Abstract
Background
Millions of Americans with suspected coronary artery disease undergo non-invasive cardiac stress testing annually. Downstream procedures and subsequent outcomes among symptomatic patients without known coronary disease referred for stress testing are not well characterized in contemporary community practice.
Methods
We examined administrative insurance billing data from a national insurance provider from November 2004 through June 2007. After excluding patients with prior cardiac disease or chest pain evaluation, we identified 80,676 people age 40–64 years with outpatient cardiac stress testing within 30 days after an office visit for chest pain. We evaluated rates of invasive coronary angiography, coronary revascularization, and cardiovascular events after stress testing.
Results
Within 60 days, only 8.8% of stress test patients underwent cardiac catheterization and only 2.7% underwent revascularization; within one year only 0.5% suffered death, myocardial infarction, or stroke. There were marked geographic variations in one-year rates of catheterization (3.8–14.8%) and revascularization (1.2–3.0%) across 20 hospital referral regions.
Conclusions
In this large national cohort of middle-aged patients without previously coded cardiac diagnosis who were referred for stress testing after outpatient chest pain evaluation, few proceeded to invasive angiography or revascularization, and subsequent cardiovascular events were infrequent.
INTRODUCTION
Chest pain is a common indication for outpatient evaluation in the United States, with 500,000 American adults age 45 or older newly diagnosed with stable angina pectoris annually.1 In such patients, non-invasive cardiac stress testing, including exercise treadmill testing (ETT), stress echocardiography and nuclear myocardial perfusion imaging (MPI) can provide valuable diagnostic and prognostic information.2–4
Non-invasive testing can most meaningfully guide further care, specifically regarding the need for invasive cardiac catheterization and/or revascularization, in patients with intermediate (10–90%) pre-test likelihood of obstructive coronary artery disease (CAD)5, as recommended by ACC/AHA clinical practice guidelines and appropriate use criteria. These guidelines recommend conservative care for patients with lower likelihood and direct invasive evaluation in those with higher likelihood of obstructive CAD.6–10
Significant increases in medical imaging utilization have led to concerns about possible overuse of diagnostic testing, with associated detrimental economic and health effects.11–13 Simultaneously, there is speculation that increased non-invasive testing could improve care and limit unnecessary invasive procedures.14–16
Despite these unresolved issues, patterns of downstream procedures and clinical outcomes after stress testing in patients without a prior diagnosis of CAD have not been well described. Therefore, we report rates of angiography, revascularization, and clinical events after elective stress testing among symptomatic middle-aged outpatients in a large national claims database.
METHODS
Data Source
We analyzed administrative claims data from United Healthcare (UHC) for 17.7 million covered individuals from November 2004 to June 2007. Hospital claims included International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9) diagnosis and procedures codes, Current Procedure Terminology (CPT) procedure codes, dates of service, discharge disposition (including vital status) and facility zip code. Physician claims included ICD-9 and CPT codes, service dates, and provider zip code.
The geographic locations of stress test providers were sorted into 306 previously developed hospital referral regions, defined by zip code.17
Study Population
Inclusion
To identify stable patients referred for stress testing to evaluate chest pain, the initial cohort included all individuals aged 40–64 years with a qualifying non-invasive cardiac stress test within 30 days after a coded outpatient chest pain encounter [stress tests identified by CPT codes (ETT: 93015-18; nuclear stress MPI: 78460-61, 78464, 78465, 78472-73, 78481, 78483, 78491-92, stress echocardiography: 93350) and outpatient chest pain visits defined by ICD-9 codes (angina: 413.x; chest pain: 786.5, 786.50, 786.51, or 786.59)].
ETT and echocardiographic testing performed on the same day were considered a single stress echocardiography event; to account for multi-day protocols, a nuclear MPI procedure within 24 hours of ETT was considered a single stress nuclear event.
Exclusion
Patients were required to have at least 180 days of continuous UHC coverage before their index stress test so that diagnoses and procedures coded in episodes prior to the index stress test could be captured. We excluded patients with any prior inpatient diagnosis of CAD or heart failure, or any outpatient physician claim diagnosis of CAD or heart failure more than 30 days prior to the index stress test by ICD-9 code [410.x, 411.x, 412.x, 413.x, 414.x, 428.x, 786.5x (except 786.52)]. Patients with procedure codes for previous coronary catheterization or revascularization procedure prior to the index stress test or with a stress test diagnosis of pre-operative cardiovascular exam were excluded [cardiac catheterization: identified by CPT codes 93510, 93508, 93526, 93539-40, 93543, or 93545, or ICD-9 codes 37.22, 37.23, or 88.53-88.57; percutaneous coronary intervention (PCI) identified by CPT codes 92980-2, 92984, 92995-6, 92973, or G0290-1, or ICD-9 codes 00.66, or 36.0x; coronary artery bypass grafting (CABG): identified by CPT codes 33510-14, 33516, 33533-36, 33517-19, or 33521-23, or ICD-9 codes 36.1x, 36.2x, 36.31, or 36.32; preoperative cardiovascular exam identified by ICD-9 code V72.81]. Patients under 40 years of age were excluded; patients 65 and older were excluded because many in this age group had incomplete UHC claims data due to concurrent Medicare coverage.
