Abstract
AIM: To investigate the outcome of living donor liver transplantation (LDLT) recipients transplanted with small-for-size grafts (SFSGs).
METHODS: Between November 2001 and December 2010, 196 patients underwent LDLT with right lobe liver grafts at our center. Recipients were divided into 2 treatment groups: group A with an actuarial graft-to-recipient weight ratio (aGRWR) < 0.8% (n = 45) and group B with an aGRWR ≥ 0.8% (n = 151). We evaluated serum liver function markers within 4 wk after transplantation. We also retrospectively evaluated the outcomes of these patients for potential effects related to the recipients, the donors and the transplantation procedures based upon a review of their medical records.
RESULTS: Small-for-size syndrome (SFSS) developed in 7 of 45 patients (15.56%) in group A and 9 of 151 patients (5.96%) in group B (P = 0.080). The levels of alanine aminotransferase and aspartate aminotransferase in group A were higher than those in group B during early period after transplantation, albeit not significantly. The cumulative 1-, 3- and 5-year liver graft survival rates were 82.22%, 71.11% and 71.11% for group A and 81.46%, 76.82%, and 75.50% for group B patients, respectively (P = 0.623). However, univariate analysis of risk factors associated with graft survival in group A demonstrated that the occurrence of SFSS after LDLT was the only significant risk factor affecting graft survival (P < 0.001). Furthermore, multivariate analysis of our data did not identify any additional significant risk factors accounting for poor graft survival.
CONCLUSION: Our study suggests that LDLT recipients with an aGRWR < 0.8% may have liver graft outcomes comparable to those who received larger size grafts. Further studies are required to ascertain the safety of using SFSGs.
Keywords: Living donor liver transplantation, Right lobe, Actuarial graft-to-recipient weight ratio, Small-for-size graft, Small-for-size syndrome
Core tip: The relative size of a liver graft is a determinant factor for successful adult-to-adult living donor liver transplantation. However, the long-term outcome and the risk factors associated with poor graft survival in recipients undergoing right lobe living donor liver transplantation using small-for-size grafts are poorly understood. In the present study, we compared the short-term and long-term outcomes of living donor liver transplantation recipients with an actuarial graft-to-recipient weight ratio < 0.8% or ≥ 0.8% and analyzed potential risk factors associated with liver graft survival when small-for-size grafts were utilized.
INTRODUCTION
Living donor liver transplantation (LDLT) is considered a valuable approach to the problem of the shortage of cadaveric donor organs. Liver graft size is a determinant factor of successful adult-to-adult LDLT, in that graft size is known to impact patient post-transplant survival[1-4] and poor outcome has been associated with small-for-size grafts (SFSGs)[1,3,5,6] according to previous studies. SFSG has been tentatively described in retrospective studies as the liver graft weight relative to the recipient’s body weight or to the estimated whole liver weight and generally defined as a graft-to-recipient weight ratio (GRWR) < 0.8% or as a standard liver weight ratio < 40%. To meet the functional demands of the liver of the recipients and improve the recipient outcome in adult LDLT, a large graft size is recommended while maintaining the safety of the donor as the first priority. Based upon a worldwide systematic review, donor morbidity ranges from 0% to 100% with a median value of 16.1%[7]. To strike a balance between the prevention of SFSG sequelae and donor safety, many surgical strategies have been introduced. Initial efforts were aimed at directly increasing the liver graft volume by auxiliary transplantation[8], dual grafts[9-11], conversion from left liver grafts to right liver grafts[12] and recently increasing the donor’s body weight[13,14]. Subsequently, efforts attempted to maximize the graft blood outflow through the inclusion of the middle hepatic vein (MHV) or reconstruction of the MHV tributaries[15] combined with various refinements of the anastomotic technique. The third approach, which is now flourishing in some adult LDLT programs, involves modulation of the portal inflow to graft, including splenic artery ligation or embolization[16,17], splenectomy[18], and partial portosystemic shunting[19-23]. The incidence of small-for-size syndrome (SFSS) and early graft failure have been reported to be substantially reduced thanks to the techniques above.
In the present study, we compared the surgical outcomes of LDLT recipients with an actuarial graft-to-recipient weight ratio (aGRWR) < 0.8% or ≥ 0.8% and analyzed the potential risk factors affecting liver graft survival when SFSGs were utilized.
MATERIALS AND METHODS
Between January 2001 and December 2010, 196 patients underwent adult-to-adult living donor liver transplantation using right lobe liver grafts at West China Hospital, Sichuan University Medical School, Chengdu, China. Information concerning the transplant recipient [age, sex, Model for End-Stage Liver Disease (MELD) score, Child-Pugh score, hospitalization duration, aGRWR, ABO-compatibility, pre-transplant complications], the transplant donor [age, sex, body mass index (BMI)], the surgical procedure (ahepatic duration, MHV drainage, inflow modulation, type of biliary reconstruction), and the postoperative period (postoperative bleeding, acute rejection, vascular complications, biliary complications, SFSS) were gathered from the Chinese Liver Transplantation Registration for the analysis of potential risk factors associated with graft survival. Recipients were divided into two treatment groups: group A with an aGRWR < 0.8% (n = 45) and group B with an aGRWR ≥ 0.8% (n = 151). We also evaluated serum liver function markers such as aspartate aminotransferase (AST), alanine aminotransferase (ALT), international normalized ratio (INR) and total bilirubin (TB) within 4 wk after transplantation (Figure 1).
Figure 1.
