Abstract
Objective
To estimate incidence and lifetime risk of diagnosed symptomatic knee OA and age of diagnosis of knee OA based on self-reports in the US population.
Methods
We estimated incidence of diagnosed symptomatic knee OA in the US by combining data on age-, sex-, and obesity-specific prevalence from the 2007–2008 National Health Interview Survey (NHIS) with disease duration estimates derived from the Osteoarthritis Policy (OAPol) Model, a validated computer simulation model of knee OA. We used the OAPol Model to estimate the mean and median ages of diagnosis and lifetime risk.
Results
The estimated incidence of diagnosed symptomatic knee OA was highest among adults aged 55 to 64, ranging from 0.37% per year for non-obese males to 1.02% per year for obese females. The estimated median age of knee OA diagnosis was 55 years. The estimated lifetime risk was 13.83%, ranging from 9.60% for non-obese males to 23.87% in obese females. About 9.29% of the US population is diagnosed with symptomatic knee OA by age 60.
Conclusion
The diagnosis of symptomatic knee OA occurs relatively early in life suggesting that prevention programs should be offered relatively early in the life course. Further research is needed to understand the future burden of healthcare utilization resulting from earlier diagnosis of knee OA.
Keywords: knee osteoarthritis, incidence, prevalence, age of diagnosis, lifetime risk
INTRODUCTION
Knee osteoarthritis (OA) is a painful, disabling condition that affects an estimated 9.3 million US adults.1 Because no disease-modifying treatments are available, treatments for symptomatic knee OA focus on symptom relief and functional restoration, including physical therapy, medications, joint injections, and total knee replacement.2–5
For decades, knee OA had been viewed as a disease mostly affecting older persons. However, recent evidence documents increased incidence of two key risk factors for knee OA – traumatic knee injury6 and obesity,7,8 particularly in younger persons.9,10 Evolving data point to a high prevalence of posttraumatic knee OA in younger persons.11–13 This trend and the increasing prevalence of obesity among children are likely to lead to increased rates of osteoarthritis in young adults.14
Most population-based data on knee OA prevalence and incidence refer to studies conducted in the mid-1990s.15–17 In fact, the two most recent studies reporting incidence of symptomatic knee OA in the US were published 17 years ago in 1995.15,16 Furthermore, although obesity is a known risk factor, no studies have reported age- and sex-specific knee OA incidence separately for obese and non-obese persons. Determining the age of diagnosis of symptomatic knee OA is critical to understanding the trajectory of decrements in quality of life and utilization of health services.
We sought to use national self-reported data to derive current age-, sex-, and obesity-stratified estimates of the incidence of diagnosed symptomatic knee OA, to estimate the mean and median ages of diagnosis, and to estimate the lifetime risk of diagnosis among 25 year olds representative of the US population.
MATERIALS AND METHODS
Analytic overview
The incidence of diagnosed symptomatic knee OA was derived as a ratio of disease prevalence odds to disease duration (see Technical Appendix, Section 2 for a more detailed explanation of incidence calculations).18 We used self-reported data from the 2007–2008 NHIS to estimate the prevalence of diagnosed symptomatic knee OA.19 Disease duration was derived using the Osteoarthritis Policy (OAPol) Model, a published and validated computer simulation model of knee OA natural history and management.20,21 Incidence was estimated within 10-year age groups and was further stratified by sex and obesity status. We then used the OAPol Model and our newly derived incidence estimates to determine mean and median ages of diagnosis of symptomatic knee OA in the US. To model disease duration, cohorts were initialized with knee OA, and the output of interest was life expectancy. To model age of diagnosis, cohorts were initialized without knee OA, and the output of interest was the development of OA, or cumulative incidence of OA.
OAPol Model
The OAPol Model is a validated, state transition Monte Carlo computer simulation model of the natural history of knee OA.20,21 ‘State transition’ implies that the natural history and clinical management of knee OA are characterized as a series of annual transitions between health states. The health states are characterized by age, sex, obesity, comorbidities, and knee OA disease severity among those who develop the condition. The model uses a set of transition probabilities to determine each individual’s sequence of annual transitions among different health states, which may include diagnosis of symptomatic knee OA. Every subject without knee OA is considered to be at risk for knee OA. The risk is governed by age, sex, and obesity (defined by BMI ≥ 30 kg/m2).
In addition to capturing the risk of knee OA, the model tracks chronic comorbidities that increase mortality either directly (coronary heart disease, cancers, and obesity), and/or by increasing the risk of other chronic diseases (diabetes mellitus and obesity). Each simulated person is followed by the model until death. Death can occur from any health state. All-cause mortality rates were obtained from CDC life tables.19,22–24 Rates specifically attributable to chronic comorbidities (coronary heart disease, cancer, and obesity) were then removed from the mortality from the life tables to estimate the mortality rates for healthy individuals, for all age and sex strata. The mortality rates attributable to specific comorbidities were then separately applied to persons with the corresponding comorbidities.
Further details of the OAPol Model structure have been published elsewhere,20,21 and can also be found in Technical Appendix, Section 1.
Estimating current prevalence of diagnosed symptomatic knee OA
We used self-reported data from the 2007–2008 NHIS to estimate the prevalence of diagnosed symptomatic knee OA.19 The NHIS is a cross-sectional survey that is representative of the civilian non-institutionalized population residing in the United States. The adult sample, which was the basis of our analysis, was comprised of persons 18 years of age or older. For the purposes of our analysis, we included persons that were 25 years of age or older.
