Abstract
In this work, we report a new nanofiber construct based on electrospun blends of gelatin and gelatin-dendrimer conjugates. Highly branched star-shaped polyamidoamine (PAMAM) dendrimer G3.5 was covalently conjugated to gelatin via EDC/NHS chemistry. Blends of gelatin and gelatin-dendrimer conjugates mixed with various loading levels of silver acetate (0, 0.83, 1.65, and 3.30% w/w) were successfully electrospun into nanofiber constructs (NCs). The NCs were further converted into semi-interpenetrating networks (sIPNs) with photoreactive polyethylene glycol diacrylate (Mn=575 gmol-1) (PEG DA575). They were characterized in terms of fiber morphology, diameter, pore size, permeability, degradation, and mechanical properties. The resulting sIPN NCs retained nanofiber morphology, possessed similar fiber diameters to counterpart NCs, and gained improved structural stability. The sIPN NCs also showed good swelling capacity owing to porous structures and were permeable to aqueous solutions. Silvercontaining sIPN NCs allowed sustained silver release and showed antimicrobial activity against two common types of pathogens—Staphylococcus aureus and Pseudomonas aeruginosa. Incorporation of dendrimers into the gelatin nanofibers through covalent conjugation not only expands drug loading capacity of nanofiber constructs but provides tremendous flexibility for developing multifunctional electrospun dressing materials.
Keywords: Electrospinning, Dendrimer, Gelatin-dendrimer hybrid, Nanotechnology, Wound care
Introduction
The wound healing cascade is a sequence of five typical local events: acute inflammation, chronic inflammation, growth of granulation tissue, foreign body reaction, and remodeling, during which specific “cues”, e.g., cells and bioactive molecules, are involved and orchestrated in a spatial and temporal fashion. Therefore, wound dressing materials are expected to not only provide basic function of covering wounds from infection but also possess controlled structures and properties as well as adaptability to accommodate functions essential for wound healing.1 Current FDA-approved wound dressings can be classified into three major categories based on properties and modes of action: passive products that are traditional dressings such as gauze, interactive products that are generally permeable to oxygen and water but impermeable to bacteria and commonly applied to wounds that do not produce significant amounts of exudates, and bioactive products that deliver bioactive substances beneficial to wound healing although some products are composed of substances that inherently promote wound healing. In particular, bioactive products have been developed primarily based on natural polymers including gelatin, collagen, noncollagenous proteins, proteoglycans, alginates, and chitosan.2, 3 Nonetheless, few existing dressing materials can satisfactorily fulfill multiple purposes or be capable of delivering multiple cues to complement wound healing. Development of effective dressings requires the underlying dressing material to be structurally adaptable and possess controlled characteristics.
In this work, we report a novel highly adaptable biocompatible platform composed of electrospun blends of gelatin and gelatin-dendrimer conjugates, which is further intertwined with a cross-linked polyethylene glycol (PEG) network to form semi-interpenetrating networks (sIPNs).4 This new nanofiber construct has adaptable structure and compelling properties. Gelatin is a biocompatible and degradable substance and has been widely used to develop wound dressings.2 Dendrimers are highly branched macromolecules composed of a central core, internal branches and a number of reactive surface groups.5-7 It is the presence of numerous surface groups that makes dendrimers suitable carriers for carrying high drug payloads and/or multifunctionality.8-11 Dendrimers can complex with silver to exert higher antimicrobial activity than silver alone.12, 13 PAMAM dendrimers themselves exhibit strong anti-inflammatory activity.14 Electrospinning enables a high degree of control over a wide range of structural features and properties of the construct such as shape, porosity, modulus, degradation, fiber size and orientation.15 Fabricating polymers into nanofiber constructs with electrospinning to mimic the features of extracellular matrix (ECM) has become an important approach in wound dressing development and drug delivery.16-23 In addition, nanoscale surface features and topography of nanofibers regulate cell behaviors, such as adhesion, proliferation, and infiltration, which are desirable features for wound healing. Incorporation of dendrimers into the gelatin nanofibers through covalent conjugation not only expands drug loading capacity of nanofiber constructs but provides tremendous flexibility for developing multifunctional electrospun dressing materials. Overall, we envision that this unique dendrimer-gelatin nanofiber construct can be tailored to deliver state-of-the-art therapeutics as they are developed to treat different types of wounds including chronic wounds, burns, and skin cancers.
Because bacterial infection is commonly associated with wound healing, silver as an integral component was incorporated into the electrospun dressing. Silver-containing nanofiber constructs (NCs) on the basis of blends of gelatin (G) and gelatin-dendrimer conjugates (G-D) were fabricated via electrospinning and further processed with PEG diacrylate to form sIPN NCs. They were characterized in terms of fiber morphology, diameter, pore size, permeability, degradation, and mechanical properties. Furthermore, silver release kinetics and antimicrobial properties of the dressing materials were assessed.