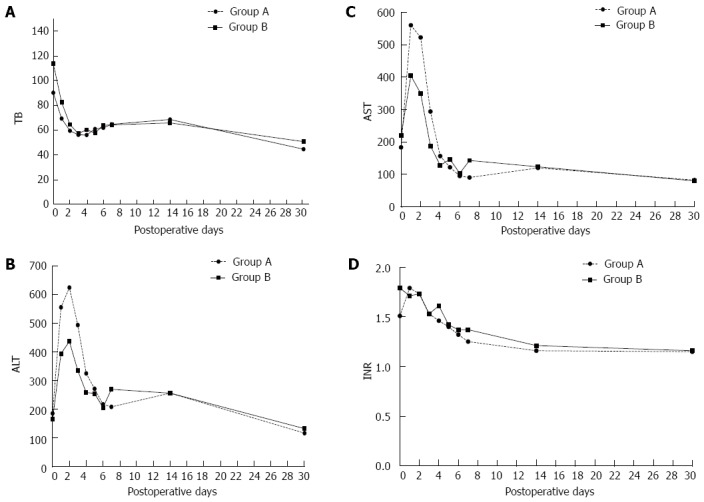
Serial changes of liver function markers (total bilirubin, alanine aminotransferase, aspartate aminotransferase, international normalized ratio) in all patients after living donor liver transplantation. A: TB; B: ALT; C: AST; D: INR. No intergroup difference regarding postoperative levels of TB, ALT, AST and INR was obtained (P = 0.739, P = 0.631, P = 0.853, P = 0.570, respectively). TB: Total bilirubin; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; INR: International normalized ratio.
Surgical procedure
Intraoperative ultrasonography was performed to confirm adequate hepatic venous anatomy of the donor and to verify the transection plane before donor hepatectomy. Intraoperative cholangiography was also performed before donor hepatectomy. Donor hepatectomy was carried out using a Cavitron ultrasonic surgical aspirator (CUSA System 200; Valleylab Inc., Boulder, CO) and bipolar electrocautery. After donor hepatectomy, grafts were flushed with 2 L of iced University of Wisconsin solution and actuarial graft weights were measured. MHV tributary reconstruction was performed with cryopreserved iliac vessels when the diameter of the MHV tributaries was > 5 mm or when dominant congestion of the right anterior segment was suggested by the clamping test. MHV tributary drainage was established in 124 recipients consisting of MHV tributary reconstruction (n = 110) and MHV trunk inclusion (n = 14). The surgical procedure and outflow reconstruction technique have been carefully described in our previous studies[24]. After removing the recipient liver, grafts were orthotopically transplanted using a piggyback technique. End-to-end right portal vein anastomosis was made using 5-0 prolene continuous sutures. An allowance of 1 cm for growth was planned at the time of the knotting of sutures. Thereafter, hepatic artery anastomosis was performed using a micro-vascular technique with 9-0 prolene interrupted sutures. Bile duct reconstruction was performed by either duct-to-duct anastomosis (n = 180) or Roux-en-Y hepaticojejunostomy (n = 16). Concurrent splenectomy was indicated in 11 graft recipients when severe hypersplenism was observed based upon preoperative white blood cell count < 2.00 × 109/L and platelet count < 30 × 109/L.
SFSS
SFSS is generally characterized by the appearance of cholestasis, prolonged coagulopathy, intractable ascites and, in the worse cases, gastrointestinal bleeding or renal failure at the end of the first week post-transplantation[2,3]. In clinical practice, SFSS has been defined by a total bilirubin value > 10 mg/dL with or without ascites formation of more than 1L/d on postoperative day 14[25]. Patients identified with any graft dysfunction, such as biliary or vascular complications or rejection, were excluded from the study and not labeled as presenting with SFSS regardless of the GRWR values. All organ donors voluntarily donated part of their liver. This study was approved by the Ethics Committee of our hospital.
Statistical analysis
The significance of the difference between the two treatment groups was assessed by Student’s t test, the Mann-Whitney U test, and the χ2 test. The Kaplan-Meier method and Cox’s regression test were used to calculate the probability of graft survival after LDLT. Intergroup differences in graft survival rates were compared using the log-rank test. Univariate analysis of potential risk factors for graft survival was performed using the log-rank test for categorical variables and Cox’s regression model for continuous variables. Multivariate analysis of potential risk factors for graft survival was performed using the Cox proportional hazards model. Statistical significance was accepted for a P value < 0.05. Statistical analysis was carried out using SPSS version 17.0.
RESULTS
Demographic characteristics of the recipient, donor and surgical procedure factors in LDLT patients
The demographic characteristics of the recipient and donor and the surgical procedure factors in LDLT patients are summarized in Table 1. With respect to recipient factors, no differences were observed between the treatment groups in terms of age, sex, Child-Pugh scores, pre-transplant complications, ABO-compatibility, ICU stay and hospitalization time after LDLT. The mean MELD scores and median follow-up time were significantly lower in group A (13.87 ± 9.09 vs 17.19 ± 9.97, P = 0.012 and 48.8 mo vs 57.8 mo, P = 0.033, respectively). The mean aGRWR values were also significantly lower in group A (0.71% ± 0.07% vs 0.99% ± 0.15%, P = 0.033). Five patients received liver grafts with an aGRWR value under 0.60%, with the lowest aGRWR value being 0.51%. Liver malignancy was the most common indication for transplantation in group A, while viral cirrhosis was the main etiology in group B. No differences were identified between the two treatment groups regarding the operative time, intraoperative blood loss, ahepatic duration, outflow and inflow modulation as well as biliary reconstruction techniques.
Table 1.