Survey participants were considered to have prevalent knee OA if they: 1) answered “Yes” to the questions “Have you ever been told by a doctor or other health professional that you have some form of arthritis, rheumatoid arthritis, gout, lupus, or fibromyalgia?” and “During the past 30 days, have you had any symptoms of pain, aching, or stiffness in or around a joint?”; 2) specified the knee as an affected joint; and 3) did not report having rheumatoid arthritis, lupus, fibromyalgia, or gout. We then used a logistic regression model that accounted for the complex survey design to derive age-, sex-, and obesity-stratified population-based prevalence estimates of the prevalence of self-reported diagnosed symptomatic knee OA. The regression model, built using SAS 9.2 (SAS Institute, Cary, NC), included age, age2, age3 and age4 and the interaction between obesity and sex.
Similar algorithms for classifying survey respondents as having symptomatic knee OA have shown specificity greater than 90%,25,26 which indicates that these algorithms perform well in terms of classifying patients without symptomatic knee OA. Since self-reported data may likely overstate the true prevalence of diagnosed symptomatic knee OA, we used published data on the positive predictive value (PPV) of self-reported diagnosed symptomatic knee OA to reduce the prevalence estimates and the corresponding 95% CIs. Specifically, we assumed that the PPV was lower for those younger than age 60 (PPV=66.0%)27 compared with those ages 60 and older (PPV=80.7%).26 For instance, for a non-obese male aged 44 years with self-reported prevalence of symptomatic knee OA at 0.0374, the PPV-adjusted prevalence was estimated at 0.0247, or 66.0% of the self-reported prevalence. Similarly, for an obese female aged 64 years, the predicted prevalence would be 80.7% of the self-reported prevalence of 0.2864, or 0.2311. These reduced prevalence data and 95% CIs were used as benchmarks for the calibration of OA incidence.
Estimating incidence of diagnosed symptomatic knee OA
We estimated the incidence rates of diagnosed symptomatic knee OA as a ratio of the NHIS 2007–2008, PPV-adjusted prevalence estimates to disease duration.18 Because there is no permanent cure for knee OA, to derive disease duration we used the OAPol Model to simulate life expectancy within each decade of age, further stratified by sex and obesity (for details see Technical Appendix, Section 3). Incidence rates within 10-year age groups between 25 and 85 years were then estimated by dividing PPV-adjusted prevalence rates by model-derived disease durations.
Disease duration was calculated as an average 10-year survival of persons affected by OA, diagnosed within each year of the decade, assuming constant rates of diagnosis in every year of a given decade. The analysis was conducted for the cohort with race/sex and obesity distributions of persons affected by knee OA in the US as well as separately for each age/sex/race and obesity cohort with knee OA.
The calculated incidence estimates were calibrated to generate model-based prevalences that fell within the 95% CIs of the prevalence estimates derived from NHIS 2007–2008 (for details see Technical Appendix, Section 4). 95% CIs for incidence estimates were calculated empirically based on model results. Using a normal approximation of the Poisson distribution, empirical incidence rates were calculated as the number of new cases of diagnosed knee OA from model output divided by the time ‘at risk’. Time ‘at risk’ was defined as the sum of years alive without knee OA within each decade.
Estimating lifetime risk of diagnosed symptomatic knee OA and average age at diagnosis
Using the OAPol Model and our calculated incidence rates, we estimated the lifetime risk of diagnosed symptomatic knee OA from age 25, defined as the cumulative probability of being diagnosed with symptomatic knee OA over the lifetime for US adults in each of the five cohorts described below. The lifetime risk was calculated by dividing the cumulative number of incident cases of diagnosed symptomatic knee OA within each cohort predicted by the OAPol Model by the size of the initial population at risk for the disease reported by US Census data. We used the OAPol Model to estimate the mean and median ages of symptomatic knee OA diagnosis using the derived incidence rates. Mean age was obtained by constructing a distribution by age of all incident cases of diagnosed symptomatic knee OA based on the OAPol Model and calculating a mean of this distribution (for details see Technical Appendix, Section 5). Median age represented the age at which 50% of those ultimately diagnosed with symptomatic knee OA had been diagnosed (i.e. the 50% mark of the cumulative distribution function of incident cases). Mean age is reported to one tenth of a year; median age is reported as an integer.
Populations under consideration
Using the OAPol Model, we simulated five cohorts that were followed from age 25 until death: 1) non-obese females; 2) obese females; 3) non-obese males; 4) obese males; and 5) the general US population. For non-obese cohorts, mean BMI at age 25 was 25.0 kg/m2 with a standard deviation (SD) of 0.5 kg/m2. For obese cohorts, mean BMI was 34.5 kg/m2 with a SD of 1.5 kg/m2. For the general US population, BMI distributions, stratified by sex and race/ethnicity, were derived from the 2005–2008 National Health and Nutrition Examination Survey (NHANES) and ranged from a mean BMI of 26.7 kg/m2 with a SD of 6.1 kg/m2 for white, non-hispanic males aged 25 to a mean BMI of 30.2 kg/m2 with a SD of 8.8 kg/m2 for black, non-hispanic females at age 25 (Table 1).28,29 The distributions of sex and race/ethnicity within each cohort were derived from 2009 US Census data.30 Prevalence rates of comorbid conditions, including cancer, coronary heart disease, and diabetes mellitus, were stratified by age, sex, and race/ethnicity and were derived from the 2005–2008 NHANES (Table 1).28,29 Incidence rates for comorbidities were calculated as the ratio of prevalence odds to disease duration. We assumed that chronic comorbid conditions were not cured in the model, so life expectancies of persons with these comorbidities were used as a measure of disease duration. Dividing prevalence odds derived from NHANES 2005–2008 data by estimated disease duration led to estimates of the comorbid incidence rates used in the model, stratified by age, sex, and race/ethnicity (Table 1).
