Abstract
Objective
Osteoarthritis (OA) is a debilitating chronic condition requiring long-term treatment of pain and functional impairment. Our objective was to characterize studies addressing management of OA-related pain with respect to the breadth of interventions, trial duration and size, outcome measures, and funding sources.
Design
We identified studies focused on ‘pain’ and ‘osteoarthritis’ from ClinicalTrals.gov and abstracted data on study status, sample size, design, funding source, duration, outcomes measured, and interventions evaluated. We examined associations among intervention type, funding source, sample size, duration, and outcomes measured.
Results
We identified 287 registered studies, of which 69% investigated pharmacologic interventions, 11% behavioral interventions, and 5% surgical procedures or devices, while the remainder examined other types of interventions. Eighty-seven percent evaluated knee OA. The average sample size was 290 subjects and average study duration was 7.4 months, with 52% using durations ≤ 3 months and 21% ≥ 12 months. Industry funded 64% of studies, either fully or partially. Of 180 completed studies, 139 were pharmacologic studies. Of these, 34 (24%) posted results to the registry. Among the studies funded by industry, 60% had durations < 3 months as compared with 36% among non-industry funded studies (p<0.0001). Behavioral intervention trials tended to be of longer duration than pharmacologic trials and were less likely to be funded by industry.
Conclusion
Over half of OA pain studies and > 80% of those funded by industry used trial durations of less than 6 months. Future studies should take into consideration the need for long-term pain management for OA when designing trial protocols.
Keywords: pain, clinical trials, osteoarthritis, registry
INTRODUCTION
Osteoarthritis (OA) affects an estimated 151 million persons worldwide and nearly 27 million adults in the United States alone1,2. Roughly one-third of American adults over the age of 65 suffer from OA, with OA of the knee, hand, and hip constituting the three most common forms of the condition2. Symptomatic knee OA has emerged as a particularly prevalent and debilitating form of the disease, becoming the most common form of OA among persons over the age of 65 as well as a leading cause of disability in the US2–4. OA also imposes a mounting burden on American healthcare resources, accounting for more than 974,000 hospital admissions and $46.6 billion in aggregate healthcare expenses in 20105.
The impact of OA on patients and healthcare systems is intensified by the progressive nature of the disorder and its attendant chronic pain6,7. Because there is currently no OA treatment that reverses the course of structural progression, OA management strategies focus on reduction of OA-related pain and improvement of functional status8,9. This management approach involves a sequence of non-pharmacologic, pharmacologic, and surgical treatment strategies10,11.
Despite the chronic, progressive nature of OA, most studies evaluating the efficacy of pharmacologic regimens in persons with OA are typically conducted with follow-up periods of less than one year, and many studies report treatment durations of less than six months12–14. Published studies of the efficacy of behavioral and other non-pharmacologic interventions (e.g. physical therapy) are also frequently conducted over short-term time frames15–18. Published data on pharmacologic interventions show substantial but incomplete pain control and raise concerns about the safety of commonly-prescribed nonsteroidal anti-inflammatory drugs (NSAIDs), which are associated with serious adverse events such as gastrointestinal bleeding and perforation8,19–21. Traditionally regarded as a condition of the elderly, OA is diagnosed at increasingly earlier ages, heightening the tension between the need to safely manage pain over the long-term and an evidence-base focused on short-term efficacy22–24.
Several contextual factors may affect the design and outcome of studies of OA-related pain management. First, many of these studies are sponsored by manufacturers of analgesic medications or other agents. It is well established that funding source may affect trial findings and the way these results are reported25,26. Heterogeneity of outcome measures also adds to the challenges of translating results of the studies into interpretable, patient-relevant metrics that would assist patients and their physicians in shared decision making19,27,28.
To address these issues of standardization, compliance with study protocol, and minimization of bias in trial publications, Congress mandated the creation of ClinicalTrials.gov, a publically accessible database of clinical trials. This repository has become the most established and largest registry of clinical trials worldwide29–31. The registry serves several purposes, including fulfilling ethical obligations to convey research designs and findings to the scientific and lay communities, documenting design features before the trial is conducted (e.g. primary outcome measure, sample size), permitting comparison of published findings with original design features, and optimizing resource allocation by reducing the number of duplicate studies30–33.
We sought to survey the current state of OA-related ongoing or recently completed clinical studies registered with ClinicalTrials.Gov to portray the landscape of studies on OA pain management with respect to study design and duration, treatments under investigation, funding sources, and the timeliness of reporting study findings to the public. We examined whether studies funded by industry differ from those funded by government, hospitals, and/or foundations with respect to duration, treatments under study, and study design. These data will help clinicians, funding agencies, and policymakers both anticipate potential changes in pain treatment options for persons with OA as well as identify challenges or gaps in current research that should be filled by the next generation of studies on pain management in OA.
METHODS
Study Selection
To identify clinical trials evaluating pain management in OA, we used the national clinical trial registry ClinicalTrials.gov. ClinicalTrials.gov has over 100,000 trials registered to the website as of 2010 from approximately 140 countries29–31,34. The two keywords ‘osteoarthritis’ and ‘pain’ were used to restrict the search to trials registered on the website that included both terms in their descriptions of evaluated conditions. Search results were then abstracted from the website into a database listing each trial along with its associated data elements, which are defined below under “Data Elements” as well as in Table 1. Data abstraction was conducted between October and November 2012.
Table 1.