Clinical features of the recipients of liver grafts with an actuarial graft-to-recipient weight ratio < 0.8% and those with an actuarial graft-to-recipient weight ratio ≥ 0.8% n (%)
| Variable | Total (n = 196) | Group A (n = 45) | Group B (n = 151) | P value |
| Recipient factors | ||||
| Age (yr) | 42.60 ± 9.05 | 42.60 ± 8.90 | 42.60 ± 9.13 | 0.673 |
| Sex (male/female) | 171/25 | 40/5 | 131/20 | 0.706 |
| MELD score | 16.42 ± 9.86 | 13.87 ± 9.09 | 17.19 ± 9.97 | 0.012a |
| Child-Pugh score | 7.71 ± 2.12 | 7.27 ± 2.08 | 7.85 ± 2.20 | 0.118 |
| aGRWR (%) | 0.93 ± 0.18 | 0.71 ± 0.07 | 0.99 ± 0.15 | < 0.001a |
| ABO-compatibility (yes/no) | 161/35 | 36/9 | 125/26 | 0.669 |
| Median follow-up (mo) | 54.3 | 48.8 | 57.8 | 0.033a |
| 2001-2005/2006-2010 | 22/174 | 2/43 | 20/131 | 0.101 |
| ICU stay (h) | 307.32 ± 305.86 | 277.07 ± 270.91 | 316.34 ± 315.81 | 0.189 |
| Hospitalization time (d) | 39.45 ± 25.21 | 38.84 ± 22.49 | 39.64 ± 26.03 | 0.970 |
| Pre-transplant complications | ||||
| Encephalopathy | 21 (10.71) | 4 (8.89) | 17 (11.26) | 0.860 |
| GI bleeding | 12 (6.12) | 4 (8.89) | 8 (5.30) | 0.598 |
| Peritonitis | 8 (4.08) | 0 | 8 (5.30) | 0.251 |
| Uncontrolled ascites | 37 (18.88) | 7 (15.56) | 30 (19.87) | 0.516 |
| Renal insufficiency | 7 (3.57) | 0 | 7 (4.64) | 0.311 |
| Etiology | < 0.001a | |||
| Hepatic malignancy | 89 (45.41) | 28 (62.22) | 61 (40.40) | 0.010 |
| Viral cirrhosis | 66 (33.67) | 7 (15.56) | 59 (39.07) | 0.003 |
| Non-viral cirrhosis | 11 (5.61) | 4 (8.89) | 7 (4.64) | 0.472 |
| Cholestasis | 12 (6.12) | 1 (2.22) | 11 (7.28) | 0.374 |
| Acute liver failure | 11 (5.61) | 4 (8.89) | 8 (5.30) | 0.598 |
| Other | 6 (3.06) | 1 (2.22) | 5 (3.31) | 0.710 |
| Donor factors | ||||
| Age (yr) | 34.97 ± 10.20 | 34.29 ± 9.97 | 35.18 ± 10.30 | 0.727 |
| Sex (male/female) | 121/75 | 32/13 | 89/62 | 0.140 |
| Body mass index | 23.00 ± 2.55 | 22.96 ± 3.15 | 23.01 ± 2.36 | 0.654 |
| Relationship (blood-related/non-blood-related) | 20.41%/79.59% | 13.33%/86.67% | 22.52%/77.48% | 0.180 |
| 40/156 | 6/39 | 34/117 | ||
| Operative factors | ||||
| Operative time (min) | 659.02 ± 132.78 | 662.24 ± 113.65 | 658.05 ± 138.30 | 0.472 |
| Blood loss (mL) | 2183.93 ± 2022.02 | 2014.44 ± 1765.92 | 2234.44 ± 2095.04 | 0.444 |
| Ahepatic duration (min) | 93.36 ± 41.82 | 87.47 ± 30.94 | 95.11 ± 44.49 | 0.412 |
| MHV tributary drainage1 | 124 (63.27) | 31 (68.89) | 93 (61.69) | 0.373 |
| Concurrent splenectomy | 11 (5.61) | 3 (6.67) | 8 (5.30) | 0.726 |
| Types of biliary reconstruction2 | 180/16 | 44/1 | 136/15 | 0.178 |
| Complications after LDLT | ||||
| Postoperative bleeding | 8 (4.08) | 1 (2.22) | 7 (4.64) | 0.773 |
| Acute rejection | 9 (4.59) | 2 (4.44) | 7 (4.64) | 0.957 |
| Vascular complications | 7 (3.57) | 1 (2.22) | 6 (3.97) | 0.922 |
| Biliary complications | 21 (10.71) | 5 (11.11) | 16 (10.60) | 0.922 |
| SFSS | 16 (8.16) | 7 (15.56) | 9 (5.96) | 0.080 |
MHV tributary drainage includes MHV tributary reconstruction and MHV trunk inclusion;
Types of biliary reconstruction type includes duct-to-duct anastomosis and Roux-en-Y hepaticojejunostomy. GI: Gastrointestinal; MELD: Model for End-Stage Liver Disease; aGRWR: Actuarial graft-to-recipient weight ratio; MHV: Middle hepatic vein; LDLT: Living donor liver transplantation; SFSS: Small-for-size syndrome.
P < 0.05.
The mean age of the donors was 34.97 ± 10.20 years, and 121 (61.73%) of them were males. The 165 donations from blood relatives were from offspring-to-parent (n = 40), sibling-to-sibling (n = 61), parent-to-offspring (n = 13), and others (n = 42). Non-related donors were spouses (n = 35), in-laws (n = 2), and altruistic volunteers (n = 3). All organ donors were ABO compatible with their recipients. The mean donor BMI was 23.00 ± 2.55.
Postoperative complications and graft survival rates
Postoperative complications are listed in Table 1. No differences were identified with regard to postoperative bleeding, acute rejection, vascular complications, and biliary complications. Recipients with SFSGs appear to be more susceptible to SFSS after LDLT than those with larger grafts (15.56% vs 5.96%, P = 0.080), although not significantly. The postoperative serum TB, ALT, AST and INR in all patients gradually returned to the normal range during the hospital stay. The levels of ALT and AST in group A were higher than those in group B during early period after transplantation, albeit not significantly. The cumulative 1-, 3- and 5-year graft survival rates were 82.22%, 71.11%, and 71.11% in group A and 81.46%, 76.82%, and 75.50% in group B, respectively (P = 0.623, Figure 2).
Figure 2.