Materials and Methods
Materials
Polyamidoamine (PAMAM) dendrimer generation 3.5 (G3.5) (core: ethylene diamine (EDA); G = 3.5; [dendri-poly(amidoamine)-(COONa)64]), 10 wt% solution in methanol) was purchased from Dendritech (Midland, MI). 1,1,1,3,3,3 Hexafluoro-2-propanol (HFP) was purchased from TCI America (Portland, OR). Agar, aqueous silver standard for ICP-OES, dimethoxyphenylacetophenone (DMPA), 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC), ethanol (denatured), ethyl ether (anhydrous), gelatin (porcine skin- type A, ∼300 g bloom), ninhydrin, N-hydroxysuccinimide (NHS), peptone, phosphate buffered saline (PBS), poly (ethylene glycol) diacrylate (Mn=575 g/mol, ρ=1.12 g/mL at 25 °C) (PEG DA575), silver acetate, sodium bicarbonate (NaHCO3), sodium chloride, and tryptone were purchased from Sigma-Aldrich (St. Louis, MO).
Synthesis of Gelatin-Dendrimer Conjugates (G-D)
Gelatin-dendrimer conjugates were obtained by covalently coupling PAMAM dendrimer G3.5 to gelatin. Briefly, a gelatin solution was prepared by dissolving 20 mg of gelatin in 20 ml of 0.1N NaHCO3 solution and stirring it at 80 °C until a clear solution formed. Upon the removal of methanol from PAMAM dendrimer G3.5 stock solution via rotary evaporation, G3.5 (20.7 mg) was redissolved in 2 ml of deionized water and then reacted with 3 mg of NHS and 5 mg of EDC while stirring for 24 h to yield G3.5-NHS esters. Finally, the G3.5-NHS solution (approximately 2 ml) was added to the gelatin solution (approximately 20 ml), and the mixture was vigorously stirred for 4 h while being kept in an ice water bath. Following centrifugation, the gelatin-dendrimer conjugates in supernatant were collected and precipitated out of cold ether followed by dialysis in a dialysis tubing of 12-14 kDa MWCO and freeze drying. Ninhydrin assay was performed to examine the percentage of primary amines in the gelatin backbone before and after conjugation.24
Fabrication of NCs via Electrospinning
All NCs were fabricated under the same electrospinning conditions. In general, 1 g of G/G-D blends alone or mixed with various amounts of silver acetate (Table 1) was suspended in 10 ml of HFP followed by thorough mixing for 24 h on a shaker plate. The electrospinning solution was then loaded into a 10 ml Becton Dickinson syringe and placed in a KD Scientific syringe pump. The syringe pump was set to deliver the solution at a rate of 5 ml/h. A voltage of 25 kV was applied to the 18 gauge blunt-tip needle of the syringe by a high voltage power supply (Spellman CZE1000R) (Spellman High Voltage Electronics Corporation, Hauppauge, NY). A stainless steel mandrel (7.5cm×2.5cm×0.5cm, L×W×T) was placed approximately 125 mm from the needle tip and rotated at ∼500 rpm for fiber collection. After electrospinning, the resulting mat was carefully removed from the mandrel, placed in a fume hood for degassing and stored in a desiccator.
Table 1. Permeability and pore size of the sIPN NCs.
| sIPN NC | Silver content (w/w) | Permeability (Darcy) | Pore size (μm) |
|---|---|---|---|
| G/G-D/Ag(0) | 0 | 2.4±1.2 | 0.8±0.2 |
| G/G-D/Ag(1) | 0.83% | 0.2±0.0 | 0.2±0.0 |
| G/G-D/Ag(2) | 1.65% | 0.8±1.3 | 0.4±0.4 |
| G/G-D/Ag(3) | 3.30% | 0.6±0.9 | 0.3±0.3 |
G/G-D blends composed of 96 w% G (gelatin) and 4 w% G-D (gelatin-dendrimer conjugates); Silver: silver acetate
Fabrication of sIPN NCs
Rectangular samples (7.5 cm×5 cm) were cut from nanofiber mats. A 2 ml of ethanol solution containing 100 μl of PEG DA575 and 4 mg of DMPA was poured onto the sample and allowed to stay for about 30 min. The samples were then exposed to UV light (UVP Blak-Ray Long Wave Lamp, 100 Watts) for 2 min on each side and then air dried.4
Characterization
Scanning electron microscopy images of nanofiber samples were taken on a Zeiss EVO 50 XVP scanning electron microscope (SEM). UTHSCSA Image tool Version 3.0 was utilized to determine fiber diameter on the basis of 30 fibers randomly chosen in the image. Porosity of construct (n=3) was calculated using the following formula: Porosity = (1-ρscaffold)/ρmaterial ×100% where ρscaffold is calculated construct density and ρmaterial is known material density (1.41 g/cm3 for collagen). Construct permeability and pore size were determined following a method described by Sell et al.25 Tensile testing was conducted on the MTS Bionix 200®- Mechanical testing system with a 100 N load cell in conjunction with TestWorks 4.0 software. Dog-bone shaped samples (n=6) were cut out from each construct and then placed in the metal grips of the mechanical testing system to be tested at a rate of 10 mm/min.26 Peak stress, strain at break, and modulus were obtained.