Data Parameters of Catalogued Clinical Trials
| Parameter | Description |
|---|---|
| Design of Trial | The design of the study according to allocation of interventions and masking. Studies were specifically evaluated for whether or not they were interventional or observational; if interventional, whether they used randomization, and whether the masking type was open label, single-blind, or double-blind. |
| Drug Application | For pharmacologic interventions, the method of application or consumption. Included oral, topical and injection (intraarticular, intradermal, intravenous, and subcutaneous). |
| Drug Classification* | For pharmacologic interventions, the class of drug evaluated. Included biologics, non-opioid analgesics, inhibitors (such as p38 MAKP inhibitors and Trpv inhibitors), NSAIDs, nutraceuticals, opioids, steroids, and viscosupplements. Drugs that could not be classified into one of these groups were classified under the group ‘other’ and further classified as ‘centrally-acting’ or ‘peripherally-acting’ based on their mechanism of action, such as the centrally-acting pharmaceutical duloxetine. |
| Duration of Follow-Up | Anticipated time period over which each enrolled subject would be followed. These time periods included pre- or post-intervention wash-out or tapering periods and were defined by the latest reported time of follow-up. Duration lengths were entered into the database as days. |
| Enrollment | Number of participants enrolled in a study. |
| Funder Name | Specific name of the sponsor(s) that provided funding for the study. |
| Funder Type* | Sponsors were classified into one of three categories: (1) industry, (2) academic or clinical institutions, and (3) government entities (including NIH) or foundations. |
| Geographic Location | Country affiliated with the study. All countries associated with each trial were entered into the database and divided into the following three groups for analysis: United States, Europe, and Other. (Puerto Rico was included in the United States classification.) |
| Intervention Name | Specific name of drug, technique, or behavioral intervention investigated. If more than one intervention was evaluated or if another intervention was used as a control, all interventions described in the trial profile were entered into the database. |
| Intervention Type* | Intervention evaluated for OA-pain management. Included pharmacologic, behavioral, surgical procedures or devices, and ‘other’ interventions. Pharmacologic interventions included nutraceuticals. Interventions listed as ‘other’ included interventions that could not be categorized into these four types, such as electrotherapy, acupuncture, and thermal treatments. |
| Joint | The joint affected with OA, such as the knee, hip, or spine. |
| NCT Number | Identification number for each clinical trial registered with ClinicalTrials.gov. |
| Outcome Measures | The instruments used to measure outcomes related to pain, functionality, and/or quality of life. Trials that used WOMAC, KOOS/KSS, or a visual analogue scale were specifically identified. All trials measuring pain intensity on a numeric scale were classified as using VAS. Both primary and secondary outcome measures related to pain, function, and quality of life were entered into the database. |
| Publications | Citations for publications associated with each trial, if available. Publications were additionally sought by looking up the NCT number of each trial on PubMed. |
| Recruitment Status | Indicated whether trials were currently open or closed to recruitment. For those studies open to recruitment, studies were listed as not yet recruiting, recruiting, enrolling by invitation, or active but not recruiting. For those studies closed to recruitment, studies were listed as completed, suspended, terminated, or withdrawn. |
| Results Posted | Identified which completed studies officially reported having results on ClinicalTrials.gov, not whether or not these studies in fact had results or publications related to their protocol. |
| Study Completion Date | Date on which final data collection has already or is expected to occur. |
| Study Start Date | Date on which enrollment to the trial has already or is expected to commence. |
| Title of Trial | Protocol title supplied by study investigator. |
The three categorical data parameters Intervention Type, Funder/Sponsor Type, and Drug Classification were defined and applied by the investigators in the course of constructing the database. Further details are described in the body of the manuscript.
From the trials returned by our query in ClinicalTrials.gov, we included only those trials that fulfilled the following three inclusion criteria: (1) OA was listed as either the primary condition or one of at most two underlying conditions contributing to chronic pain; (2) trials evaluated the efficacy and/or safety of interventions intended to manage OA-related pain; and (3) if the efficacy and/or safety of pain management methods were not addressed, the study had to focus on understanding the origin or progression of OA-related pain (observational studies).
We implemented three exclusion criteria. (1) Trials were excluded if they completed enrollment earlier than 1997 since such trials may not reflect contemporary management. Because the time period in which subjects were recruited varied widely, the date a trial completed enrollment was determined to be more pertinent than the date enrollment commenced. (2) Trials that evaluated more than two underlying pain-related conditions were not considered. Such trials investigating OA along with other underlying causes of pain were determined to be too broad in focus to give an accurate picture of research on OA-related pain. (3) Trials were excluded if they evaluated pain unrelated to OA. For example, trials that evaluated pain associated with the interventions themselves (e.g. pain associated with a specific surgical procedure) rather than the impact of these interventions on OA-related pain were deemed tangential to this analysis.
Data Elements
We abstracted the following data elements from the ClinicalTrials.Gov registry: protocol title, NCT registration number, study start and completion dates for enrollment, joint affected, intervention name and type, study duration, study design, outcome measures, sample size, funding source, geographic location of the study, trial recruitment status, whether or not results were reported in the registry, publications arising from the results of the trial, and, for those trials examining pharmacologic interventions, the class of drug used in the study as well as the method of drug application. Detailed descriptions and classification of each data element are presented in Table 1.
The categories for pharmacologic regimen types and details of study design were developed by consensus between two co-authors (JNK, EL). When information on any parameter was not directly accessible from the ClinicalTrials.Gov registry, publications associated with the trials themselves were used to determine the additional details of the study protocol. In addition to recording publication citations listed on ClinicalTrials.Gov, publications were sought using the NCT number of a trial on PubMed.
We classified interventions as behavioral, pharmacologic, surgical/device, and ‘other’. Device interventions included surgical devices such as implants. Nonsurgical devices (e.g. electromagnetic therapy) were classified as ‘other.’ The pharmacologic intervention category included nutraceuticals as a subcategory.
The pharmacologic interventions were divided in the following groups: biologics, non-opioid analgesics, inhibitors (such as p38 mitogen-activated protein kinase (MAKP) inhibitors and transient receptor potential vanilloid (TRPV) inhibitors), NSAIDs, nutraceuticals, opioids, steroids, and viscosupplements. For those drugs that could not be adequately classified into one of these eight groups, an ‘other’ group was created and further divided into ‘centrally-acting’ (e.g. duloxetine) or ‘peripherally-acting’ based on the drug’s mechanism of action. Those pharmacologic interventions that could not be classified into any of these categories were labeled ‘not otherwise specified’.