Cardiac computed tomography angiography and stress magnetic resonance imaging studies were not included due to very low utilization rates (<1%) during the study period.
Outcomes
Invasive coronary angiography and revascularization procedures were identified using CPT and ICD-9 codes (as above for cardiac catheterization, PCI, and CABG). Myocardial infarction (MI), and stroke were identified using ICD-9 codes in inpatient claims (MI: 410.00–410.70, 410.01–410.71; stroke: 430.x-432.x, 434.x). Death dates were provided by UHC, based on inpatient deaths and Social Security Death Master File.
Statistical Analysis
We evaluated demographic characteristics and comorbid conditions of patients and hospital referral region of stress test providers overall and by initial stress test modality. Comorbid conditions were defined using published algorithms18 and based on inpatient and physician claims in the six months preceding the index stress test. Continuous variables are described as means with interquartile ranges; categorical variables are presented as percentages. We assessed cardiac catheterization, revascularization, and clinical event rates using cumulative incidence functions, treating death as a competing risk and accounting for administrative censoring due to staggered entry. Cumulative incidence curves were stratified and compared by initial stress test type and by age/sex groupings. Age and sex adjusted rates of catheterization and of revascularization one year after stress testing were estimated for referral regions using Cox proportional hazard models. The correlation between the one-year catheterization rate and the proportion of patients undergoing catheterization who had PCI or CABG within one year of stress was also assessed graphically and using the Pearson correlation coefficient..
Population mortality rates, by year of age and sex, were obtained for the year 2006 for the United States (US),19 and proportionally weighted to match the age and sex of the study cohort. Expected US population death rates were derived for comparison with observed death rates in the study cohort.
All statistical analyses were performed using SAS 9.2 (SAS Institute, Cary, North Carolina) and R 2.11.1 (R Foundation for Statistical Computing, Vienna, Austria). The Duke University Institutional Review Board reviewed and approved the study design.
RESULTS
Patient Cohort
We identified 80,676 people aged 40 to 64 years (53% women, mean age 51.5 years) without prior cardiac diagnosis or evaluation referred for outpatient stress testing to evaluate chest pain without acute coronary syndrome (Figure I). Fifty-four percent were referred for nuclear stress MPI, 21% underwent stress echocardiography, and 25% had ETT without imaging as their initial test. (Table I)
Figure I.
Study population.
Table I.
Patient characteristics and location at time of non-invasive cardiac stress testing, by test type
| All (N=80,676) |
Nuclear Stress MPI (N=43,700) |
Stress ECHO (N=16,879) |
ETT (N=20,009) |
P-value | |
|---|---|---|---|---|---|
| Age (mean, interquartile range) | 51.5 (46, 57) | 52.1 (47, 57) | 50.8 (45, 56) | 50.7 (45, 56) | <.001 |
| Female (%) | 53 | 54 | 57 | 46 | <.001 |
| Diabetes | 9.9 | 12.0 | 7.0 | 7.9 | <.001 |
| Hypertension | 38.9 | 43.3 | 34.5 | 33.2 | <.001 |
| Hyperlipidemia | 38.1 | 40.7 | 34.1 | 36.0 | <.001 |
| Cerebrovascular Disease | 2.4 | 3.0 | 2.1 | 1.6 | <001 |
| Peripheral Vascular Disease | 1.9 | 2.2 | 1.2 | 1.5 | <.001 |
| Chronic Pulmonary Disease | 7.4 | 8.1 | 6.4 | 6.7 | <.001 |
| Hospital Referral Region* (%) | (% of Total) | (% of Stress Tests in Referral Region)† | <.001 | ||
| Atlanta (2,647) | 3 | 57 | 9 | 34 | |
| Charlotte (914) | 1 | 49 | 34 | 17 | |
| Columbus (1,814) | 2 | 64 | 19 | 17 | |
| Dallas (4,037) | 5 | 32 | 47 | 21 | |
| Denver (1,138) | 1 | 50 | 10 | 40 | |
| East Long Island (1,591) | 2 | 65 | 10 | 25 | |
| Ft. Lauderdale (1,164) | 1 | 79 | 11 | 10 | |
| Ft. Worth (1,684) | 2 | 38 | 39 | 23 | |
| Houston (5,320) | 7 | 61 | 3 | 36 | |
| Kansas City (1,419) | 2 | 38 | 51 | 11 | |
| Los Angeles (962) | 1 | 21 | 31 | 48 | |
| Manhattan (1,375) | 2 | 48 | 19 | 33 | |
| Miami (1,340) | 2 | 87 | 7 | 6 | |
| Milwaukee (1,339) | 2 | 54 | 24 | 21 | |
| Nashville (893) | 1 | 65 | 6 | 29 | |
| Orlando (1,987) | 2 | 73 | 3 | 23 | |
| Phoenix (1,437) | 2 | 50 | 14 | 36 | |
| Raleigh (942) | 1 | 53 | 39 | 8 | |
| St. Louis (2,812) | 4 | 55 | 33 | 12 | |
| Tucson (993) | 1 | 17 | 24 | 59 | |
| ALL OTHERS (44,744) | 56 | 55 | 21 | 24 | |
MPI = myocardial perfusion imaging; ECHO = echocardiography; ETT = exercise treadmill testing.
