Abstract
Epithelial cells establish apical and basolateral (BL) membranes with distinct protein and lipid compositions. To achieve this spatial asymmetry, the cell utilizes a variety of mechanisms for differential sorting, delivery and retention of cell surface proteins. The EGF receptor (EGFR) and its ligand, amphiregulin (AREG), are transmembrane proteins delivered to the BL membrane in polarized epithelial cells. Herein, we show that the cytoplasmic domain of AREG contains dominant BL sorting information; replacement of the cytoplasmic domain of apically targeted NGFR with the cytoplasmic domain of AREG redirects the chimera to the BL surface. Using sequential truncations and site-directed mutagenesis of the AREG cytoplasmic domain, we identify a novel BL sorting motif consisting of a single leucine C-terminal to an acidic cluster (EEXXXL). In AP-1B-deficient cells, newly synthesized AREG is initially delivered to the BL surface like in AP-1B-expressing cells. However, in these AP-1B-deficient cells, recycling of AREG back to the BL surface is compromised, leading to its appearance at the apical surface. These results show that recycling, but not delivery, of AREG to the BL surface is AP-1B-dependent.
Keywords: amphiregulin, EGF receptor, basolateral, μ1B, AP-1B, MDCK, LLC-PK1
Introduction
Sheets of polarized epithelial cells line all body cavities, forming a physical barrier between external or luminal environments and the internal milieu. Creation and maintenance of this barrier requires the cells to establish biochemically and functionally diverse membrane surfaces with distinct protein and lipid compositions (1). The apical membrane, which is exposed to external or luminal environments, acts as a protective barrier that also allows for the regulated uptake of nutrients and ions. The lateral membrane is exposed to neighboring cells and functions in cell-cell contact and communication, while the basal membrane contacts the extracellular matrix and functions in cell-matrix contact. Together, these domains comprise the basolateral (BL) membrane, which regulates ion gradients, signal transduction, and cell adhesion (2). Because each surface serves a unique function critical for overall barrier integrity and cell function, the cell has evolved a complex network of signals, proteins, and compartments to establish and maintain the distinct membrane surfaces (3, 4). Understanding the mechanisms by which epithelial cells establish and maintain polarity is crucial to understanding normal organ function and how loss of polarity can lead to disease (5, 6).
One family of proteins distributed to the cell surface in a polarized manner is the epidermal growth factor receptor (EGFR) and its cognate ligands (7). EGFR is a transmembrane tyrosine kinase receptor involved in proliferation, migration, and cell survival, and is critical for epithelial wound repair and homeostasis (8–10). Seven mammalian EGFR ligands have been identified (EGF, amphiregulin [AREG], transforming growth factor-α [TGFα], heparin-binding EGF-like growth factor [HBEGF], betacellulin, epiregulin [EREG] and epigen [EPGN]), all of which are produced as type 1 transmembrane proteins that are delivered to the cell surface where they are cleaved by metalloproteases to release mature, soluble ligands (7). Ligands bind and activate the EGFR with differing affinities and intensities in a paracrine, autocrine, or juxtacrine manner (7, 11).
In polarized epithelial cells, EGFR, TGFα and AREG are selectively delivered to the BL surface (12–14). EGF is delivered to both the apical and BL membranes, but is selectively cleaved by BL metalloproteases, leading to steady state apical accumulation of membrane-bound EGF (15). The cytoplasmic domain of EGF contains a PXXP BL sorting motif, mutation of which results in loss of EGF at the BL surface, but not the apical surface, suggesting EGF contains separate apical and BL sorting motifs (16).
Our lab previously reported that TGFα is preferentially delivered to the BL surface through a mechanism that depends on the myristoylated cargo recognition and targeting (CaRT) protein Naked2 (NKD2) (13, 17). TGFα-containing vesicles are coated by NKD2 after emergence from the trans-Golgi network (TGN) and are delivered to the BL corner of polarized MDCK cells where they fuse with the plasma membrane in a NKD2 myristoylation-dependent manner (17). shRNA knockdown of NKD2 reduces cell surface expression of TGFα and leads to accumulation of TGFα-containing vesicles in the cytoplasm (18). TGFα is the only EGFR ligand known to require NKD2 for polarized BL trafficking.
AREG is delivered to the BL surface of polarized epithelial cells (14), although by a different mechanism than EGF and TGFα. Previous studies by our lab showed that removal of the AREG cytoplasmic domain results in the appearance of apical and BL membrane-associated AREG, suggesting that this protein lacks a separate apical sorting determinant in the extracellular and transmembrane domains (19).
The present study demonstrates that the AREG cytoplasmic domain contains dominant BL sorting information, sufficient to redirect the apically targeted 75-kDa human nerve growth factor receptor (NGFR) to the BL surface. AREG BL trafficking is directed by a novel mono-leucine-based motif (EEXXXL) that differs from similar motifs found in CD147 and stem cell factor (SCF) (20, 21). Initial delivery of AREG to the BL surface is AP-1B independent. However, AP-1B is required for recycling back to the BL surface.
Results
The cytoplasmic domain of AREG contains a dominant BL sorting motif
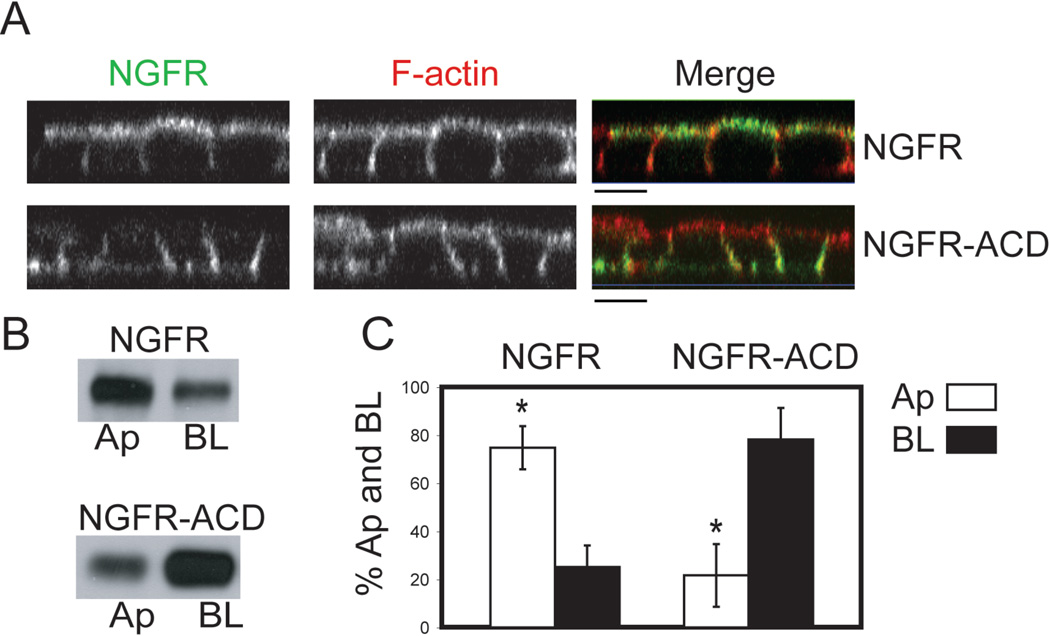
Previous work from our lab showed that AREG contains BL sorting information within the last 27 amino acids of its 31 amino acid cytoplasmic domain, since deletion of these amino acids results in its non-polar cell surface distribution in MDCK cells (19). In contrast, NGFR, which is normally localized to the apical surface of polarized MDCK cells, contains an extracellular apical sorting determinant consisting of an O-glycosylated region (22, 23). To determine whether the cytoplasmic domain of AREG is sufficient for BL delivery, a chimeric protein consisting of the extracellular and transmembrane domains of NGFR, and the cytoplasmic domain of AREG (NGFR-ACD) was generated and stably expressed in MDCK cells. Confocal immunofluorescence microscopy using an antibody specific to the ectodomain of NGFR showed NGFR on the apical surface, while NGFR-ACD was mainly present on the BL surface of polarized MDCK cells (Figure 1A). Domain-selective cell surface biotinylation showed 75% of NGFR was on the apical surface, whereas 78% of the NGFR-ACD chimera was BL (Figure 1B and C). These data indicate that the AREG cytoplasmic domain contains a BL sorting motif that overrides the NGFR apical sorting determinant, and therefore is a dominant BL sorting motif. Interestingly, CD147, which contains a canonical mono-leucine BL sorting motif (EDDXXXXXL), is unable to redirect NGFR to the BL surface (20, 24).
Figure 1. The cytoplasmic domain of AREG redirects NGFR from the apical (Ap) to the basolateral (BL) surface of polarized MDCK cells.
MDCK cells stably expressing either full-length NGFR or the NGFR extracellular and transmembrane domains fused to the AREG cytoplasmic domain (NGFR-ACD) were polarized on Transwell filters (TEER>200 Ω/cm2) and then analyzed for steady state cell surface distribution of NGFR using an NGFR ectodomain-specific antibody (ME20.4). (A) Polarized cells were stained for NGFR (green) under non-permeabilized conditions followed by permeabilization with 0.1% Triton and F-actin (red) staining. Analysis was performed by confocal microscopy. Bar, 10 µm. (B) Polarized cells were selectively biotinylated on the Ap or BL surface, immunoprecipitated with ME20.4, separated by SDS-PAGE, transferred to nitrocellulose and then probed with streptavidin-HRP, or (C) with streptavidin-IRDye680. Results were scanned on an Odyssey scanner and quantitated using Odyssey software (n=3). Columns marked with an * are significantly different.