Swelling Studies
To mimic clinical conditions, simulated wound fluid (SWF) was used for swelling studies. SWF consisted of 50% calf serum and 50% maximum recovery diluent (0.1% w/v peptone and 0.9% w/v sodium chloride).27 Samples (2.5×2.5 cm, n=3) were weighed (Wd) and then immersed in 5 ml of SWF at room temperature. At pre-determined time points up to 48h, the samples were taken out of the fluid, blot dried and weighed. Equilibrium swelling ratio (%) is calculated as , where Wd is the mass of dry sample and Ws is the mass of swollen sample when no further weight increase was observed.
In Vitro Degradation Studies
Construct degradation at 37 °C in three types of media including SWF, Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), and conditioned cell medium was investigated. Conditioned cell medium was DMEM supplemented with 10% FBS conditioned by confluent BJ-hTERT fibroblasts for 24 h. Samples (1×1 cm, n=3) were immersed in 1.5 ml of medium for various lengths of time (6, 12, or 24h), taken out, centrifuged, freeze dried, and weighed. Mass loss (%) is defined as , where Wo is the initial mass of the dry construct and Wd is the mass of the residue after incubation and freeze-drying.
Silver Release Studies
Silver-containing nanofiber constructs (2.5×2.5 cm) were incubated in 20 ml of PBS at 37°C. At pre-determined time points, 10 ml of PBS was collected for silver content analysis. Meanwhile, 10 ml of fresh PBS was added to maintain the volume of the medium for continuous observation. Silver content was quantified by inductively coupled plasma-optical emission spectroscopy (ICP-OES).28, 29 The Peppas-Sahlin equation was applied for data fitting to understand silver release mechanism.30
Antimicrobial Activity Assessment
The antimicrobial activity of silver-containing nanofiber scaffolds against two common wound pathogens—gram positive Staphylococcus aureus (strain N315) and gram negative Pseudomonas aeruginosa (strain PA01)—was assessed. Sample scaffold (2.5×2.5 cm) was incubated in a test tube containing 10 ml of 105 diluted bacterial solution inoculated in SWF at 37 °C. Aliquots (100 μl) were withdrawn at 4 h, 24 h, and 48 h and then plated on luria agar plates. The plates were incubated overnight at 37 °C. The bacterial colonies present on the plate were counted and expressed as cfu/ml (colony-forming units per milliliter).
Statistical Analysis
Statistical analysis was based on analysis of variance (ANOVA) using SigmaPlot 12. Holm-Sidak method was applied for subgroup pairwise comparison. A p-value of less than 0.05 was considered statistically significant.
Results
Preparation of Electrospun Blends of Gelatin and Gelatin-Dendrimer Conjugates
Nearly 25% surface amine groups of PAMAM dendrimer G3.5 was activated with EDC/NHS chemistry to form G3.5-NHS ester. Lysine and arginine constitute 13∼16% of total amino acids in gelatin. They readily react with NHS esters and diminish in quantity as a result of coupling reaction. According to the ninhydrin assay, the percentage of free amines in G-D conjugates was only 44% of those found in unmodified gelatin, confirming the success of coupling of dendrimer to gelatin. There are many factors that affect structure of nanofiber constructs and properties, such as voltage applied, mandrel rotating speed, air gap distance, diameter of the needle and the rate at which the polymer solution is delivered.31 To examine effects of silver content in the polymer blends on nanofiber structure and properties, the electrospinning conditions were kept stable.
According to SEM images (Figure 1, top panel), blends of gelatin and gelatin-dendrimer conjugates alone or containing various amounts of silver acetate were successfully electrospun to form nanofiber constructs under the conditions specified in this work. The gelatin-based NCs were further treated with PEG DA575 in the presence of photoinitiator DMPA and formed an sIPN NC upon UV light exposure.4 The resultant sIPN NCs retain nanofiber morphology (Figure 1, bottom panel). The fiber diameter of the NCs ranges from 3.20 to 5.89 μm, and that of the sIPN NCs varies from 4.08 to 6.98 μm (Figure 2). According to statistical analysis, the mean fiber diameter of each type of silver-containing NCs (G/G-D/Ag(1), G/G-D/Ag(2), and G/G-D/Ag(3)) did not change significantly after having been cross-linked with PEG DA575. In contrast, the effect of cross-linking on non-silver-containing NC G/G-D/Ag(0) was more pronounced. The mean fiber diameter of NC G/G-D/Ag(0) is 3.20 μm. After forming an sIPN with PEG DA575, the mean fiber diameter significantly increases by 52% to 4.85 μm (p<0.05).
