| CREA (µmol/L)1 | 0.92 ± 0.28 | 0.94 ± 0.27 | -0.692 | 0.489 | |
| ICG R15 (%)1 | 7.2 ± 4.3 | 8.7 ± 5.5 | -2.598 | 0.010 | |
| INR1 | 1.09 ± 0.10 | 1.07 ± 0.11 | 1.305 | 0.192 | |
| HBsAg1 | 0.201 | 0.654 | |||
| Positive | 268 | 72 | |||
| Negative | 112 | 26 | |||
| CTP grade1 | 7.735 | 0.007 | |||
| A | 351 | 81 | |||
| B | 29 | 17 | |||
| MELD score1 | 5.8 ± 3.8 | 6.2 ± 3.4 | -0.981 | 0.327 | |
| IBL (mL) | 731 ± 790 | 972 ± 953 | -2.309 | 0.022 | |
| IBT (mL) | 427 ± 764 | 649 ± 943 | -2.157 | 0.033 | |
| RS | 2.4 ± 1.0 | 3.8 ± 1.4 | -9.852 | 2. 0e-17 |
All parameters were measured one day before liver resection. BIL: Total bilirubin; ALB: Albumin; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; CREA: Creatinine; ICG-R15: The indocyanine green retention rate at 15 min; INR: International normalized ratio; HBsAg: Hepatitis B surface antigen; CTP: Child-Turcotte-Pugh; MELD: Model for end-stage liver disease; IBL: Intraoperative blood loss; IBT: Intraoperative blood transfusion; RS: Resected segments.
Table 5.
Multivariate analysis of the hepatic damage score
| Variable | β | SE | Wald | P value | Exp (β) |
95%CI |
|
| Lower | Upper | ||||||
| ICG-R15 | 0.176 | 0.03 | 35.080 | 3.2e-9 | 1.192 | 1.125 | 1.263 |
| RS | 1.292 | 0.142 | 83.158 | 7.6e-20 | 3.639 | 2.757 | 4.803 |
ICG-R15: The indocyanine green retention rate at 15 min; RS: Resected segments.
Figure 3.
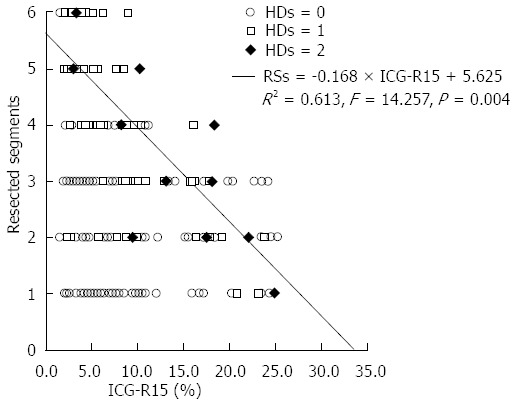
Relationship between the number of resected segments and pre-operative the indocyanine green retention rate at 15 min values for 478 patients. An equation was established based on eleven patients who had a hepatic damage score of 2. HDs: Hepatic damage score; RS: Resected segment;.ICG: Indocyanine green.
DISCUSSION
PLF is a serious complication of extended liver resection and often leads to patient deaths. Although conventional routine laboratory analyses, including TBIL, albumin, alanine aminotransferase, aspartate aminotransferase and INR, can be easily performed during the perioperative period, accurate determination of remnant liver function without delay remains difficult in hepatic resection patients because of the operative complexity. Different independent risk factors such as the model for end-stage liver disease score[21], fibrosis[22] and hepatic steatosis[23] have been reported in previous studies; however, because of the different definitions of PLF, it is difficult to compare the data across these studies and evaluate the factors contributing to PLF. Therefore, the establishment of a reliable and standardized definition of PLF with strong predictive power for the incidence and outcomes of patients who have undergone liver resection is of great significance.