Determination of the amino acids within the cytoplasmic domain necessary for the BL localization of AREG
Having established the presence of a dominant BL sorting motif within the cytoplasmic domain of AREG, we set out to determine the specific amino acid sequence that contains the necessary AREG BL sorting information. Sequential cytoplasmic domain truncation mutants of AREG were constructed with EGFP fused to their C-termini (Figure 2A). MDCK cells stably expressing these chimeras were selected by flow cytometric sorting for high surface expression of AREG. The apical/BL distribution of the truncation mutants was analyzed by confocal immunofluorescence microscopy (Figure 2B) and domain-selective cell surface biotinylation (Figure 2C). Full-length AREG and AREG with 6-amino acids removed from the cytoplasmic domain (25 aa) were 95% and 93% localized to the BL surface, respectively. However, truncating the cytoplasmic domain by 17-amino acids (14 aa) resulted in only 57% of total surface AREG localized to the BL surface, a statistically significant difference compared to wild-type AREG and 25 aa mutant AREG. Further truncation of the AREG cytoplasmic domain (4 aa) did not impart significant additional apical localization. Combined, these results indicate that BL sorting information resides within amino acids 236 (14 aa) through 246 (25 aa) of the AREG cytoplasmic domain.
Figure 2. AREG cytoplasmic domain (ACD) truncations reveal that residues 236–246 contain BL sorting information.
(A) Amino acid composition of ACD and points of truncation. MDCK cells stably expressing ACD truncations that were C-terminally EGFP-tagged were generated and used to determine the region of the tail that contains BL sorting information. Cells were polarized on Transwell filters and (B) surface stained for AREG (red) followed by permeabilization and F-actin (blue) staining. Analysis was performed by confocal microscopy. The AREG-EGFP channel is shown to demonstrate the EGFP tag does not disrupt cell surface delivery of AREG. Bars, 10 µm. (C) Polarized cells were selectively biotinylated on the Ap or BL surface, immunoprecipitated with anti-AREG mAb, separated by SDS-PAGE, transferred to nitrocellulose and then probed with streptavidin-IRDye680. Results were scanned on an Odyssey scanner and quantitated using Odyssey software (n=3). Columns marked with an * are significantly different from WT.
Identification of a mono-leucine based BL sorting motif
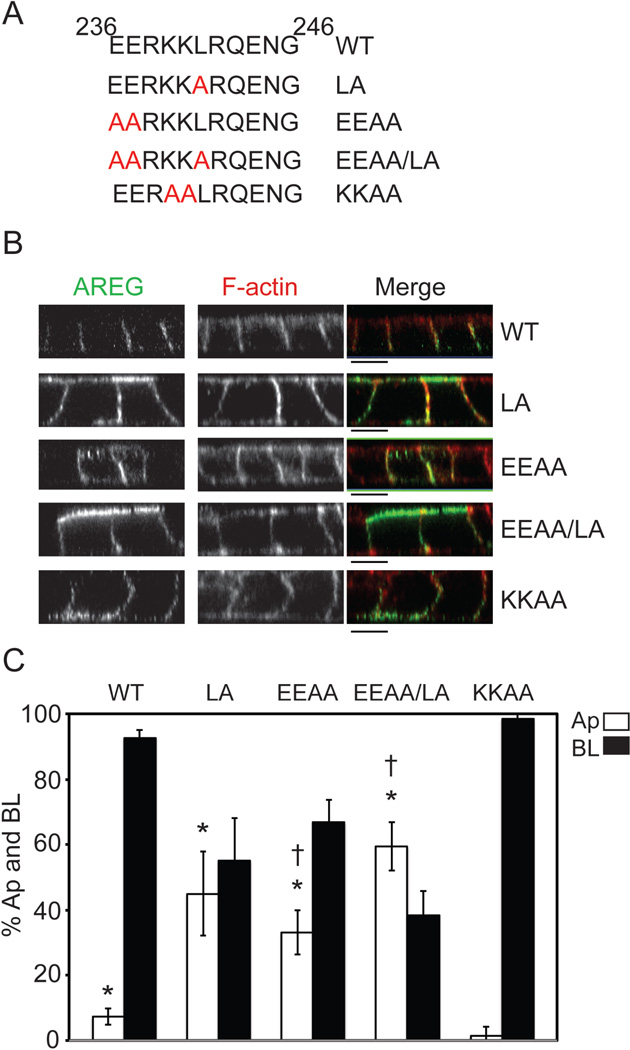
Analysis of amino acids 236 through 246 (EERKKLRQENG) revealed no obvious canonical BL sorting motifs. Comparison of this region across species shows it to be 73% identical and 91% similar, making it the most conserved region of the AREG cytoplasmic domain (Figure 3). This region contains an acidic cluster, a basic cluster and a mono-leucine. Mono-leucine BL sorting motifs consisting of an acidic cluster N-terminal of a mono-leucine (EDDXXXXXL) have been reported for two other BL proteins, SCF and CD147 (20, 21). Although this exact motif is not present in the cytoplasmic domain of AREG, we further analyzed the possibility that AREG may contain a variation of the consensus mono-leucine BL sorting motif. Using PCR-based site-directed mutagenesis, individual amino acids were substituted with alanine (A) residues (Figure 4A). When the single leucine (L) residue was changed to alanine (L241A [LA]), AREG polar BL distribution was lost (Figure 4B) with 45% of total surface AREG distributed to the apical surface at steady state (Figure 4C). When the two conserved acidic glutamine (E) residues were changed to alanine residues (EE236, 237AA [EEAA]), 33% of AREG was detected on the apical surface at steady state (Figure 4C). These changes in AREG apical distribution are both significantly different from the 7% of steady state wild-type AREG detected on the apical surface. Combination of these two mutations (EEAA/LA) resulted in 59% of total surface AREG localized at the apical membrane at steady state (Figure 4C). To exclude the possibility that the charge of the region plays a role in BL sorting (25), we mutated the two positive lysine (K) residues (KK239, 240AA [KKAA]) to alanine residues. Like wild-type AREG this mutant protein exhibited predominant BL localization with 98% present on the BL surface (Figure 4C). These results indicate that both the acidic cluster and the mono-leucine in the cytoplasmic domain of AREG, which are both highly conserved across species (Figure 3), are critical for BL sorting of AREG.
Figure 3. Schematic diagram of full-length AREG and amino acid composition of the AREG cytoplasmic domain.
AREG is synthesized as a 252- amino acid type 1 transmembrane glycoprotein. Beginning with a signal sequence (1–19), AREG contains an N-glycosylated pro-region (20–100) that is required for proper folding and secretion. Following the pro-region is mature AREG (101–184) consisting of a heparin-binding domain (HBD) and an EGF domain. The EGF domain contains the conserved spacing of six cysteine residues that is preserved in all EGFR ligands. The 31-amino acid cytoplasmic domain (222–252) is aligned with other species with the conserved residues highlighted in red.
Figure 4. Amino acid substitutions within residues 236–246 identify a mono-leucine-based sorting signal.
(A) Amino acid composition of AREG cytoplasmic domain (ACD) between residues 236 and 246. Specific amino acid mutations are indicated in red. MDCK cells stably expressing the ACD mutants were generated and used to determine the BL sorting motif. Cells were polarized on Transwell filters and (B) surface stained for AREG (green) followed by permeabilization and F-actin (red) staining. Analysis was performed by confocal microscopy. Bars, 10 µm. (C) Polarized cells were selectively biotinylated on the Ap or BL surface, immunoprecipitated with anti-AREG mAb, separated by SDS-PAGE, transferred to nitrocellulose, and then probed with streptavidin-IRDye680. Results were scanned on an Odyssey scanner and quantitated using Odyssey software (n=3). Columns marked with an * are significantly different from WT. Columns marked with a † are significantly different from each other.