Data unavailable for 124 patients.
May not sum to 100 due to rounding.
Patients referred for nuclear MPI tended to be older (mean age 52.1 years vs. 50.8 years for echocardiography and 50.7 years for ETT, p<0.001). Stress echocardiography and nuclear MPI patients were more likely to be female than patients undergoing ETT alone (57%, 54%, and 46%, respectively, p<0.001). Compared to those referred to stress ECHO and ETT, patients referred for nuclear MPI testing had higher rates of comorbid diagnoses including diabetes, hypertension, hyperlipidemia, cerebrovascular disease, peripheral vascular disease, and chronic pulmonary disease (p<0.001).
Test locations were distributed across 306 hospital referral regions; the 20 most common referral regions accounted for 44% of the population. Initial stress test distribution varied across the 20 largest referral regions, with rates of nuclear MPI ranging from 17% to 87%, and stress echocardiography from 3% to 51% (p<0.001).
Downstream Procedures and Clinical Events
Figure II displays cumulative rates of procedures and clinical events over time through one year following stress testing. Within 60 days of stress testing, only 8.8% of patients underwent subsequent invasive coronary angiography and only 2.7% had received coronary revascularization. One-year rates of myocardial infarction, stroke, and death, were 0.2%, 0.1%, and 0.2%, respectively.
Figure II.
Cumulative incidence of (a) procedures and (b) clinical events after stress testing.
Cath indicates cardiac catheterization; revasc indicates coronary revascularization.
Factors Associated with Outcomes
Tables IIa and IIb show one-year catheterization, revascularization, and clinical event cumulative incidence rates stratified by initial stress test modality and by patient age and sex, respectively. Men, older patients, and those referred for nuclear studies were more likely to be referred for subsequent invasive coronary angiography and revascularization, and were more likely to have adverse outcomes.
Table II.
1-year event rates (%) by test type (a) and patient demographics (b)
| a. | |||||
|---|---|---|---|---|---|
| All (N=80,676) |
Nuclear Stress MPI (N=43,700) |
Stress ECHO (N=16,879) |
ETT (N=20,097) |
P- value |
|
| Procedures | |||||
| Catheterization | 10.5 | 13.81 | 6.4 | 6.90 | <.001 |
| Revascularization | 3.3 | 4.1 | 2.3 | 2.3 | <.001 |
| PCI | 2.5 | 3.1 | 1.6 | 1.8 | <.001 |
| CABG | 0.9 | 1.21 | 0.7 | 0.6 | <.001 |
| Clinical Events | |||||
| Death/MI/Stroke | 0.5 | 0.6 | 0.4 | 0.4 | .004 |
| Death | 0.2 | 0.2 | 0.1 | 0.1 | <.001 |
| MI | 0.2 | 0.3 | 0.2 | 0.2 | .32 |
| Stroke | 0.1 | 0.1 | 0.1 | 0.1 | .43 |
| b. | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| All (N=80,676) |
Men (N=38,085) |
Women (N=42,591) |
|||||||
| Men N=38,085 |
Women N=42,591 |
P- value |
40–54y N=25,380 |
55–64y N=12,705 |
P- value |
40–54y N=26,380 |
55–64y N=16,211 |
P-value | |
| Procedures | |||||||||
| Catheterization | 12.9 | 8.5 | <.001 | 10.1 | 18.5 | <.001 | 7.0 | 10.8 | <.001 |
| Revascularization | 5.5 | 1.3 | <.001 | 3.4 | 9.5 | <.001 | 0.8 | 2.2 | <.001 |
| PCI | 4.0 | 1.0 | <.001 | 2.7 | 6.7 | <.001 | 0.6 | 1.7 | <.001 |
| CABG | 1.6 | 0.3 | <.001 | 0.9 | 3.0 | <.001 | 0.2 | 0.6 | <.001 |
| Clinical Events | |||||||||
| Death/MI/Stroke | 0.7 | 0.3 | <.001 | 0.5 | 1.0 | <.001 | 0.2 | 0.4 | .011 |
| Death | 0.2 | 0.1 | .002 | 0.1 | 0.4 | <.001 | 0.1 | 0.2 | .004 |
| MI | 0.4 | 0.1 | <.001 | 0.3 | 0.7 | <.001 | 0.1 | 0.1 | .18 |
| Stroke | 0.1 | 0.1 | .25 | 0.1 | 0.1 | .39 | 0.1 | 0.1 | .64 |
CABG = coronary artery bypass grafting; ECHO = echocardiography; ETT = exercise treadmill testing; MI = myocardial infarction; MPI = myocardial perfusion imaging; PCI = percutaneous coronary intervention.
