Abstract
There is growing interest in measuring social disability as a core element of autism spectrum disorders in medication trials. We conducted a secondary analysis on the Aberrant Behavior Checklist Social Withdrawal subscale using data from two federally-funded, multi-site, randomized trials with risperidone. Study 1 included 52 subjects assigned to placebo and 49 subjects to risperidone under double-blind conditions. Study 2 included 49 subjects assigned to risperidone only and 75 subjects assigned to risperidone plus parent training. After 8 weeks of treatment, all active treatments were superior to placebo (effect sizes ranging from 0.42 to 0.65). The findings suggest that the Social Withdrawal subscale may be a useful measure of social disability in acute treatment trials.
Introduction
Autism Spectrum Disorders (ASDs) may affect as many as 11 per 1000 children (Centers for Disease Control, 2012). This estimate from the 2008 survey conducted by the Centers for Disease Control reflects a 78% increase in estimated prevalence since a previous survey conducted in 2002 using similar study methods (Centers for Disease Control, 2012). The investigators indicate that improved identification of children with average intellectual capacity and minority children as cases of ASD at least partially explains this apparent rise in prevalence. In addition to the core features of social disability, communication delay and repetitive behavior, children with ASDs may also exhibit hyperactivity, aggression, tantrums, self-injury, sleep disturbance and anxiety symptoms (Lecavalier, 2006; Levy, Mandel & Schultz, 2009). Although estimates vary, depending on the sources of sample, many children with ASDs have intellectual disability and virtually all have adaptive skill deficits (Carter et al., 1998). Accumulated data over the past 2 decades support the central role of genetics in the etiology of ASDs with heritability estimates as high as 90% (Levy, Mandel & Schultz, 2009). In a large population twin study of 12-year-olds, Robinson and colleagues (2011) examined the heritability of autistic traits as measured on a 30-item, parent-rated scale that encompasses social disability, impaired communication and repetitive behavior. In the highest 1% of the distribution on this quantitative measure, heritability was 53% for girls and 72% for boys. Although these findings support the notion of an autism spectrum, the underlying genetics are complex and continue to be elusive (O'Roak & State, 2008). Based on available evidence from neuroimaging studies, ASDs may share a common disruption in brain networks that interfere with social perception and social communication (Levy, Mandel & Schultz, 2009). Emerging findings in molecular neuroscience hold promise and may provide credible leads for psychopharmacology. For example, encouraging advances in the pathophysiology of developmental disorders such as Fragile X offer a path for drug development based on underlying neurobiology (Dolen et al., 2010).
Multisite psychopharmacological treatments in children with ASDs over the past decade have focused on specific treatment targets such as tantrums, aggression, self-injury (Research Units on Pediatric Psychopharmacology (RUPP) Autism Network, 2002), hyperactivity (RUPP Autism Network 2005); or repetitive behavior (King et al., 2009). The results of these trials provide guidance for clinical practice. Moreover, these trials provide models for designing and conducting clinical trials in this population that can influence policy. For example, risperidone and aripiprazole are now approved by the Food and Drug Administration (FDA) for the treatment of children with autistic disorder accompanied by serious behavioral problems (tantrums, aggression, self-injury) (Marcus et al., 2009; Owen et al., 2009; RUPP Autism Network, 2002;Shea et al., 2004). This successful regulatory pathway with drugs already on the market entailed selection of a clinically meaningful target problem, an acceptable study design to test the efficacy and safety of the drug, as well as the use of a reliable and valid outcome measure that aptly reflects the clinical target. The parent-rated, 15-item Aberrant Behavior Checklist (ABC) Irritability subscale, which covers tantrums, aggression and self-injury, was the primary outcome measure in these trials (see below). Although these serious behavioral problems are not core features of ASDs, they interfere with the child's activities of daily living and confer additional burden on families (Scahill et al., 2012). FDA approval of a drug for core features of ASDs such as social disability will require empirical evidence on how to measure this central element of ASDs (Posey, Erickson & McDougle, 2008). The purpose of this report is to examine the ABC Social Withdrawal subscale as an outcome for social disability in children with autism spectrum disorders.