Loss of AP-1B affects the polarized distribution of AREG at steady state
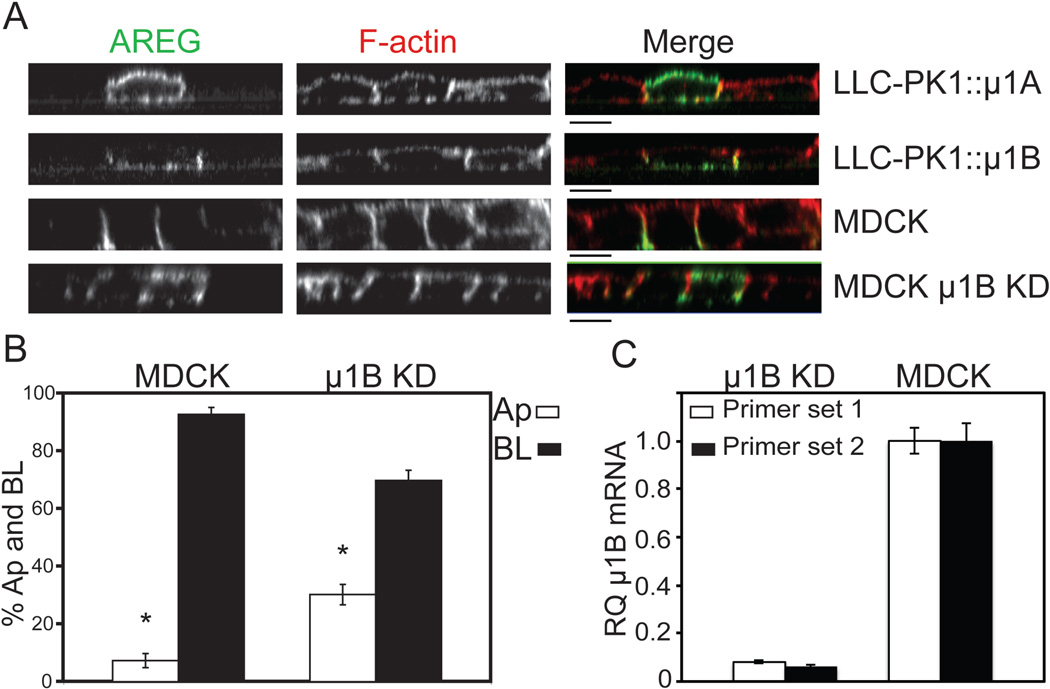
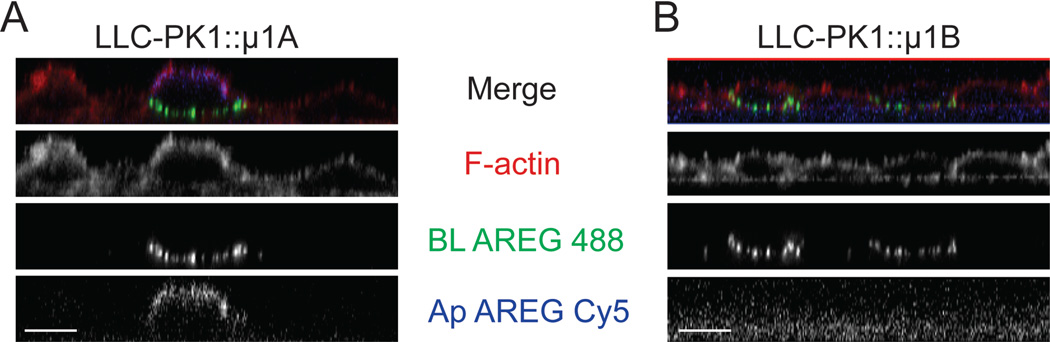
Heterotetrameric adaptor protein complexes (AP-1A, AP-1B, AP-2, AP-3A, AP-3B, AP-4) selectively incorporate cargo proteins and facilitate vesicle formation (26). Of the known AP complexes, only AP-1B and AP-4 have been implicated in BL sorting (27). AP-1B expression is epithelial cell-specific and only differs from the ubiquitous AP-1A by the medium (μ1B) subunit (28). AP-1B is localized to recycling endosomes and plays a role in biosynthetic delivery and recycling of BL proteins (29–32). Polarizing epithelial LLC-PK1 cells do not express endogenous AP-1B and, consequently, mis-sort proteins dependent on AP-1B for BL delivery (33). To determine the role of AP-1B in AREG BL delivery, clonal lines of LLC-PK1 cells expressing either pCB6∷ μ1A (LLC-PK1∷ μ1A) or pCB6∷ μ1B (LLC-PK1∷ μ1B) were transiently transfected with wild-type AREG and analyzed by confocal immunofluorescence microscopy (Figure 5A). In the LLC-PK1∷ μ1A cells, which lack AP-1B, AREG was detected on the apical and BL surface, suggesting a role for AP-1B in maintaining the polar BL distribution of AREG (Figure 5A). As expected, parental LLC-PK1 cells had similar apical and BL distribution of AREG (data not shown). In the LLC-PK1∷ μ1B cells, AREG was restricted to the BL surface and was undetectable on the apical surface (Figure 5A). These results strongly support the involvement of AP-1B in AREG BL localization. Because AREG was detected on both surfaces in LLC-PK1∷ μ1A cells at steady state, we further characterized the AREG distribution using domain-selective cell surface biotinylation. For these experiments, we used a clonal line of MDCK cells expressing shRNA against μ1B (31) because previous work has shown that LLC-PK1 cells are not ideal for biotinylation studies (33). The clonal MDCK μ1B knockdown cell line was transfected with AREG, the cells selected with G418 and enriched by flow cytometric sorting to produce a pool of μ1B knockdown MDCK cells expressing AREG. Knockdown of μ1B in the clonal line has been previously characterized (31); this was confirmed in these AREG-expressing cells by quantitative RT-PCR, which showed a 93% reduction in μ1B message compared to parental MDCK cells (Figure 5C). AREG immunoreactivity was detected on the apical surface of polarized μ1B knockdown MDCK cells (Figure 5A). To quantitate the cell surface distribution of AREG in the μ1B knockdown MDCK cells at steady state, cells were polarized on Transwell filters and selectively biotinylated on either the apical or BL surface. The μ1B knockdown MDCK cells expressed 30% of the total cell surface AREG on the apical surface at steady state, a two-fold increase over parental MDCK cells expressing endogenous wild-type μ1B (Figure 5B). This result, along with the presence of AREG on the apical surface of LLC-PK1∷ μ1A cells, demonstrates that AP-1B is required for steady state BL localization of AREG.
Figure 5. Loss of AP-1B results in apical distribution of AREG.
μ1B-deficient LLC-PK1 cells stably expressing either the μ1B (LLC-PK1∷μ1B) or μ1A (LLC-PK1∷μ1A) subunit and MDCK cells stably expressing shRNA against the μ1B subunit of AP-1B (μ1B KD) were used to demonstrate the role of AP-1B in the cell surface distribution of AREG. The cells were transiently transfected with AREG and then polarized on Transwell filters. (A) The polarized monolayer was surface stained for AREG (green) followed by permeabilization and F-actin (red) staining. Analysis was performed by confocal microscopy. Bars, 10 µm. (B) AREG-expressing parental and μ1B KD MDCK cells polarized on Transwell filters were selectively biotinylated on the Ap or BL surface, immunoprecipitated with AREG mAb, separated by SDS-PAGE, transferred to nitrocellulose, and then probed with streptavidin-IRDye680. Results were scanned on an Odyssey scanner and quantitated using Odyssey software (n=3). Columns marked with an * are significantly different. (C) Knockdown of μ1B in μ1B KD cells was confirmed by quantitative RT-PCR using two different primer sets. Relative quantity= RQ.
Biosynthetic delivery of AREG to the BL surface is dependent on the mono-leucine sorting motif and independent of AP-1B
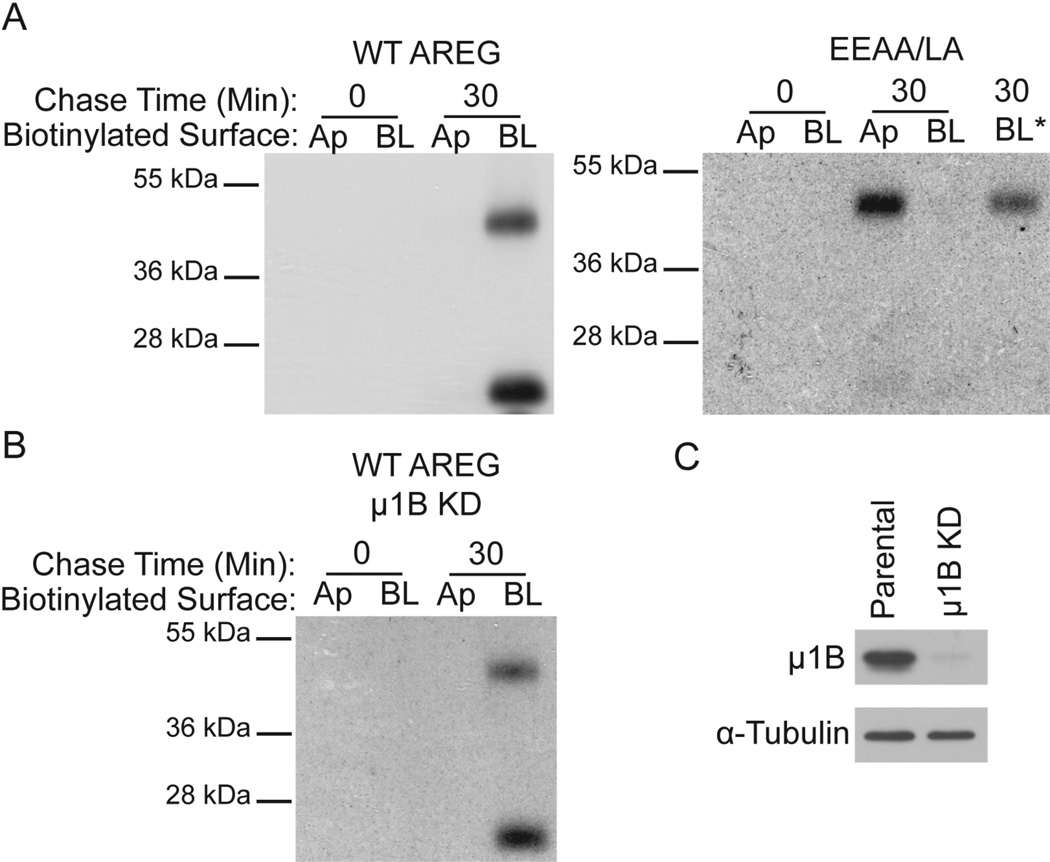
Figure 4 shows that under steady state conditions, mutation of the mono-leucine motif within the AREG cytoplasmic domain (EEAA/LA) directs approximately 60% of AREG to the apical surface and 40% to the BL surface (Figure 4). To determine if delivery of newly synthesized AREG to the BL surface is dependent on the mono-leucine motif, we performed metabolic labeling pulse chase experiments, followed by domain-selective cell surface biotinylation. MDCK cells expressing wild-type AREG or EEAA/LA mutant AREG were polarized on Transwell filters. The cells were metabolically labeled with Trans35S label for 20 minutes, chased for 0 or 30 minutes, followed by domain-selective cell surface biotinylation. The results show that wild-type AREG is detected only on the BL surface at 30 minutes (Figure 6A), supporting a previously published study from our lab (19). In marked contrast, the EEAA/LA mutant AREG is detected on the apical surface at 30 minutes, demonstrating the requirement of this mono-leucine motif in AREG for biosynthetic BL delivery (Figure 6A). Treatment with the broad-spectrum metalloprotease inhibitor batimastat resulted in detection of BL EEAA/LA mutant AREG, suggesting that newly synthesized mutant AREG is also delivered to the BL surface, but appears to be rapidly cleaved under these experimental conditions (Figure 6A). These results are consistent with the conclusion that direct BL biosynthetic delivery of AREG is dependent on the mono-leucine motif within the cytoplasmic domain.