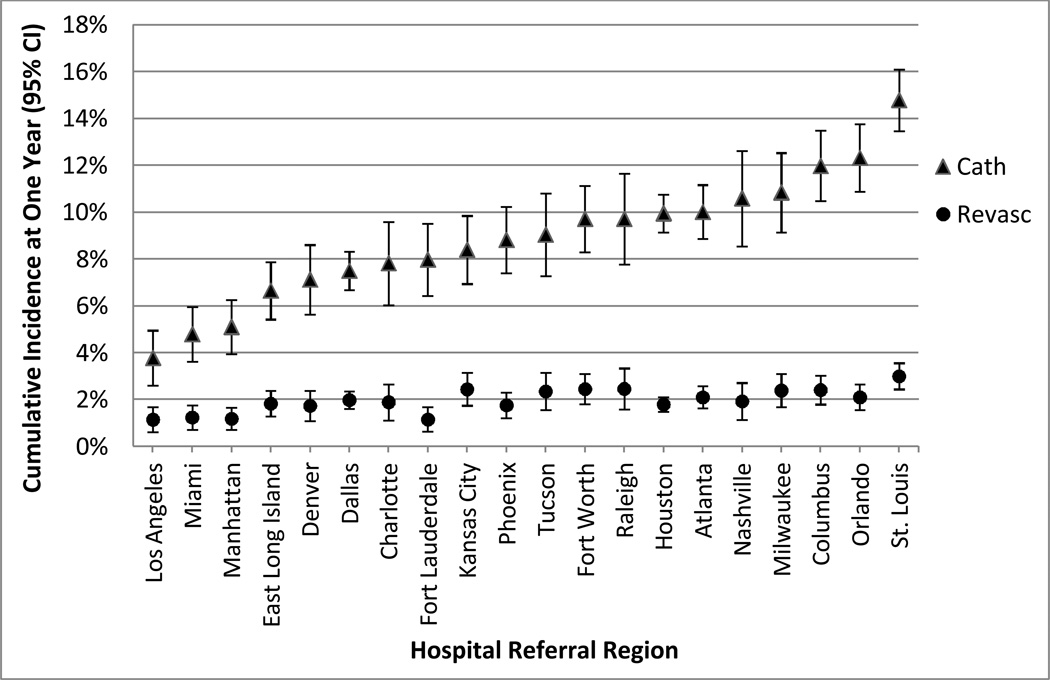
Rates of invasive coronary angiography and revascularization (PCI and CABG) after stress testing varied across the 20 largest hospital referral regions. After adjustment for age and gender, the estimated catheterization rate at one year after stress testing ranged from 3.8% to 14.8% among regions, and revascularization rates ranged from 1.2% to 3.0%. Figure III. Across regions, the proportion of patients who underwent revascularization relative to those who underwent cardiac catheterization ranged from 15% to 31%, with an inverse correlation to regional catheterization rate (correlation coefficient = −0.57, p=0.007).
Figure III.
One-year cumulative procedure rates, by hospital referral region, adjusted for age and sex. One-year adjusted procedure rates for the overall study cohort were 9.9% for catheterization and 2.2% for coronary revascularization.
Cath indicates cardiac catheterization; revasc indicates coronary revascularization.