Methods
Subjects
We used data from two federally-funded, multisite, randomized clinical trials (Aman et al., 2009; RUPP Autism Network, 2002). These trials were approved by each institutional review board and informed consent from the primary caregiver was obtained prior to data collection. Minors, who were developmentally able provided assent. In the first trial (RUPP 1), subjects with autistic disorder (age 5 to 17 years) were randomized to risperidone (N=49) or placebo (N=52) under double-blind conditions (RUPP Autism Network, 2002). In the second trial (RUPP 2), children (age 4 to 14 years) with ASDs (autistic disorder, Asperger's disorder and Pervasive Developmental Disorder – Not Otherwise Specified (PDD-NOS) were randomly assigned to open-label risperidone only (n=49) or risperidone plus parent training (n=75) (Aman et al., 2009; Scahill et al., 2012).
Procedures
Prior to randomization, subjects were evaluated by an experienced clinical team (e.g., child psychiatrist, psychologist and nurse practitioner) using standard methods. The comprehensive assessment included medical history and physical examination, developmental and psychiatric histories, as well as ASD diagnostic and behavioral assessments to confirm study eligibility. The ASD diagnosis was based on clinical interview, observation and supported by Autism Diagnostic Interview-Revised (Lord et al., 1997). The trials accepted children with a wide range of IQ. To confirm that IQ was 35 or greater, we used several different tests based on the child's ability (Aman et al., 2009; RUPP Autism Network, 2002; see Supplemental material). Twelve subjects (4.4%) could not be tested due to lack of cooperation. Because several different tests were employed, children were classified categorically (e.g., average intelligence [≥ 70] or intellectually disabled [<70]).
Both trials required subjects to be healthy and medication-free (7 to 28 days depending on the prior medication). Children on stable anticonvulsant medication for seizure control who were seizure-free for at least six months were eligible. Both trials required the presence of serious behavioral problems (e.g., tantrums, aggression and self-injury in any combination) as evidenced by a score of 18 or higher on the parent-rated ABC Irritability subscale and a clinician rating of at least Moderate on the Clinical Global Impression Severity scale (Aman et al., 2009; RUPP Autism Network, 2002). Children with a co-existing psychiatric disorder requiring treatment (e.g., psychosis, bipolar disorder, depression, anxiety disorder, obsessive-compulsive disorder, attention deficit hyperactivity disorder) were excluded.
Once randomized, subjects were assessed weekly for eight weeks for safety and dose adjustment. A set of common outcome measures was used in the two trials (Table 1 in Supplement shows the schedule of common of measures used in these trials). Outcome assessments were conducted every two weeks. In both trials, subjects were followed by two clinicians who were blind to treatment assignment during the eight week trial. The treating clinician monitored adverse effects and adjusted the medication dose. In both trials, the risperidone dose schedule was based on weight. For example, children between 20 and 45 kg started with a single bedtime dose of 0.5 mg. On study day 4, this dose was increased to 0.5 mg twice a day. Thereafter the dose could be increased in 0.5 mg increments over four weeks to a maximum of 2.5 mg in divided doses (1.0 mg in the morning and 1.5 mg at bedtime). Children below 20 kg started with 0.25 mg and followed a slower upward adjustment. The treating clinician could delay scheduled increases of reduce the dose to manage suspected adverse effects. An independent evaluator, who did not engage in any discussion of adverse effects, rated the CGI.
Measures Used in This Report
Survey Edition of the Vineland Adaptive Behavior Scales (Vineland)
The Vineland is a semi-structured, parent interview that measures the child's competence in communication, daily living skills, and socialization. The scale is standardized assessment for age and gender adjusted with population mean of 100 ± 15. It is a commonly used measure of adaptive functioning in children with developmental disabilities with excellent reliability and validity for each domain (Sparrow, Balla & Cicchetti, 1984).