Figure 1.
SEM images of NCs and sIPN NCs based on electrospun blends of gelatin and gelatin-dendrimer conjugates containing various amounts of silver acetate. Bars: 10 μm.
Figure 2.
Fiber diameters of NCs and sIPN NCs. * indicates p<0.05, # indicates no significant difference.
We observed that silver content affects fiber diameter of electrospun blends. In particular, increasing the loading level of silver tends to cause fiber diameter to increase. Although silver loading effect on fiber diameter increase is negligible when 0.83% w/w silver is loaded to the construct, a further increase of silver loading from 0.83% w/w to 1.65% w/w results in a mean fiber diameter of 6.70 μm for NC G/G-D/Ag(2), which is 64% higher than that of NC G/G-D/Ag(1) (p<0.05). NC G/G-D/Ag(3) containing 3.30% w/w silver also has a large mean fiber diameter, which was comparable to that of NC G/G-D/Ag(2).
Mechanical Properties
Peak stress, strain at break, and modulus of the NCs and their counterparts in form of sIPN were determined and compared. Peak stress is the maximum stress that a construct can withstand before breaking. As shown in Figure 3A, the mean peak stress of the NCs ranges from 1.3 to 2.1 MPa and that of the sIPN NCs ranges from 1.2 to 1.6 MPa. Except for a signficant reduction in peak stress for sIPN NC G/G-D/Ag(2) as compared to NC G/G-D/Ag(2), the rest sIPN NCs display a similar peak stress to their corresponding NCs.
Figure 3.
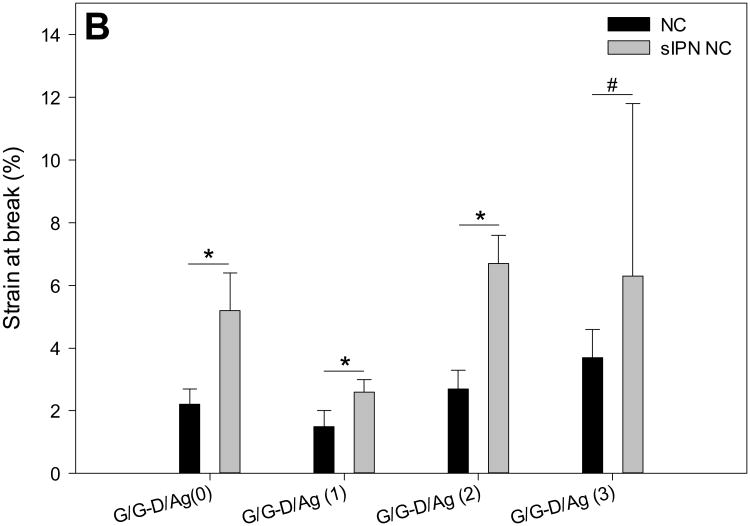
Mechanical properties of NCs and sIPN NCs including peak stress (A), strain at break (B), and modulus (C). * indicates p<0.05, # indicates no significant difference.
Strain at break depicts the ductility and brittleness of the construct as well as reveals the elongation of the construct at its breaking point.32 As opposed to their counterparts, sIPN NCs G/G-D/Ag(0), G/G-D/Ag(1), and G/G-D/Ag(2) are able to tolerate significantly more elongation before they break (Figure 3B). For instance, the strain at break of NC G/G-D/Ag(0) increased from 2.2±0.5% to 5.2±1.2% after cross-linking, representing a 1.4-fold increase (p<0.05). Strain at break of sIPN NC G/G-D/Ag(1) and sIPN NC G/G-D/Ag(2) increased by 73% (p<0.05) and 148% (p<0.05), respectively. The enhanced strain at break is attributed to the addition of a PEG network and enhanced chain entanglement in the fiber. Although cross-linking did not cause significant increase in strain at break for NC G/G-D/Ag(3), its strain at break before cross-linking was already significantly higher other three groups (p<0.05).
