Abstract
Background
Investigations have attempted to modify the outcome of tubular injury by either ameliorating renal tubular damage or promoting tubular regeneration in the case of acute tubular necrosis.
Objectives
We investigated the protective effect of Eprex an erythropoietin analogue on tubular injury induced by gentamicin (GM).
Materials and Methods
Forty male Wistar rats were randomly divided into four groups. In group 1,rats were served as a sham group. In group 2, rats were injected intraperitoneally with 100 mg/kg of GM for 10 consecutive days (positive control group) and then were sacrificed. In group 3, rats received GM for 10 days then Eprex 100U/kg was injected intraperitoneally for the next 10 days and then they were sacrificed at the day 20th. In group 4 rats were injected a combination of GM (80 mg/kg) and Eprex 100U/kg intraperitoneally for 10 days and then were sacrificed.
Results
The results indicated that, Eprex prevented the increase in serum creatinine (Cr) and blood urea nitrogen (BUN). The effect of Eprex on damage score, showed that co-administration of GM and Eprex (group 3 and 4) reduced the kidney tissue damage compared to positive control group (P<0.05). This result indicat that Eprex potentially can reduce or prevent the kidney tissue damage.
Conclusions
Ameliorative effect of Eprex when the drug was given in combination with GM and also when the drug was applied after GM–induced tubular damage, revealed the renoprotective potency of Eprex. Eprex is a promising drug to prevent or attenuate tubular damage induced by GM or other nephrotoxic agents which act through the same mechanisms as gentamicin.
Keywords: Gentamicin, Tubular toxicity, Erythropoietin
1. Background
Acute renal failure (ARF) is characterized by a rapid decline in glomerular filtration rate over hours to several days, and the retention of nitrogenous waste products (1,2). The mortality rate of patients with ARF is remained 25-70%, despite the use of various pharmacologic agents (2-5). Indeed, ARF frequently occurs in critically ill patients and is an independent risk factor for poor outcome. The prevention of kidney injury in intensive care remains a great challenge as specific nephroprotective therapies are still lacking (6-8). Investigations have been attempted to modify the outcome of this disease especially in patients suffering from tubular injury by either ameliorating renal tubular damage or promoting tubular regeneration in the case of acute tubular necrosis. However, to date, none of these modalities has made any appreciable clinical trend on the high mortality and morbidity rates associated with this condition (9). Unfortunately, in the clinical setting, most cases of ARF are not identified until sometime after the insult has already occurred. Thus, the clinical utility of any therapeutic agent for ARF would be greatly enhanced if delayed administration of the drug still proved to be effective in renoprotection. Several growth factors have been identified to regulate regeneration and repair (9,10). Erythropoietin was first characterized as a hematopoietic factor, and it has been used clinically for the treatment of anemia (10-12). Erythropoietin is a cytokine that is produced primarily in the kidneys by renal cortical fibroblasts (10,11), and which regulates the differentiation and proliferation of erythroid progenitor cells. However, due to in-vitro human recombinant erythropoietin stimulates endothelial cell proliferation (10-12) and angiogenesis (10,13,14), erythropoietin effects may not be limited to bone marrow cells. Moreover, it has been shown to exert pleiotropic properties, such as cytoprotection, anti-inflammation and antiapoptosis, in the central nervous, kidney and liver (12-15). Studies also suggested that erythropoietin may act directly on damaged tubular cells and stimulates their regeneration (9,15). It has been shown that erythropoietin receptors are found on renal tubular epithelial, mesangial and endothelial cells. This effect may be through activation signaling pathways to prevent apoptosis and/or stimulate reparative proliferation of the injured cells (9,14,15). Gentamicin (GM) is widely known to induce ARF as a result of renal tubular epithelial cell injury by direct acute tubular necrosis which is primarily localized to the proximal tubule. Nephrotoxicity is manifested by increased concentrations of plasma urea nitrogen, serum creatinine and various histological aspects of renal tissue (16-19). Mechanisms of renal injury include binding to anionic phospholipids and altering the function and structure of cellular and intracellular membranes (17-20). Furthermore, ATP depletion from either mitochondrial damage or direct inhibition of mitochondrial oxidative phosphorylation (17-21) and reactive oxygen species (ROS) production are other mechanism take part in nephrotoxicity(18-20). Most studies reported previously were designed to administer drugs before or at the same time of renal insult (18-24). However, most therapeutic agents are usually administered after the expression of clinical diseases. Indeed, most cases of acute renal failures are not identified until the insult has already occurred. Hence, the clinical utility of any therapeutic agent for this disease would be greatly enhanced if delayed administration of the drug still proved to be renoprotective. Whether delayed treatment with erythropoietin exerts similar benefits in ischemic, or toxic renal injury is still unknown.