Mortality Comparison
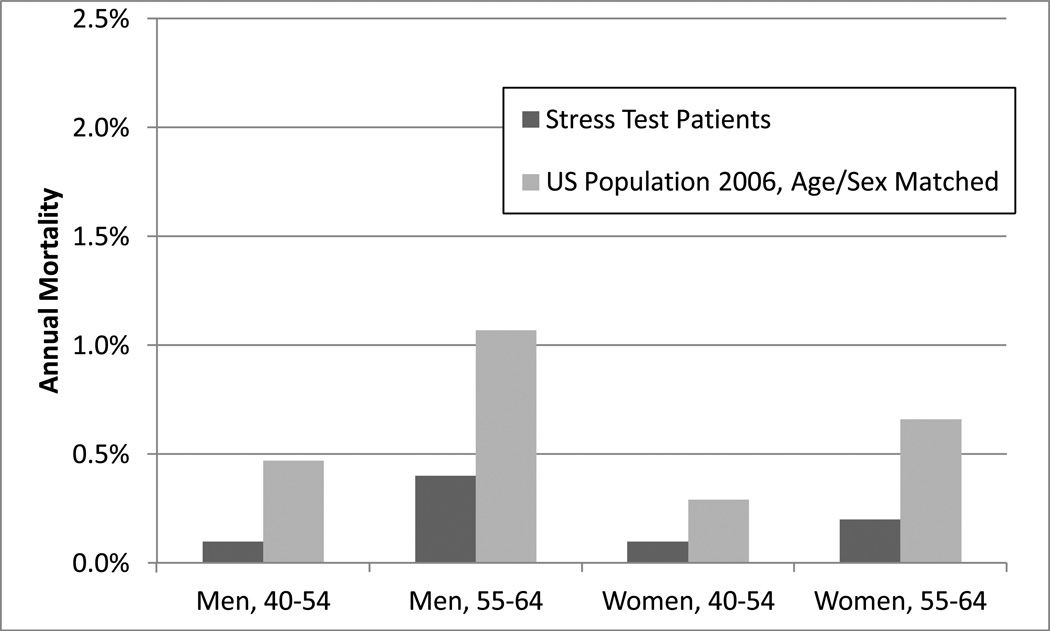
We compared mortality rates of the study cohort, stratified by age and sex, to age and sex matched US population mortality rates for 2006. In each strata, the study cohort had a numerically lower mortality rate than the general US population. Figure IV.
Figure IV.
Mortality rate at one year, stress test patients and United States population.
DISCUSSION
In this nationwide cohort of 80,676 middle-aged patients undergoing elective stress testing within 30 days after outpatient chest pain evaluation, less than 10% of patients proceeded to invasive coronary angiography, and only 1 in 37 (2.7%) received coronary revascularization within 60 days of stress testing. There were very low subsequent rates of adverse clinical events, with only 1 in 200 patients (0.5%) suffering MI, stroke, or death within one year.
This is the largest study of its type, to our knowledge, to report rates of downstream procedures and outcomes after elective, non-invasive evaluation of chest pain in a geographically dispersed United States population of patients without known cardiac disease. Previous single center reports and smaller registries looking at comparable populations have been published. For example, the event rate in the current study was lower than that noted in a registry of 3031 European patients (mean age 61 years, 42% female) newly diagnosed with stable angina by a cardiologist (one third of whom had negative stress testing and one third of whom had angiographically-proven obstructive CAD) in which the one-year rate of either myocardial infarction or death was 2.3%.20 Hachamovitch et al studied 2,200 consecutive patients without known CAD (mean age 61 years, 60% men) referred to Cedars Sinai for nuclear MPI testing and described 60-day catheterization and revascularization rates of 8.4% and 4.0% respectively. Excluding patients with early revascularization, they reported a 0.6% death rate and 1.2% non-fatal MI rate after 566 days of follow up.3
Consistent with known demographic and clinical factors,14,21,22 increasing age and male sex were associated with higher risk of downstream events and procedures. Patients referred for initial nuclear stress MPI testing were almost twice as likely to undergo catheterization, revascularization, or to suffer downstream events as those referred for stress echocardiography or ETT. Because there is no appropriate comparator group in our data set, we provided the age and sex matched mortality rates for the general, unselected United States population (Figure IV) as a point of reference. It is important to note that patients in the study cohort were all privately insured without known cardiac disease and capable of undergoing stress testing, and may therefore carry a better prognosis than the general population.
Pretest Likelihood of CAD
Although we were unable to assess directly pre-stress-test likelihood of CAD in individual patients, the low downstream event rates (all-cause mortality 0.2%/year and myocardial infarction 0.2%/year) are consistent with low prevalence and low pre-test probability of significant CAD on a population level. For comparison, Bangalore et al have described 3,259 consecutive patients (mean age 60 years, 48% men) referred for stress echocardiography at Columbia University’s St. Luke’s-Roosevelt Hospital Center and stratified by pre-test probability of coronary artery disease using a validated software algorithm that included age, sex, chest pain type, and cardiac risk factors. Those in the low pretest probability group (<15%) had annual combined cardiac death and MI rates of 0.8%, while those in the intermediate (15–85%) and high (>85% or known CAD) pretest probability groups had combined annual rates of 1.6% and 3.3%, respectively.23
Because a primary goal of non-invasive testing is to screen for patients who can safely avoid invasive angiography, low catheterization or event rates are not necessarily indicative of poor test performance or improper patient selection. However, Bayesian principles dictate that in a population with low prevalence of obstructive coronary artery disease, the use of screening tests with imperfect specificity can lead to more false positive than true positive results, with many patients without true disease referred for invasive catheterization.24 Specificity rates for current forms of noninvasive testing in intermediate risk populations are estimated at 71% for ETT, 77–84% for stress echocardiography and 62–88% for nuclear MPI.25–27
A recent analysis of almost 400,000 elective invasive angiography patients without known CAD in a national diagnostic cardiac catheterization registry demonstrated obstructive CAD in only 38% of patients.16 Over 84% had undergone some form of noninvasive testing prior to catheterization. The low rate of revascularization in our study, only 3.3% after one year in the overall population and less than one-third (31.4%) of the patients referred for catheterization, suggests that in this population a low prevalence of obstructive disease and low pretest risk for CAD could contribute to a low rate of obstructive CAD observed at catheterization, even after stress testing.