Funding source categories were categorized as industry, the National Institutes of Health (NIH), other government, academic/clinical institutions, and foundations. If a study received funding from more than one type of sponsor, all funding sources and types of funding were recorded. ClinicalTrials.gov did not provide data on changes in funding source over the course of the study nor on the specific components of the study that were supported by particular funders.
Data Validation
Initially all data were abstracted by one co-author (EED). The stratification of pharmacologic interventions was guided by a clinician author with a clinical and research focus in OA (JNK). To ensure data abstraction accuracy, we conducted a validation study in which a second author (MED) performed an independent data abstraction for a random 10% of studies. As study duration was particularly relevant for this analysis, two coauthors (EED, MED) independently abstracted data on duration from all studies and compared and resolved any discrepancies. Using information from the registry on details of the study design, we created a study design quality index as a summative measure of two components: randomization and blinding. Randomization was coded as a binary variable (randomized or not). Blinding was scored from 0 to 2, with 0 corresponding to no blinding, 1 to single blinding and 2 to double blinding. The index varied from 0 if the study was not randomized and not blinded to 3 if study was randomized and double blinded. Details of the methodology and quality of study randomization and blinding were not available in ClinicalTrials.gov and thus were not taken into account in the design quality index.
Data Analysis
We first performed a descriptive analysis of the distributions of continuous variables and frequency of categorical variables. We used the Student t-test to compare study duration and sample size between industry-supported studies and studies supported by non-industry sources. We also examined whether certain types of studies (e.g. pharmacologic) more frequently have industry support than other types (e.g. behavioral). We constructed multivariate logistic regression analyses to identify study features that were independently associated with industry funding. We also examined factors associated with posting results as required by the Food and Drug Administration for completed pharmacologic studies.
RESULTS
Description of studies
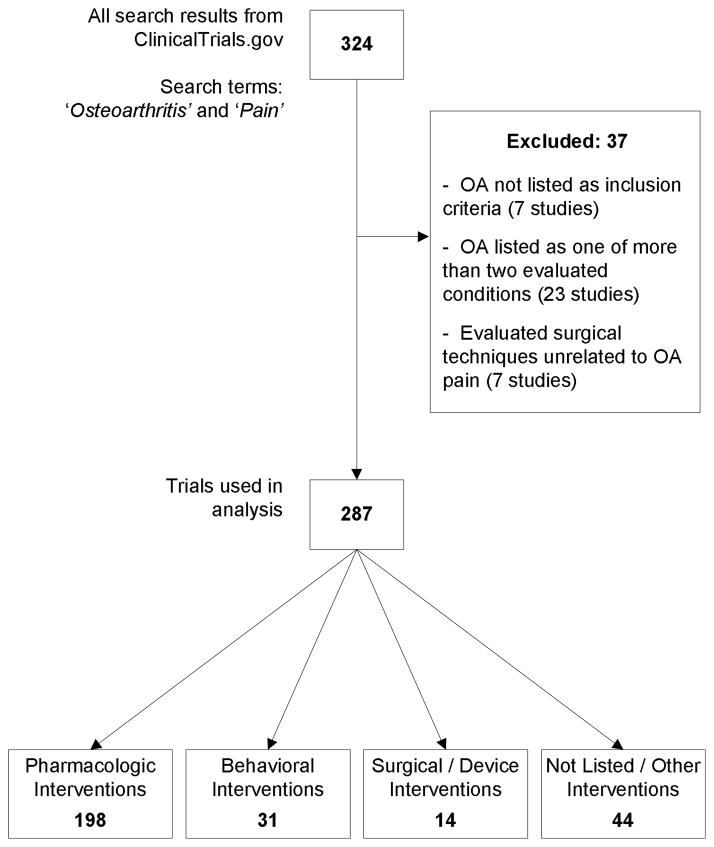
The initial search identified 324 studies registered at ClinicalTrials.Gov. Thirty-seven studies met one or more of our exclusion criteria. The remaining 287 studies were used in the current investigation (Figure 1). Among them, 198 (69%) focused on pharmacologic interventions, 31 (11%) on behavioral interventions and 14 (5%) on surgical/device interventions. Another 44 studies (15%) were categorized as ‘other,’ including 9 that did not provide sufficient information to categorize. Seventy percent of the studies focused solely on knee OA. Two hundred thirty-six (83%) used a randomized design. One hundred and eighty studies (63%) were listed as ‘Completed.’ Of the 60 still listed as ‘Open,’ 43 were actively recruiting. Among open studies, 30 (50%) investigated pharmacologic interventions. Quality scores varied, with 16% of studies having a score of 0 (worst) and 65% 3 (best).
Figure 1. Collection and Exclusion of Clinical Trials.
This figure depicts the process by which trials were collected from ClinicalTrials.gov. The initial search results were examined and studies that did not focus on treatments for OA pain were excluded. The included trials are further separated by the type of intervention they evaluate: pharmacologic, behavioral, surgical, or other.