Aberrant Behavior Checklist (ABC)
The ABC is a 58-item, informant-based scale comprising five subscales: I. Irritability (includes agitation, aggression and self-injurious behaviors, 15 items); II. Social Withdrawal (16 items); III. Stereotypic Behaviors (7 items); IV. Hyperactivity, 16 items (includes over-activity and impulsiveness); and V. Inappropriate Speech (4 items) (Aman et al., 1985). The parent-rated ABC is reliable and valid with normative data in developmentally disabled populations (Brown et al., 2002). The Irritability and Hyperactivity subscales have demonstrated sensitivity to change with treatment and has been used as outcome measures in several trials (Aman et al., 2009; RUPP Autism Network, 2002; RUPP Autism Network, 2005). In this secondary analysis, we focus on the parent-rated Social Withdrawal subscale because it captures observable behaviors on response to interaction initiated by others (e.g., “is difficult to reach, contact or get through to;” withdrawn; prefers solitary activities”) and the extent to which the child initiates interaction (e.g., “shows few social reactions to others;” “does not try to communicate by words or gestures”).
The Clinical Global Impression scale for Severity (CGI-S)
(Guy, 1976) is a 7-point scale ranging from non-symptomatic (score of 1) to extreme (score of 7). The score of 3 was used to describe uncomplicated autism; 4 (Moderate) was used in both trials as the benchmark indicating the for treatment intervention. In this study, the CGI-S was rated by clinicians with at least master's level of education who were trained to reliability.
Child Symptom Inventory (CSI)
The CSI is a 132-item, parent-rated scale based on DSM-IV disorder categories (Gadow, DeVincent & Schneider, 2008). Items are scored from 0 (never) to 3 (very often). The subscales can be scored dimensionally (total of the 0 to 3 scores per disorder category). Alternatively, item scores of 2 and 3 may be tallied to identify a symptom count within each disorder category. The 132-item CSI is a reliable and valid screening instrument for a full range of psychiatric disorders in youth and has been used in the ASD populations. In a previous analysis, we identified 20 anxiety items that included symptoms of separation anxiety, generalized anxiety and social phobia (Sukhodolsky et al., 2008). Also embedded in the CSI is a 12-item autism spectrum scale. This scale includes items on social and communication deficits as well as repetitive behavior. Only data from these two scales of the CSI are included in the current report.
Analytic Plan
We compared baseline values on the Aberrant Behavior Checklist subscales, CSI PDD and Anxiety scales, Vineland scores, age, percentage of males and percentage of subjects in the intellectually disabled range (IQ < 70) to check for group differences. As a further exploration of baseline values, we examined the correlation of the Social Withdrawal subscale with the other ABC subscales, Vineland scores as well as CASI ASD and Anxiety scales. Using parent-rated ABC collected at baseline, Weeks 2, 4, 6 and 8, we compared the change on the Social Withdrawal subscale across the four treatment groups with a linear mixed effect model. This model included a fixed effect of groups (four levels), time (continuous) and interaction between groups and time. Random effects of subject and time were modeled by using AR1 autoregressive covariance structure. The model was adjusted for IQ (< 70 versus ≥70), age and gender. The first trial included a higher percentage of children with autistic disorder than the second trial (100% vs 63.5%), therefore, the model was also adjusted for diagnosis (autistic disorder or not). Because previous analyses did not show site by treatment interaction, site was not included in the model (Aman et al., 2009; RUPP Autism Network, 2002). Time course was plotted using the LSMEANS function in SAS to show adjusted mean estimates with standard error bars. Analyses were performed in SAS version 9.2 (SAS Institute Inc, Cary, NC).
Results
The combined sample of 225 (187 boys and 38 girls) included 182 subjects with autistic disorder; 35 subjects with pervasive developmental disorder-not otherwise specified and 8 subjects with Asperger's disorder. One hundred sixty (n=160) were white; 27 were black; 18 were Hispanic; 11 were Asian; parents listed 9 subjects as “other” on the racial/ethnicity question. Fifty three subjects were in regular education, 146 were a special education program, 1 in a residential school and 23 were not in school or were in home school (see Table 2 of the Supplement for more detail on baseline characteristics).