2. Objectives
In this study we aimed firstly to investigate the possible protecting effect of erythropoietin on GM-induced renal toxicity in rats and secondly to examine whether post treatment of erythropoietin for tubular cell regeneration would be still beneficial.
3. Materials and Methods
Our study is a randomized controlled trial, approved by the ethical committee of Shahrekord University of Medical Sciences.
2. Objectives
Human recombinant erythropoietin [Eprex (Epoetin Alfa), Janssen Cilag Ltd, Switzerland] was provided as a 2,000 IU/mL solution. The GM and erythropoietin treatment protocols used in the present study have been reported previously (25,26).
3.2. Animal preparation
Study samples including 40 male Wistar rats with a weight range of 200-250 g were purchased from Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran. All animals were similarly handled in the animal house of our research center, had free access to food and water. They were housed in a temperature and humidity controlled environment (25 ± 3 ° C, 50-60%), with a 12 hrs dark-light cycle (lights on at 7.00 AM) and allowed free access to pelleted diet and tap water. Their general health state and activity were monitored closely during the experiment. The animal experimentation was conducted in accordance with the National Institute of Health guide for the careful use of laboratory animals (27). They were allocated randomly to 4 groups (10 rats in each) .The study groups were assigned the following regimens:
Group 1: Rats in this group were served as a sham group. They were kept in the same condition as others without receiving drugs for 10 days and then were sacrificed.
Group 2: Rats in this group were injected intraperitoneally with 100 mg/kg of GM for 10 consecutive days (positive control group) and then were sacrificed.
Group 3: Rats in this group were received GM (100mg/kg) for 10 days then Eprex 100U/kg was injected intraperitoneally for the next 10 days and then the rats were sacrificed at the day 20th.
Group 4: Rats in this group were injected intraperitoneally with a combination of GM (100mg/kg) and Eprex 100U/kg intraperitoneally for 10 days and then were sacrificed.
On the day one (before experiment) and on the final day (day of sacrificing) serum samples were obtained to measure urea nitrogen (BUN) and creatinine (Cr) for all the rats. Blood samples were obtained and then rats were sacrificed by injecting ketamine (i.p.) and kidneys were removed immediately for histological examinations.
3.3. Determination of blood urea nitrogen (BUN) and serum creatinine level
BUN and serum creatinine (Cr) level was measured by colorimetric method using commercial kits by an auto analyzer.
3.4. Histopathological evaluations
The animal kidneys were removed, then fixed in formalin for 12 hours and processed for histopathological study. Four 2-3 μm-thick paraffin sections were stained with hematoxylin and eosin for light microscope examination using conventional protocol (28). Histopathological studies were performed under a light microscope. Slides were coded and were examined by a Nephropathologist (HN) who was blinded to the treatment groups. All specimens were examined for six morphologic parameters including epithelial cell vacuolization, degeneration, tubular cell flattening, hyaline cast, tubular dilatation and debris materials in tubular lumen on a semi-quantitative score from 1 to 5. The score of zero was assigned to the normal tissue without damage (29,30).