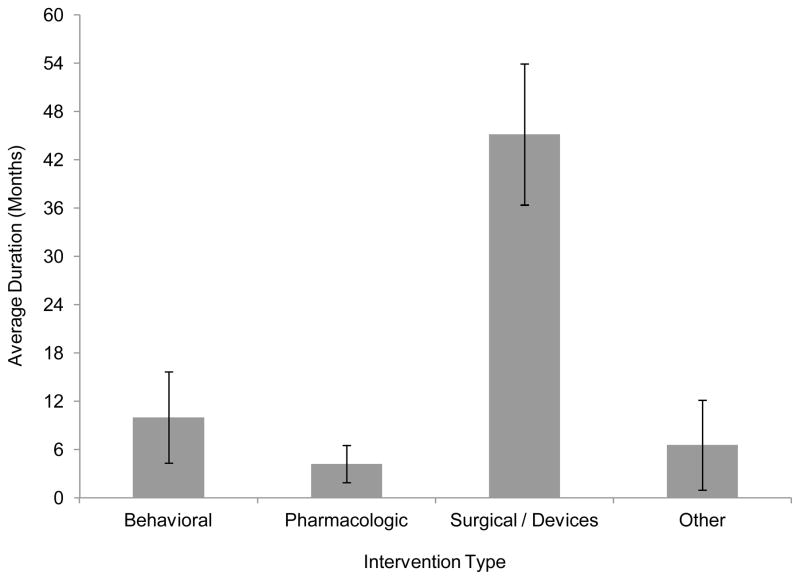
The sample size varied depending on intervention type, with pharmacologic interventions enrolling a greater number of subjects (mean enrollment of 281 subjects) compared to behavioral (171 subjects), surgical (114 subjects) and other (115 subjects). The overall p-value comparing sample size of studies of pharmacologic interventions (mean 281 subjects) vs. those of non-pharmacologic interventions (138 subjects) was <0.0001. Study duration ranged widely from 4.2 months on average in pharmacologic studies to 45 months on average in surgical studies (p<0.0001; Figure 2).
Figure 2. Average Study Duration by Type of Intervention Investigated.
This figure shows average clinical trial study duration, stratified by intervention type (behavioral, pharmacologic, surgical procedures and devices, or other). Average duration is reported in months. The 95% confidence intervals are indicated in the graph.
Outcomes varied widely across studies. The visual analog scale (VAS) was the most frequently used measure of pain severity, reported by 68% of studies, followed by the Pain Scale of the Western Ontario and McMaster Universities (WOMAC) Arthritis Index, which was reported in 50% of studies. Fewer than 10% of studies measured pain using other scales including the Knee Osteoarthritis Outcome Scales (KOOS) and the Knee Society Scale. One or more aspects of quality of life were ascertained in 33% of studies and functional status in 64%. Behavioral intervention studies were more likely to ascertain quality of life than studies of other types of interventions (odds ratio (OR) 2.35, 95% confidence interval (CI): 1.11–4.98). Behavioral intervention studies were also less likely to use VAS as a measure of outcome (OR 0.18, 95% CI: 0.08–0.41). Pharmacologic studies were more likely to use WOMAC (OR 1.98, 95% CI: 1.19–3.30) compared to non-pharmacologic studies.
Among pharmacologic studies, 51(26%) studied NSAIDS, 69 (35%) opioids, 19 (10%) novel inhibitors, 19 (10%) nutraceuticals, and 19 (10%) focused on peripherally-acting analgesics. Steroids, non-opioid analgesics, biologics, and viscosupplementation were each examined by less than 7% of registered studies.
One hundred fifty-six studies (55%) were conducted in the US, an additional 60 studies (21%) in Europe, and another 46 studies (16%) in other regions of world. Twenty-four studies were done in multiple geographic areas. In all geographic areas, more than two-thirds of studies focused on pharmacologic interventions. Seventeen percent of studies conducted in the US focused on behavioral interventions compared to 7% in Europe and 2% in other parts of the world. Studies of surgical/device interventions were more prominent in Europe, comprising 17% of studies registered in Europe compared to about 2% in other parts of the world.
Role of funding sources
One hundred sixty-five studies (57%) were funded solely by industry, 17 (6%) reported being funded solely by government and foundation sources, and 63 studies (22%) reported clinical and academic institutions as funding sources. The rest of the studies reported multiple sources of funding. Among pharmacologic studies, those funded by industry tended to be larger (mean enrollment of 303 versus 176, p=0.0187) and shorter (3.6 months average duration vs. 7.3 months, p<0.0001) than those not funded by industry (Table 3). Studies funded by industry tended to have higher quality index scores, with a mean quality index of 2.4 (out of 3.0) compared to 2.0 for non-industry funded studies. This difference in methodological rigor between industry and non-industry funded studies diminished when looking only at pharmacologic studies, which tended to have higher quality index scores overall compared to non-pharmacologic studies (mean quality index of 2.6 for pharmacologic studies vs. 1.6 for non-pharmacologic, p<0.0001). Accordingly, the subset of industry and non-industry funded pharmacologic studies had similar mean quality scores of 2.5 and 2.6, respectively (Table 3). Because there was very little difference between the quality scores of industry and non-industry funded pharmacological trials, this stratified analysis indicated that the overall higher quality of industry funded studies was confounded by study type.
Table 3.
Description of Pharmacologic Studies by Funding Source
| Funded by Industry: | Yes n=165 | % | No n=33 | % | p value |
|---|---|---|---|---|---|
| Status | 0.0016 | ||||
| Closed | |||||
| Completed | 121 | 73% | 18 | 55% | |
| Active, Not Recruiting | 3 | 2% | 2 | 6% | |
| Enrolling by Invitation | 0 | 0% | 1 | 3% | |
| Suspended | 1 | 1% | 0 | 0% | |
| Terminated | 21 | 13% | 1 | 3% | |
| Open | |||||
| Recruiting | 14 | 8% | 6 | 18% | |
| Not Yet Recruiting | 5 | 3% | 5 | 15% | |
| Duration | 0.0004 | ||||
| Less than 3 Months | 99 | 63% | 10 | 30% | |
| 3 to 6 Months | 39 | 25% | 10 | 30% | |
| 6 to 12 Months | 4 | 3% | 4 | 12% | |
| Greater than 12 Months | 15 | 10% | 9 | 27% | |
| Design | |||||
| Randomized | 139 | 85% | 32 | 97% | 0.0670 |
| Enrollment Mean (SD) | 303 | (276) | 176 | (306) | 0.0187 |
| Quality Score Mean (SD) | 2.5 | (1.0) | 2.6 | (0.8) | 0.6506 |
| Focus of study | |||||
| Novel Compounds* | 35 | 21% | 9 | 27% | 0.4457 |
| NSAIDs | 45 | 27% | 6 | 18% | 0.2769 |
| Opioids | 66 | 40% | 3 | 9% | 0.0007 |
| Outcomes | |||||
| VAS | 129 | 78% | 19 | 58% | 0.0131 |
| WOMAC | 98 | 59% | 11 | 33% | 0.0061 |
| KOOS | 2 | 1% | 5 | 15% | <0.0001 |
| QOL | 47 | 28% | 15 | 45% | 0.0556 |
Includes novel inhibitors, biologics, and centrally- or peripherally-acting compounds.