Table 1 presents baseline clinical characteristics by treatment group. The two samples were similar on many measures, though there were a few significant differences. The RUPP 2 sample was slightly younger, had a lower percentage of children with intellectual disability and higher Vineland scores. Compared to the RUPP 1 sample, the RUPP 2 sample also had a higher percentage of subjects rated as Moderate on the CGI-S (Moderate vs > Moderate), a lower percentage of subjects in full time special education, and slightly higher scores on parent-rated ABC Hyperactivity subscale (See supplemental material for details). The higher scores on the ABC Hyperactivity subscale notwithstanding, these differences suggest that the RUPP 2 sample was slightly less impaired than the subjects in RUPP 1.
Table 1. Baseline characteristics across four groups in RUPP Autism Network Risperidone Trials.
| Risperidone (N=49) | Placebo (N=52) | Risperidone (N=49) | Risperidone + PT (N=75) | |
|---|---|---|---|---|
| Mean Age (SD) | 8.6 (2.97) | 9.1 (2.6) | 7.50 (2.80) | 7.38 (2.21) |
| N (%) | N (%) | N (%) | N (%) | |
| Males | 39 (80) | 43 (83) | 40 (82) | 65 (87) |
| ASD Diagnosis | ||||
| Autistic Disorder | 49 (100%) | 52 (100%) | 32 (65.3) | 49 (65.3) |
| PDD-NOS | 0 | 0 | 13 (26.5) | 22 (29.3) |
| Asperger's | 0 | 0 | 4 (8.2) | 4 (5.3) |
| IQ* | ||||
| ≥ 70 | 11 (23.9) | 6 (13.3) | 23 (46.9) | 46 (63.0) |
| < 70 | 35 (76.1) | 39 (86.7) | 26 (53.1) | 27 (37.0) |
| CGI | ||||
| Moderate | 9 (18) | 9 (17) | 14 (28.6) | 25 (33.3) |
| Marked | 27 (55) | 28 (57) | 19 (38.8) | 33(44.0) |
| Severe | 12 (24) | 12 (24) | 15 (30.6) | 17 (22.7) |
| Extreme | 1 (2) | 0 | 1(2.0) | 0 (0.0) |
| Vineland (standard scores) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) |
| Communication | 45.0 (16.7) | 42.0 (14.3) | 53.2(19.94) | 61.1 (20.95) |
| Socialization | 49.1 (16.6) | 47.4 (10.1) | 53.5 (14.41) | 59.5 (15.01) |
| Daily Living | 40.8 (21.0) | 34.0 (15.6) | 41.1 (19.81 | 50.8 (18.49) |
| ABC | ||||
| Irritability | 26.2 (7.9) | 25.5 (6.6) | 29.7 (6.1) | 29.3 (7.0) |
| Social Withdrawal | 16.4 (8.2) | 16.1 (8.7) | 17.1 (8.5) | 15.2 (9.0) |
| Stereotypy | 10.6 (4.9) | 9.0 (4.4) | 10.6 (5.5) | 7.59 (5.2) |
| Hyperactivity | 31.8 (9.6) | 32.3 (8.5) | 36.1 (6.9) | 35.3 (9.3) |
| Inappropriate Speech | 4.8 (4.1) | 6.5 (3.1) | 6.37 (4.0) | 5.75 (3.4) |
| CSI | ||||
| PDD | 24.3 (6.9) | 23.2 (7.7) | 23.2 (7.0) | 22.0 (7.7) |
| Anxiety | 14.5 (9.7) | 13.8 (7.6) | 14.6 (9.5) | 17.2 (10.3) |
IQ missing on 12 subjects; 10 subjects in RUPP 1 and 2 subjects in RUPP 2.