3.5. Statistical Analysis
Data were expressed as mean ± SEM. One way ANOVA was applied to compare the serum BUN and Cr levels between the groups. To compare the pathological damage score between the groups, Mann-Whitney or Kruskal-Wallis was applied. P<0.05 were considered statistically significant.
4. Results
4.1. The effect of Eprex on BUN and Cr levels:
Compared to others groups, the results indicated that GM increased the levels of BUN and Cr significantly (P<0.05). However, Eprex prevented this increment. No significant difference in serum BUN and Cr levels was detected in GM plus Eprex treated rats when compared with sham group (table 1).
Table 1. Serum levels of BUN and Cr in 4 groups of experiment.
| Group | BUN (mg/dl) | Cr (mg/dl) | ||||
| before | after | Δ | before | after | Δ | |
| 1 | 21.80±0.49 | 22.60±0.65 | 0.8±0.72 | 0.54±0.02 | 0.55±0.02 | 0.01±0.02 |
| 2 | 21.75±0.43 | 77.90±16.28* | 56±16.23* | 0.54±0.01 | 1.55±0.33* | 1.01±0.33* |
| 3 | 21.80±0.49 | 31.60±1.08 | 9.8±1.17 | 0.54±0.02 | 0.59±0.03 | 0.05±0.02 |
| 4 | 21.78±0.55 | 45.22±2.09 | 23.4±2.09 | 0.54±0.02 | 0.81±0.03 | 0.26±0.04 |
| P value | n.s | <0.01 | <0.001 | n.s | <0.01 | <0.01 |
| *: Significant difference from other groups. Group 1; sham, group 2: GM treated for 10 days (positive control), group 3: GM treated for 10 days following by Eprex treated for 10 days, group 4; GM plus Eprex treated for 10 days. Ns: non–significant, ?: concentration difference (after-before) | ||||||
4.2. The effect of Eprex on damage score
GM induced kidney damage (p<0.05). Co-administration of GM and Eprex (group 3 and 4) reduced the kidney tissue damage when compared with positive control group (P<0.05). This result indicated that Eprex potentially can reduce or prevent the kidney tissue damage (Figure 1).
Figure 1.
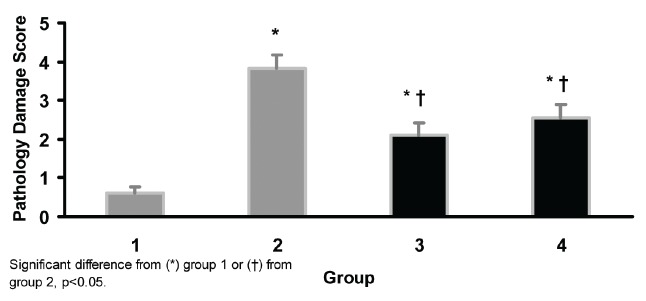
Kidney tissue damage score in 4 groups of experiments. Group 1: sham; group 2: gentamicin treated for 10 days (positive control); group 3: gentamicin treated for 10 days following Eprex treatment for 10 days, group 4; gentamicin plus Epex treated for 10 days.