Figure 6. BL delivery of newly synthesized AREG is dependent on the mono-leucine sorting signal and independent of AP-1B.
(A) MDCK cells expressing wild-type (WT) AREG or EEAA/LA mutant AREG and (B) μ1B knockdown MDCK cells (μ1B KD) expressing WT AREG were polarized on Transwell filters. The cells were labeled with Trans35S label for 20 minutes followed by 0 or 30 minutes chase. After the chase, the cells were selectively biotinylated on the Ap or BL surface and AREG was immunoprecipitated with anti-AREG mAb. AREG immunoprecipitates were eluted and affinity purified with streptavidin-agarose to capture cell surface AREG, separated by SDS-PAGE, and analyzed by autoradiography. BL* indicates pre-treatment with the broad-spectrum metalloprotease inhibitor batimastat (10µM). (C) Lysates from MDCK cells used above expressing endogenous μ1B (Parental) and μ1B KD cells were separated by SDS-PAGE, transferred to nitrocellulose and then blotted for canine μ1B, stripped and re-blotted for α-tubulin as a loading control.
In addition, we sought to test whether AP-1B is involved in biosynthetic delivery of AREG to the BL surface. In the absence of μ1B expression, AREG is present on the BL surface, but is also detected on the apical membrane (Figure 5), suggesting maintenance of AREG on the BL surface is AP-1B dependent. To exclude the possibility that AP-1B is involved in biosynthetic delivery of AREG to the BL surface, we performed similar metabolic labeling pulse chase followed by domain-selective cell surface biotinylation experiments in μ1B knockdown MDCK cells. These results show that newly synthesized AREG is present at the BL, but not apical, surface at 30 minutes (Figure 6B), similar to μ1B-expressing MDCK cells. We confirmed the reduction of μ1B protein level by western blotting of μ1B-expressing (Parental) and μ1B knockdown (μ1B KD) MDCK cells used in the metabolic pulse chase experiment (Figure 6C). Taken together, these data demonstrate that direct BL biosynthetic delivery of AREG is dependent on the AREG mono-leucine motif and independent of AP-1B.
Loss of AP-1B results in inappropriate recycling of post-endocytic AREG to the apical surface in fully polarized LLC-PK1 cells
The presence of steady state AREG on the apical surface of polarized epithelial cells lacking AP-1B, along with the observation that AREG BL biosynthetic delivery is AP-1B-independent, led us to ask what role AP-1B plays in the predominant BL distribution of AREG. Previous studies have shown that AP-1B is localized to the recycling endosomes and participates in both biosynthetic delivery and recycling of proteins to the BL surface (29–32). Because the majority of steady state cell surface AREG was detected on the BL surface of polarized MDCK cells lacking AP-1B (Figure 5B) and the AREG biosynthetic route is AP-1B-independent (Figure 6B), we hypothesized that recycling of post-endocytic AREG back to the BL surface was AP-1B dependent. To test this hypothesis we used LLC-PK1∷ μ1A and LLC-PK1∷ μ1B cells transfected with AREG and asked if an AREG-specific antibody applied only to the BL surface could be detected on the apical surface of cells in the presence or absence of AP-1B. The LLC-PK1∷ μ1A and LLC-PK1∷ μ1B cells were polarized on Transwell filters and the integrity of the monolayer was determined by both transepithelial electrical resistance (TEER) (>400 Ω/cm2) and the ability to inhibit diffusion of 3000 MW dextran Texas Red across the monolayer (see Materials and Methods). Once the monolayer was intact, the cells were incubated for 30 minutes at 37°C with an AREG ectodomain-specific antibody in the BL compartment only. This provided the antibody access to AREG only on the BL surface and ample time for antibody-bound AREG to be endocytosed and then transit through the recycling endosomes back to the cell surface. The cells were then washed and incubated for 10 minutes at room temperature with two secondary antibodies, anti-mouse-Cy5 on the apical surface only and anti-mouse-Alexa488 on the BL surface only. Primary antibody-bound AREG was detected on the apical surface of 80% of LLC-PK1∷ μ1A cells with positive BL AREG staining (Figure 7A), suggesting these cells, which lack AP-1B, inappropriately recycle AREG from the BL membrane to the apical surface. However, in LLC-PK1∷ μ1B cells, primary antibody-bound AREG was detected on the apical surface in only 35% of BL AREG-positive cells (Figure 7B), suggesting the importance of AP-1B in recycling of AREG back to the BL surface. These data support our hypothesis that AREG is endocytosed from the BL surface and mis-recycled to the apical surface in AP-1B deficient cells.
Figure 7. BL labeled AREG appears at Ap surface of μ1B-deficient LLC-PK1 cells.
AREG primary antibody was added only to the BL compartment of polarized μ1B-deficient LLC-PK1 cells stably expressing μ1A (LLC-PK1∷μ1A) or μ1B (LLC-PK1∷μ1B) and incubated at 37°C for 30 minutes. Cells were washed and then selectively incubated with Alexa488-conjugated secondary antibody in the BL compartment and Cy5-conjugated secondary antibody in the Ap compartment for 10 minutes at RT. (A) BL primary antibody-bound AREG was detected at the Ap surface in 80% of LLC-PK1∷μ1A cells. Only AREG positive cells were scored that had detectable BL Alexa488 secondary antibody; n=100. (B) A majority of AREG expressing LLC-PK1∷μ1B cells did not have Ap staining (only 35% showing apical Cy5 staining); n=93. The individual channels of the X–Z axis are displayed. Bar, 10 µm.
Discussion
Previous work from our lab demonstrated the importance of the cytoplasmic domain for polar distribution of AREG (19). However, the requirements for polarized BL AREG delivery were not described. The present study extends those observations to show the AREG cytoplasmic domain contains a dominant BL sorting motif consisting of a mono-leucine C-terminal to an acidic cluster, and show that the adaptor protein, AP-1B, is necessary to maintain the polar BL distribution of AREG.
The 31-amino acid cytoplasmic domain of AREG is 35% identical across species (Figure 3). We showed that the cytoplasmic domain is sufficient for BL distribution (Figure 1), and using cytoplasmic domain truncation mutants, we identified amino acids 236–246 in the AREG cytoplasmic domain as necessary for the polarized BL distribution of AREG (Figure 2). These amino acids constitute the most conserved region of the cytoplasmic domain that is 73% identical and 91% similar across species (Figure 3). In addition, extending the deletion to 4 aa did not significantly disrupt the polarized distribution of AREG further than the 14 aa (Figure 2C). Of note, this deleted region (amino acids 226–235) is only 22% identical across species. However, we cannot exclude the possibility that additional information in the juxtamembrane region contributes to the steady state BL localization of AREG.
We previously reported that the juxtamembrane region of TGFα was sufficient to maintain its BL distribution (34). There are similarities between the amino acid composition of the AREG (QLRRQYVRK) and TGFα (HCCQVRKH) juxtamembrane regions. However, in contrast to TGFα, the presence of this region alone in AREG does not maintain polar BL distribution (Figure 2) (34). Also, unlike TGFα, AREG BL delivery is not dependent on NKD2, which, in part, binds the juxtamembrane region of TGFα (17). The AREG juxtamembrane region does contain tyrosine residues at positions 227 and 231. Because tyrosine residues are established constituents of BL sorting motifs (35, 36), we mutated these tyrosine residues individually and in combination to alanine residues, but no change in AREG polarity was observed (Table 1 and data not shown). In addition, we confirmed that removal of all but 4-amino acids from the cytoplasmic domain still resulted in 35% of total surface AREG being distributed to the BL surface (Figure 2C) (19). We believe this indicates the absence of an apical sorting determinant within the ectodomain of AREG.
Table 1. AREG cytoplasmic domain and its mutants.