Table 1.
Baseline Demographic and Clinical Characteristics of US Adults Aged 25 Years
| Initial age | 25.0 | Source | ||
| Race/ethnicity (all cohorts) | Male | Female | 2009 US Census Population Estimates30 | |
| White, non-Hispanic (%) | 63.7 | 65.0 | ||
| Hispanic (%) | 20.9 | 18.8 | ||
| Black, non-Hispanic (%) | 15.4 | 16.1 | ||
| Mean BMI (standard deviation) (kg/m2) | ||||
| Cohort | Male | Female | ||
| Non-obese | 25.0 (0.5) | 25.0 (0.5) | Maximum: 29.9̄ | |
| Obese | 34.5 (1.5) | 34.5 (1.5) | Minimum: 30.0 | |
| Overall US Population | ||||
| White, non-Hispanic | 26.7 (6.1) | 26.8 (7.7) | NHANES 2005–200828,29 | |
| Hispanic | 27.6 (6.0) | 28.0 (6.7) | ||
| Black, non-Hispanic | 27.7 (6.2) | 30.2 (8.8) | ||
| Comorbid condition | Prevalencea | Incidencea | Relative risk of mortalitya,b,c | NHANES 2005–200828,29 |
| Cancer | 0.0% – 5.7% | 0.0% – 6.4% | 1.0 – 30.0 | |
| Coronary heart disease | 0.0% – 0.3% | 0.0% – 4.4% | 1.0 – 36.6 | |
| Diabetes mellitus | 1.5% – 7.0% | 0.0% – 5.1% | 1.0d | |
Prevalence of comorbid conditions is stratified by sex, race/ethnicity, and obesity status. Incidence and relative risks of mortality for comorbid conditions are stratified by age, sex, race/ethnicity, and obesity status. The ranges of values across these stratifications are presented in this table.
For individuals with more than one of the comorbid conditions listed here, the model assigns only the maximum of the relative risks of mortality associated with those separate conditions (i.e. relative risks are not cumulative; an individual with a 1.5 relative risk of mortality due to coronary heart disease and a 3.5 relative risk of mortality due to cancer will experience a relative risk of mortality of 3.5).
The relative risk of mortality due to obesity ranged from 1.0 to 2.5.
While diabetes mellitus does not directly increase the risk of mortality in the model, individuals with diabetes mellitus are at increased risk for developing coronary heart disease, which can increase the risk of mortality.
RESULTS
Estimated prevalence of diagnosed symptomatic knee OA
Age-, sex-, and obesity-stratified estimates of the prevalence of diagnosed symptomatic knee OA are presented in Table 2. For non-obese males, the estimated prevalence ranged from 0.74% among those aged 25 to 34 to 12.94% in those older than 85 years. For non-obese females, estimated prevalence ranged from 0.88% for the youngest age group (25 to 34 years) to 14.97% among those older than 85 years of age. For obese males, the estimated prevalence ranged from 1.54% in the youngest group to 23.54% in the oldest group. Obese females had the highest estimated prevalence ranging from 2.41% for the youngest group to 32.45% in the oldest age group.
Table 2.
Estimated Prevalence and Incidence of Diagnosed Symptomatic Knee OA by Age, Sex, and Obesity Status
| Obesity status | Sex | Age | Estimated prevalence (%)(using NHIS 2007–08 data) (95% CI) | Estimated incidence (annual, %) (95% CI) |
|---|---|---|---|---|
| Non-obese | Male | 25–34 | 0.74 (0.61, 0.89) | 0.12 (0.12, 0.12) |
| 35–44 | 1.74 (1.51, 2.00) | 0.13 (0.12, 0.13) | ||
| 45–54 | 3.61 (3.21, 4.06) | 0.22 (0.22, 0.22) | ||
| 55–64 | 6.70 (6.09, 7.37) | 0.37 (0.37, 0.38) | ||