These results are consistent with the possibility that patients with lower than recommended likelihood of disease are being referred for stress testing. However, without test results (unavailable in this administrative data set), we cannot exclude other possible explanations. These include high rates of conservative management without invasive angiography or revascularization even for patients with positive or even high-risk findings on non-invasive testing. Alternatively, inadequacies in pre-test likelihood models in this population, imperfect application of available methods for disease likelihood estimation, or intentional referral of patients with low likelihood of disease for non-invasive testing for other reasons, such as valid patient or physician preference, medico-legal pressure, financial considerations, or other causes, could also contribute.
Geographic Variation
The Dartmouth Atlas Project and other studies17,28–30 have demonstrated wide geographic variation in the use of cardiovascular imaging procedures, with increased use of non-invasive testing associated with increased invasive testing, but not with improvements in outcomes.
In the current study, although we cannot address population stress testing rates, there was almost four-fold variation in adjusted catheterization rates after stress testing across the 20 largest hospital referral regions studied, from 3.8% in Los Angeles to 14.8% in St. Louis. Revascularization rates among stress test patients demonstrated similar variation, ranging from 1.2% to 3.0% (Figure III). There were also geographic variations in selection of initial test with, for example, a 5-fold variation in the percent of patients referred to nuclear stress MPI as the initial test (17% in Tucson versus 87% in Miami).
Limitations
Given the retrospective nature of this study and use of an administrative data set, results should be considered descriptive and hypothesis generating. Stress test and catheterization results were not available and were inferred from subsequent patterns of care. For this reason, we chose a conservative one-year time period for reporting of downstream procedures, in addition to evaluating more clinically meaningful 60-day procedure rates. Likewise, history of cardiovascular disease, comorbid conditions, and indications for testing and presence of symptoms were derived from coding on insurance billing documents, and details of the clinical presentation were not available.
Patients referred directly to catheterization without non-invasive stress testing after outpatient chest pain visits were not included in our cohort, potentially removing a higher risk group from the study population. We do not know the total number of chest pain patients, so generalizability of these findings to patients with chest pain not referred for stress testing is not possible.
Clinical event (MI and stroke) rates were identified from inpatient claims data, and some events may not have been recorded. Mortality data was derived from recorded inpatient deaths and from the Social Security Death Master File, which may not fully capture all deaths. Prior validation work, however, suggests that the vast majority of deaths should be ascertained in this way.31–33 Event rates at one year may not fully reflect longer-term risk in this middle-aged cohort. Clustering of events and procedures, especially MI and revascularization, early in the follow up period, however, supports the validity of one-year follow up to capture meaningful outcomes after evaluation of chest pain.
While our study drew from a large, geographically dispersed cohort, it only included subscribers of one national insurance company and the markets in which it operates. Therefore, the results may not be generalizable to other health payment mechanisms or other markets in the country. Geographic variations only reflect patterns among patients insured by UHC, and may not reflect general practice patterns in a given area. Due to incomplete claims data in UHC subscribers who are also enrolled in Medicare, our findings are limited to those under 65 years of age.
Certain assumptions are implicit in the interpretation of our data. By limiting our cohort to patients without prior cardiac history who received stress testing within 30 days after an outpatient visits for chest pain, we assumed that stress testing was ordered primarily to aid in the evaluation of possible obstructive CAD in this population of symptomatic patients. Our analysis cannot account for other indications for testing, the prognostic data derived from exercise testing or stress imaging beyond detection of inducible ischemia, or for medico-legal pressures to perform testing; nor should these results be taken to minimize the value inherent in a negative stress test finding for clinical decision making or patient reassurance.
Clinical Implications and Conclusions
Our findings demonstrate that in real-world practice, symptomatic middle-aged patients without known CAD referred for elective stress testing were unlikely to require revascularization or have adverse events at one year. These results underscore the need for accurate assessment of pre-test likelihood of disease and clinical events to guide referral to non-invasive stress testing.