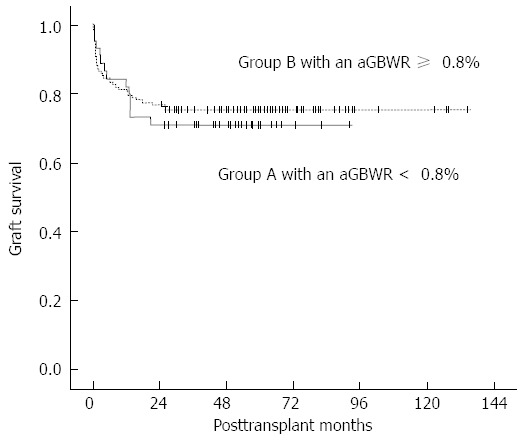
Cumulative 1-, 3- and 5-year graft survival rates. The cumulative graft survival rates were 82.22%, 71.11% and 71.11% in group A and 81.46%, 76.82% and 75.50% in group B, respectively (P = 0.623). aGRWR: Actuarial graft-to-recipient weight ratio
Risk factors for graft survival in recipients with SFSGs
Factors associated with liver graft survival in patients with SFSGs were also analyzed. The following deceased patients were excluded from further analysis due to causes unrelated to the graft function: one death by suicide, one death from an intestinal fistula, one from pneumonia, one from respiratory failure, one from intracranial bleeding and four deaths from recurrence of hepatocellular carcinoma. Graft failure occurred in four patients at 0-13 mo. Variables related to graft survival were evaluated by univariate analysis, and the results are summarized in Table 2. The occurrence of SFSS after LDLT was a significant risk factor for graft survival by univariate analysis in group A (P < 0.001) Patients with SFSS after LDLT have significantly lower graft survival rates than those without (33.3% vs 100%, P < 0.001). Recipients who had prolonged ICU stay after LDLT tended to have shorter graft survival than those did not (HR = 1.002, 95%CI: 1.000-1.003, P = 0.065). Patients with MHV drainage appeared to have better graft survival than those without (95.8% vs 75.0%, P = 0.064). These risk factors were further assessed by multivariate analysis, however, none of the differences were found to be significant.
Table 2.
Risk factors for graft survival in patients undergoing living donor liver transplantation with small-for-size graft, results of the univariate analysis
| Variable | P value |
| Recipient factors | |
| Age (yr) | 0.942 |
| Sex (male/female) | 0.410 |
| MELD score | 0.150 |
| Child-Pugh score | 0.754 |
| GRWR (%) | 0.545 |
| ABO-compatibility | 0.185 |
| 2001-2005/2006-2010 | 0.634 |
| ICU stay (h) | 0.065 |
| Hospitalization time (d) | 0.868 |
| Pre-transplant complications | |
| Encephalopathy | 0.688 |
| GI bleeding | 0.688 |
| Peritonitis | - |
| Uncontrolled ascites | 0.563 |
| Renal insufficiency | - |
| Etiology | |
| Hepatic malignancy | - |
| Viral cirrhosis | - |
| Non-viral cirrhosis | - |
| Cholestasis | - |
| Acute liver failure | - |
| Other | - |
| Donor factors | |
| Age (yr) | 0.094 |
| Sex (male/female) | 0.201 |
| Body mass index | 0.253 |
| Relationship | 0.537 |
| Operative factors | |
| Operative time (min) | 0.245 |
| Blood loss (mL) | 0.310 |
| Ahepatic duration (min) | 0.605 |
| MHV tributary drainage1 | 0.064 |
| Concurrent splenectomy | 0.358 |
| Types of biliary reconstruction2 | 0.730 |
| Complications after LDLT | |
| Postoperative bleeding | 0.730 |
| Acute rejection | 0.730 |
| Vascular complications | 0.730 |
| Biliary complications | 0.469 |
| SFSS | < 0.001a |
Middle hepatic vein (MHV) tributary drainage includes MHV tributary reconstruction and MHV trunk inclusion;
Types of biliary reconstruction type includes duct-to-duct anastomosis and Roux-en-Y hepaticojejunostomy. LDLT: Living donor liver transplantation; MELD: Model for end-stage liver disease; GRWR: Graft-to-recipient weight ratio; GI: Gastrointestinal; ICU: Intensive care unit.
P < 0.05.
DISCUSSION
Along with the increased utilization of right lobe LDLT, controversy regarding the pathogenesis, clinical manifestations and management of SFSS has been rising in recent years[2]. Early evidence indicated that liver graft size is an independent predictor of mortality[5,26,27]. While an increasing number of successful transplantation cases with SFSGs have been reported in recent years[2,28], their results demonstrate statistically significant differences, hence it is needed to reevaluate the incidence and clinical relevance of SFSGs-related issues.
In the present study, we compared the surgical outcomes of recipients with an aGRWR < 0.8% and those with an aGRWR ≥ 0.8%. The cumulative 1-, 3- and 5-year graft survival rates were 82.22%, 71.11% and 71.11% in group A and 81.46%, 76.82% and 75.50% in group B, respectively. Moreover, the incidence of postoperative complications was similar between the two groups. We found no evidence of inferior outcomes with smaller size grafts compared to larger size living donor grafts. Although some centers reported that the graft survival rates after LDLT with a right lobe graft range from 83% to 88%[29,30] of controls, evidence from Moon et al[28] and Selzner et al[31] has showed that the outcomes with smaller size grafts were not inferior to larger size living donor grafts or even full-size deceased donor grafts. Nevertheless, our findings with SFSGs with an aGRWR < 0.8% were found to be inferior to the above results. Potential explanations include the following: (1) the majority of our transplant recipients received LDLT for liver cancer (Table 1), which led to early cancer recurrence and patient death; (2) patients with liver cancer usually exhibit normal liver function based on serological testing before LDLT and hence are assigned a low MELD score; and (3) full-size deceased donor liver transplantation was considered first instead of LDLT for those in critical condition at our center.
Some studies have demonstrated that a high MELD score was not a predictor of graft survival in recipients with SFSGs[28,32], while others found that patients with a low MELD score[33] or Child class A patients[34] are associated with reduced mortality in patients who received either SFSGs or large grafts. However, in the present study, transplant recipients with smaller liver grafts were found to have low MELD scores but reduced survival rates. Thus, we were unable to draw the conclusion that a high MELD score is a potential risk factor for survival of those with SFSGs although no statistical significance was reached. We attribute this result to a high proportion of patients with liver cancer but normal organ function indicated for transplantation.