| 65–74 | 9.83 (9.01, 10.71) | 0.20 (0.19, 0.20) | ||
| 75–84 | 11.64 (10.51, 12.88) | 0.13 (0.12, 0.13) | ||
| 85+ | 12.94 (10.86, 15.34) | 0.04 (0.04, 0.04) | ||
| Female | 25–34 | 0.88 (0.73, 1.05) | 0.14 (0.14, 0.14) | |
| 35–44 | 2.06 (1.83, 2.31) | 0.15 (0.14, 0.15) | ||
| 45–54 | 4.26 (3.90, 4.64) | 0.27 (0.27, 0.27) | ||
| 55–64 | 7.85 (7.22, 8.52) | 0.43 (0.43, 0.43) | ||
| 65–74 | 11.44 (10.48, 12.47) | 0.27 (0.27, 0.27) | ||
| 75–84 | 13.50 (12.44, 14.65) | 0.16 (0.16, 0.16) | ||
| 85+ | 14.97 (13.04, 17.12) | 0.06 (0.06, 0.06) | ||
| Obese | Male | 25–34 | 1.54 (1.26, 1.87) | 0.25 (0.24, 0.25) |
| 35–44 | 3.58 (3.12, 4.11) | 0.24 (0.24, 0.24) | ||
| 45–54 | 7.25 (6.49, 8.09) | 0.44 (0.43, 0.44) | ||
| 55–64 | 13.00 (11.76, 14.33) | 0.64 (0.64, 0.65) | ||
| 65–74 | 18.48 (16.71, 20.39) | 0.32 (0.32, 0.33) | ||
| 75–84 | 21.48 (19.33, 23.78) | 0.17 (0.17, 0.18) | ||
| 85+ | 23.54 (20.24, 27.19) | 0.05 (0.05, 0.05) | ||
| Female | 25–34 | 2.41 (2.02, 2.88) | 0.37 (0.37, 0.38) | |
| 35–44 | 5.53 (4.93, 6.21) | 0.40 (0.39, 0.40) | ||
| 45–54 | 10.93 (10.01, 11.92) | 0.57 (0.57, 0.58) | ||
| 55–64 | 18.94 (17.52, 20.44) | 1.02 (1.01, 1.02) | ||
| 65–74 | 26.20 (24.25, 28.23) | 0.41 (0.40, 0.41) | ||
| 75–84 | 29.94 (27.73, 32.23) | 0.28 (0.27, 0.28) | ||
| 85+ | 32.45 (28.79, 36.31) | 0.10 (0.10, 0.10) |
Estimated incidence of diagnosed symptomatic knee OA
For non-obese males, estimated disease duration within each decade ranged from 5.48 years for the youngest age group to 3.39 years for the 85+ age group. For obese males, estimated disease duration ranged from 5.47 years to 3.39 years for the 25–34 year olds and 85+ year olds, respectively. For non-obese and obese females, estimated disease duration ranged from 5.49 years among those aged 25–34 to 3.89 years among those over 85 years old.
Estimated annual incidences of diagnosed symptomatic knee OA, stratified by age, sex, and obesity, are presented in Table 2. Estimated incidence ranged from 0.04% per year in non-obese males over 85 years old to 1.02% per year in obese females aged 55 to 64 years. For all sex and obesity status combinations, incidence peaked at ages 55 to 64 and was lowest among those older than age 85. For non-obese males, incidence ranged from 0.04% to 0.37% per year. For non-obese females, incidence ranged from 0.06% to 0.43% per year. Among obese males, incidence ranged from 0.05% per year to 0.64% per year. For obese females, incidence ranged from 0.10% to 1.02% per year.
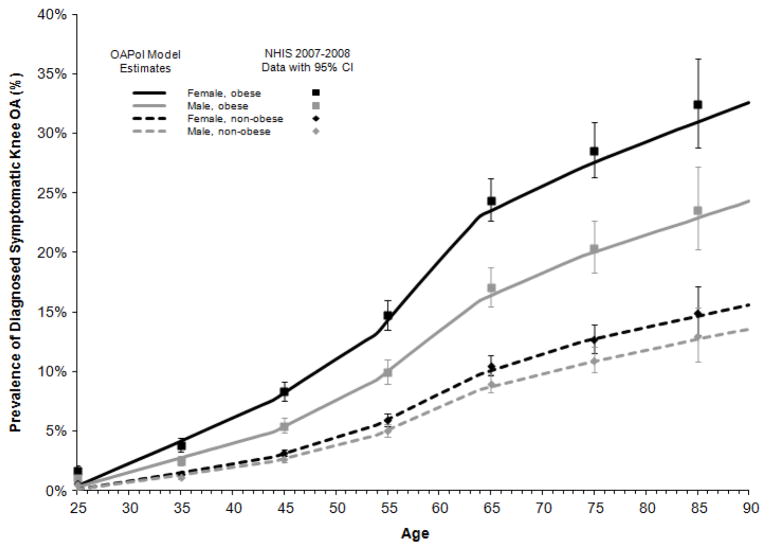
Results of the internal validation analysis using our current incidence estimates are presented in Figure 1. Within each 10-year age group, the model-based prevalence estimates fell within the 95% CI of the NHIS data for each cohort, defined by sex and obesity status.
Figure 1. Estimated Prevalence of Diagnosed Symptomatic Knee OA by Age in the US (Internal Validation of OAPol Model Estimates Using NHIS 2007–2008 Data).
Dashed curves represent prevalence among non-obese persons and solid curves represent prevalence among obese persons. Female prevalence is in black; male prevalence is in gray. Prevalence from NHIS is depicted by squares for obese and diamonds for non-obese persons and accompanied by 95% confidence intervals.