Several investigators from major hepatobiliary centers around the world have proposed arbitrary criteria to define PLF[2,3,15,16,24,25]. The “50-50 criteria”, as proposed by Balzan et al[15], was an accurate predictor of PLF, and they reported a sensitivity of 69.6% and a specificity of 98.5%. However, in a large retrospective study, the “50-50 criteria” only showed a sensitivity of 50% for the prediction of PLF-related death in a cohort of patients without underlying liver disease who had undergone major hepatic resection[16]. A peak bilirubin (p-TBIL) of 7.0 mg/dL was identified by Mullen et al[16] as a high cut-off value for the prediction of liver failure-related death. However, clinically, the p-TBIL was reached in most of the patients over a long period of time or even on the day of death. For all these reasons, simply using the TBIL or INR level to determine remnant liver function is unsuitable.
TBIL-r1 and INR-r1 are ratios that can reflect dynamic changes in TBIL and INR and can precisely predict remnant liver function. Increases in both TBIL-r1 and INR-r1 reflect a decrease in the synthetic and excretory functions of the liver. In contrast, a decrease in these values indicates recovery of the synthetic and excretory functions of the liver. Thus, in the present study, we focused on the use of TBIL-r1 and INR-r1 to define PLF. The combination of TBIL-r1 and INR-r1 on POD 5 had a strong predictive power for liver failure-related death (sensitivity = 92.9%, specificity = 90.1%). This finding suggests to calculate the HDs of every patient through the cut-off value detected by the ROC analysis, with a higher HDs indicating a higher risk of liver failure-related death after liver resection. Furthermore, the HDs which reflects the degree of metabolic functional impairment after resection as mild (HDs = 0), reversible hepatic “dysfunction” (HDs = 1) to fatal hepatic failure (HDs = 2), was validated as a precise predictor of PLF by survival analysis.
The HDs can provide a precise prognosis that can be calculated after liver resection (on POD 5); therefore, we analyzed the preoperative and perioperative predictors of the HDs and identified ICG-R15 and RSs as independent predictors. ICG, which is eliminated by the liver without extrahepatic metabolism and excretion, is an ideal dye to reflect hepatic reserve[26]. Previous studies have suggested a relationship between the preoperative ICG clearance test and the postoperative outcome[12,27-29]. In addition, liver volume can also reflect hepatic reserve, which can be used to assess the operative risk prior to hepatectomy and guide the ideal treatment[12]. However, it is difficult to determine the amount of liver parenchyma that can be resected in an individual patient based solely on the ICG-R15 value. Therefore, we calculated the liner relationship between the ICG-R15 and RSs values of patients with an HDs of 2 which we regarded as fatal hepatic failure. The regression equation was RSs = -0.168 × ICG-R15 + 5.625 (r2 = 0.613, F = 14.257, P = 0.004). Using this equation, a safe resection can be predicted based on the individual’s ICG-R15 prior to hepatectomy. Hepatectomy will be safer if the number of resected segments is lower than the value calculated by the equation. Conversely, if the resection volume exceeds the calculated value, the risk associated with liver hepatectomy will increase. Furthermore, using this equation, when zero segments are resected, the ICG-R15 is equal to 33%. Therefore, if the ICG-R15 is greater than 33%, PLF is more likely to occur. However, if the ICG-R15 is 0%, the RS value will equal 5.625. This indicates that in patients with good liver function, a maximum of 5.625 liver segments can be safely resected. Nonetheless, successful outcomes for hepatectomy are dependent not only on the ICG-R15 and the number of resected segments but also on hemostasis, a meticulous surgical technique and skilled perioperative care.
In conclusion, the present study proposed and verified a criterion that can be used in the postoperative period to accurately predict the outcomes of patients who underwent liver resection. Although confirmation of the results of this study requires a prospective validation, we recommend that the degree of metabolic functional impairment after resection be defined as mild (HDs = 0), reversible hepatic “dysfunction” (HDs = 1) and fatal hepatic failure (HDs = 2). Furthermore, we detected a relationship between ICG-R15 and the number of resected segments in patients with an HDs of 2. On this basis, we identified preoperative criteria for selecting patients for hepatectomy to increase the safety of hepatic resection.