According to Kiuchi et al[2] and Moon et al[28], donor age > 50 years or ≥ 44 years was associated with poorer graft survival in patients with SFSGs. The advantages of selecting a young donor have been described in many studies, which include the non-impaired regenerative potential of both the donated liver graft and the donor liver remnant[35-38], which facilitates antiviral treatment[39] as well as the rapid convalescence of both the recipient and the donor. Therefore, the selection of younger donors is still favored in our practice although donor age was not found to be a risk factor in the present study.
Liver graft sinusoidal pressure is the major determinant of clinically evident SFSS, as reviewed by Ikegami et al[40]. SFSG would be jeopardized and clinically evident SFSS would ensue under such circumstances as graft congestion or absence of inflow modulation. SFSG is already at risk of developing elevated sinusoidal pressure due to over-regeneration of the graft and excessive portal flow/graft volume ratio[2]. As a result, either inflow or outflow modulation to relieve portal hyperperfusion and graft congestion is paramount in LDLT with SFSGs. MHV tributary reconstruction or inclusion of the MHV trunk[25,40-43] is also essential to improve venous drainage of the right anterior segment of the liver and hence relieve congestion and reduce graft loss. In the present study, greater than half of the transplant recipients with smaller size grafts received MHV tributary drainage. Their graft survival rates tended to be higher than those without in group A (P = 0.064), albeit not significantly. We hypothesize that other factors such as imbalance among portal vein inflow, hepatic vein outflow and functional liver mass, donor age, parenchymal factors and the indication for the LDLT procedure may influence graft survival of patients with SFSGs undergoing MHV tributary reconstruction.
Additionally, splenectomy, splenic arterial modulation and portocaval shunt have been shown to benefit LDLT patients with SFSGs in overcoming SFSS by decreasing portal vein pressure[17,18,21,22,25,40,44]. Only a small proportion of our recipients underwent concurrent splenectomy, based upon the severity of hypersplenism rather than the portal pressure as adopted by many other centers[17,18,45]. We did not evaluate the portal vein pressure or the portal vein flow rate in our study, and our study population was small. Therefore, we cannot draw any conclusion regarding the benefit of splenectomy in this study. Further study is warranted at our center to clarify the effect of concurrent splenectomy on the outcome of patients with SFSGs. More effort in inflow modulation research is also needed at our center.
Interestingly, 9 of 16 recipients who developed SFSS in our series had an aGRWR ≥ 0.8%. These data suggest that factors other than liver graft size contribute significantly to the development of SFSS and that an aGRWR ≥ 0.8% is not always a prerequisite to achieving excellent outcomes. However, it is worth noting that the incidence of SFSS (15.56%) in group A was much higher than that reported by other centers[28,31]. We believe that the explanation is most likely multifactorial and that aggressive reconstruction of the MHV tributaries including those < 5 mm in diameter, the use of extended right lobe liver, the selection of younger donors when possible, the development of an inflow modulation program and prompt effective treatment to SFSS would be beneficial. Attenuation of portal hyperperfusion is generally recommended for the treatment of SFSS because it can be achieved without performing another laparotomy. Delayed splenic artery occlusion or embolization[46-49] and intravenous somatostatin combined with oral propranolol[50] have also been reported to effectively treat SFSS after LDLT with SFSGs.
We conclude that LDLT recipients with smaller graft size may yield comparable outcomes to those with larger graft size. However, multivariate analysis did not identify any significant risk factors associated with graft survival in transplant recipients with SFSGs. Further studies are required to ascertain the safety of using small-for-size grafts.
ACKNOWLEDGMENTS
We thank American Journal Experts for language editing and the China Liver Transplant Registry System for providing all of the data.
COMMENTS
Background
Liver graft size is considered a determinant factor for successful adult-to-adult living donor liver transplantation (LDLT) in that inadequate graft size is thought to be associated with small-for-size syndrome (SFSS). Early evidence has also showed that graft size was a predictor of post-transplant mortality. Thus, a larger graft size is favored at the risk of compromising donor safety.
Research frontiers
The problem of liver graft size is one of the major factors limiting the expansion of the use of adult-to-adult LDLT. SFSS often develops when the graft-to-recipient weight ratio (GRWR) is < 0.8%. The research focus is to develop surgical strategies capable of achieving favorable outcomes in recipients with small-for-size grafts (SFSGs) while ensuring donor safety.
Innovations and breakthroughs
Problems such as SFSS often occur in LDLT recipients with a GRWR < 0.8% because the grafts cannot meet the functional demands of the recipients. An increasing number of successful transplantation cases with SFSGs have been reported in recent years as a result of technical innovations. However, the issue of sufficient graft volume remains contentious in LDLT; in particular, the factors that affect graft survival after LDLT with an SFSG with a GRWR < 0.8% are poorly understood. Therefore, authors compared the outcomes of adult-to-adult LDLT patients who were transplanted with right lobe grafts with an actuarial GRWR < 0.8% and those using right lobe grafts with an actuarial GRWR ≥ 0.8%. Authors also attempted to uncover the risk factors that may influence graft survival after adult-to-adult LDLT with SFSGs.
Applications
This study results suggest that an SFSG can be safely used in adult-to-adult right lobe LDLT under some circumstances.
Terminology
SFSS is generally characterized by the appearance of cholestasis, prolonged coagulopathy, and intractable ascites and, in the worse cases, gastrointestinal bleeding or renal failure at the end of the first week post-transplantation without any identified causes of graft dysfunction. An SFSG has been described tentatively in retrospective studies as the liver graft weight relative to the recipient body weight or to the estimated whole liver weight and is generally defined as a GRWR < 0.8% or as a standard liver volume < 40%.
Peer review
The outcome of patients undergoing right lobe living donor liver transplantation with small-for-size grafts is a good study.