Estimated age of diagnosis of symptomatic knee OA
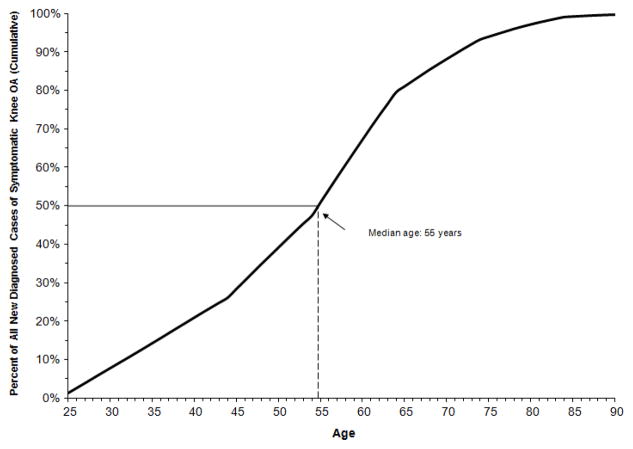
Using current demographic, obesity, and comorbidity profiles representative of the general population in the US, the estimated mean age of symptomatic knee OA diagnosis was 53.5 (SD = 14.4). The estimated median age of diagnosis of symptomatic knee OA was 55 years (Figure 2).
Figure 2. Estimated Age of Diagnosis among Persons with Symptomatic Knee OA.
The black curve is the cumulative incidence using calculated and calibrated incidence estimates. The vertical dashed line denotes the median age of diagnosis (i.e. the age by which 50% of cases have been diagnosed).
Lifetime risk of diagnosed symptomatic knee OA
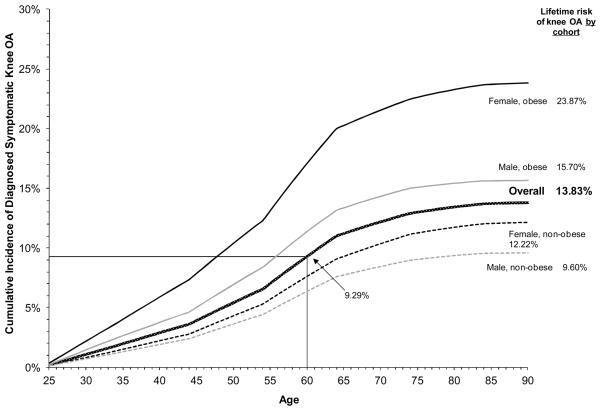
The lifetime risk of diagnosed symptomatic knee OA from age 25 in the US population was estimated at 13.83%, with a 9.29% risk of having diagnosed symptomatic knee OA by age 60 (Figure 3). In obese persons, lifetime risk of diagnosed knee OA was estimated at 19.67% compared to 10.85% for non-obese persons. In females, the lifetime risk was estimated at 16.34% compared to 11.42% in males. In sex- and obesity-stratified cohorts, we estimated the lifetime risk to be highest in obese females at 23.87% and lowest in non-obese males at 9.60%.
Figure 3. Estimated Cumulative Incidence of Diagnosed Symptomatic Knee OA from Age 25 in the US Population, Stratified by Sex and Obesity Status.
The patterned dark gray curve represents the overall cumulative incidence of diagnosed symptomatic knee OA from age 25. Dashed curves represent the cumulative incidence among non-obese persons; solid curves represent the cumulative incidence among obese persons. Female cumulative incidence is in black; male cumulative incidence is in light gray. The cumulative incidence by the end of life represents the lifetime risk for each cohort, as denoted in the right margin. The point of intersection of the thin black horizontal and vertical lines emphasizes the cumulative incidence for the general US population by age 60.
DISCUSSION
We used the OAPol Model, a state transition Monte Carlo computer simulation model, combined with national data from the 2007–2008 NHIS on the prevalence of diagnosed symptomatic knee OA, to estimate the annual incidence of diagnosed symptomatic knee OA in the US. We estimated that incidence peaked during ages 55 to 64; was higher among obese persons than non-obese persons; and, adjusting for obesity, was higher among females than males. The estimated median age of knee OA diagnosis was 55 years of age. Our incidence estimates were derived with an approach that relates incidence, prevalence odds and disease duration (prevalence odds/disease duration = incidence rate). Prevalence was obtained from a national population-based cross-sectional study. To ensure a ‘stable population’ we considered prevalence to be stable within 10-year age intervals. This method is widely used when longitudinal data are not available.31,32
This study adds important insights to research on OA incidence. Until now a report from the mid-1990s provided the most recently published estimates of incidence of diagnosed symptomatic knee OA.15–17 Our contemporary estimates are consistent with prior literature in showing higher incidence of diagnosed knee OA among females16 and among obese individuals.8,33,34
However, the estimated rates of diagnosed knee OA incidence differ somewhat from prior studies published 10–20 years ago. The study by Oliveria et al., published in 1995 based on data from the Fallon Community Health Plan, used medical records to estimate annual incidence of diagnosed knee OA. Authors found that incidence of diagnosed symptomatic knee OA increases with increasing age until age 80.16 The key difference in estimated incidence between our study and the study reported by Oliveria et al. in the 1990s lies in distributional shift. In our study, diagnosed OA incidence peaked in an earlier age group (55–64), consistent with current trends where use of total knee replacements (TKR) occurs earlier in life, with 40% of TKR recipients being younger than 65 years of age.35–38
Our estimates also provide insight into the differential impact of obesity on diagnosed OA incidence across age and sex strata. A recent study from the Johnston County Osteoarthritis Project in North Carolina found that lifetime risk of symptomatic knee OA from age 45 was nearly 45%,39 considerably greater than our estimate of 14%. Several differences in study design and population may explain this difference. First, the Johnston County study utilized radiographs to define symptomatic knee OA. Unpublished data from the Johnston County Osteoarthritis Project indicate that only about 2/3 of those identified as having symptomatic knee OA reported being diagnosed with OA by a health professional. Second, the current study projected lifetime risk from an earlier age (25 years), while the Johnston County study estimated lifetime risk from age 45.39 Third, differences between the study populations in Johnston County and the NHIS may also account for some of the differences in estimated lifetime risk. Indeed, when we ran a cohort through the model using the sex, race, and BMI distributions, as well as the incidence, prevalence, and progression of symptomatic knee OA, representative of the Johnston County population, the lifetime risk for developing knee OA increased from 14% to 38% -- similar to the 45% risk reported in the Johnston County study. The remaining differences may reflect the fact that while the Johnston County Study had good follow-up, not all study participants returned for the follow-up assessment. As in many cohorts, those participants who developed OA were more likely to return for follow-up assessments, increasing estimates of OA risk.