5. Discussion
In this study we found that Eprex could attenuate GM-induced tubular toxicity. Numerous studies have been attempted to modify the outcome of acute tubular injury by either ameliorating tubular injury or promoting tubular regeneration (9,10,31). While erythropoietin receptors are expressed on renal tubular epithelial cells (15,31), it is possible that the systemic administration of erythropoietin may also provide protection against acute renal damage (15,31). Erythropoietin has also been found to ameliorate toxic renal injury caused by Cisplatin (31,32). Similarly, its renoprotective effect in ischemia–reperfusion renal injury, as the most common cause of ARF in the community was also reported (15,31). It seems that erythropoietin is not solely a hormone charged with regulating the proliferation and differentiation of erythroid progenitor cells (33,34). Its erythropoietic function is mediated by anti-apoptotic pathways, mainly involving Akt and the Bcl-2 gene family in bone marrow. Thereby, it facilitates the maturation and differentiation of erythroid progenitor cells (10,15). The effect is a compensatory adaptation to renal tissue hypoxia by augmenting the oxygen-carrying capacity of the blood (31). Recently, erythropoietin has been reported to have several important biological effects in addition to the stimulation of erythropoiesis (8-11). Dang et al. found that renal tubular cells possess erythropoietin receptor reduces high glucose-induced oxidative stress in renal tubular cells (35). They have also found that erythropoietin inhibited high glucose-induced renal tubular cell apoptosis. In their study, this protective effect was dependent on the reduction of Bax/caspase-3 expression and elevation of Bcl-2 expression. They suggested that erythropoietin can inhibit high glucose-induced renal tubular cell apoptosis by direct effect on anti-oxidative stress and that erythropoietin receptor may play a key role in this process (35). Erythropoietin is reduced kidney dysfunction by decreasing apoptosis. Also, erythropoietin has been shown to reduce the expression of pro-inflammatory mediators, TNF-alpha and IL-2, in renal injury and reverse the effect of endotoxin on the antioxidant, renal superoxide dismutase. These anti-inflammatory properties of erythropoietin also suggested the involvement of the NF-kB pathway through its kidney protection (15). While, erythropoietin is an extremely potent stimulator of endothelial progenitor cells, its function is partly dependent on nitric oxide bioavailability. Erythropoietin activates endothelial nitric oxide synthase, and this effect on the endothelium may be critical for its renal protective effect. Erythropoietin limits acute kidney injury in part by stimulating vascular repair and by increasing tubular cell proliferation (31,36,37). These findings suggested that erythropoietin may exert a protective effect via an interaction with the microvasculature, too (15,31,36,37). The cytoprotective and mitogenic effects of erythropoietin on proximal and distal tubular epithelial cells may explain the observation of a modest reduction in tubular cast formation in erythropoietin -treated rats following renal injury. Decreased cell sloughing and cast formation may be due to reduction in intra-tubular obstruction and a subsequent amelioration of renal functional impairment (31). Previous investigators have also shown that erythropoietin exerts significant mitogenic and anti-apoptotic actions in non-renal and non-erythroid cells, including endothelial and gastric mucosal cells and similarly in myoblasts and Leydig cells (31,37). To explain the mechanisms toward erythropoietin-related renal protection, several hypotheses have been described. Rjiba-Touati et al. found that the protective effect of erythropoietin against Cisplatin-induced oxidative stress and nephrotoxicity in rat kidney may be mediated through decreased oxidative damage induced by Cisplatin. Recombinant human erythropoietin reduced malondialdehyde and protein carbonyl levels and also prevented glutathione depletion and ameliorated the increased catalase activity induced by Cisplatin treatment (38). They also found that, recombinant human erythropoietin restored creatinine and blood urea nitrogen levels increased by Cisplatin. They concluded that recombinant human erythropoietin administration especially in pretreatment condition protected rats against Cisplatin-induced renal oxidative stress and nephrotoxicity(39). In another study conducted by Rjiba-Touati et al. to find the protective effect of erythropoietin against Cisplatin-induced apoptosis in rat kidney, a reduction of apoptosis by up regulation of antiapoptotic protein expressions, down regulation of pro apoptotic protein levels, and reduction of caspase-3 activity in male Wistar rats was seen (40). Hence it seems that the protective action of Eprex on the kidney probably is not directly related to its hematopoietic effects (14). Furthermore, various beneficial effects of erythropoietin in preventing aristolochic acid-induced apoptosis in the cultured cells were investigated by Wang et al. (41). To understand the efficacy of pretreatment with erythropoietin in the attenuation of radio contrast-induced acute renal failure in rats, Goldfarb et al. have conducted a study on twenty-two male rats. It their work, rats were subjected to saline (controls) or erythropoietin injections (3000 U/kg and 600 U/kg, 24 and 2 h before the induction of radio contrast-induced nephropathy, respectively. They found that erythropoietin pre-treatment prevents renal dysfunction in a rat model of contrast-induced nephropathy (42). In our study post-treatment of GM-induced renal toxicity in group 4 had the same efficacy with rats in group 3, where there was a combination therapy of GM and Eprex. Thus we could assume that erythropoietin treatment is still effective when renal injuries happen.