Footnotes
Supported by National Science and Technology Major Project of China, No. 2008ZX10002-025 and No. 2008ZX10002-026
P- Reviewers: Asonuma K, Hatipoglu S S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Wu HL
References
- 1.Kiuchi T, Kasahara M, Uryuhara K, Inomata Y, Uemoto S, Asonuma K, Egawa H, Fujita S, Hayashi M, Tanaka K. Impact of graft size mismatching on graft prognosis in liver transplantation from living donors. Transplantation. 1999;67:321–327. doi: 10.1097/00007890-199901270-00024. [DOI] [PubMed] [Google Scholar]
- 2.Kiuchi T, Tanaka K, Ito T, Oike F, Ogura Y, Fujimoto Y, Ogawa K. Small-for-size graft in living donor liver transplantation: how far should we go? Liver Transpl. 2003;9:S29–S35. doi: 10.1053/jlts.2003.50198. [DOI] [PubMed] [Google Scholar]
- 3.Sugawara Y, Makuuchi M. Small-for-size graft problems in adult-to-adult living-donor liver transplantation. Transplantation. 2003;75:S20–S22. doi: 10.1097/01.TP.0000046616.76542.DF. [DOI] [PubMed] [Google Scholar]
- 4.Lo CM, Liu CL, Fan ST. Portal hyperperfusion injury as the cause of primary nonfunction in a small-for-size liver graft-successful treatment with splenic artery ligation. Liver Transpl. 2003;9:626–628. doi: 10.1053/jlts.2003.50081. [DOI] [PubMed] [Google Scholar]
- 5.Fan ST, Lo CM, Liu CL, Yong BH, Wong J. Determinants of hospital mortality of adult recipients of right lobe live donor liver transplantation. Ann Surg. 2003;238:864–869; discussion 869-870. doi: 10.1097/01.sla.0000098618.11382.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thuluvath PJ, Yoo HY. Graft and patient survival after adult live donor liver transplantation compared to a matched cohort who received a deceased donor transplantation. Liver Transpl. 2004;10:1263–1268. doi: 10.1002/lt.20254. [DOI] [PubMed] [Google Scholar]
- 7.Middleton PF, Duffield M, Lynch SV, Padbury RT, House T, Stanton P, Verran D, Maddern G. Living donor liver transplantation--adult donor outcomes: a systematic review. Liver Transpl. 2006;12:24–30. doi: 10.1002/lt.20663. [DOI] [PubMed] [Google Scholar]
- 8.Inomata Y, Kiuchi T, Kim I, Uemoto S, Egawa H, Asonuma K, Fujita S, Hayashi M, Tanaka K. Auxiliary partial orthotopic living donor liver transplantation as an aid for small-for-size grafts in larger recipients. Transplantation. 1999;67:1314–1319. doi: 10.1097/00007890-199905270-00004. [DOI] [PubMed] [Google Scholar]
- 9.Lee SG, Park KM, Hwang S, Lee YJ, Kim KH, Ahn CS, Choi DL, Joo SH, Jeon JY, Chu CW, et al. Adult-to-adult living donor liver transplantation at the Asan Medical Center, Korea. Asian J Surg. 2002;25:277–284. doi: 10.1016/S1015-9584(09)60192-5. [DOI] [PubMed] [Google Scholar]
- 10.Chen Z, Yan L, Li B, Zeng Y, Wen T, Zhao J, Wang W, Xu M, Yang J. Prevent small-for-size syndrome using dual grafts in living donor liver transplantation. J Surg Res. 2009;155:261–267. doi: 10.1016/j.jss.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 11.Soejima Y, Taketomi A, Ikegami T, Yoshizumi T, Uchiyama H, Yamashita Y, Meguro M, Harada N, Shimada M, Maehara Y. Living donor liver transplantation using dual grafts from two donors: a feasible option to overcome small-for-size graft problems? Am J Transplant. 2008;8:887–892. doi: 10.1111/j.1600-6143.2008.02153.x. [DOI] [PubMed] [Google Scholar]
- 12.Wachs ME, Bak TE, Karrer FM, Everson GT, Shrestha R, Trouillot TE, Mandell MS, Steinberg TG, Kam I. Adult living donor liver transplantation using a right hepatic lobe. Transplantation. 1998;66:1313–1316. doi: 10.1097/00007890-199811270-00008. [DOI] [PubMed] [Google Scholar]
- 13.Oniscu GC, Wigmore SJ. Increasing donor body weight to prevent small-for-size syndrome in living donor liver transplantation. World J Surg. 2010;34:2409–2410. doi: 10.1007/s00268-010-0671-5. [DOI] [PubMed] [Google Scholar]
- 14.Liu C, Lee RC, Loong CC, Hsia CY, Yeh YC, Chiou SY. Increasing donor body weight to prevent small-for-size syndrome in living donor liver transplantation. World J Surg. 2010;34:2401–2408. doi: 10.1007/s00268-010-0656-4. [DOI] [PubMed] [Google Scholar]
- 15.de Villa VH, Chen CL, Chen YS, Wang CC, Lin CC, Cheng YF, Huang TL, Jawan B, Eng HL. Right lobe living donor liver transplantation-addressing the middle hepatic vein controversy. Ann Surg. 2003;238:275–282. doi: 10.1097/01.SLA.0000081093.73347.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ito T, Kiuchi T, Yamamoto H, Oike F, Ogura Y, Fujimoto Y, Hirohashi K, Tanaka AK. Changes in portal venous pressure in the early phase after living donor liver transplantation: pathogenesis and clinical implications. Transplantation. 2003;75:1313–1317. doi: 10.1097/01.TP.0000063707.90525.10. [DOI] [PubMed] [Google Scholar]
- 17.Umeda Y, Yagi T, Sadamori H, Matsukawa H, Matsuda H, Shinoura S, Mizuno K, Yoshida R, Iwamoto T, Satoh D, et al. Effects of prophylactic splenic artery modulation on portal overperfusion and liver regeneration in small-for-size graft. Transplantation. 2008;86:673–680. doi: 10.1097/TP.0b013e318181e02d. [DOI] [PubMed] [Google Scholar]