Compared to a community sample of 484 children in developmentally handicapped classroom settings, the subjects from these RUPP trials had elevated scores on the Social Withdrawal subscale at baseline (see Brown, Aman & Havercamp, 2002). For example, Brown and colleagues observed that boys in these special education settings had a mean score of 6.45 ± 7.14 on the Social Withdrawal subscale compared to 16.2± 8.67 in the combined RUPP samples. Baseline scores on the Social Withdrawal subscale in our sample were significantly correlated with other ABC subscales (Irritability = 0.30; Stereotypy = 0.45, p < .01 for both), CSI scales (Anxiety= 0.23; PDD= 0.35, p < .01 for both), Vineland Standard scores (Daily Living = −0.23; Socialization = −0.21; Communication= −0.21, p < .01 for all three). Although significant, these small to medium correlations suggest that the Social Withdrawal subscale is measuring a separate construct (see Table 3 of Supplement for more details on correlations with the ABC Social Withdrawal subscale and other measurers).
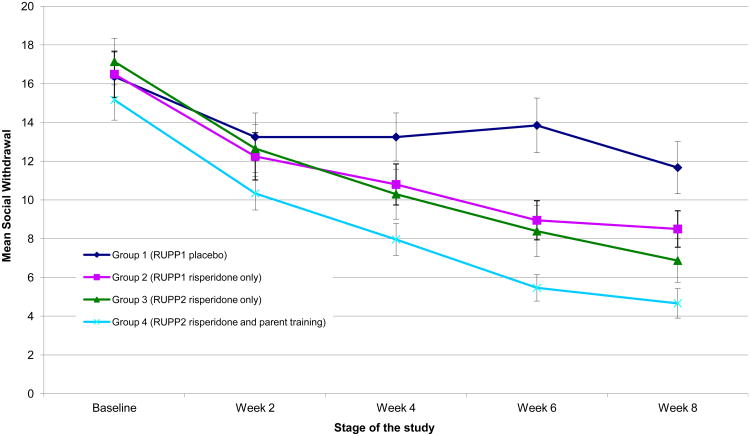
After eight weeks of treatment, Social Withdrawal subscale scores declined in all four groups, but significant group differences did emerge. Figure 1 shows the change from baseline in Group 1 (placebo in RUPP 1), Group 2 (double-blind risperidone in RUPP 1); Group 3 (risperidone only in RUPP 2) and Group 4 (risperidone plus parent training in RUPP 2). There was a significant group-by-time interaction (F(3,778)=5.49; p=0.001). Post hoc pair-wise comparisons showed significant differences between Group 1 and all active treatment groups: Group 2 (F(1, 314)=3.82; p=0.05; effect size = 0.42); Group 3 (F(1,337)=10.63; p=0.001; effect size = 0.65 ) and Group 4 (F(1, 429)=15.15; p=0.0001; effect size = 0.65). There were no significant differences between any of the active treatment groups (see Figure 1).
Figure 1. Change in the LS Mean of ABC Social Withdrawal subscale over time for the four study groups.
ABC=Aberrant Behavior Checklist; RUPP1 and RUPP2 = data from the first and second risperidone trials by the Research Units on Pediatric Psychopharmacology Autism Network. Error bars report standard errors of the mean.
Discussion
Risperidone was the first medication approved by the FDA for the treatment of serious behavioral problems in children with autism. This was followed three years later by approval of aripiprazole. The primary outcome measure for the trials that supported FDA-approval was the 15-item, parent-rated Irritability subscale of the Aberrant Behavior Checklist (ABC). The ABC also includes a 16-item Social Withdrawal subscale, which was elevated in the participants of these two RUPP trials. After eight weeks of treatment, all three groups of children treated with risperidone showed improvement in social disability compared to placebo. This analysis was not intended to test the efficacy of risperidone for social disability in children with autism spectrum disorders. The primary purpose was to evaluate the utility of the ABC Social Withdrawal subscale as an endpoint in acute treatment trials. The measure showed sensitivity to change with incremental precision (effect sizes 0.42 to 0.65).