Aminoglysosides-induced nephrotoxicity is typically characterized by tubular necrosis (43). It seems that free radicals may be involved (20). Since, erythropoietin induces erythrocytosis by suppressing erythroid progenitor cell apoptosis through the Janus-activated kinase/signal transducers and activators of transcription pathway (20,44) and apoptosis contributes to GM-induced nephrotoxicity, we could speculate that the attenuation of GM-induced nephrotoxicity by Eprex treatment of is mediated through it’s antiapoptotic actions as well. In a randomized, placebo-controlled, clinical trial of preoperative erythropoietin in 71 patients who were underwent elective coronary artery bypass graft surgery, renoprotective effects of erythropoietin was reported (45). Erythropoietin was given at a dose of 300 U/kg IV immediately preoperatively and was associated with a reduction in the incidence of acute kidney injury from 29% to 8% and was improved postoperative renal function as indicated by a smaller increase in serum Cr and a smaller decline in estimated glomerular filtration rate postoperatively. Moore et al. assessed the effect of erythropoietin in ICU patients deemed at risk for acute kidney injury (15). Erythropoietin was given at 500 U/kg IV when a high GGT × ALP product was detected and repeated 24 hours later. The primary outcome of this study was the increase in the average percentage of creatinine from baseline to its peak over 7 days. In contrast to the previous study, in a trial conducted by Endre et al., they found no renoprotective effect of erythropoietin (46). The reasons for these contradictory findings may be due to the differences in the design and the methods of the investigation.
6. Conclusions
This study showed the renoprotective effect of Eprex, when the drug is given in combination with GM. Moreover, the protective effect of Eprex was observed when the drug was applied after GM–induced tubular damage and was revealed that the drug was still effective after installation of tissue damage. Eprex is a promising renoprotective drug to prevent or attenuate tubular damage induced by GM or other nephrotoxic agents which act through the same mechanisms as gentamicin.
Author’s contributions
MRK and HN designed and performed the research. MN analyzed data and wrote some parts of paper. AB, AG, HR, SMAS, MBG, FGA and MRA provided extensive intellectual contribution and reviewed the manuscript. HN and MRK prepared the manuscript.
Conflict of interest
The author declared no competing interests.
Funding/Support
This study was granted by the research deputy of Shahrekord University of Medical Sciences (grant #994).
Acknowledgments
The authors wish to thank staffs of Medical Plants Research Center of Shahrekord University of Medical Sciences.
Implication for health policy/practice/research/medical education:
This article presents that Eprex as an analogue of erythropoietin is a promising renoprotective drug to prevent or attenuate tubular damage induced by gentamicin.