Results of multivariate analyses revealed that pharmacologic studies funded by industry were more likely than studies funded by non-industry sources to: be conducted in the US (adjusted OR 9.06, 95% CI: 2.78–29.58), investigate opioids either as active treatment or comparator (OR 23.79, 95% CI: 4.15–136.50), and use WOMAC to measure outcomes (OR 8.58, 95% CI: 2.64–27.91). Further, these analyses showed that industry funded studies were less likely to measure quality of life (OR 0.20, 95% CI: 0.06–0.65) and more likely to be of short duration (<3 months as compared to >12 months, OR 24.18, 95%CI: 4.13–141.41).
Factors associated with posting results
One hundred eighty studies had status ‘Closed: Completed’. Only 34 of these closed and completed studies (19%) had results posted. All completed studies that had results posted were funded by industry. Furthermore, none of the behavioral studies have had their results posted. Among 121 pharmacologic industry funded studies that had been completed, only 34 (28%) had results posted directly to the registry.
DISCUSSION
Pain management is a paramount priority for patients with OA and their clinicians35,36. In an attempt to anticipate the pipeline of pain management interventions currently under study, we examined features of ongoing studies of OA pain management as reflected in the ClinicalTrials.Gov repository. Our findings indicate that despite the fact that OA pain management poses a decades-long challenge for each patient with OA and his or her provider, 80% of trials of pain management interventions are less than one year in duration and half are three months or less. Industry funded trials are especially likely to be of short duration.
Few systematic evaluations of the methodology of OA-related research have been reported previously. Three studies published since 2001 have examined specific methodological aspects of research focused on managing OA-related pain37–39. Two of these studies focused specifically on the challenge of interpreting the clinical relevance of study results. Farrar et al. evaluated the 11-point numerical rating scale as an outcome measure used to measure pain and pain efficacy and called for standardization of pain outcome measures across research studies in order to improve the clinical relevance of OA-related research overall38. Moore et al. conducted a meta-analysis of randomized control trials investigating the efficacy of NSAIDs39. These authors also focused on the duration of the trials, noting that while NSAIDs are intended for long-term use in the treatment of OA-related pain, studies of these agents in OA were generally conducted over 12 weeks. Boutron et al. focused on methodological differences between non-pharmacologic and pharmacologic interventions, noting (as we did) that pharmacologic studies had greater methodological rigor as they were more likely to be controlled, randomized, and single- or double-blinded37. The role of funding source in the development of pain control interventions has been noted in published literature, most notably in studies evaluating the efficacy of glucosamine and chondroitin. Vlad et al. and Wandel et al. conducted meta-analyses that examined the sources of funding for trials investigating glucosamine or chondroitin in the treatment of OA, observing that studies supported by industry frequently reported greater effect sizes for both drugs in the reduction of pain than studies without industry support25,26.
Guidelines and recommendations from professional organizations such as Osteoarthritis Research Society International (OARSI) constitute another segment of the published literature focused on examining and improving methods in OA-related research10,11,27,28,40–44. Indeed, OARSI, the American College of Rheumatology (ACR), the European League of Associations of Rheumatology (EULAR), and the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) have each issued recommendations or reports indicating how researchers should conduct OA-related studies. These evaluations have generally pointed out that validating and standardizing outcome measures might improve the clinical applicability of trials of OA pain management28,40,42,44. While these reports typically used outcome measures that examine pain, functional ability, and patient global assessments, these publications also noted the need for better, validated outcome measures to examine quality of life28,40,42,44.
The rich ClinicalTrials.gov database comprises a key strength of this study, as the database provides insight into therapies in the pipeline and documents key study characteristics such as funding source and various design features. As the most extensive and established clinical trial registry available, ClinicalTrials.gov is used increasingly by researchers to investigate methods and trends in clinical research30. However, the database also imposes limitations that should be considered in interpreting our findings. First, we do not know what proportion of all studies of OA pain management are in fact registered on ClincalTrials.gov, nor whether there are systematic differences between registered and unregistered trials. Similarly, by restricting our search terms to ‘osteoarthritis’ and ‘pain’, several relevant studies may have been overlooked. Before any data abstraction, an initial search was conducted using only the term ‘osteoarthritis,’ yielding over 1,300 studies in the search results. In order to maintain a focus on pain management in OA, we limited the final search in the registry to those tagged with the terms ‘osteoarthritis’ and ‘pain.’ Because we do not know how thoroughly or consistently studies in the database are tagged with these descriptors, it is possible that we excluded studies that would have been relevant to our analysis.
Because information for trial profiles is provided directly by the trial investigators, we also do not know how compliant investigators are with requests to update their listings in ClinicalTrials.gov regularly30,34. For example, only 19% of studies listed as closed and completed had posted study findings to the registry. We cannot distinguish whether the other studies had not analyzed their data or simply had not posted the results. Moreover, registry data are not scrutinized for accuracy. Investigators may make errors in creating their trial listings on ClinicalTrials.gov, though the resulting misclassification is likely non-differential with respect to the hypotheses addressed in this paper. Finally, the sparse detail provided by the ClinicalTrials.gov registry and protocols limited our understanding of the nature of these study parameters. For example, study profiles clearly stated whether or not a trial was funded by industry and clearly indicated whether or not a trial was randomized or observational in nature. However, protocols gave no indication about the point at which studies became funded by a particular source or what proportion of funding was derived from each source. Similarly, protocols listing a trial as double-blinded and randomized did not provide information on how randomization was conducted or blinding ensured. Because protocol parameters were typically described in this binary fashion (that is, a trial either possessed a certain characteristic or did not), we accepted the meaning of these parameters at face value.