- 18.Yoshizumi T, Taketomi A, Soejima Y, Ikegami T, Uchiyama H, Kayashima H, Harada N, Yamashita Y, Kawanaka H, Nishizak T, et al. The beneficial role of simultaneous splenectomy in living donor liver transplantation in patients with small-for-size graft. Transpl Int. 2008;21:833–842. doi: 10.1111/j.1432-2277.2008.00678.x. [DOI] [PubMed] [Google Scholar]
- 19.Huang JW, Yan LN, Chen ZY, Wu H, Lu Q, Xu YL, Prasoon P, Zeng Y. Hemi-portocaval shunt: a simple salvage maneuver for small-for-size graft during living donor liver transplantation: a case report. Chin Med J (Engl) 2011;124:2231–2233. [PubMed] [Google Scholar]
- 20.Yamada T, Tanaka K, Uryuhara K, Ito K, Takada Y, Uemoto S. Selective hemi-portocaval shunt based on portal vein pressure for small-for-size graft in adult living donor liver transplantation. Am J Transplant. 2008;8:847–853. doi: 10.1111/j.1600-6143.2007.02144.x. [DOI] [PubMed] [Google Scholar]
- 21.Oura T, Taniguchi M, Shimamura T, Suzuki T, Yamashita K, Uno M, Goto R, Watanabe M, Kamiyama T, Matsushita M, et al. Does the permanent portacaval shunt for a small-for-size graft in a living donor liver transplantation do more harm than good? Am J Transplant. 2008;8:250–252. doi: 10.1111/j.1600-6143.2007.02045.x. [DOI] [PubMed] [Google Scholar]
- 22.Ikegami T, Imura S, Arakawa Y, Shimada M. Transient portocaval shunt for a small-for-size graft in living donor liver transplantation. Liver Transpl. 2008;14:262; author reply 263. doi: 10.1002/lt.21307. [DOI] [PubMed] [Google Scholar]
- 23.Taniguchi M, Shimamura T, Suzuki T, Yamashita K, Oura T, Watanabe M, Kamiyama T, Matsushita M, Furukawa H, Todo S. Transient portacaval shunt for a small-for-size graft in living donor liver transplantation. Liver Transpl. 2007;13:932–934. doi: 10.1002/lt.21080. [DOI] [PubMed] [Google Scholar]
- 24.Liu B, Yan LN, Wang WT, Li B, Zeng Y, Wen TF, Xu MQ, Yang JY, Chen ZY, Zhao JC, et al. Clinical study on safety of adult-to-adult living donor liver transplantation in both donors and recipients. World J Gastroenterol. 2007;13:955–959. doi: 10.3748/wjg.v13.i6.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Imura S, Shimada M, Ikegami T, Morine Y, Kanemura H. Strategies for improving the outcomes of small-for-size grafts in adult-to-adult living-donor liver transplantation. J Hepatobiliary Pancreat Surg. 2008;15:102–110. doi: 10.1007/s00534-007-1297-3. [DOI] [PubMed] [Google Scholar]
- 26.Sugawara Y, Makuuchi M, Takayama T, Imamura H, Dowaki S, Mizuta K, Kawarasaki H, Hashizume K. Small-for-size grafts in living-related liver transplantation. J Am Coll Surg. 2001;192:510–513. doi: 10.1016/s1072-7515(01)00800-6. [DOI] [PubMed] [Google Scholar]
- 27.Chui AK, Rao AR, Island ER, Lau WY. Critical graft size and functional recovery in living donor liver transplantation. Transplant Proc. 2004;36:2277–2278. doi: 10.1016/j.transproceed.2004.08.036. [DOI] [PubMed] [Google Scholar]
- 28.Moon JI, Kwon CH, Joh JW, Jung GO, Choi GS, Park JB, Kim JM, Shin M, Kim SJ, Lee SK. Safety of small-for-size grafts in adult-to-adult living donor liver transplantation using the right lobe. Liver Transpl. 2010;16:864–869. doi: 10.1002/lt.22094. [DOI] [PubMed] [Google Scholar]
- 29.Marcos A. Right-lobe living donor liver transplantation. Liver Transpl. 2000;6:S59–S63. doi: 10.1053/jlts.2000.19011. [DOI] [PubMed] [Google Scholar]
- 30.Broelsch CE, Malagó M, Testa G, Valentin Gamazo C. Living donor liver transplantation in adults: outcome in Europe. Liver Transpl. 2000;6:S64–S65. doi: 10.1053/jlts.2000.19015. [DOI] [PubMed] [Google Scholar]
- 31.Selzner M, Kashfi A, Cattral MS, Selzner N, Greig PD, Lilly L, McGilvray ID, Therapondos G, Adcock LE, Ghanekar A, et al. A graft to body weight ratio less than 0.8 does not exclude adult-to-adult right-lobe living donor liver transplantation. Liver Transpl. 2009;15:1776–1782. doi: 10.1002/lt.21955. [DOI] [PubMed] [Google Scholar]
- 32.Yi NJ, Suh KS, Lee HW, Shin WY, Kim J, Kim W, Kim YJ, Yoon JH, Lee HS, Lee KU. Improved outcome of adult recipients with a high model for end-stage liver disease score and a small-for-size graft. Liver Transpl. 2009;15:496–503. doi: 10.1002/lt.21606. [DOI] [PubMed] [Google Scholar]
- 33.Li C, Wen TF, Yan LN, Li B, Yang JY, Xu MQ, Wang WT, Wei YG. Risk factors for in-hospital mortality of patients with high model for end-stage liver disease scores following living donor liver transplantation. Ann Hepatol. 2012;11:471–477. [PubMed] [Google Scholar]
- 34.Ben-Haim M, Emre S, Fishbein TM, Sheiner PA, Bodian CA, Kim-Schluger L, Schwartz ME, Miller CM. Critical graft size in adult-to-adult living donor liver transplantation: impact of the recipient’s disease. Liver Transpl. 2001;7:948–953. doi: 10.1053/jlts.2001.29033. [DOI] [PubMed] [Google Scholar]