Please cite this paper as: Rafieian-Kopaei M, Nasri H, Nematbakhsh M, Baradaran A, Gheissari A, Rouhi H, Ahmadi Soleimani SM, Baradaran-Ghahfarokhi M, Ghaed-Amini F, Ardalan MR.Erythropoietin ameliorates gentamycin-induced renal toxicity: A biochemical and histopathological study. J Nephropathology. 2012; 1(2): 109-116, DOI:10.5812/nephropathol.7533
References
- 1.Prokai A, Fekete A, Banki NF, Muller V, Ver A, Degrell P. et al. Renoprotective effect of erythropoietin in rats subjected to ischemia/reperfusion injury: gender differences. Surgery . 2011;150(1):39–47. doi: 10.1016/j.surg.2011.02.019. [DOI] [PubMed] [Google Scholar]
- 2.Heyman SN, Rosenberger C, Rosen S. Acute kidney injury: lessons from experimental models. Contrib Nephrol . 2011;169:286–96. doi: 10.1159/000313957. [DOI] [PubMed] [Google Scholar]
- 3.Khajehdehi P. Turmeric: Reemerging of a neglected Asian traditional remedy. Journal of Nephropathology. 2012;1(1) doi: 10.5812/jnp.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uz E, Uz B, Kaya A, Akdeniz D, BavbekRuzgaresen N, HilmiTurgut F. et al. Protective effect of erdosteine on cyclosporine induced chronic nephrotoxicity in rats. Nephro-Urology Monthly . 2011;3(04):280–4. [Google Scholar]
- 5.Rosen S, Stillman IE. Acute tubular necrosis is a syndrome of physiologic and pathologic dissociation. J Am Soc Nephrol . 2008;19(5):871–5. doi: 10.1681/ASN.2007080913. [DOI] [PubMed] [Google Scholar]
- 6.Tayebi Khosroshahi H. Short history about renal transplantation program in Iran and the world: Special focus on world kidney day 2012. Journal of Nephropathology. 2012;1(1) doi: 10.5812/jnp.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tolou-Ghamari Z. Nephro and neurotoxicity, mechanisms of rejection: A review on Tacrolimus and Cyclosporin in organ transplantation. J Nephropathology . 2012;1(1):23–30. doi: 10.5812/jnp.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernhardt WM, Eckardt KU. Physiological basis for the use of erythropoietin in critically ill patients at risk for acute kidney injury. Curr Opin Crit Care . 2008;14(6):621–6. doi: 10.1097/MCC.0b013e328317ee82. [DOI] [PubMed] [Google Scholar]
- 9.Johnson DW, Pat B, Vesey DA, Guan Z, Endre Z, Gobe GC. Delayed administration of darbepoetin or erythropoietin protects against ischemic acute renal injury and failure. Kidney Int . 2006;69(10):1806–13. doi: 10.1038/sj.ki.5000356. [DOI] [PubMed] [Google Scholar]
- 10.Johnson DW, Forman C, Vesey DA. Novel renoprotective actions of erythropoietin: new uses for an old hormone. Nephrology (Carlton) . 2006;11(4):306–12. doi: 10.1111/j.1440-1797.2006.00585.x. [DOI] [PubMed] [Google Scholar]
- 11.Provatopoulou ST, Ziroyiannis PN. Clinical use of erythropoietin in chronic kidney disease: outcomes and future prospects. Hippokratia . 2011;15(2):109–15. [PMC free article] [PubMed] [Google Scholar]
- 12.Chateauvieux S, Grigorakaki C, Morceau F, Dicato M, Diederich M. Erythropoietin, erythropoiesis and beyond. Biochem Pharmacol . 2011;82(10):1291–303. doi: 10.1016/j.bcp.2011.06.045. [DOI] [PubMed] [Google Scholar]
- 13.RezaTamadon M, Khatibinezhad A, Ghorbani R, Soleimani A, Malek F, Jalalzadeh M. et al. The impact of human recombinant erythropoietin on renal function in patients with chronic kidney disease. Nephro-Urology Monthly . 2011;3(02):114–6. [Google Scholar]
- 14.De Beuf A, D’Haese PC, Verhulst A. Epoetin delta as an antifibrotic agent in the remnant kidney rat: a possible role for transforming growth factor beta and hepatocyte growth factor. Nephron Exp Nephrol . 2010;115(3):e46–59. doi: 10.1159/000313830. [DOI] [PubMed] [Google Scholar]