The 31-amino acid cytoplasmic domain of AREG (222–252) is aligned with the mutant AREG protein sequences. Alanine substitutions are highlighted red. Wild-type and mutant AREG protein surface localization is noted and is based on the analysis performed in Figure 2, Figure 3, and data not shown. Basolateral localization is indicated as BL and non-polar delivery to both surfaces is indicated as AP/BL.
| Domain Mutation | Amino Acid Composition | Localization |
|---|---|---|
| WT AREG | BL | |
| 25aa AREG | BL | |
| 14aa AREG | AP/BL | |
| 4aa AREG | AP/BL | |
| L241A | AP/BL | |
| EE236/237AA | AP/BL | |
| EEL236/237/241A | AP/BL | |
| KK239/240AA | BL | |
| Y231A | BL | |
| Y227A | BL | |
| Y227/231A | BL |
Canonical BL sorting motifs (YXXπ, FXNPXY, [DE]XXXL[LI]) (35, 37) are not present within amino acids 236–246 (EERKKLRQENG) of AREG. However, a less well-characterized BL sorting motif consisting of a mono-leucine is present. The consensus motif for a mono-leucine BL sorting signal consists of a single leucine five residues C-terminal to an acidic cluster (EEDXXXXXL) and is present in the only other two proteins identified to contain mono-leucine BL sorting motifs, CD147 and SCF (20,21, 38). Although AREG does not contain this exact consensus motif, it does contain a mono-leucine C-terminal to an acidic cluster (EEXXXL). The AREG BL sorting motif differs from CD147 not only in the consensus motif, but also in its dominance over the NGFR apical sorting determinant (Figure 1). CD147 and SCF can both redirect the apical reporter Tac to the BL surface, but CD147 is incapable of redirecting NGFR to the BL surface (20,21, 24). CD147 also has a much more pronounced apical distribution compared to AREG after mutation of the mono-leucine, with 80% of L252A CD147 distributed apically compared to 45% of L241A AREG (Figure 4C) (20). This may indicate additional BL sorting information is present in the AREG cytoplasmic domain, although removal of the AREG cytoplasmic domain results in only 65% apical distribution (Figure 2C). Also, removal of the AREG cytoplasmic domain does not result in a statistically significant difference in apical distribution compared to the 59% apical distribution of the mono-leucine motif mutant (EEAA/LA) (Figure 4C). Another commonality between SCF and CD147 is their formation of homo- and heterodimers, respectively. SCF homodimerization is necessary for efficient cell surface expression while CD147 heterodimerization with the proton-coupled monocarboxylate transporter 1 (MCT1) directs the localization of MCT1 (24, 39). It is unknown if AREG dimerizes.
The AREG mono-leucine BL sorting motif is critical for the polarized BL biosynthetic delivery of AREG as demonstrated by metabolic labeling pulse chase domain-selective cell surface biotinylation (Figure 6A). Mutation of the mono-leucine motif (EEAA/LA) results in delivery of newly synthesized EEAA/LA AREG to both apical and BL surfaces with accumulation on the apical surface. Because AREG is more rapidly cleaved from the BL surface than the apical surface (19), pre-treatment with the broad-spectrum metalloprotease inhibitor batimastat is necessary for detection of newly synthesized EEAA/LA AREG delivered to the BL surface. Batimastat is not necessary for detection of BL wild-type AREG because all of the wild-type AREG is delivered to the BL surface whereas only a portion of the EEAA/LA is delivered to the BL surface. However, over time enough EEAA/LA AREG does accumulate on the BL surface to result in approximately 40% of total surface EEAA/LA AREG to be on the BL surface at steady state (Figure 4C). The dramatic difference in surface distribution of newly synthesized EEAA/LA AREG compared to wild-type AREG highlights the importance of the mono-leucine sorting motif in AREG BL delivery.
The adaptor protein AP-1B is not necessary for the delivery of newly synthesized wild-type AREG to the BL surface (Figure 6B). After a 30-minute chase, wild-type AREG is detectable only on the BL surface of μ1B knockdown MDCK cells. Pre-treatment with batimastat is not necessary to detect BL AREG in μ1B knockdown cells, suggesting all of the AREG is delivered to the BL surface, similar to μ1B-expressing MDCK cells. Therefore, polar steady state BL distribution of AREG is AP-1B-dependent, in that newly synthesized AREG is delivered to the BL surface in AP-1B-deficient cells and, subsequently, mis-recycled to the apical membrane after internalization (Figure 5, 6 and 7). This suggests a role for AP-1B in the recycling of AREG and not in the biosynthetic delivery in fully polarized MDCK cells, as previous studies have demonstrated for LDLR, transferrin receptor (TfR), and Coxsackie-adenovirus receptor (31,32, 38–40). These previous studies demonstrated an AP-1B-independent biosynthetic route for TfR in a mature, fully polarized monolayer, but an AP-1B-dependent trans-endosomal biosynthetic route in an immature, recently confluent MDCK monolayer (31). It should be noted that all our experiments were performed on mature fully polarized monolayers.
To investigate the possibility that AP-1B affects AREG polar BL distribution due to regulation of AREG recycling, we performed an antibody binding and transcytosis assay in LLC-PK1∷ μ1A and LLC-PK1∷ μ1B cells transfected with AREG. Because the integrity of the monolayer is important in this type of assay and LLC-PK1 cells are known to form incomplete monolayers, we used stringent criteria to validate the monolayer was fully intact, performing the assay only on monolayers that prevented the diffusion of a molecule 50 times smaller than mouse IgG (3000 MW dextran Texas Red) across the monolayer. Our results support the hypothesis that post-endocytic AREG is mis-sorted to the apical surface in AP-1B-deficient cells (Figure 7), as has been shown for the LDLR and Coxsackie-adenovirus receptor (29, 40). Future work will determine how AP-1B interacts with AREG.
Mutant EEAA/LA AREG appears to have aberrant biosynthetic delivery to the apical membrane, suggesting this region is involved in biosynthetic BL delivery. However, AP-1B does not seem to be involved in AREG biosynthetic delivery because newly synthesized wild-type AREG is present on the BL surface and undetectable on the apical surface in MDCK cells with μ1B knocked down. These results are consistent with the idea that an adaptor protein other than AP-1B recognizes the mono-leucine motif and is required for BL delivery of AREG. Currently, μ1B is thought to mainly interact with tyrosine-based sorting motifs (27,36, 41). However, mutation of the two tyrosine residues in the AREG cytoplasmic domain does not disrupt AREG BL distribution in MDCK cells (Table 1). AREG may interact with AP-1B through a linker/adaptor protein, as has been recently reported for LDLR and the autosomal recessive hypercholesterolemia protein (ARH) (42). Extensive efforts by our lab have yet to identify an interacting protein for the AREG cytoplasmic domain. In the case of LDLR, ARH interacts with the proximal FXNPXY motif in the LDLR cytoplasmic domain (43). One possible explanation as to why mutation of the AREG tyrosine residues does not produce the same phenotype as knockdown of μ1B is that the tyrosine residues are also involved in the endocytosis of AREG. Mutation of these residues could prevent post-endocytic AREG from reaching recycling endosomes where mis-sorting to the apical membrane can occur (Figure 7). However, the AREG tyrosine motifs do not resemble the well-characterized NPXY internalization motif or the YXXπ motif demonstrated to interact with the medium subunit, μ2, of AP-2 (44–47). AREG does contain a YXXXXEE motif, similar to the proximal BL sorting motif in LDLR, but mutation of the acidic residues in the LDLR motif did not affect endocytosis (45). Mutation of the tyrosine in the LDLR proximal motif results in apical distribution of truncated LDLR (48), while the Y231A mutation in AREG did not affect BL delivery. Also, if this YXXXXEE motif in AREG acts as a BL signal, we would expect the L241A mutant to maintain polar BL distribution, as is the case if only one BL motif in LDLR is mutated (48). Future work to determine, the AREG motif recognized by AP-1B, the AREG endocytosis motif, and identification of interacting proteins for the cytoplasmic domain of AREG will be addressed.
The goal of this study was to identify the components necessary for the polarized BL distribution of AREG. We have demonstrated the presence of a dominant BL sorting motif in the cytoplasmic domain of AREG that consists of a mono-leucine and an acidic cluster (EEXXXL) and differs from previously described mono-leucine motifs. We have also revealed a role for AP-1B in the maintenance of AREG polarity.
Materials and Methods
Reagents and Antibodies
Cell culture media was purchased from Media Tech Inc (Manassas, VA) and fetal bovine serum from Hyclone Laboratories (Logan, UT). All chemicals were purchased from Sigma (St. Louis, MO) unless otherwise stated. Sulfo-NHS-LC-Biotin and micro-BSA protein assay kits were purchased from Pierce Biotechnology (Rockford, IL). All electrophoresis reagents were purchased from Bio-Rad Laboratories (Hercules, CA). Rainbow markers were purchased from Bio-Rad or Fermentas Life Sciences (Glen Burnie, MD). ECL reagents were purchased from Perkin Elmer (Waltham, MA). Nitrocellulose was purchased from Whatman (Dassel, Germany). DNA mini-prep and cleanup kits were purchased from Qiagen Sciences (Hilden, Germany). Protein G agarose beads were purchased from Invitrogen (Carlsbad, CA). All conjugated secondary antibodies were purchased from Jackson ImmunoResearch Laboratories Inc. (West Grove, PA). Labeled phalloidin was purchased from Invitrogen (Eugene, OR). Mouse monoclonal antibody 6R1C2.4 against human AREG was previously described (19,49, 50). Anti-NGFR p75 (ME20.4) mouse monoclonal antibody was purchased from Santa Cruz Biotechnology, Inc (Santa Cruz, CA). Streptavidin-IR-Dye680 was purchased from LI-COR Biosciences (Lincoln, NE). μ1B-specific antibodies that do not recognize μ1A were produced in rabbits immunized with a synthetic peptide (CK-EKEEVEGRPPIGV) corresponding to the carboxyterminal 366–271 residues of human μ1B conjugated with mollusk Concholepas concholepas hemocyanin (Blue Carrier, Biosonda Biotechnology, Santiago, Chile) (32). The antibody was affinity purified using the same peptide linked to Affi15 resin (Bio-Rad). Bound antibodies were eluted with 0.1M of Glycine pH 2.5 and promptly neutralized with 1M Tris pH 9.