COMMENTS
Background
Postoperative liver failure (PLF) is a serious complication of extended liver resection and often leads to patient deaths. However, the definitions of PLF are not uniform, making it difficult to assess the efficacy of interventions designed to reduce the incidence of PLF and to compare outcome data across studies. Therefore, it is of great importance to establish a reliable and standardized definition of PLF that can precisely predict early liver failure-related mortality and morbidity after liver resection in a timely fashion.
Research frontiers
With advances in surgical techniques, the extended hepatectomy and complex liver resection have increased, which has been followed by an increase in the number of postoperative liver failure-related death. In the field of liver surgery, the current focus of research is the establishment of a standard definition of PLF which can precisely predict liver failure-related death in a timely fashion and can be used in studies analyzing the dangerous parameters to avoid postoperative liver failure-related death before hepatectomy.
Innovations and breakthroughs
Previous studies have proposed arbitrary criteria to define PLF by focusing on total bilirubin (TBIL) and the international normalized ratio (INR). For example, the “50-50 criteria” were proposed by Balzan et al and the “peak bilirubin (p-TBIL) of 7.0 mg/dL criteria” were proposed by Mullen et al. However, either the predictive power was not sufficiently strong or the untimely prediction hampered these criteria, which are widely used in the clinical diagnosis and prediction of liver failure-related death. To overcome these disadvantages, we used an arbitrarily defined score, termed the hepatic damage score (HDs), to establish a reliable and standardized definition of PLF. The HDs was derived from the postoperative changes in TBIL and INR as the TBIL and INR values on postoperative day (POD) X divided by the values on POD 1 (TBIL-r1 and INR-r1, respectively), which combined can precisely predict early liver failure-related mortality after liver resection in a timely fashion. Furthermore, the HDs reflects the degree of metabolic functional impairment after resection, classifying it as mild (HDs = 0), reversible hepatic “dysfunction” (HDs = 1) or fatal hepatic failure (HDs = 2), and was validated to be an ideal definition of PLF.
Applications
The findings of their study suggest a method for precisely predicting liver failure-related death in a timely fashion and defining the degree of metabolic functional impairment after resection as mild (HDs = 0), reversible hepatic “dysfunction” (HDs = 1) and fatal hepatic failure (HDs = 2). Furthermore, the relationship between ICG-R15 and the number of resected segments in patients with an HDs of 2 can help select patients for hepatectomy and increase the safety of hepatic resection.
Terminology
TBIL-r1 and INR-r1 are ratios reflecting the changes in TBIL and INR compared with POD 1. These ratios reflect the dynamic changes in TBIL and INR and can precisely predict remnant liver function. Increases in both TBIL-r1 and INR-r1 reflect decreases in the synthetic and excretory functions of the liver. In contrast, decreases in these values indicate the recovery of these functions.
Peer review
This is an interesting article on a large group of patients who underwent liver resection and is an attempt to predict mortality and morbidity due to liver failure post-resection. The authors use some novel measures to identify predictors.