The modulus of sIPN NC G/G-D/Ag(2) decreases significantly by 68% (p<0.05) from 104.8 ±19.0 MPa to 33.8±8.9 MPa (Figure 3C). The modulus of the remaining sIPN NCs is similar to that of their non-cross-linked counterparts. The peak stress and modulus reduction of sIPN NC G/G-D/Ag(2) is presumably attributed to its higher permeability and larger pore size (Table 1) as peak stress and modulus of porous materials are inversely related to porosity and pore size.33
Porosity, Permeability, Swelling and Degradation Studies
Non-cross-linked gelatin-based NCs are vulnerable to degradation. Macroscopic visual dissolution of the structure in aqueous solutions is immediate. Therefore, we applied PEG DA575 to convert gelatin NCs into sIPN NCs for stability improvement. Only sIPN NCs were further characterized and discussed in context of dressing material development in the remaining portion of the work.
Porosity is the measure of void space within the constructs. The porosity of the sIPN NCs varies from 78 to 90% (Figure 4), and there is no significant difference among the sIPN NCs. This range of porosity is adequate for a wound dressing or regenerative construct to promote moisture and oxygen exchange.34 Permeability is the measure of the ease of flow of fluid through the construct. Permeability of the sIPN NCs falls in a range of 0.2 to 2.4 Darcy, however, with large variations (Table 1). The constructs have a range of mean pore size from 0.2 to 0.8 μm.
Figure 4.
Porosity of sIPN NCs. No significant difference among the groups.
The swelling studies were carried out in SWF, which replicates the clinical conditions. As shown in Figure 5, all the constructs possessed a high swelling capacity. In particular, the equilibrium swelling ratio of the sIPN NCs varies from 594% to 797% and there are no signficant differences between groups. The stability of the sIPN NCs was tested in SWF as well as regular cell culture medium (i.e., DMEM) and cell-conditioned DMEM. The mass loss of the construct during incubation was quantified. sIPN NC G/G-D was tested as an example and the mass loss profiles are presented in Figure 6. It loses 30% of its original mass in 6 h, 54% in 12 h, and completely disintegrates into SWF in 24 h. sIPN NC G/G-D has a similar degradation profile in DMEM. In contrast, DMEM conditioned with fibroblasts significantly accelerates degradation of sIPN NC G/G-D. In particular, incubation in cell-conditioned medium causes sIPN NC G/G-D to lose its 77% mass in 6 h and completely dissolve in 12 h, suggesting that the construct degrades in cell-conditioned medium at one fold faster rate.
Figure 5.
Equilibrium swelling ratios of sIPN NCs in SWF at room temperature. No significant difference among the groups.
Figure 6.
In vitro degradation of sIPN NC G/G-D at 37 °C following incubation for various amounts of time (6, 12, and 24 h) in SWF, DMEM supplemented with 10% FBS, and fibroblast-conditioned DMEM supplemented with 10% FBS.
Silver Release Kinetics and Antimicrobial Assay
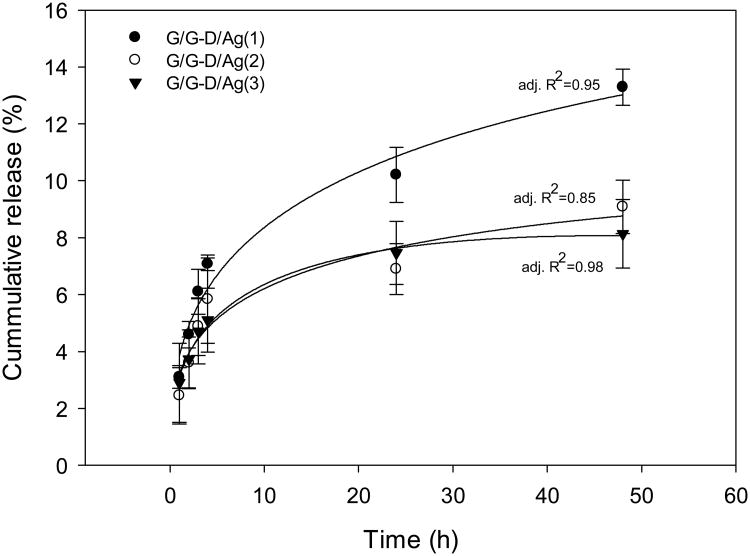
We first examined silver release kinetics to confirm whether silver ions can be released from the sIPN NCs. Release of silver from the sIPN NCs is attributed to the combination of passive silver diffusion and construct degradation. Although the construct degrades completely in 24 h, silver release lasted much longer. It was observed that only 8-13% of silver has been released at 48 h (Figure 7). This was attributed to strong electrostatic interactions of silver and dendrimers conjugated to the gelatin fiber even after construct completely degraded. The Peppas-Sahlin equation describes drug release mechanism following a coupled Fickian diffusion/macromolecular relaxation model.30, 35 The release in the first 48 h release was studied. The modeling results show that the Peppas-Sahlin equation fits the data well, thus confirming the diffusion/relaxation effect on silver release.