Our estimates suggest that persons aged 55 to 64 today are at highest risk for a new diagnosis of symptomatic knee OA, with incidence of diagnosed disease tapering off in older ages. If these differences are indicative of secular trends, they may reflect increased likelihood of diagnosis earlier in life rather than earlier onset of biological disease. An increase in the rate of diagnosed cases could result from increased patient awareness of knee OA, as well as a heightened inclination to diagnose knee OA on the part of physicians. Because rigorous comparisons between our findings and the study by Oliveria and colleagues are limited by methodological differences and heterogeneity in the study populations, further research is needed to understand secular trends in the incidence and diagnosis of symptomatic knee OA.
Our findings have important implications for disease prevention and health care utilization. The early median age of diagnosis of symptomatic knee OA (55 years) suggests that public health officials should introduce prevention strategies relatively early in the life course. Policymakers should implement prevention strategies aimed at reducing obesity and the risk of knee injury, two major risk factors for knee OA. Furthermore, the early age of diagnosis of symptomatic knee OA may yield high levels of lifetime health care utilization and costs. In the last decade, the mean age of persons undergoing TKR has decreased from 69 to 66 years and utilization of TKR has tripled among US adults aged 45 to 64.38 Whether health outcomes improve as a result of early diagnosis of symptomatic knee OA offers a rich area for future research.
The results from this study should be viewed within the context of certain assumptions and limitations in our approach. First, the definition of OA poses challenges.40 The incidence estimates reported here depend on the method used to define diagnosed symptomatic knee OA. To derive population-based national data we relied on a national, self-reported survey (NHIS 2007–2008). Diagnosed symptomatic knee OA was defined based on responses to several questions asked in the survey. Certain questions pertain more to recent knee pain than to chronic knee pain, which would serve as a better indicator of knee OA prevalence. However, similar algorithms to the one we used have specificity greater than 90%,25,26 and the sample size and national scope of the NHIS offer advantages over smaller, geographically-limited observational studies. Nevertheless, to account for possible misclassification in the self-reported prevalence of diagnosed symptomatic knee OA from the 2007–2008 NHIS survey data, we applied positive predictive values from published studies to the younger and older age groups.25–27 We could not identify any published data on negative predictive values in relation to under-reporting of diagnosed symptomatic knee OA, therefore our estimates of lifetime risk of diagnosed knee OA are conservative. Second, we assumed constant annual incidence of symptomatic knee OA within 10-year age strata. While a more precise method might have captured a smoother age distribution, incidence of the disease is unlikely to differ enough across any 10-year age group to have a meaningful effect on our estimates.
Our estimates suggest that diagnosis of symptomatic knee OA occurs early in the life course (median age 55 years), with incidence peaking between ages 55 and 64. Early diagnosis of symptomatic knee OA may yield future increases in health care utilization and medical costs associated with knee OA. Physicians and policymakers can use our findings to direct resources toward preventing risk factors for knee OA. Policymakers and planners can also use our estimates to prepare for the potential future burden on the US health care system resulting from the early age of diagnosis of symptomatic knee OA.
Supplementary Material
SIGNIFICANCE AND INNOVATIONS.
We provide estimates of the incidence of diagnosed symptomatic knee OA in the US using self-reported population-based national data from 2007–2008.
The estimated mean age of diagnosis of symptomatic knee OA was 53.5 years and the estimated median age of diagnosis was 55 years.
With half of cases of symptomatic knee OA diagnosed by age 55, the burden of future healthcare utilization for knee OA may be high.
Acknowledgments
Supported by: NIH/NIAMS R01 AR053112, K24 AR057827, K23 AR054095, P60 AR47782, and T32 AR 055885, VA Connecticut Healthcare System (Dr. Suter), and Centers for Medicare & Medicaid Services, an agency of the U.S. Department of Health and Human Services HHSM-500-2008-0025I/HHSM-500-T0001 (Dr. Suter).
We would like to thank Dr. Edward H. Yelin for helpful comments on a previous draft of the manuscript.