We conclude that while the pipeline contains a wide range of interventions for pain management in OA, the studies of these therapies are generally performed over short time frames. The trial findings will therefore be difficult to apply to long-term pain management strategies39. Industry funded studies are particularly likely to focus on the short term. These findings reveal a tension between the design of research studies and the needs of patients attempting to effectively manage a chronic disease. Indeed, the trends described here suggest that many questions about long-term pain management for patients with OA will not be addressed fully by the generation of studies currently underway. The implications for researchers, methodologists, and policy-makers are clear: in order to attend to the needs and concerns of patients and their physicians, designing trials that better reflect the patient experience may be an effective next step. The shorter trial durations typical of studies underway reflect funders’ logical impulse to limit investment in the development of any particular therapeutic agent. As long as regulatory agencies require evidence of short-term efficacy for approval, industry will not be incented to conduct the longer-term studies that would capture the challenges experienced by patients with chronic disease.
Our work accordingly underscores the utility of alternative research methodologies in addressing the unique problems associated with long-term pain management. Pragmatic randomized controlled trials are one methodologic strategy that inherently emphasizes the use of real-world clinical settings within a trial in order to produce outcomes with direct clinical relevance for patients and their healthcare providers45. Researchers may also employ observational cohort or case-controlled studies in order to gain different insights into effective ways to manage pain in ‘real-world’ clinical settings46. Researchers may also make use of entirely different methodological paradigms such as computer simulation modeling, which enables researchers to extend the findings of short-term studies to long-term patient care47.
The methods noted above are but a subset of a broad and diverse array of research methodologies that may permit researchers to engage the challenges posed by studies of chronic disease. Through the identification of current limitations in the body of OA research and the judicious use of alternative research approaches to comprehensively evaluate OA pain management, researchers, policymakers, and clinicians may be better able to optimize patient care.
Table 2.
Description of Studies on Osteoarthritis and Pain Registered in ClinicalTrials.Gov
| U.S. | Europe | Other Parts of the World | International Collaborations | Overall* | |
|---|---|---|---|---|---|
| Trial Status | |||||
| Registered | 156 | 60 | 46 | 24 | 286 |
| Closed | 123 | 46 | 33 | 24 | 226 |
| Completed | 96 | 38 | 29 | 17 | 180 |
| Active, Not Recruiting | 11 | 4 | 0 | 1 | 16 |
| Enrolling by Invitation | 1 | 0 | 1 | 0 | 2 |
| Suspended | 1 | 0 | 0 | 0 | 1 |
| Terminated | 13 | 3 | 2 | 6 | 24 |
| Withdrawn | 1 | 1 | 1 | 0 | 3 |
| Open | 33 | 14 | 13 | 0 | 60 |
| Not Yet Recruiting | 10 | 1 | 6 | 0 | 17 |
| Recruiting | 23 | 13 | 7 | 0 | 43 |
| Intervention Type | |||||
| Behavioral | 26 | 4 | 1 | 0 | 31 |
| Pharmacologic | 104 | 38 | 33 | 22 | 197 |
| Surgical / Devices | 2 | 10 | 1 | 1 | 14 |
| Other | 18 | 7 | 9 | 1 | 35 |
| Not Listed | 6 | 1 | 2 | 0 | 9 |
| Funded by Industry | |||||
| Yes | 105 | 36 | 19 | 24 | 184 |
| No | 51 | 24 | 27 | 0 | 102 |
Because one study did not have geographic data, only 286 studies were included in this table.
Acknowledgments
ROLE OF THE FUNDING SOURCE
Supported by NIH/NIAMS Grants K24 AR 057827, P60 47782 and R01 AR053112
Footnotes
CONFLICT OF INTEREST
The authors do not have any conflict of interest with respect to the context of this paper.
AUTHOR CONTRIBUTIONS
Conception and design: Losina
Analysis and interpretation of the data: Losina, Dervan, Daigle, Katz
Drafting of the article: Losina, Dervan, Katz
Critical revision of the article for important intellectual content: Losina, Dervan, Katz, Daigle
Final approval of the article: Losina, Dervan, Katz, Daigle
Provision of study materials or patients: Losina
Statistical expertise: Losina
Obtaining of funding: Losina
Collection and assembly of data: Losina, Dervan, Daigle
Contributor Information
Elena Losina, Email: elosina@partners.org.
Elizabeth E. Dervan, Email: edervan@partners.org.
Meghan E. Daigle, Email: medaigle@partners.org.
Jeffrey N. Katz, Email: jnkatz@partners.org.