Figure 7.
Release of silver from silver-containing sIPN NCs at 37 °C. The Peppas-Sahlin equation30 was applied to fit the data points.
Antimicrobial activity of silver-containing sIPN NCs following release was tested against two common types of wound pathogens, gram positive Staphylococcus aureus and gram negative Pseudomonas aeruginosa. Inhibition of bacteria growth by the sIPN NCs following different incubation periods (4, 24, and 48 h) was assessed. Bacterial colonies were quantified to indicate the presence of live bacteria in the aliquots that were plated. After overnight incubation at 37 °C, the bacterial colonies formed on the agar plate (Figure 8) and were then counted. The results are summarized in Table 2.
Figure 8.
Assessment of antimicrobial activity of sIPN NCs against Staphylococcus aureus (A) and Pseudomonas aeruginosa (B). sIPN NCs were incubated in diluted bacterial suspensions. Aliquots (100 μl) were withdrawn at 4 h, 24 h, and 48 h and then plated on luria agar plates. The plates were incubated overnight at 37 °C. The bacterial colonies present on the plate were imaged and counted.
Table 2. Antimicrobial activity of the sIPN NCs.
| Colonies on agar plate | ||||||
|---|---|---|---|---|---|---|
| Treatment | Staphylococcus aureus(gram-positive) | Pseudomonas aeruginosa (gram-negative) | ||||
|
| ||||||
| 4 h | 24 h | 48 h | 4 h | 24 h | 48 h | |
|
|
|
|||||
| Control | 1×108 cfu/ml | 2.4×108 cfu/ml | lawn | lawn | lawn | lawn |
| sIPN NC G/G-D/Ag(0) | 6×107 cfu/ml | lawn | lawn | lawn | lawn | lawn |
| sIPN NC G/G-D/Ag(1) | 1.5×107 cfu/ml | none | none | none | none | none |
| sIPN NC G/G-D/Ag(2) | none | none | none | none | none | none |
| sIPN NC G/G-D/Ag(3) | none | none | none | none | none | none |
The control group was Staphylococcus aureus suspension without silver treatment. After 4 h-culture, the bacterial density was 1×108 cfu/ml. Having been cultured for 24 h, the bacterial medium generated a bacterial density of 2.4×108 cfu/ml. 48 h culture led to the formation of a bacterial lawn on the agar plate, reflecting unrestrained log growth of the bacteria. Similar to the control group, the growth of Staphylococcus aureus was not inhibited by the plain construct (i.e., sIPN NC G/G-D/Ag(0)). The formation of a bacterial lawn following 24 h incubation with the dressing may suggest that bacterial growth was accelerated by nutrient enrichment as a result of addition of a gelatin-based dressing. In contrast, silver-containing dressings successfully inhibited bacterial growth. Staphylococcus aureus growth was completely inhibited by sIPN NC G/G-D/Ag(2) and sIPN NC G/G-D/Ag(3) in 4 h. Similar inhibition results were also observed following 24 h- and 48 h- incubation. There was slight growth of bacteria incubated with sIPN NC G/G-D/Ag(1) for 4 h likely due to an insufficient silver concentration in the culture medium following a short release period. Longer incubation periods made sIPN NC G/G-D/Ag(1) more effective against bacterial growth. A complete bacterial growth inhibition was oberved following 24 h- and 48 h-incubation. All the silver-containing dressings were found to be effective in inhibiting growth of Pseudomonas aeruginosa.
Discussion
Electrospun nanofibrous dressing possesses several compelling structural features including a high surface-to-volume ratio, tunable porosity, and flexibility to conform over a wide variety of sizes and shapes. Such electrospun constructs can be fabricated from natural and synthetic polymers to mimic the extracellular matrix to promote tissue regeneration. Also, therapeutic moieties such as drugs and growth factors can be incorporated into the constructs for enhanced treatment. Therefore, electrospun constructs are ideal for dressing material development.36
The gelatin-dendrimer nanofiber dressing reported herein integrates advantages of both dendrimers and nanofibers for wound healing and drug delivery. The constructs have a unique structural configuration in which covalently bound dendrimer is evenly distributed along the dimension of the nanofiber. The addition of dendrimer provides functional sites for attachment of drugs and their controlled release. Converting electrospun gelatin nanofibers into sIPN with PEG diacrylate successfully improved the structural stability and mechanical properties of the construct.
Although gelatin NCs degrade instantly in water, they are stable in ethanol and swell slightly. Ethanol diffuses through interfibrillar spaces and may be absorbed by fibers. In the meantime, solute PEG DA dissolved in ethanol can be passively brought into the porous fiber construct and may penetrate into individual fibers to a certain extent. Following this incubation process and UV-curing, the fiber construct is then intertwined with a cross-linked PEG network. The resulting sIPN NCs became more stable, making them suitable for sustained silver release and water absorption.