Cells and Cell Culture
MDCK II cells were obtained from Enrique Rodriguez-Boulan (Cornell University Medical College, Ithaca, NY). MDCK μ1B KD cells were obtained from Enrique Rodriguez-Boulan and were previously described (31). LLC-PK1∷ μ1A and LLC-PK1∷μ1B cells were previously described (33). Cells were cultured as previously described (13). Cells were grown on 12 mm or 24 mm Transwells (0.4 µm pores, Corning Inc.) as previously described (19).
AREG Constructs
Human AREG cDNA encoding wild-type pro-AREG was obtained from Dr. Greg Plowman (Sugen, Redwood City, CA) (51) and Dr. Gary Shipley (Oregon Health Sciences University, Portland, OR) (52) and expressed in a pCB6 vector (50). All untagged constructs were expressed in a pCB6 vector (53). All EGFP tagged constructs were expressed in the Clontech vector pEGFP-N1 (GenBank Accession #U55762). Human NGFR cDNA was obtained from Dr. Andre Le Bivic (54). A chimera of the extracellular and transmembrane domains of NGFR with the cytoplasmic domain of AREG (NGFR-ACD) was constructed by creating a BsmI site at the transmembrane-cytoplasmic domain junction of NGFR. The cytoplasmic domain of NGFR was removed with a BsmI/XbaI digest. Using PCR, AREG cytoplasmic domain fragment was ligated to the NGFR to create an NGFR-ACD chimera. AREG cytoplasmic domain truncations and amino acid mutations were obtained by PCR QuikChange® site-directed mutagenesis of wild-type pro-AREG in a pCB6 vector as per the manufacture’s instructions (Stratagene Catalog# 200518). All DNA constructs were confirmed by sequencing prior to use.
Domain-Selective Cell Surface Biotinylation
Cells were plated on 12 mm Transwell inserts at a cell density of 1×105 cells/Transwell. Four days after plating, the transepithelial electrical resistance (TEER) for each Transwell was confirmed to be >200 Ω/cm2. Cells were then treated with 5 mM sodium butyrate overnight. On day five, the cells were washed three times with cold PBS containing 0.1 mM CaCl2 and 1.0 mM MgCl2 (1xPBS-CM) on ice. All subsequent steps were done on ice or at 4°C. Either the apical or BL cell surface was biotinylated with 0.5 mg/ml biotin in 1xPBS-CM. Cells were incubated with biotin for 20 minutes, then used biotin was removed and replaced with fresh biotin for an additional 20 minutes. The biotin was quenched with five washes of 1xPBS-CM, 0.2% BSA, 100 mM glycine followed by two washes with 1xPBS-CM. Filters were cut from the inserts and placed in 250 µl 1%NP-40 lysis buffer (50 mM Tris-HCl pH 8.0, 150 mM NaCl, 1% NP40, 2 mM EDTA) plus protease inhibitor cocktail (Sigma P2714) and rotated for 30 minutes. Cell lysates were transferred to new eppendorf tubes and centrifuged for 15 minutes at 13,000 RPM. The supernatants were then transferred to new tubes and rotated for 1 hour with 10 µl 50% slurry recombinant Protein G agarose beads to pre-clear the samples. The protein concentration of each sample was determined using a BCA protein assay. Equal protein concentration of each sample was transferred to a new tube with 1 ug of mouse anti-AREG antibody (6R1C2.4) and rotated overnight. 20 µl of recombinant Protein G agarose bead slurry was added to each sample and rotated for 3 hours. Beads were gently pelleted and washed three times with 1 ml lysis buffer. The final pellet was resuspended in 20 µl 1× bromophenol blue sample buffer (2× sample buffer: 125 mM Tris-HCl pH 6.8, 2% Glycerol, 4% SDS (w/v), 0.05% bromophenol blue) and heated at 75°C for 10 minutes. Proteins were separated by 12.5% SDS-PAGE and electrophoretically transferred to nitrocellulose membranes. All subsequent steps were performed with filtered PBS. Membranes were blocked overnight with 1xPBS, 3% BSA. Membranes were probed for 30 minutes at room temperature (RT) with streptavidin-IR-Dye680 diluted 1:25000 in 1xPBS, 3% BSA, 0.1% Tween20, 0.01% SDS. Membranes were then washed three times with 1xPBS, 0.1% Tween and two times with 1xPBS before imaged on an Odyssey® infrared imaging system (LI-COR Biosciences). Odyssey® software (version 3.0) was used to determine the integrated intensity of each band. The integrated intensities for all the bands in both the apical and BL lanes were added together to obtain a “total cell surface” value [(apical integrated intensity)+(BL integrated intensity)=total]. The integrated intensities of the bands in either the apical or BL lanes were divided by the total cell surface value to give the percentage of total value [(apical integrated intensity/total)×100= percentage of total on apical surface].
Metabolic Labeling and Pulse-chase Analysis Followed by Domain-selective Cell Surface Biotinylation
Trans35S label (Met-Cys) was purchased from ICN Biomedicals (Costa Mesa, CA). Five hundred thousand MDCK cells were seeded on 24 mm Transwell filters and grown for five days until the TEER reached 200 Ω/cm2 cells at which time they were treated with 5 mM sodium butyrate overnight. The next day filters were starved for 30 minutes with DMEM (-cys, -met) at 37°C. One hundred microliter puddles of DMEM (-cys, -met) containing Trans35S label (2 mCi/ml) were spotted on to Parafilm and filters were placed on top of these puddles and incubated for 20 minutes at 37°C. The label was then washed with DMEM (10% bovine growth serum, BGS) containing 10 times excess L-cysteine and L-methionine at 18°C, and then chased with DMEM/BGS containing 10 times excess L-cysteine and L-methionine for varying periods of time at 37°C. After the chase, filters were placed in 1xPBS-CM, 0.2% BSA at 4°C and processed for domain-selective cell surface biotinylation as described above. After the biotinylation, cells were lysed in 1% NP-40 lysis buffer and the lysates were precleared with 40 µl 50% protein G agarose bead slurry (2hr, 4°C). Twenty µl of anti-AREG antibody pre-coated beads (1ug antibody/µl beads) were added and incubated overnight at 4°C. Beads were washed three times with lysis buffer followed by elution in 40 µl buffer containing 5% SDS by boiling for 5 minutes. Supernatant was removed and diluted with additional 460 µl lysis buffer. Twenty µl of 50% streptavidin agarose bead slurry were added to each sample and incubated for 4 hr at 4°C. Beads were again washed three times with lysis buffer. Samples were finally boiled for 5 minutes in 20 µl 2× loading buffer containing 65 mM DTT, resolved on 10% SDS-PAGE, dried, and exposed to autoradiographic film to detect labeled cell surface AREG (n=3).
Immunofluorescence and Confocal Microscopy
LLC-PK1 cells were processed and stained for microscopic analysis as previously described (33). Cells were plated on 12 mm Transwell inserts at a cell density of 1×105 cells/Transwell. Cells were grown on Transwells for four days and reached appropriate TEER (MDCK >200 Ω/cm2, LLC-PK1 >400 Ω/cm2) before staining. MDCK cells were washed three times with ice cold PBS-CM then stained for 1 hour on ice with primary antibody diluted in 1xPBS-CM. Cells were washed six times with ice-cold 1xPBS-CM prior to fixation with 4% paraformaldehyde/PBS-CM for 15 minutes on ice then quenched with 50 mM ammonium chloride in 1x PBS-CM for 10 minutes. Cells were washed and blocked in 1x PBS-CM, 1% BSA, 0.2% fish skin gelatin (blocking buffer) three times over 1 hour. Secondary antibodies were diluted 1:200 in blocking buffer and incubated on cells for 1 hour. Cells were washed three times followed by a 10 minute wash with blocking buffer plus 0.1% Triton X-100. Cells were incubated with labeled phalloidin diluted 1:200 in blocking buffer for 30 minutes prior to three washes with blocking buffer and mounting in ProLong® Gold (Invitrogen P36934). Immunofluorescence imaging was acquired on a Zeiss LSM 510 confocal microscope (Zeiss Microscope Imaging, Inc., Thornwood, NY) using a 40× objective with a 2× zoom at 1024×1024 resolution. Contents of image window were exported as Tiff files using LSM Image Browser software (Version 4.2.0.121, Carl Zeiss GmbH Jena 1997–2006; Zeiss Microscope Imaging, Inc., Thornwood, NY). Tiff files were processed and cropped using Adobe Photoshop software (Version 12.0). Levels for each channel were independently modified and include all available data.
Transcytosis Assay
LLC-PK1 cells were plated on 12 mm Transwell inserts at a cell density of 1×105 cells/Transwell. Cells were grown on Transwells for six days and reached appropriate TEER (>400 Ω/cm2). Integrity of the monolayer was confirmed using 3000 MW dextran Texas Red® (Invitrogen D3328) diluted to 63 µg/ml in phenol red free complete media. Labeled dextran was added to the apical compartment of the Transwell only and incubated at 37°C for 30 minutes. 100 µl of media was removed from the BL compartment and replaced with fresh media every five minutes. The diffusion of the dextran across the monolayer was measured using a Synergy 4 BioTek plate reader and Gen5 OLE Automation software (Version 1.06.10) (BioTek Instruments, Inc., Winooski, VT). Once the monolayer was determined to be intact, mouse anti-AREG antibody (6R1C2.4) diluted 4 µg/ml in serum free media was added to the BL compartment only for 30 minutes at 37°C. Cells were washed three times with RT 1xPBS-CM, 1% BSA to remove unbound antibody. In 1xPBS-CM, 1% BSA, Alexa-488 conjugated anti-mouse secondary antibody (1:200) was added to the BL compartment only and Cy5 conjugated anti-mouse secondary antibody (1:200) was added to the apical compartment only. Cells were incubated with secondary antibodies for 10 minutes at RT, washed three times with 1xPBS-CM, 1% BSA, then fixed with 4% paraformaldehyde/PBS-CM for 15 minutes at RT. Cells were washed three times with 1xPBS-CM, 1% BSA then one 15 minute wash with 1xPBS-CM, 1% BSA, 0.1% Triton X-100. F-actin was stained with phalloidin-Texas Red® diluted 1:200 in 1xPBS-CM, 1% BSA for 30 minutes followed by three washes with 1xPBS-CM, 1% BSA then mounted with ProLong® Gold. Images were acquired as described above.