The approval of risperidone and aripiprazole for the treatment of children with autism focused on target behaviors of tantrums, aggression and self-injury rather than core features of the disorder. However, the approval of these medications has prompted interest in pursuing clinical trials in autism focused on social interaction, communication, repetitive behavior or all three domains. The pressing need for usable, reliable and valid measures that are sensitive to change has been noted by the 2011 strategic plan of the Interagency Autism Coordinating Committee (2011) and advocacy organizations (Autism Speaks, 2011). The FDA has emphasized the importance of patient reported outcomes (US Food and Drug Administration, 2007) in trials intended to support an indication. In youth with developmental disabilities, however, we are compelled to rely on caregiver reports. In recognition of this constraint, the FDA recommends that informant-based measures focus on observable behavior. The Social Withdrawal subscale does indeed include observable behaviors that reflect the child's interaction with others, i.e., the frequency of the child's response to invitation from others and initiation of social interaction. In this combined sample at baseline, the Social Withdrawal subscale showed small to medium correlations with several other scales suggesting that it measures a unique construct. The subscale has normative data in developmentally delayed youth, which can be used to set a benchmark of severity pretreatment and a benchmark for clinically meaningful change (Brown, Aman & Havercamp, 2002). In small pilot trials of adults with Fragile X and adults with autism using drugs presumed to affect the glutamatergic system, the Social Withdrawal subscale has been used as a primary outcome measure (Jacquemont et al., 2011; Veenstra-VanderWeele , et al. 2011). Our results indicate that the Social Withdrawal subscale is sensitive to change. A factor analysis in a sample of 630 subjects with Fragile X generally supported the existing ABC factor structure (Sansone et al., 2012). Notable exceptions were the Hyperactivity and Social Withdrawal subscales. These investigators also identified a four-item social avoidance factor nested in the current Social Withdrawal subscale. It may be argued that social avoidance is separate from social indifference, but these problems may also co-occur in children with ASDs. In addition, use of a four-item scale as an outcome measure in clinical trials seems ill-advised.
There are several limitations of this secondary analysis. First, although the two trials used similar entry criteria and random assignment, this was not a four-group randomized trial. Second, placebo-control was only present in the first trial. Thus, the placebo control group in RUPP 1 may be different in unknown ways from the participants in RUPP 2 (Groups 3 and 4 in this analysis). Third, there was a large treatment effect of risperidone on disruptive behavior in both trials. The observed improvement on the Social Withdrawal subscale may reflect a “halo effect” in this sample of children with serious behavioral problems. Nonetheless, the findings indicate that the Social Withdrawal subscale may be a useful endpoint in acute treatment trials focused on social disability in children with autism spectrum disorders. The increased recognition of autism spectrum disorders underscores the need for better outcome measurement in the social domain. The Social Withdrawal subscale may also be useful in clinical settings to measure improvement with social skills training programs.
Supplementary Material
Acknowledgments
NIMH: Ann Wagner, Ph.D., William R. Harlan, M.D. , IU: Jon T. Diener, B.S.; Kelly A. Ernsperger, L.C.S.W.; Joy M. Fairbanks, M.S.; Jennifer E. Mullett, R.N.; Marianna R. Zaphiriou, B.A. OSU: Kristy Hall-Reel, M.A.; Amanda Wilkes, M.A.; Kristina Humphries, M.S.; Lorelai Ark, M.A.; Susan Thompson; Yale Karen Bearss, PhD, Allison Gavaletz, BA, Kathy Koenig, MSN, Caitlin Tillberg, BA, Karol Katz, MS, Lily Katsovich, MS, MBA, Mary Ellen Pachler. MSN. For support as Scientific Advisors: Andrew C. Leon, Ph.D., Weill Medical College of Cornell University Jose Alvir, Dr.P.H., New York University Child Study Center; CT Gordon, M.D., (Private Practice, Rockville, Maryland); Sandra Harris, Ph.D., Rutgers University, State University of New Jersey; Henrietta Leonard, M.D., Brown University; Susan Swedo, M.D., NIMH Intramural Research Program; Richard Todd, Ph.D., M.D., Washington University School of Medicine. DSMB
This work was funded by National Institute of Mental Health by the following RUPP grants and contracts: Yale, N01MH70009, U10MH66764 (Dr Scahill); Ohio State University, N01MH80011, U10MH66768 (Dr Aman); Indiana University, N01MH70001, U10MH66766 (Dr McDougle); N01MH70010 (Dr McCracken); and. Johnson & Johnson Pharmaceutical Research & Development provided active risperidone and placebo for RUPP 1 and risperidone for RUPP 2. This publication was also supported by the Yale CTSA, UL1 RR024139, IU CTSA UL1 RR025761, OSU CTSA UL1 RR025755 from the National Center for Research Resources (NCRR); K24 MH001805 (Dr McCracken) from the National Institute of Mental Health, and by the Korczak Foundation, Amsterdam, the Netherlands (Dr Scahill).