Footnotes
P- Reviewer: Therapondos G S- Editor: Zhai HH L- Editor: Wang TQ E- Editor: Wang CH
References
- 1.Schwartz M, Roayaie S, Konstadoulakis M. Strategies for the management of hepatocellular carcinoma. Nat Clin Pract Oncol. 2007;4:424–432. doi: 10.1038/ncponc0844. [DOI] [PubMed] [Google Scholar]
- 2.Imamura H, Seyama Y, Kokudo N, Maema A, Sugawara Y, Sano K, Takayama T, Makuuchi M. One thousand fifty-six hepatectomies without mortality in 8 years. Arch Surg. 2003;138:1198–1206; discussion 1206. doi: 10.1001/archsurg.138.11.1198. [DOI] [PubMed] [Google Scholar]
- 3.Vauthey JN, Pawlik TM, Abdalla EK, Arens JF, Nemr RA, Wei SH, Kennamer DL, Ellis LM, Curley SA. Is extended hepatectomy for hepatobiliary malignancy justified? Ann Surg. 2004;239:722–730; discussion 730-732. doi: 10.1097/01.sla.0000124385.83887.d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wei AC, Tung-Ping Poon R, Fan ST, Wong J. Risk factors for perioperative morbidity and mortality after extended hepatectomy for hepatocellular carcinoma. Br J Surg. 2003;90:33–41. doi: 10.1002/bjs.4018. [DOI] [PubMed] [Google Scholar]
- 5.Choti MA, Sitzmann JV, Tiburi MF, Sumetchotimetha W, Rangsin R, Schulick RD, Lillemoe KD, Yeo CJ, Cameron JL. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg. 2002;235:759–766. doi: 10.1097/00000658-200206000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Charny CK, Jarnagin WR, Schwartz LH, Frommeyer HS, DeMatteo RP, Fong Y, Blumgart LH. Management of 155 patients with benign liver tumours. Br J Surg. 2001;88:808–813. doi: 10.1046/j.0007-1323.2001.01771.x. [DOI] [PubMed] [Google Scholar]
- 7.Foster JH, Berman MM. Solid liver tumors. Major Probl Clin Surg. 1977;22:1–342. [PubMed] [Google Scholar]
- 8.Belghiti J, Regimbeau JM, Durand F, Kianmanesh AR, Dondero F, Terris B, Sauvanet A, Farges O, Degos F. Resection of hepatocellular carcinoma: a European experience on 328 cases. Hepatogastroenterology. 2002;49:41–46. [PubMed] [Google Scholar]
- 9.The Liver Cancer Study Group of Japan. Predictive factors for long term prognosis after partial hepatectomy for patients with hepatocellular carcinoma in Japan. The Liver Cancer Study Group of Japan. Cancer. 1994;74:2772–2780. doi: 10.1002/1097-0142(19941115)74:10<2772::aid-cncr2820741006>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 10.Shimada M, Takenaka K, Fujiwara Y, Gion T, Shirabe K, Yanaga K, Sugimachi K. Risk factors linked to postoperative morbidity in patients with hepatocellular carcinoma. Br J Surg. 1998;85:195–198. doi: 10.1046/j.1365-2168.1998.00567.x. [DOI] [PubMed] [Google Scholar]
- 11.Belghiti J, Hiramatsu K, Benoist S, Massault P, Sauvanet A, Farges O. Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg. 2000;191:38–46. doi: 10.1016/s1072-7515(00)00261-1. [DOI] [PubMed] [Google Scholar]
- 12.Schindl MJ, Redhead DN, Fearon KC, Garden OJ, Wigmore SJ. The value of residual liver volume as a predictor of hepatic dysfunction and infection after major liver resection. Gut. 2005;54:289–296. doi: 10.1136/gut.2004.046524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jarnagin WR, Gonen M, Fong Y, DeMatteo RP, Ben-Porat L, Little S, Corvera C, Weber S, Blumgart LH. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg. 2002;236:397–406; discussion 406-407. doi: 10.1097/01.SLA.0000029003.66466.B3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schroeder RA, Marroquin CE, Bute BP, Khuri S, Henderson WG, Kuo PC. Predictive indices of morbidity and mortality after liver resection. Ann Surg. 2006;243:373–379. doi: 10.1097/01.sla.0000201483.95911.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balzan S, Belghiti J, Farges O, Ogata S, Sauvanet A, Delefosse D, Durand F. The “50-50 criteria” on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Ann Surg. 2005;242:824–828, discussion 828-829. doi: 10.1097/01.sla.0000189131.90876.9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mullen JT, Ribero D, Reddy SK, Donadon M, Zorzi D, Gautam S, Abdalla EK, Curley SA, Capussotti L, Clary BM, et al. Hepatic insufficiency and mortality in 1,059 noncirrhotic patients undergoing major hepatectomy. J Am Coll Surg. 2007;204:854–862; discussion 862-864. doi: 10.1016/j.jamcollsurg.2006.12.032. [DOI] [PubMed] [Google Scholar]