Quantitative RT-PCR
Two sets of primers were used to determine the mRNA level of canine μ1B in μ1B knockdown MDCK cells. Primer set 1 (RealTimePrimers.com) (annealing and extension: 61°C, 45 seconds) contained the forward primer 5’-ACA AGA CGG TGG AGG TTT TC -3’ and reverse primer 5’-CCT GCT GCG TGA TGT ACT CT -3’. Primer set 2 (Sigma) (annealing and extension: 65°C, 45 seconds) was self-designed and contained the forward primer 5’-CCT GAT CAG CCG CAA CTA CAA GG -3’ and reverse primer 5’-GTA CTC AGA GAA AAC CTC CAC CG -3’. Primers for canine beta-actin (RealTimePrimers.com) (annealing and extension: 61°C, 45 seconds) contained the forward primer 5’-CCC AGA TCA TGT TCG AGA CT -3’ and reverse primer 5’-CAT GAG GTA GTC GGT CAG GT -3’. Platinum® SYBR® Green qPCR supermix-UDG (Invitrogen Cat # 11733-038) was used as per the manufacture’s instructions. Samples were run and analyzed on a StepOnePlus real-time PCR system with StepOne software version 2.1 (Applied Biosystems).
Statistical Analysis
Analysis of variance was used to compare apical distribution of AREG in polarized MDCK cells. Tukey’s honestly significant difference was used for pair-wise comparisons. Statistical significance was declared for p<0.05.
Acknowledgements
This work was funded by NCI grant CA046413 to RJC. JDG was supported by the Biochemical and Chemical Training for Cancer Research NCI training grants 2 T32 CA009582. Experiments and data analysis were performed in part through the use of the VUMC Cell Imaging Shared Resource (supported by NIH grants CA68485, DK20593, DK58404, HD15052, DK59637 and EY08126). Generation of anti-μ1B antibodies was funded by a CONICYT grant# PFB-12/2007 to AG.
Special thanks to Enrique Rodriguez-Boulan for providing the MDCK μ1B knockdown cells, Gregory D. Ayers in the VICC Division of Cancer Biostatistics for statistical analysis, and Michelle Demory Beckler for reviewing the manuscript.
References
- 1.Martin-Belmonte F, Mostov K. Regulation of cell polarity during epithelial morphogenesis. Curr Opin Cell Biol. 2008;20(2):227–234. doi: 10.1016/j.ceb.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Rodriguez-Boulan E, Nelson WJ. Morphogenesis of the polarized epithelial cell phenotype. Science. 1989;245(4919):718–725. doi: 10.1126/science.2672330. [DOI] [PubMed] [Google Scholar]
- 3.Datta A, Bryant DM, Mostov KE. Molecular regulation of lumen morphogenesis. Curr Biol. 2011;21(3):R126–R136. doi: 10.1016/j.cub.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Folsch H. Regulation of membrane trafficking in polarized epithelial cells. Curr Opin Cell Biol. 2008;20(2):208–213. doi: 10.1016/j.ceb.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stein M, Wandinger-Ness A, Roitbak T. Altered trafficking and epithelial cell polarity in disease. Trends Cell Biol. 2002;12(8):374–381. doi: 10.1016/s0962-8924(02)02331-0. [DOI] [PubMed] [Google Scholar]
- 6.Tanos B, Rodriguez-Boulan E. The epithelial polarity program: machineries involved and their hijacking by cancer. Oncogene. 2008;27(55):6939–6957. doi: 10.1038/onc.2008.345. [DOI] [PubMed] [Google Scholar]
- 7.Harris RC, Chung E, Coffey RJ. EGF receptor ligands. Exp Cell Res. 2003;284(1):2–13. doi: 10.1016/s0014-4827(02)00105-2. [DOI] [PubMed] [Google Scholar]
- 8.Wells A. EGF receptor. Int J Biochem Cell Biol. 1999;31(6):637–643. doi: 10.1016/s1357-2725(99)00015-1. [DOI] [PubMed] [Google Scholar]
- 9.Jost M, Kari C, Rodeck U. The EGF receptor - an essential regulator of multiple epidermal functions. Eur J Dermatol. 2000;10(7):505–510. [PubMed] [Google Scholar]
- 10.Konturek PC, Konturek SJ, Brzozowski T, Ernst H. Epidermal growth factor and transforming growth factor-alpha: role in protection and healing of gastric mucosal lesions. Eur J Gastroenterol Hepatol. 1995;7(10):933–937. doi: 10.1097/00042737-199510000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Shoyab M, Plowman GD, McDonald VL, Bradley JG, Todaro GJ. Structure and function of human amphiregulin: a member of the epidermal growth factor family. Science. 1989;243(4894 Pt 1):1074–1076. doi: 10.1126/science.2466334. [DOI] [PubMed] [Google Scholar]
- 12.Hobert M, Carlin C. Cytoplasmic juxtamembrane domain of the human EGF receptor is required for basolateral localization in MDCK cells. J Cell Physiol. 1995;162(3):434–446. doi: 10.1002/jcp.1041620316. [DOI] [PubMed] [Google Scholar]
- 13.Dempsey PJ, Coffey RJ. Basolateral targeting and efficient consumption of transforming growth factor-alpha when expressed in Madin-Darby canine kidney cells. The Journal of biological chemistry. 1994;269(24):16878–16889. [PubMed] [Google Scholar]
- 14.Damstrup L, Kuwada SK, Dempsey PJ, Brown CL, Hawkey CJ, Poulsen HS, Wiley HS, Coffey RJ., Jr Amphiregulin acts as an autocrine growth factor in two human polarizing colon cancer lines that exhibit domain selective EGF receptor mitogenesis. British journal of cancer. 1999;80(7):1012–1019. doi: 10.1038/sj.bjc.6690456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dempsey PJ, Meise KS, Yoshitake Y, Nishikawa K, Coffey RJ. Apical enrichment of human EGF precursor in Madin-Darby canine kidney cells involves preferential basolateral ectodomain cleavage sensitive to a metalloprotease inhibitor. The Journal of cell biology. 1997;138(4):747–758. doi: 10.1083/jcb.138.4.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Groenestege WM, Thebault S, van der Wijst J, van den Berg D, Janssen R, Tejpar S, van den Heuvel LP, van Cutsem E, Hoenderop JG, Knoers NV, Bindels RJ. Impaired basolateral sorting of pro-EGF causes isolated recessive renal hypomagnesemia. The Journal of clinical investigation. 2007;117(8):2260–2267. doi: 10.1172/JCI31680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li C, Franklin JL, Graves-Deal R, Jerome WG, Cao Z, Coffey RJ. Myristoylated Naked2 escorts transforming growth factor alpha to the basolateral plasma membrane of polarized epithelial cells. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(15):5571–5576. doi: 10.1073/pnas.0401294101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li C, Hao M, Cao Z, Ding W, Graves-Deal R, Hu J, Piston DW, Coffey RJ. Naked2 acts as a cargo recognition and targeting protein to ensure proper delivery and fusion of TGF-alpha containing exocytic vesicles at the lower lateral membrane of polarized MDCK cells. Molecular biology of the cell. 2007;18(8):3081–3093. doi: 10.1091/mbc.E07-02-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown CL, Coffey RJ, Dempsey PJ. The proamphiregulin cytoplasmic domain is required for basolateral sorting, but is not essential for constitutive or stimulus-induced processing in polarized Madin-Darby canine kidney cells. The Journal of biological chemistry. 2001;276(31):29538–29549. doi: 10.1074/jbc.M102114200. [DOI] [PubMed] [Google Scholar]
- 20.Deora AA, Gravotta D, Kreitzer G, Hu J, Bok D, Rodriguez-Boulan E. The basolateral targeting signal of CD147 (EMMPRIN) consists of a single leucine and is not recognized by retinal pigment epithelium. Molecular biology of the cell. 2004;15(9):4148–4165. doi: 10.1091/mbc.E04-01-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wehrle-Haller B, Imhof BA. Stem cell factor presentation to c-Kit. Identification of a basolateral targeting domain. The Journal of biological chemistry. 2001;276(16):12667–12674. doi: 10.1074/jbc.M008357200. [DOI] [PubMed] [Google Scholar]
- 22.Le Bivic A, Sambuy Y, Patzak A, Patil N, Chao M, Rodriguez-Boulan E. An internal deletion in the cytoplasmic tail reverses the apical localization of human NGF receptor in transfected MDCK cells. The Journal of cell biology. 1991;115(3):607–618. doi: 10.1083/jcb.115.3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yeaman C, Le Gall AH, Baldwin AN, Monlauzeur L, Le Bivic A, Rodriguez-Boulan E. The O-glycosylated stalk domain is required for apical sorting of neurotrophin receptors in polarized MDCK cells. The Journal of cell biology. 1997;139(4):929–940. doi: 10.1083/jcb.139.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Castorino JJ, Deborde S, Deora A, Schreiner R, Gallagher-Colombo SM, Rodriguez-Boulan E, Philp NJ. Basolateral sorting signals regulating tissue-specific polarity of heteromeric monocarboxylate transporters in epithelia. Traffic. 2010 doi: 10.1111/j.1600-0854.2010.01155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolff SC, Qi AD, Harden TK, Nicholas RA. Charged residues in the C-terminus of the P2Y1 receptor constitute a basolateral-sorting signal. J Cell Sci. 2010;123(Pt 14):2512–2520. doi: 10.1242/jcs.060723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakatsu F, Ohno H. Adaptor protein complexes as the key regulators of protein sorting in the post-Golgi network. Cell Struct Funct. 2003;28(5):419–429. doi: 10.1247/csf.28.419. [DOI] [PubMed] [Google Scholar]