REFERENCES
- 1.Rosamond W, Flegal K, Furie K, et al. Heart disease and stroke statistics--2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117(4):e25–e146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 2.Mark DB, Shaw L, Harrell FE, Jr, et al. Prognostic value of a treadmill exercise score in outpatients with suspected coronary artery disease. N Engl J Med. 1991;325(12):849–853. doi: 10.1056/NEJM199109193251204. [DOI] [PubMed] [Google Scholar]
- 3.Hachamovitch R, Berman DS, Kiat H, et al. Exercise myocardial perfusion SPECT in patients without known coronary artery disease: incremental prognostic value and use in risk stratification. Circulation. 1996;93(5):905–914. doi: 10.1161/01.cir.93.5.905. [DOI] [PubMed] [Google Scholar]
- 4.Picano E, Severi S, Michelassi C, et al. Prognostic importance of dipyridamole-echocardiography test in coronary artery disease. Circulation. 1989;80(3):450–457. doi: 10.1161/01.cir.80.3.450. [DOI] [PubMed] [Google Scholar]
- 5.Pauker SG, Kassirer JP. The threshold approach to clinical decision making. N Engl J Med. 1980;302(20):1109–1117. doi: 10.1056/NEJM198005153022003. [DOI] [PubMed] [Google Scholar]
- 6.Klocke FJ, Baird MG, Lorell BH, et al. ACC/AHA/ASNC guidelines for the clinical use of cardiac radionuclide imaging--executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASNC Committee to Revise the 1995 Guidelines for the Clinical Use of Cardiac Radionuclide Imaging) J Am Coll Cardiol. 2003;42(7):1318–1333. doi: 10.1016/j.jacc.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 7.Cheitlin MD, Armstrong WF, Aurigemma GP, et al. ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography--summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASE Committee to Update the 1997 Guidelines for the Clinical Application of Echocardiography) J Am Coll Cardiol. 2003;42(5):954–970. doi: 10.1016/s0735-1097(03)01065-9. [DOI] [PubMed] [Google Scholar]
- 8.Douglas PS, Khandheria B, Stainback RF, et al. ACCF/ASE/ACEP/AHA/ASNC/SCAI/SCCT/SCMR 2008 appropriateness criteria for stress echocardiography: a report of the American College of Cardiology Foundation Appropriateness Criteria Task Force, American Society of Echocardiography, American College of Emergency Physicians, American Heart Association, American Society of Nuclear Cardiology, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance endorsed by the Heart Rhythm Society and the Society of Critical Care Medicine. J Am Coll Cardiol. 2008;51(11):1127–1147. doi: 10.1016/j.jacc.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 9.Gibbons RJ, Balady GJ, Bricker JT, et al. ACC/AHA 2002 guideline update for exercise testing: summary article. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines) J Am Coll Cardiol. 2002;40(8):1531–1540. doi: 10.1016/s0735-1097(02)02164-2. [DOI] [PubMed] [Google Scholar]
- 10.Hendel RC, Berman DS, Di Carli MF, et al. ACCF/ASNC/ACR/AHA/ASE/SCCT/SCMR/SNM 2009 Appropriate Use Criteria for Cardiac Radionuclide Imaging: A Report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the American Society of Nuclear Cardiology, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the Society of Cardiovascular Computed Tomography, the Society for Cardiovascular Magnetic Resonance, and the Society of Nuclear Medicine. J Am Coll Cardiol. 2009;53(23):2201–2229. doi: 10.1016/j.jacc.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 11.Einstein AJ, Moser KW, Thompson RC, et al. Radiation dose to patients from cardiac diagnostic imaging. Circulation. 2007;116(11):1290–1305. doi: 10.1161/CIRCULATIONAHA.107.688101. [DOI] [PubMed] [Google Scholar]
- 12.Iglehart JK. The new era of medical imaging--progress and pitfalls. N Engl J Med. 2006;354(26):2822–2828. doi: 10.1056/NEJMhpr061219. [DOI] [PubMed] [Google Scholar]
- 13.Lauer MS. Elements of danger--the case of medical imaging. N Engl J Med. 2009;361(9):841–843. doi: 10.1056/NEJMp0904735. [DOI] [PubMed] [Google Scholar]
- 14.Diamond GA. A clinically relevant classification of chest discomfort. J Am Coll Cardiol. 1983;1(2 Pt 1):574–575. doi: 10.1016/s0735-1097(83)80093-x. [DOI] [PubMed] [Google Scholar]
- 15.Lin GA, Dudley RA, Lucas FL, et al. Frequency of stress testing to document ischemia prior to elective percutaneous coronary intervention. JAMA. 2008;300(15):1765–1773. doi: 10.1001/jama.300.15.1765. [DOI] [PubMed] [Google Scholar]
- 16.Patel MR, Peterson ED, Dai D, et al. Low diagnostic yield of elective coronary angiography. N Engl J Med. 2010;362(10):886–895. doi: 10.1056/NEJMoa0907272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The Dartmouth Atlas of Health Care 1998. Lebanon, NH: American Hospital Association; 1998. The Center for the Evaluative Clinical Sciences DMS. [PubMed] [Google Scholar]
- 18.Birman-Deych E, Waterman AD, Yan Y, et al. Accuracy of ICD-9-CM codes for identifying cardiovascular and stroke risk factors. Med Care. 2005;43(5):480–485. doi: 10.1097/01.mlr.0000160417.39497.a9. [DOI] [PubMed] [Google Scholar]
- 19.SSA. Social Security Online: Period Life Table, 2006. [Accessed December 10, 2010]; http://www.ssa.gov/OACT/STATS/table4c6.html.