Footnotes
Disclaimer: The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
Disclosures: Dr. Scahill: Roche, consultant; Pfizer, consultant; Brachet, consultatnt, BioMarin, consultant. Shire, research support; Roche, research support; Pfizer, research support. Dr. Aman: Roche, consultant; Bristol-Meyers Squibb, consultant, research grant; Forest, consultant; Pfizer, consultant; Supernus, consultant; Johnson & Johnson, research grant. Dr. McDougle: Bristol-Myers Squibb, consultant, research grant, speaker's bureau; Forest Research Institute, consultant. Dr. McCracken: Hoffman-Roche, consultant; BioMarin, consultant; Shionogi, consultant; Novartis, consultant; Noven, consultant; PharmaNet consultant; Bristol Myers Squibb, consultant; Seaside Therapeutics, consultant, research support; Dr. Arnold: AstraZeneca, advisory board; Biomarin, advisory board; CureMark, research funding; Lilly, research funding; Noven, advisory board ; Seaside therapeutics, advisory board; Shire, research funding. Dr. Tierney : BioMarin, consultant. Ms. Deng, Dr. Dziura and Dr. Vitiello report no financial relationships with commercial interests.
References
- Aman MG, McDougle CJ, Scahill L, et al. Medication and Parent Training in Children With Pervasive Developmental Disorders and Serious Behavior Problems: Results from a Randomized Clinical Trial. J Amer Acad of Child Adolesc Psychiatry. 2009;48(12):1143–54. doi: 10.1097/CHI.0b013e3181bfd669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aman MG, Singh NN, Stewart AW, Field CJ. Psychometric characteristics of the Aberrant Behavior Checklist. Am J Ment Defic. 1985;89:492–502. [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th, text rev. DSMIV-TR; Washington, DC: 2000. [Google Scholar]
- Autism Speaks. Autism Speaks announces a major effort to develop new effective medical treatments for people with ASD. [accessed September 18,2012];2011 http://blog.autismspeaks.org/2011/02/15/science-effective-medical-treatments.
- Brown EC, Aman MG, Havercamp SM. Factor analysis and norms for parent ratings on the Aberrant Behavior Checklist - Community for young people in special education. Res Dev Disabil. 2002;23:45–60. doi: 10.1016/s0891-4222(01)00091-9. [DOI] [PubMed] [Google Scholar]
- Carter AS, Volkmar FR, Sparrow SS, et al. The Vineland Adaptive Behavior Scales: supplementary norms for individuals with autism. J Autism Dev Disord. 1998;28(4):287–302. doi: 10.1023/a:1026056518470. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Prevalence of the Autism Spectrum Disorders (ASDs) in Multiple Areas of the United States, 2004 and 2006. 2009 http://www.cdc.gov/ncbddd/autism/states/ADDMCommunityReport2009.pdf.
- Dolen G, Carpenter RL, Ocain TD, Bear MF. Mechanism-based approaches to treating fragile X. Pharmacology & Therapeutics. 2010;127(2010):78–93. doi: 10.1016/j.pharmthera.2010.02.008. [DOI] [PubMed] [Google Scholar]
- Gadow KD, DeVincent C, Schneider J. Predictors of Psychiatric Symptoms in Children with an Autism Spectrum Disorder. J Autism Dev Disord. 2008;38:1710–1720. doi: 10.1007/s10803-008-0556-8. [DOI] [PubMed] [Google Scholar]
- Interagency Autism Coordinating Committee. 2011 IACC Strategic Plan for Autism Spectrum Disorder Research. 2011 Jan; Retrieved from the Department of Health and Human Services Interagency Autism Coordinating Committee website at http://iacc.hhs.gov/strategic-plan/2011/index.shtml.