- 27.Folsch H. The building blocks for basolateral vesicles in polarized epithelial cells. Trends Cell Biol. 2005;15(4):222–228. doi: 10.1016/j.tcb.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 28.Ohno H, Tomemori T, Nakatsu F, Okazaki Y, Aguilar RC, Folsch H, Mellman I, Saito T, Shirasawa T, Bonifacino JS. Mu1B, a novel adaptor medium chain expressed in polarized epithelial cells. FEBS Lett. 1999;449(2–3):215–220. doi: 10.1016/s0014-5793(99)00432-9. [DOI] [PubMed] [Google Scholar]
- 29.Gan Y, McGraw TE, Rodriguez-Boulan E. The epithelial-specific adaptor AP1B mediates post-endocytic recycling to the basolateral membrane. Nat Cell Biol. 2002;4(8):605–609. doi: 10.1038/ncb827. [DOI] [PubMed] [Google Scholar]
- 30.Folsch H, Pypaert M, Maday S, Pelletier L, Mellman I. The AP-1A and AP-1B clathrin adaptor complexes define biochemically and functionally distinct membrane domains. The Journal of cell biology. 2003;163(2):351–362. doi: 10.1083/jcb.200309020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gravotta D, Deora A, Perret E, Oyanadel C, Soza A, Schreiner R, Gonzalez A, Rodriguez-Boulan E. AP1B sorts basolateral proteins in recycling and biosynthetic routes of MDCK cells. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(5):1564–1569. doi: 10.1073/pnas.0610700104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cancino J, Torrealba C, Soza A, Yuseff MI, Gravotta D, Henklein P, Rodriguez-Boulan E, Gonzalez A. Antibody to AP1B adaptor blocks biosynthetic and recycling routes of basolateral proteins at recycling endosomes. Molecular biology of the cell. 2007;18(12):4872–4884. doi: 10.1091/mbc.E07-06-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Folsch H, Ohno H, Bonifacino JS, Mellman I. A novel clathrin adaptor complex mediates basolateral targeting in polarized epithelial cells. Cell. 1999;99(2):189–198. doi: 10.1016/s0092-8674(00)81650-5. [DOI] [PubMed] [Google Scholar]
- 34.Dempsey PJ, Meise KS, Coffey RJ. Basolateral sorting of transforming growth factor-alpha precursor in polarized epithelial cells: characterization of cytoplasmic domain determinants. Exp Cell Res. 2003;285(2):159–174. doi: 10.1016/s0014-4827(03)00035-1. [DOI] [PubMed] [Google Scholar]
- 35.Bonifacino JS, Traub LM. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu Rev Biochem. 2003;72:395–447. doi: 10.1146/annurev.biochem.72.121801.161800. [DOI] [PubMed] [Google Scholar]
- 36.Carmosino M, Valenti G, Caplan M, Svelto M. Polarized traffic towards the cell surface: how to find the route. Biol Cell. 2010;102(2):75–91. doi: 10.1042/BC20090134. [DOI] [PubMed] [Google Scholar]
- 37.Rodriguez-Boulan E, Musch A. Protein sorting in the Golgi complex: shifting paradigms. Biochim Biophys Acta. 2005;1744(3):455–464. doi: 10.1016/j.bbamcr.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 38.Gonzalez A, Rodriguez-Boulan E. Clathrin and AP1B: key roles in basolateral trafficking through trans-endosomal routes. FEBS Lett. 2009;583(23):3784–3795. doi: 10.1016/j.febslet.2009.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paulhe F, Wehrle-Haller M, Jacquier MC, Imhof BA, Tabone-Eglinger S, Wehrle-Haller B. Dimerization of Kit-ligand and efficient cell-surface presentation requires a conserved Ser-Gly-Gly-Tyr motif in its transmembrane domain. FASEB J. 2009;23(9):3037–3048. doi: 10.1096/fj.09-129577. [DOI] [PubMed] [Google Scholar]
- 40.Diaz F, Gravotta D, Deora A, Schreiner R, Schoggins J, Falck-Pedersen E, Rodriguez-Boulan E. Clathrin adaptor AP1B controls adenovirus infectivity of epithelial cells. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(27):11143–11148. doi: 10.1073/pnas.0811227106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fields IC, Shteyn E, Pypaert M, Proux-Gillardeaux V, Kang RS, Galli T, Folsch H. v-SNARE cellubrevin is required for basolateral sorting of AP-1B-dependent cargo in polarized epithelial cells. The Journal of cell biology. 2007;177(3):477–488. doi: 10.1083/jcb.200610047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kang RS, Folsch H. ARH cooperates with AP-1B in the exocytosis of LDLR in polarized epithelial cells. The Journal of cell biology. 2011 doi: 10.1083/jcb.201012121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Traub LM. Tickets to ride: selecting cargo for clathrin-regulated internalization. Nat Rev Mol Cell Biol. 2009;10(9):583–596. doi: 10.1038/nrm2751. [DOI] [PubMed] [Google Scholar]
- 44.Chen WJ, Goldstein JL, Brown MS. NPXY, a sequence often found in cytoplasmic tails, is required for coated pit-mediated internalization of the low density lipoprotein receptor. The Journal of biological chemistry. 1990;265(6):3116–3123. [PubMed] [Google Scholar]
- 45.Matter K, Yamamoto EM, Mellman I. Structural requirements and sequence motifs for polarized sorting and endocytosis of LDL and Fc receptors in MDCK cells. The Journal of cell biology. 1994;126(4):991–1004. doi: 10.1083/jcb.126.4.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ohno H, Stewart J, Fournier MC, Bosshart H, Rhee I, Miyatake S, Saito T, Gallusser A, Kirchhausen T, Bonifacino JS. Interaction of tyrosine-based sorting signals with clathrin-associated proteins. Science. 1995;269(5232):1872–1875. doi: 10.1126/science.7569928. [DOI] [PubMed] [Google Scholar]
- 47.Owen DJ, Evans PR. A structural explanation for the recognition of tyrosine-based endocytotic signals. Science. 1998;282(5392):1327–1332. doi: 10.1126/science.282.5392.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matter K, Hunziker W, Mellman I. Basolateral sorting of LDL receptor in MDCK cells: the cytoplasmic domain contains two tyrosine-dependent targeting determinants. Cell. 1992;71(5):741–753. doi: 10.1016/0092-8674(92)90551-m. [DOI] [PubMed] [Google Scholar]
- 49.Piepkorn M, Underwood RA, Henneman C, Smith LT. Expression of amphiregulin is regulated in cultured human keratinocytes and in developing fetal skin. J Invest Dermatol. 1995;105(6):802–809. doi: 10.1111/1523-1747.ep12326567. [DOI] [PubMed] [Google Scholar]
- 50.Brown CL, Meise KS, Plowman GD, Coffey RJ, Dempsey PJ. Cell surface ectodomain cleavage of human amphiregulin precursor is sensitive to a metalloprotease inhibitor. Release of a predominant N-glycosylated 43-kDa soluble form. The Journal of biological chemistry. 1998;273(27):17258–17268. doi: 10.1074/jbc.273.27.17258. [DOI] [PubMed] [Google Scholar]
- 51.Plowman GD, Green JM, McDonald VL, Neubauer MG, Disteche CM, Todaro GJ, Shoyab M. The amphiregulin gene encodes a novel epidermal growth factor-related protein with tumor-inhibitory activity. Molecular and cellular biology. 1990;10(5):1969–1981. doi: 10.1128/mcb.10.5.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cook PW, Mattox PA, Keeble WW, Pittelkow MR, Plowman GD, Shoyab M, Adelman JP, Shipley GD. A heparin sulfate-regulated human keratinocyte autocrine factor is similar or identical to amphiregulin. Molecular and cellular biology. 1991;11(5):2547–2557. doi: 10.1128/mcb.11.5.2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brewer CB, Roth MG. A single amino acid change in the cytoplasmic domain alters the polarized delivery of influenza virus hemagglutinin. The Journal of cell biology. 1991;114(3):413–421. doi: 10.1083/jcb.114.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Monlauzeur L, Rajasekaran A, Chao M, Rodriguez-Boulan E, Le Bivic A. A cytoplasmic tyrosine is essential for the basolateral localization of mutants of the human nerve growth factor receptor in Madin-Darby canine kidney cells. The Journal of biological chemistry. 1995;270(20):12219–12225. doi: 10.1074/jbc.270.20.12219. [DOI] [PubMed] [Google Scholar]