References
- 1.Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: Part II. Arthritis Rheum. 2008;58(1):26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hochberg MC. Proposed 2011 American College of Rheumatology recommendations for the use of non-pharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip and knee. Paper presented at: Seminars in Arthritis and Rheumatism; 2011. [Google Scholar]
- 3.Hochberg MC, Altman RD, Brandt KD, et al. Guidelines for the medical management of osteoarthritis. Part II. Osteoarthritis of the knee. American College of Rheumatology. Arthritis Rheum. 1995 Nov;38(11):1541–1546. doi: 10.1002/art.1780381104. [DOI] [PubMed] [Google Scholar]
- 4.Richmond J, Hunter D, Irrgang J, et al. American academy of orthopaedic surgeons clinical practice guideline on the treatment of osteoarthritis (OA) of the knee. J Bone Joint Surg Am. 2010 Apr;92(4):990–993. doi: 10.2106/JBJS.I.00982. [DOI] [PubMed] [Google Scholar]
- 5.Zhang W, Nuki G, Moskowitz RW, et al. OARSI recommendations for the management of hip and knee osteoarthritis: Part III: changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthritis Cartilage. 2010 Apr;18(4):476–499. doi: 10.1016/j.joca.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 6.Wilder FV, Hall BJ, Barrett JP, Lemrow NB. History of acute knee injury and osteoarthritis of the knee: a prospective epidemiological assessment: The Clearwater Osteoarthritis Study. Osteoarthritis Cartilage. 2002;10(8):611–616. doi: 10.1053/joca.2002.0795. [DOI] [PubMed] [Google Scholar]
- 7.Cooper C, Snow S, McAlindon TE, et al. Risk factors for the incidence and progression of radiographic knee osteoarthritis. Arthritis Rheum. 2000 May;43(5):995–1000. doi: 10.1002/1529-0131(200005)43:5<995::AID-ANR6>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 8.Niu J, Zhang YQ, Torner J, et al. Is obesity a risk factor for progressive radiographic knee osteoarthritis? Arthritis Rheum. 2009 Mar 15;61(3):329–335. doi: 10.1002/art.24337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Electronic Injury Surveillance System (NEISS) [Accessed August 17, 2012.];US Consumer Product Safety Commission; 2000–2009. https://www.cpsc.gov/cgibin/NEISSQuery/home.aspx.
- 10.Wang Y, Beydoun MA. The obesity epidemic in the United States--gender, age, socioeconomic, racial/ethnic, and geographic characteristics: a systematic review and meta-regression analysis. Epidemiol Rev. 2007;29:6–28. doi: 10.1093/epirev/mxm007. [DOI] [PubMed] [Google Scholar]
- 11.Brown TD, Johnston RC, Saltzman CL, Marsh JL, Buckwalter JA. Posttraumatic osteoarthritis: a first estimate of incidence, prevalence, and burden of disease. J Orthop Trauma. 2006 Nov-Dec;20(10):739–744. doi: 10.1097/01.bot.0000246468.80635.ef. [DOI] [PubMed] [Google Scholar]
- 12.Roos EM. Joint injury causes knee osteoarthritis in young adults. Curr Opin Rheumatol. 2005 Mar;17(2):195–200. doi: 10.1097/01.bor.0000151406.64393.00. [DOI] [PubMed] [Google Scholar]
- 13.Sowers M, Lachance L, Hochberg M, Jamadar D. Radiographically defined osteoarthritis of the hand and knee in young and middle-aged African American and Caucasian women. Osteoarthritis Cartilage. 2000 Mar;8(2):69–77. doi: 10.1053/joca.1999.0273. [DOI] [PubMed] [Google Scholar]
- 14.Lobstein T, Baur L, Uauy R. Obesity in children and young people: a crisis in public health. Obes Rev. 2004;5:4–85. doi: 10.1111/j.1467-789X.2004.00133.x. [DOI] [PubMed] [Google Scholar]
- 15.Felson DT, Zhang Y, Hannan MT, et al. The incidence and natural history of knee osteoarthritis in the elderly. The Framingham Osteoarthritis Study. Arthritis Rheum. 1995 Oct;38(10):1500–1505. doi: 10.1002/art.1780381017. [DOI] [PubMed] [Google Scholar]
- 16.Oliveria SA, Felson DT, Reed JI, Cirillo PA, Walker AM. Incidence of symptomatic hand, hip, and knee osteoarthritis among patients in a health maintenance organization. Arthritis Rheum. 1995 Aug;38(8):1134–1141. doi: 10.1002/art.1780380817. [DOI] [PubMed] [Google Scholar]
- 17.Pereira D, Peleteiro B, Araujo J, Branco J, Santos RA, Ramos E. The effect of osteoarthritis definition on prevalence and incidence estimates: a systematic review. Osteoarthritis Cartilage. 2011;19(11):1270–1285. doi: 10.1016/j.joca.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 18.Alho JM. On prevalence, incidence, and duration in general stable populations. Biometrics. 1992;48(2):587–592. [PubMed] [Google Scholar]
- 19.2007–2008 National Health Interview Survey (NHIS) Centers for Disease Control and Prevention (CDC), and National Center for Health Statistics (NCHS), U.S. Department of Health and Human Services (DHHS); 2008. [Accessed August 17, 2012.]. http://www.cdc.gov/nchs/nhis.htm. [Google Scholar]