- 20.Daly CA, De Stavola B, Sendon JL, et al. Predicting prognosis in stable angina--results from the Euro heart survey of stable angina: prospective observational study. BMJ. 2006;332(7536):262–267. doi: 10.1136/bmj.38695.605440.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morise AP, Haddad WJ, Beckner D. Development and validation of a clinical score to estimate the probability of coronary artery disease in men and women presenting with suspected coronary disease. Am J Med. 1997;102(4):350–356. doi: 10.1016/s0002-9343(97)00086-7. [DOI] [PubMed] [Google Scholar]
- 22.Pryor DB, Shaw L, Harrell FE, Jr, et al. Estimating the likelihood of severe coronary artery disease. Am J Med. 1991;90(5):553–562. [PubMed] [Google Scholar]
- 23.Bangalore S, Gopinath D, Yao SS, et al. Risk stratification using stress echocardiography: incremental prognostic value over historic, clinical, and stress electrocardiographic variables across a wide spectrum of bayesian pretest probabilities for coronary artery disease. J Am Soc Echocardiogr. 2007;20(3):244–252. doi: 10.1016/j.echo.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 24.Diamond GA, Kaul S. Low diagnostic yield of elective coronary angiography. N Engl J Med. 2010;363(1):93. author reply 94–95. [PubMed] [Google Scholar]
- 25.Fleischmann KE, Hunink MG, Kuntz KM, et al. Exercise echocardiography or exercise SPECT imaging? A meta-analysis of diagnostic test performance. JAMA. 1998;280(10):913–920. doi: 10.1001/jama.280.10.913. [DOI] [PubMed] [Google Scholar]
- 26.Garber AM, Solomon NA. Cost-effectiveness of alternative test strategies for the diagnosis of coronary artery disease. Ann Intern Med. 1999;130(9):719–728. doi: 10.7326/0003-4819-130-9-199905040-00003. [DOI] [PubMed] [Google Scholar]
- 27.Schuijf JD, Poldermans D, Shaw LJ, et al. Diagnostic and prognostic value of non-invasive imaging in known or suspected coronary artery disease. Eur J Nucl Med Mol Imaging. 2006;33(1):93–104. doi: 10.1007/s00259-005-1965-y. [DOI] [PubMed] [Google Scholar]
- 28.Lucas FL, DeLorenzo MA, Siewers AE, et al. Temporal trends in the utilization of diagnostic testing and treatments for cardiovascular disease in the United States, 1993–2001. Circulation. 2006;113(3):374–379. doi: 10.1161/CIRCULATIONAHA.105.560433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lucas FL, Siewers AE, Malenka DJ, et al. Diagnostic-therapeutic cascade revisited: coronary angiography, coronary artery bypass graft surgery, and percutaneous coronary intervention in the modern era. Circulation. 2008;118(25):2797–2802. doi: 10.1161/CIRCULATIONAHA.108.789446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pilote L, Califf RM, Sapp S, et al. Regional variation across the United States in the management of acute myocardial infarction. GUSTO-1 Investigators. Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries. N Engl J Med. 1995;333(9):565–572. doi: 10.1056/NEJM199508313330907. [DOI] [PubMed] [Google Scholar]
- 31.Cowper DC, Kubal JD, Maynard C, et al. A primer and comparative review of major US mortality databases. Ann Epidemiol. 2002;12(7):462–468. doi: 10.1016/s1047-2797(01)00285-x. [DOI] [PubMed] [Google Scholar]
- 32.Flory J, Yinong YX, Gurol I, et al. Place of death: U.S. trends since 1980. Health Aff (Millwood) 2004;23(3):194–200. doi: 10.1377/hlthaff.23.3.194. [DOI] [PubMed] [Google Scholar]
- 33.Schisterman EF, Whitcomb BW. Use of the Social Security Administration Death Master File for ascertainment of mortality status. Popul Health Metr. 2004;2(1):2. doi: 10.1186/1478-7954-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]