Introduction
Taking creatine supplements, as a possible performance enhancing substance, is quite common among body builders and power lifters (1). Various researches have shown that oral creatine supplementation appears to be safe even at dose of 20 g/day for 5 days, and maintenance doses of less than 3 g/day (1) while, other reports have denoted the nephrotoxic effects of these products (2,3). At this study, a previously healthy man who has developed interstitial nephritis and renal failure shortly after taking ceatine monohydrate supplement is reported.
Case
A 32-year-old man presented with 2 weeks history of nausea and weakness. He had the history of creatine monohydrate consumption for body building purpose for 3 weeks during the last month, while consuming intermittent dose of 20 g/day for 3 days and maintenance dose of 1 g/day for three weeks. He had not had any other past medical history, family history, and/or kidney disease. Physical characteristics of the case were 65 kg weight and 160/100 mmHg blood pressure. The initial laboratory studies were as follows; serum urea; 111 mg/dL (10-20 mg/dL), serum creatinine; 4.3 mg/dL, uric acid; 8 mg/dL, potassium; 5.6 meq/L and serum sodium; 148 meq/L. Blood gas analysis revealed; 7.29 PH (normal 7.35–7.45), hemoglobin was 10 g/dL with 30.5% hematocrit level Urinalysis revealed proteinuria, and 24 hours protein excretion was 850 mg/day. Serology examination revealed a negative result for antinuclear antibody (ANA), Anti-double-stranded DNA and Antineutrophil cytoplasmic antibodies (ANCA). Serologic examinations for hepatitis B, hepatitis C and HIV infection were negative. During hospitalization, his serum creatinine level increased to 6.2 mg/dL. Kidney sonography showed normal sized kidneys (120 mm right kidney and 118 mm left kidney in length) with slightly increased echogenicity. The renal biopsy revealed normal glomeruli with mononuclear interstitial infiltrations and focal tubular disappearance. There were few sloughed epithelial cells, and cellular debris in the tubular lumina (Fig. 1-A and 1-B). However, immune complex deposition was not identified in immunofluorescence study. Considering the above-mentioned features, renal biopsy was mostly consistent with interstitial nephritis.
Figure 1(A, B).Renal biopsy showing normal glomerulus with mononuclear interstitial infiltrations and tubular disappearance. [H & E]. Magnifications: x400 in A and B.
A.
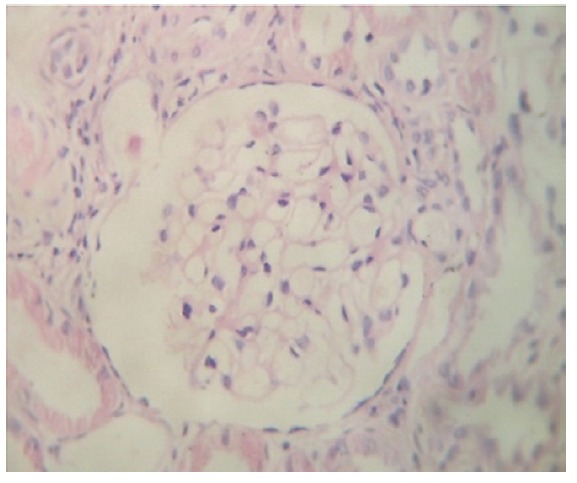
B.
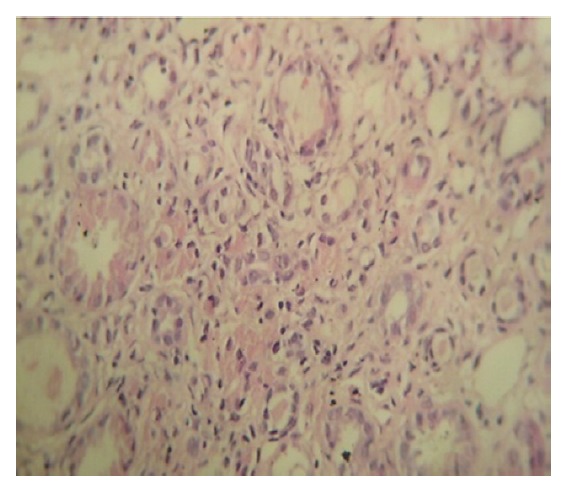
Patient has received two consecutive days of Methylprednisolon pulse therapy (500 mg/day) followed by 60 mg daily Prednisolon, which was finally tapered off over the next six weeks. After starting corticosteroid therapy, patient’s serum creatinine level decreased to 1.8 mg/dL and his general condition improved profoundly.
Discussion
In this case, the patient developed renal dysfunction and interstitial nephritis after taking creatine monohydrate. Patient had no history of renal disease or using any other nephrotoxic substance. After stopping the creatine supplement and starting treatment with corticosteroid, his renal function improved reasonably.
In literature, creatine supplementation associated with renal dysfunction and acute tubular necrosis have been reported in a few patients (2,3). Secondary, focal segmental glomerulosclerosis has been reported in athletes using anabolic androgenic steroids (AAS), (4). Combination of AAS abuse with Creatine monohydrate, and high-protein diet is a common combination among body-builders and this combination could increase the risk of renal damage.
In conclusion, even low doses of creatine monohydrate supplementation may cause kidney damage, and athletes should be warned about this possible side effect.
Author’s contributions
MRA and ZS provided extensive intellectual contribution and prepared some parts of the draft. AV reviewed the draft. MRA prepared the manuscript.
Conflict of interest
The author declared no competing interests.
Funding/Support
None declared.
Acknowledgments
The authors wish to thank to the staffs of Internal Medicine Department, Tabriz University of Medical Sciences.
Implication for health policy/practice/research/medical education:
At this study, a man with developed interstitial nephritis and renal failure, shortly after taking creatine monohydrate supplement is reported. Renal biopsy is mostly consistent with interstitial nephritis. Results of this study showed that, even low dose of creatine monohydrate supplementation may cause kidney damage, and athletes should be warned about this possible side effect.
Please cite this paper as: Ardalan MR, Samadifar Z, Vahedi A. creatine monohydrate supplement induced interstitial nephritis. J Nephropathology. 2012; 1(2): 117-120. DOI: 10.5812/nephropathol.7530
References
- 1.Bizzarini E, De Angelis L. Is the use of oral creatine supplementation safe? J Sports Med Phys Fitness . 2004;44(4):411–6. [PubMed] [Google Scholar]
- 2.Taner B, Aysim O, Abdulkadir U. The effects of the recommended dose of creatine monohydrate on kidney function. NDT plus . 2011;4(1):23–4. doi: 10.1093/ndtplus/sfq177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pritchard NR, Kalra PA. Renal dysfunction accompanying oral creatine supplements. Lancet . 1998;351(9111):1252–3. doi: 10.1016/s0140-6736(05)79319-3. [DOI] [PubMed] [Google Scholar]
- 4.Herlitz LC, Markowitz GS, Farris AB, Schwimmer JA, Stokes MB, Kunis C. et al. Development of focal segmental glomerulosclerosis after anabolic steroid abuse. J Am Soc Nephrol . 2010;21(1):163–72. doi: 10.1681/ASN.2009040450. [DOI] [PMC free article] [PubMed] [Google Scholar]