- Jacquemont S, Curie A, des Portes V, Torrioli MG, Berry-Kravis E, Hagerman RJ, et al. Epigenetic modification of the FMR1 gene in fragile X syndrome is associated with differential response to the mGluR5 antagonist AFQ056. Science Trans Medicine. 2011;3(64):64ra1. doi: 10.1126/scitranslmed.3001708. [DOI] [PubMed] [Google Scholar]
- Lecavalier L. Behavioral and emotional problems in young people with pervasive developmental disorders: Relative prevalence, effects of subject characteristics, and empirical classification. J Autism Dev Disord. 2006;36:1101–1114. doi: 10.1007/s10803-006-0147-5. [DOI] [PubMed] [Google Scholar]
- Marcus RN, Owen R, Kamen L, et al. A placebo-controlled, fixed-dose study of aripiprazole in children and adolescents with irritability associated with autistic disorder. J Amer Acad Child Adoles Psychiatry. 2009;48(11):1110–9. doi: 10.1097/CHI.0b013e3181b76658. [DOI] [PubMed] [Google Scholar]
- Levy SE, Mandell DS, Schultz RT. Autism. Lancet. 2007;374(9701):1627–38. doi: 10.1016/S0140-6736(09)61376-3. Erratum appears in Lancet. 2011 Oct 29;378(9802):1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen R, Sikich L, Marcus R, et al. A multicenter, double-blind, randomized, placebo controlled, flexible-dose, parallel-group study of aripiprazole in the treatment of irritability in children and adolescents (6–17 years) with autistic disorder. Pediatrics. 2009;124:1533–1540. doi: 10.1542/peds.2008-3782. [DOI] [PubMed] [Google Scholar]
- Posey DJ, Erickson CA, McDougle CJ. Developing drugs for core social and communication impairment in autism. Child & Adolescent Psychiatric Clinics of North America. 2008;17(4):787–801. doi: 10.1016/j.chc.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson EB, Koenen KC, McCormick MC, Munir K, Hallett V, Happe F, Plomin R, Ronald A. Evidence that autistic traits show the same etiology in the general population and at the quantitative extremes (5%, 2.5%, and 1%) Arch Gen Psychiatry. 2011;68(11):1113–21. doi: 10.1001/archgenpsychiatry.2011.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUPP Autism Network. Risperidone in children with autism and serious behavioral problems. NEJM. 2002;347(5):314–321. doi: 10.1056/NEJMoa013171. [DOI] [PubMed] [Google Scholar]
- Scahill L, McDougle CJ, Aman MG, Johnson C, Handen B, Bearss K, et al. for the Research Units on Pediatric Psychopharmacology Autism Network. Effects of risperidone and parent training on adaptive functioning in children with a pervasive developmental disorders and serious behavioral problems. J Amer Acad Child Adolesc Psychiatry. 2012;51(2):136–146. doi: 10.1016/j.jaac.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea S, Turgay A, Carrol A, et al. Risperidone in the treatment of disruptive behavioural symptoms in children with autistic and other PDDs. Pediatrics. 2004;114:e634–e641. doi: 10.1542/peds.2003-0264-F. [DOI] [PubMed] [Google Scholar]
- US Food and Drug Administration. Guidance for Industry: Patient-Reported OutcomeMeasures: Use in Medical Product Development to Support Labeling Claims. [Accessed May 4, 2007]; Available from: www.Fda.Gov/Cder/Guidance/5460dft.pdf. Federal Register: February 3, 2006, Vol. 71. Number (23) Docket no. 2006D-0044.
- Veenstra-VanderWeele J, King B, Erickson C, Ginsberg L, Melman R, Scahill L, Sikich L, McCracken JT, Rathmell B, Carpenter R, Bear M, Wang P. (2011). An open label trial of arbaclofen in autism spectrum disorder shows improvements in multiple symptom domains. Poster presentation at NCDEU. 2011 Spring [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.