As bacterial infection is commonly associated with wound healing, silver was incorporated into the construct and showed effectiveness in inhibiting bacterial growth. In general, silver exerts antimicrobial activity through release kill mechanism. Conjugated dendrimers also provide nanodomains for association with silver ions, allowing silver to exert antimicrobial activities possibly through a dual bactericidal mode of action—both release and contact killing mechanisms.37
This unique gelatin-dendrimer nanofiber construct has high adaptability and can be easily tailored to deliver various compounds needed for wound healing. Therefore, it has great potential to be customized to deliver state-of-the-art therapeutics as they are developed to treat different types of wounds. In future studies, growth factors can be incorporated into the dressing and assessed for effectiveness in promoting wound healing. The process of wound healing may require involvement of growth factors such as TGF-β, which can help promote collagen production in fibroblasts in the proliferative phase of wound healing. Correlation between cellular/tissue response and changes in material physicochemical properties will be pursued to establish criteria in optimizing nanofiber dressing structure for mediating healing in vivo.
Conclusions
Blends of gelatin and gelatin-dendrimer conjugates containing various loading levels of silver acetate were successfully electrospun into nanofiber constructs. The structural stability of the resulting nanofiber constructs was improved by converting them into sIPNs with PEG DA575 without compromising nanofiber morphology. The sIPN NCs possessed good swelling capacity owing to porous structures and were permeable to aqueous solutions. They allowed sustained silver release and showed antimicrobial activity against two common types of pathogens—Staphylococcus aureus and Pseudomonas aeruginosa. Incorporation of dendrimers into the gelatin nanofibers through covalent conjugation not only expands drug loading capacity of nanofiber constructs but provides tremendous flexibility for developing multifunctional electrospun dressing materials.
Acknowledgments
This work was supported, in part, by the National Science Foundation (CAREER Award CBET0954957). Confocal microscopy was performed in the Department of Neurobiology and Anatomy Microscopy Facility at Virginia Commonwealth University, supported, in part, with funding from NIH-NINDS Center core grant (5P30NS047463). The authors thank Dr. Ping Xu and his lab at the Philips Institute of Oral and Craniofacial Molecular Biology of Virginia Commonwealth University for assistance in antimicrobial assay.
References
- 1.Seaman S. J Am Podiatr Med Assoc. 2002;92:24–33. doi: 10.7547/87507315-92-1-24. [DOI] [PubMed] [Google Scholar]
- 2.Kao WJ. Biomaterials. 1999;20:2213–2221. doi: 10.1016/s0142-9612(99)00152-0. [DOI] [PubMed] [Google Scholar]
- 3.Ruszczak Z. Adv Drug Delivery Rev. 2003;55:1595–1611. doi: 10.1016/j.addr.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 4.Aduba DC, Hammer JA, Yuan Q, Yeudall WA, Bowlin GL, Yang H. Acta Biomater. 2013;9:6576–6584. doi: 10.1016/j.actbio.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu M, Frechet JMJ. Pharm Sci Technol Today. 1999;2:393–401. doi: 10.1016/s1461-5347(99)00203-5. [DOI] [PubMed] [Google Scholar]
- 6.Esfand R, Tomalia DA. Drug Discovery Today. 2001;6:427–436. doi: 10.1016/s1359-6446(01)01757-3. [DOI] [PubMed] [Google Scholar]
- 7.Newkome GR, Yao Z, Baker GR, Gupta VK. J Org Chem. 1985;50:2003–2004. [Google Scholar]
- 8.Tomalia DA, Baker H, Dewald J, Hall M, Kallos G, Martin S, Roeck J, Ryder J, Smith P. Polym J (Tokyo, Jpn) 1985;17:117–132. [Google Scholar]
- 9.Tomalia DA, Baker H, Dewald J, Hall M, Kallos G, Martin S, Roeck J, Ryder J, Smith P. Macromolecules. 1986;19:2466–2468. [Google Scholar]