References
- 1.World Health Organization. The global burden of disease: 2004 update. Geneva, Switzerland: WHO Press; 2008. [Google Scholar]
- 2.Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis and rheumatism. 2008 Jan;58(1):26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prevalence and Most Common Causes of Disability Among Adults - United States, 2005. Vol. 2009 Atlanta, GA: Centers for Disease Control and Prevention; May 1, 2009. [Google Scholar]
- 4.Felson DT, Lawrence RC, Dieppe PA, et al. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Intern Med. 2000 Oct 17;133(8):635–646. doi: 10.7326/0003-4819-133-8-200010170-00016. [DOI] [PubMed] [Google Scholar]
- 5.Healthcare Cost and Utilization Project (HCUP). Nationwide Inpatient Sample (NIS). Agency for Healthcare Research and Quality. [Accessed November 12, 2012];2010 http://hcupnet.ahrq.gov/
- 6.White AG, Birnbaum HG, Janagap C, Buteau S, Schein J. Direct and indirect costs of pain therapy for osteoarthritis in an insured population in the United States. J Occup Environ Med. 2008 Sep;50(9):998–1005. doi: 10.1097/JOM.0b013e3181715111. [DOI] [PubMed] [Google Scholar]
- 7.Woolf AD, Akesson K. Understanding the burden of musculoskeletal conditions. The burden is huge and not reflected in national health priorities. BMJ. 2001 May 5;322(7294):1079–1080. doi: 10.1136/bmj.322.7294.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Felson DT, Lawrence RC, Hochberg MC, et al. Osteoarthritis: new insights. Part 2: treatment approaches. Ann Intern Med. 2000 Nov 7;133(9):726–737. doi: 10.7326/0003-4819-133-9-200011070-00015. [DOI] [PubMed] [Google Scholar]
- 9.Hochberg MC, Altman RD, Brandt KD, et al. Guidelines for the medical management of osteoarthritis. Part I. Osteoarthritis of the hip. American College of Rheumatology. Arthritis and rheumatism. 1995 Nov;38(11):1535–1540. doi: 10.1002/art.1780381103. [DOI] [PubMed] [Google Scholar]
- 10.Zhang W, Moskowitz RW, Nuki G, et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2008 Feb;16(2):137–162. doi: 10.1016/j.joca.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 11.Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Arthritis and rheumatism. 2000 Sep;43(9):1905–1915. doi: 10.1002/1529-0131(200009)43:9<1905::AID-ANR1>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 12.Avouac J, Gossec L, Dougados M. Efficacy and safety of opioids for osteoarthritis: a meta-analysis of randomized controlled trials. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2007 Aug;15(8):957–965. doi: 10.1016/j.joca.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 13.Lee C, Straus WL, Balshaw R, Barlas S, Vogel S, Schnitzer TJ. A comparison of the efficacy and safety of nonsteroidal antiinflammatory agents versus acetaminophen in the treatment of osteoarthritis: a meta-analysis. Arthritis and rheumatism. 2004 Oct 15;51(5):746–754. doi: 10.1002/art.20698. [DOI] [PubMed] [Google Scholar]
- 14.Towheed TE, Hochberg MC. A systematic review of randomized controlled trials of pharmacological therapy in osteoarthritis of the knee, with an emphasis on trial methodology. Seminars in arthritis and rheumatism. 1997 Apr;26(5):755–770. doi: 10.1016/s0049-0172(97)80043-1. [DOI] [PubMed] [Google Scholar]
- 15.Christensen R, Bartels EM, Astrup A, Bliddal H. Effect of weight reduction in obese patients diagnosed with knee osteoarthritis: a systematic review and meta-analysis. Annals of the rheumatic diseases. 2007 Apr;66(4):433–439. doi: 10.1136/ard.2006.065904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fransen M, McConnell S. Land-based exercise for osteoarthritis of the knee: a metaanalysis of randomized controlled trials. The Journal of rheumatology. 2009 Jun;36(6):1109–1117. doi: 10.3899/jrheum.090058. [DOI] [PubMed] [Google Scholar]
- 17.Hernandez-Molina G, Reichenbach S, Zhang B, Lavalley M, Felson DT. Effect of therapeutic exercise for hip osteoarthritis pain: results of a meta-analysis. Arthritis and rheumatism. 2008 Sep 15;59(9):1221–1228. doi: 10.1002/art.24010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Warsi A, LaValley MP, Wang PS, Avorn J, Solomon DH. Arthritis self-management education programs: a meta-analysis of the effect on pain and disability. Arthritis and rheumatism. 2003 Aug;48(8):2207–2213. doi: 10.1002/art.11210. [DOI] [PubMed] [Google Scholar]
- 19.Hawker GA, Mian S, Bednis K, Stanaitis I. Osteoarthritis year 2010 in review: non-pharmacologic therapy. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2011 Apr;19(4):366–374. doi: 10.1016/j.joca.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 20.Towheed TE, Maxwell L, Judd MG, Catton M, Hochberg MC, Wells G. Acetaminophen for osteoarthritis. Cochrane Database Syst Rev. 2006;(1):CD004257. doi: 10.1002/14651858.CD004257.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hunter DJ. Osteoarthritis. Best Pract Res Clin Rheumatol. 2011 Dec;25(6):801–814. doi: 10.1016/j.berh.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 22.Golightly YM, Marshall SW, Callahan LF, Guskiewicz K. Early-onset arthritis in retired National Football League players. J Phys Act Health. 2009 Sep;6(5):638–643. doi: 10.1123/jpah.6.5.638. [DOI] [PubMed] [Google Scholar]
- 23.Palmieri-Smith RM, Thomas AC. A neuromuscular mechanism of posttraumatic osteoarthritis associated with ACL injury. Exerc Sport Sci Rev. 2009 Jul;37(3):147–153. doi: 10.1097/JES.0b013e3181aa6669. [DOI] [PubMed] [Google Scholar]
- 24.Losina E, Weinstein AM, Reichmann WM, et al. Lifetime risk and age of diagnosis of symptomatic knee osteoarthritis in the US. Arthritis Care & Research. 2012 doi: 10.1002/acr.21898. n/a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vlad SC, LaValley MP, McAlindon TE, Felson DT. Glucosamine for pain in osteoarthritis: why do trial results differ? Arthritis and rheumatism. 2007 Jul;56(7):2267–2277. doi: 10.1002/art.22728. [DOI] [PubMed] [Google Scholar]