- 20.Losina E, Walensky RP, Reichmann WM, et al. Impact of Obesity and Knee Osteoarthritis on Morbidity and Mortality in Older Americans. Ann Intern Med. 2011 Feb 15;154(4):217–226. doi: 10.1059/0003-4819-154-4-201102150-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holt HL, Katz JN, Reichmann WM, et al. Forecasting the burden of advanced knee osteoarthritis over a 10-year period in a cohort of 60–64 year-old US adults. Osteoarthritis Cartilage. 2011 Jan;19(1):44–50. doi: 10.1016/j.joca.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Vital Statistics System (NVSS) (accessed from Health Data Interactive) Centers for Disease Control and Prevention (CDC), and National Center for Health Statistics (NCHS), U.S. Department of Health and Human Services (DHHS); 2007. [Accessed August 17, 2012.]. http://www.cdc.gov/nchs/hdi.htm. [Google Scholar]
- 23.Arias E. United States Life Tables, 2006. Hyattsville, MD: National Center for Health Statistics; 2010. [PubMed] [Google Scholar]
- 24.Arias E. United States Life Tables by Hispanic Origin. Vital Health Stat. 2010 Oct;2(152):1–33. [PubMed] [Google Scholar]
- 25.LaValley M, McAlindon TE, Evans S, Chaisson CE, Felson DT. Problems in the development and validation of questionnaire-based screening instruments for ascertaining cases with symptomatic knee osteoarthritis: The Framingham Study. Arthritis Rheum. 2001;44(5):1105–1113. doi: 10.1002/1529-0131(200105)44:5<1105::AID-ANR191>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 26.March LM, Schwarz JM, Carfrae BH, Bagge E. Clinical validation of self-reported osteoarthritis. Osteoarthritis Cartilage. 1998;6(2):87–93. doi: 10.1053/joca.1997.0098. [DOI] [PubMed] [Google Scholar]
- 27.Giles K, Kafle R, Hocking L, et al. Validation of Self-Reported Osteoarthritis in a Postmenopausal Population and its Association with Body Weight [Abstract: 355] Osteoarthritis Cartilage. 2010 Oct;18(Supplement 2):S157. [Google Scholar]
- 28.2005–2006 National Health and Nutrition Examination Survey (NHANES) Data. Centers for Disease Control and Prevention (CDC), and National Center for Health Statistics (NCHS), U.S. Department of Health and Human Services (DHHS); 2006. [Accessed August 17, 2012.]. http://www.cdc.gov/nchs/nhanes/nhanes2005-2006/nhanes05_06.htm. [Google Scholar]
- 29.2007–2008 National Health and Nutrition Examination Survey (NHANES) Data. Centers for Disease Control and Prevention (CDC), and National Center for Health Statistics (NCHS), U.S. Department of Health and Human Services (DHHS); 2008. [Accessed August 17, 2012.]. http://www.cdc.gov/nchs/nhanes/nhanes2007-2008/nhanes07_08.htm. [Google Scholar]
- 30.Annual Estimates of the Hispanic, White, and Black Resident Populations by Sex and Age for the United States: April 1, 2000 to July 1, 2009 (NC-EST2009-04-HISP/WANH/BA) Population Division, U.S. Census Bureau; 2010. [Accessed August 17, 2012.]. http://www.census.gov/popest/national/asrh/NC-EST2009-asrh.html. [Google Scholar]
- 31.Reichenheim ME, Coutinho ES. Measures and models for causal inference in cross-sectional studies: arguments for the appropriateness of the prevalence odds ratio and related logistic regression. BMC medical research methodology. 2010;10:66. doi: 10.1186/1471-2288-10-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rothman KJ. Epidemiology: An Introduction. Oxford University Press Inc; 2012. p. 47. [Google Scholar]
- 33.Blagojevic M, Jinks C, Jeffery A, Jordan KP. Risk factors for onset of osteoarthritis of the knee in older adults: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2010 Sep 2; doi: 10.1016/j.joca.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 34.Grotle M, Hagen K, Natvig B, Dahl F, Kvien T. Obesity and osteoarthritis in knee, hip and/or hand: An epidemiological study in the general population with 10 years follow-up. BMC Musculoskelet Disord. 2008;9(1):132. doi: 10.1186/1471-2474-9-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khatod M, Inacio M, Paxton EW, et al. Knee replacement: epidemiology, outcomes, and trends in Southern California: 17,080 replacements from 1995 through 2004. Acta orthopaedica. 2008 Dec;79(6):812–819. doi: 10.1080/17453670810016902. [DOI] [PubMed] [Google Scholar]
- 36.Jain NB, Higgins LD, Ozumba D, et al. Trends in epidemiology of knee arthroplasty in the United States, 1990–2000. Arthritis Rheum. 2005 Dec;52(12):3928–3933. doi: 10.1002/art.21420. [DOI] [PubMed] [Google Scholar]
- 37.Mehrotra C, Remington PL, Naimi TS, Washington W, Miller R. Trends in total knee replacement surgeries and implications for public health, 1990–2000. Public Health Rep. 2005 May-Jun;120(3):278–282. doi: 10.1177/003335490512000310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Healthcare Cost and Utilization Project (HCUP). Nationwide Inpatient Sample (NIS). Agency for Healthcare Research and Quality. [Accessed August 17, 2012.];1997–2009 http://hcupnet.ahrq.gov/
- 39.Murphy L, Schwartz TA, Helmick CG, et al. Lifetime risk of symptomatic knee osteoarthritis. Arthritis Rheum. 2008 Sep 15;59(9):1207–1213. doi: 10.1002/art.24021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Felson DT, Niu J, Guermazi A, Sack B, Aliabadi P. Defining radiographic incidence and progression of knee osteoarthritis: suggested modifications of the Kellgren and Lawrence scale. Ann Rheum Dis. 2011 Sep 8; doi: 10.1136/ard.2011.155119. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.