- 10.Yuan Q, Fu Y, Kao WJ, Janigro D, Yang H. ACS Chem Neurosci. 2011;2:676–683. doi: 10.1021/cn200078m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuan Q, Lee E, Yeudall WA, Yang H. Oral Oncol. 2010;46:698–704. doi: 10.1016/j.oraloncology.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balogh L, Swanson DR, Tomalia DA, Hagnauer GL, McManus AT. Nano Lett. 2001;1:18–21. [Google Scholar]
- 13.Ghosh S, Banthia AK. J Biomed Mater Res, Part A. 2004;71:1–5. doi: 10.1002/jbm.a.30121. [DOI] [PubMed] [Google Scholar]
- 14.Chauhan AS, Diwan PV, Jain NK, Tomalia DA. Biomacromolecules. 2009;10:1195–1202. doi: 10.1021/bm9000298. [DOI] [PubMed] [Google Scholar]
- 15.Reneker DH, Yarin AL, Fong H, Koombhongse S. J Appl Phys. 2000;87:4531–4547. [Google Scholar]
- 16.Matthews JA, Wnek GE, Simpson DG, Bowlin GL. Biomacromolecules. 2002;3:232–238. doi: 10.1021/bm015533u. [DOI] [PubMed] [Google Scholar]
- 17.Boland ED, Matthews JA, Pawlowski KJ, Simpson DG, Wnek GE, Bowlin GL. Front Biosci. 2004;9:1422–1432. doi: 10.2741/1313. [DOI] [PubMed] [Google Scholar]
- 18.Shields KJ, Beckman MJ, Bowlin GL, Wayne JS. Tissue Eng. 2004;10:1510–1517. doi: 10.1089/ten.2004.10.1510. [DOI] [PubMed] [Google Scholar]
- 19.Rho KS, Jeong L, Lee G, Seo BM, Park YJ, Hong SD, Roh S, Cho JJ, Park WH, Min BM. Biomaterials. 2006;27:1452–1461. doi: 10.1016/j.biomaterials.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 20.Khil MS, Cha DI, Kim HY, Kim IS, Bhattarai N. J Biomed Mater Res, Part B. 2003;67:675–679. doi: 10.1002/jbm.b.10058. [DOI] [PubMed] [Google Scholar]
- 21.Ignatova M, Manolova N, Markova N, Rashkov I. Macromol Biosci. 2009;9:102–111. doi: 10.1002/mabi.200800189. [DOI] [PubMed] [Google Scholar]
- 22.Babaeijandaghi F, Shabani I, Seyedjafari E, Naraghi ZS, Vasei M, Haddadi-Asl V, Hesari KK, Soleimani M. Tissue Eng, Part A. 2010;16:3527–3536. doi: 10.1089/ten.TEA.2009.0829. [DOI] [PubMed] [Google Scholar]
- 23.Hadjiargyrou M, Chiu JB. Expert Opin Drug Delivery. 2008;5:1093–1106. doi: 10.1517/17425247.5.10.1093. [DOI] [PubMed] [Google Scholar]
- 24.Kailasan A, Yuan Q, Yang H. Acta Biomater. 2010;6:1131–1139. doi: 10.1016/j.actbio.2009.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sell S, Barnes C, Simpson D, Bowlin G. J Biomed Mater Res, Part A. 2008;85A:115–126. doi: 10.1002/jbm.a.31556. [DOI] [PubMed] [Google Scholar]
- 26.Ayres CE, Bowlin GL, Pizinger R, Taylor LT, Keen CA, Simpson DG. Acta Biomater. 2007;3:651–661. doi: 10.1016/j.actbio.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parsons D, Bowler PG, Myles V, Jones S. Wounds. 2005;17:222–232. [Google Scholar]
- 28.Mermet JM. J Anal At Spectrom. 2005;20:11–16. [Google Scholar]
- 29.Stefánsson A, Gunnarsson I, Giroud N. Anal Chim Acta. 2007;582:69–74. doi: 10.1016/j.aca.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 30.Peppas NA, Sahlin J. J Int J Pharm. 1989;57:169–172. [Google Scholar]
- 31.He C, Kim SW, Lee DS. J Controlled Release. 2008;127:189–207. doi: 10.1016/j.jconrel.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 32.Boateng JS, Matthews KH, Stevens HNE, Eccleston GM. J Pharm Sci. 2008;97:2892–2923. doi: 10.1002/jps.21210. [DOI] [PubMed] [Google Scholar]
- 33.Sevostianov I, Kushch V. Int J Solids Struct. 2009;46:4419–4429. [Google Scholar]
- 34.Lukacs GL, Haggie P, Seksek O, Lechardeur D, Freedman N, Verkman AS. J Biol Chem. 2000;275:1625–1629. doi: 10.1074/jbc.275.3.1625. [DOI] [PubMed] [Google Scholar]
- 35.Yang H, Tyagi P, Kadam RS, Holden CA, Kompella UB. ACS Nano. 2012;6:7595–7606. doi: 10.1021/nn301873v. [DOI] [PubMed] [Google Scholar]
- 36.Sill TJ, von Recum HA. Biomaterials. 2008;29:1989–2006. doi: 10.1016/j.biomaterials.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 37.Sambhy V, MacBride MM, Peterson BR, Sen A. J Am Chem Soc. 2006;128:9798–9808. doi: 10.1021/ja061442z. [DOI] [PubMed] [Google Scholar]