- 35.Tanemura A, Mizuno S, Wada H, Yamada T, Nobori T, Isaji S. Donor age affects liver regeneration during early period in the graft liver and late period in the remnant liver after living donor liver transplantation. World J Surg. 2012;36:1102–1111. doi: 10.1007/s00268-012-1496-1. [DOI] [PubMed] [Google Scholar]
- 36.Ono Y, Kawachi S, Hayashida T, Wakui M, Tanabe M, Itano O, Obara H, Shinoda M, Hibi T, Oshima G, et al. The influence of donor age on liver regeneration and hepatic progenitor cell populations. Surgery. 2011;150:154–161. doi: 10.1016/j.surg.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 37.Katsuragawa H, Yamamoto M, Katagiri S, Yoshitoshi K, Ariizumi S, Kotera Y, Takahashi Y, Takasaki K. Graft size and donor age are independent factors for graft loss in adult-to-adult living-donor liver transplantation using the left liver. J Hepatobiliary Pancreat Surg. 2009;16:178–183. doi: 10.1007/s00534-008-0026-x. [DOI] [PubMed] [Google Scholar]
- 38.Akamatsu N, Sugawara Y, Tamura S, Kaneko J, Matsui Y, Togashi J, Makuuchi M. Impact of live donor age (& gt; or=50) on liver transplantation. Transplant Proc. 2007;39:3189–3193. doi: 10.1016/j.transproceed.2007.03.116. [DOI] [PubMed] [Google Scholar]
- 39.Selzner N, Girgrah N, Lilly L, Guindi M, Selzner M, Therapondos G, Adeyi O, McGilvray I, Cattral M, Greig PD, et al. The difference in the fibrosis progression of recurrent hepatitis C after live donor liver transplantation versus deceased donor liver transplantation is attributable to the difference in donor age. Liver Transpl. 2008;14:1778–1786. doi: 10.1002/lt.21598. [DOI] [PubMed] [Google Scholar]
- 40.Ikegami T, Shimada M, Imura S, Arakawa Y, Nii A, Morine Y, Kanemura H. Current concept of small-for-size grafts in living donor liver transplantation. Surg Today. 2008;38:971–982. doi: 10.1007/s00595-008-3771-1. [DOI] [PubMed] [Google Scholar]
- 41.Wu J, Wang W, Zhang M, Shen Y, Liang T, Yu P, Xu X, Yan S, Zheng S. Reconstruction of middle hepatic vein in living donor liver transplantation with modified right lobe graft: a single center experience. Transpl Int. 2008;21:843–849. doi: 10.1111/j.1432-2277.2008.00690.x. [DOI] [PubMed] [Google Scholar]
- 42.Wu H, Yan LN, Li B, Zeng Y, Wen TF, Zhao JC, Wang WT, Yang JY, Xu MQ, Chen ZY, et al. Hepatic venous outflow reconstruction in right lobe graft without middle hepatic vein. Hepatol Res. 2007;37:1044–1051. doi: 10.1111/j.1872-034X.2007.00121.x. [DOI] [PubMed] [Google Scholar]
- 43.Chan SC, Lo CM, Ng KK, Fan ST. Alleviating the burden of small-for-size graft in right liver living donor liver transplantation through accumulation of experience. Am J Transplant. 2010;10:859–867. doi: 10.1111/j.1600-6143.2010.03017.x. [DOI] [PubMed] [Google Scholar]
- 44.Takada Y, Ueda M, Ishikawa Y, Fujimoto Y, Miyauchi H, Ogura Y, Ochiai T, Tanaka K. End-to-side portocaval shunting for a small-for-size graft in living donor liver transplantation. Liver Transpl. 2004;10:807–810. doi: 10.1002/lt.20164. [DOI] [PubMed] [Google Scholar]
- 45.Kokai H, Sato Y, Yamamoto S, Oya H, Nakatsuka H, Watanabe T, Takizawa K, Hatakeyama K. Successful super-small-for-size graft liver transplantation by decompression of portal hypertension via splenectomy and construction of a mesocaval shunt: a case report. Transplant Proc. 2008;40:2825–2827. doi: 10.1016/j.transproceed.2008.08.080. [DOI] [PubMed] [Google Scholar]
- 46.Humar A, Beissel J, Crotteau S, Cohen M, Lake J, Payne WD. Delayed splenic artery occlusion for treatment of established small-for-size syndrome after partial liver transplantation. Liver Transpl. 2009;15:163–168. doi: 10.1002/lt.21636. [DOI] [PubMed] [Google Scholar]
- 47.Singhal A, Goyal N, Gupta VV. Delayed splenic artery occlusion for treatment of established small-for-size syndrome after partial liver transplantation. Liver Transpl. 2009;15:1381–1382. doi: 10.1002/lt.21844. [DOI] [PubMed] [Google Scholar]
- 48.Yan L, Wang W, Chen Z, Lu W, Lu Q, Cheng W, Wen T, Xu M, Yang J. Small-for-size syndrome secondary to outflow block of the segments V and VIII anastomoses--successful treatment with trans-splenic artery embolization: a case report. Transplant Proc. 2007;39:1699–1703. doi: 10.1016/j.transproceed.2007.03.071. [DOI] [PubMed] [Google Scholar]
- 49.Gruttadauria S, Mandala’ L, Miraglia R, Caruso S, Minervini MI, Biondo D, Volpes R, Vizzini G, Marsh JW, Luca A, et al. Successful treatment of small-for-size syndrome in adult-to-adult living-related liver transplantation: single center series. Clin Transplant. 2007;21:761–766. doi: 10.1111/j.1399-0012.2007.00735.x. [DOI] [PubMed] [Google Scholar]
- 50.Ozden I, Imura S. Somatostatin and propranolol for the treatment of small-for-size syndrome after liver transplantation. J Hepatobiliary Pancreat Surg. 2008;15:560–561. doi: 10.1007/s00534-008-1375-1. [DOI] [PubMed] [Google Scholar]