- 17.Jalan R, Sen S, Williams R. Prospects for extracorporeal liver support. Gut. 2004;53:890–898. doi: 10.1136/gut.2003.024919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Du ZG, Li B, Wei YG, Yin J, Feng X, Chen X. A new scoring system for assessment of liver function after successful hepatectomy in patients with hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2011;10:265–269. doi: 10.1016/s1499-3872(11)60044-1. [DOI] [PubMed] [Google Scholar]
- 19.Sakka SG, Meier-Hellmann A. Non-invasive liver function monitoring by indocyanine green plasma disappearance rate in critically ill patients. Int J Intensive Care. 2002;122:66–72. [Google Scholar]
- 20.Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, de Santibañes E, Pekolj J, Slankamenac K, Bassi C, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187–196. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- 21.Cucchetti A, Ercolani G, Vivarelli M, Cescon M, Ravaioli M, La Barba G, Zanello M, Grazi GL, Pinna AD. Impact of model for end-stage liver disease (MELD) score on prognosis after hepatectomy for hepatocellular carcinoma on cirrhosis. Liver Transpl. 2006;12:966–971. doi: 10.1002/lt.20761. [DOI] [PubMed] [Google Scholar]
- 22.Shirabe K, Shimada M, Kajiyama K, Gion T, Ikeda Y, Hasegawa H, Taguchi K, Takenaka K, Sugimachi K. Clinicopathologic features of patients with hepatocellular carcinoma surviving & gt; 10 years after hepatic resection. Cancer. 1998;83:2312–2316. [PubMed] [Google Scholar]
- 23.Kooby DA, Fong Y, Suriawinata A, Gonen M, Allen PJ, Klimstra DS, DeMatteo RP, D’Angelica M, Blumgart LH, Jarnagin WR. Impact of steatosis on perioperative outcome following hepatic resection. J Gastrointest Surg. 2003;7:1034–1044. doi: 10.1016/j.gassur.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 24.Farges O, Belghiti J, Kianmanesh R, Regimbeau JM, Santoro R, Vilgrain V, Denys A, Sauvanet A. Portal vein embolization before right hepatectomy: prospective clinical trial. Ann Surg. 2003;237:208–217. doi: 10.1097/01.SLA.0000048447.16651.7B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fujii Y, Shimada H, Endo I, Morioka D, Nagano Y, Miura Y, Tanaka K, Togo S. Risk factors of posthepatectomy liver failure after portal vein embolization. J Hepatobiliary Pancreat Surg. 2003;10:226–232. doi: 10.1007/s00534-002-0820-9. [DOI] [PubMed] [Google Scholar]
- 26.Faybik P, Hetz H. Plasma disappearance rate of indocyanine green in liver dysfunction. Transplant Proc. 2006;38:801–802. doi: 10.1016/j.transproceed.2006.01.049. [DOI] [PubMed] [Google Scholar]
- 27.Mullin EJ, Metcalfe MS, Maddern GJ. How much liver resection is too much? Am J Surg. 2005;190:87–97. doi: 10.1016/j.amjsurg.2005.01.043. [DOI] [PubMed] [Google Scholar]
- 28.Wakabayashi H, Ishimura K, Izuishi K, Karasawa Y, Maeta H. Evaluation of liver function for hepatic resection for hepatocellular carcinoma in the liver with damaged parenchyma. J Surg Res. 2004;116:248–252. doi: 10.1016/j.jss.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 29.Poon RT, Fan ST. Hepatectomy for hepatocellular carcinoma: patient selection and postoperative outcome. Liver Transpl. 2004;10:S39–S45. doi: 10.1002/lt.20040. [DOI] [PubMed] [Google Scholar]