- 26.Wandel S, Juni P, Tendal B, et al. Effects of glucosamine, chondroitin, or placebo in patients with osteoarthritis of hip or knee: network meta-analysis. BMJ. 2010;341:c4675. doi: 10.1136/bmj.c4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008 Feb;9(2):105–121. doi: 10.1016/j.jpain.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 28.Bellamy N, Kirwan J, Boers M, et al. Recommendations for a core set of outcome measures for future phase III clinical trials in knee, hip, and hand osteoarthritis. Consensus development at OMERACT III. The Journal of rheumatology. 1997 Apr;24(4):799–802. [PubMed] [Google Scholar]
- 29.Viergever RF, Ghersi D. The quality of registration of clinical trials. PloS one. 2011;6(2):e14701. doi: 10.1371/journal.pone.0014701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zarin DA, Tse T, Williams RJ, Califf RM, Ide NC. The ClinicalTrials.gov results database--update and key issues. The New England journal of medicine. 2011 Mar 3;364(9):852–860. doi: 10.1056/NEJMsa1012065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Califf RM, Zarin DA, Kramer JM, Sherman RE, Aberle LH, Tasneem A. Characteristics of clinical trials registered in ClinicalTrials.gov, 2007–2010. JAMA: the journal of the American Medical Association. 2012 May 2;307(17):1838–1847. doi: 10.1001/jama.2012.3424. [DOI] [PubMed] [Google Scholar]
- 32.Ross JS, Mulvey GK, Hines EM, Nissen SE, Krumholz HM. Trial publication after registration in ClinicalTrials.Gov: a cross-sectional analysis. PLoS medicine. 2009 Sep;6(9):e1000144. doi: 10.1371/journal.pmed.1000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reynolds T. Researchers push for publication, registration of all clinical trials. Journal of the National Cancer Institute. 2003 Jun 4;95(11):772–774. doi: 10.1093/jnci/95.11.772. [DOI] [PubMed] [Google Scholar]
- 34.Zarin DA, Ide NC, Tse T, Harlan WR, West JC, Lindberg DA. Issues in the registration of clinical trials. JAMA: the journal of the American Medical Association. 2007 May 16;297(19):2112–2120. doi: 10.1001/jama.297.19.2112. [DOI] [PubMed] [Google Scholar]
- 35.Peat G, McCarney R, Croft P. Knee pain and osteoarthritis in older adults: a review of community burden and current use of primary health care. Annals of the rheumatic diseases. 2001 Feb;60(2):91–97. doi: 10.1136/ard.60.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Woolf AD, Pfleger B. Burden of major musculoskeletal conditions. Bull World Health Organ. 2003;81(9):646–656. [PMC free article] [PubMed] [Google Scholar]
- 37.Boutron I, Tubach F, Giraudeau B, Ravaud P. Methodological differences in clinical trials evaluating nonpharmacological and pharmacological treatments of hip and knee osteoarthritis. JAMA: the journal of the American Medical Association. 2003 Aug 27;290(8):1062–1070. doi: 10.1001/jama.290.8.1062. [DOI] [PubMed] [Google Scholar]
- 38.Farrar JT, Young JP, Jr, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001 Nov;94(2):149–158. doi: 10.1016/S0304-3959(01)00349-9. [DOI] [PubMed] [Google Scholar]
- 39.Moore RA, Moore OA, Derry S, Peloso PM, Gammaitoni AR, Wang H. Responder analysis for pain relief and numbers needed to treat in a meta-analysis of etoricoxib osteoarthritis trials: bridging a gap between clinical trials and clinical practice. Annals of the rheumatic diseases. 2010 Feb;69(2):374–379. doi: 10.1136/ard.2009.107805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Altman R, Brandt K, Hochberg M, et al. Design and conduct of clinical trials in patients with osteoarthritis: recommendations from a task force of the Osteoarthritis Research Society. Results from a workshop. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 1996 Dec;4(4):217–243. doi: 10.1016/s1063-4584(05)80101-3. [DOI] [PubMed] [Google Scholar]
- 41.Hochberg MC, Altman RD, Brandt KD, et al. Guidelines for the medical management of osteoarthritis. Part II. Osteoarthritis of the knee. American College of Rheumatology. Arthritis and rheumatism. 1995 Nov;38(11):1541–1546. doi: 10.1002/art.1780381104. [DOI] [PubMed] [Google Scholar]
- 42.Jordan KM, Arden NK, Doherty M, et al. EULAR Recommendations 2003: an evidence based approach to the management of knee osteoarthritis: Report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT) Annals of the rheumatic diseases. 2003 Dec;62(12):1145–1155. doi: 10.1136/ard.2003.011742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Turk DC, Dworkin RH, McDermott MP, et al. Analyzing multiple endpoints in clinical trials of pain treatments: IMMPACT recommendations. Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials. Pain. 2008 Oct 31;139(3):485–493. doi: 10.1016/j.pain.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 44.Pham T, van der Heijde D, Altman RD, et al. OMERACT-OARSI initiative: Osteoarthritis Research Society International set of responder criteria for osteoarthritis clinical trials revisited. Osteoarthritis and cartilage / OARSI, Osteoarthritis Research Society. 2004 May;12(5):389–399. doi: 10.1016/j.joca.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 45.Tunis SR, Stryer DB, Clancy CM. Practical clinical trials: increasing the value of clinical research for decision making in clinical and health policy. JAMA: the journal of the American Medical Association. 2003 Sep 24;290(12):1624–1632. doi: 10.1001/jama.290.12.1624. [DOI] [PubMed] [Google Scholar]
- 46.Black N. Why we need observational studies to evaluate the effectiveness of health care. BMJ. 1996 May 11;312(7040):1215–1218. doi: 10.1136/bmj.312.7040.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paltiel AD, Fuhlbrigge AL, Kitch BT, et al. Cost-effectiveness of inhaled corticosteroids in adults with mild-to-moderate asthma: results from the asthma policy model. The Journal of allergy and clinical immunology. 2001 Jul;108(1):39–46. doi: 10.1067/mai.2001.116289. [DOI] [PubMed] [Google Scholar]