- 15.Moore E, Bellomo R. Erythropoietin (EPO) in acute kidney injury. Ann Intensive Care . 2011;1(1):3. doi: 10.1186/2110-5820-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hariprasad G, Kumar M, Rani K, Kaur P, Srinivasan A. Aminoglycoside induced nephrotoxicity: molecular modeling studies of calreticulin-gentamicin complex. J Mol Model . 2012;18(6):2645–52. doi: 10.1007/s00894-011-1289-8. [DOI] [PubMed] [Google Scholar]
- 17.Ali BH, Al Za’abi M, Blunden G, Nemmar A. Experimental gentamicin nephrotoxicity and agents that modify it: a mini-review of recent research. Basic Clin Pharmacol Toxicol . 2011;109(4):225–32. doi: 10.1111/j.1742-7843.2011.00728.x. [DOI] [PubMed] [Google Scholar]
- 18.Tavafi M, Ahmadvand H, Toolabi P. Inhibitory effect of olive leaf extract on gentamicin-induced nephrotoxicity in rats. Iran J Kidney Dis . 2012;6(1):25–32. [PubMed] [Google Scholar]
- 19.Tavafi M, Ahmadvand H. Effect of rosmarinic acid on inhibition of gentamicin induced nephrotoxicity in rats. Tissue Cell . 2011;43(6):392–7. doi: 10.1016/j.tice.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Kopple JD, Ding H, Letoha A, Ivanyi B, Qing DP, Dux L. et al. L-carnitine ameliorates gentamicin-induced renal injury in rats. Nephrol Dial Transplant . 2002;17(12):2122–31. doi: 10.1093/ndt/17.12.2122. [DOI] [PubMed] [Google Scholar]
- 21.Juan SH, Chen CH, Hsu YH, Hou CC, Chen TH, Lin H. et al. Tetramethylpyrazine protects rat renal tubular cell apoptosis induced by gentamicin. Nephrol Dial Transplant . 2007;22(3):732–9. doi: 10.1093/ndt/gfl699. [DOI] [PubMed] [Google Scholar]
- 22.Kadkhodaee M, Khastar H, Arab HA, Ghaznavi R, Zahmatkesh M, Mahdavi-Mazdeh M. Antioxidant vitamins preserve superoxide dismutase activities in gentamicin-induced nephrotoxicity. Transplant Proc . 2007;39(4):864–5. doi: 10.1016/j.transproceed.2007.02.038. [DOI] [PubMed] [Google Scholar]
- 23.Ghaznavi R, Kadkhodaee M. Comparative effects of selective and non-selective nitric oxide synthase inhibition in gentamicin-induced rat nephrotoxicity. Arch Toxicol . 2007;81(6):453–7. doi: 10.1007/s00204-006-0157-2. [DOI] [PubMed] [Google Scholar]
- 24.Kadkhodaee M, Khastar H, Faghihi M, Ghaznavi R, Zahmatkesh M. Effects of co-supplementation of vitamins E and C on gentamicin-induced nephrotoxicity in rat. Exp Physiol . 2005;90(4):571–6. doi: 10.1113/expphysiol.2004.029728. [DOI] [PubMed] [Google Scholar]
- 25.Derakhshanfar A, Bidadkosh A, Sadeghian M. L-methionine attenuates gentamicin nephrotoxicity in male Wistar rat: pathological and biochemical findings. Iranian Journal of Veterinary Research. 2009;10(4) [Google Scholar]
- 26.Lee DW, Kwak IS, Lee SB, Song SH, Seong EY, Yang BY. et al. Post-treatment effects of erythropoietin and nordihydroguaiaretic acid on recovery from cisplatin-induced acute renal failure in the rat. J Korean Med Sci . 2009;24 Suppl:S170–5. doi: 10.3346/jkms.2009.24.S1.S170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jabbari M, Rostami Z, Jenabi A, Bahrami A, Mooraki A. Simvastatin ameliorates gentamicin-induced renal injury in rats. Saudi J Kidney Dis Transpl . 2011;22(6):1181–6. [PubMed] [Google Scholar]
- 28.Nasri H, Mortazavi M, Ghorbani A, Shahbazian H, Kheiri S, Baradaran A. et al. Oxford-MEST classification in IgA nephropathy patients: A report from Iran. Journal of Nephropathology . 2012;1(1):31–42. doi: 10.5812/jnp.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nematbakhsh M, Ashrafi F, Safari T, Talebi A, Nasri H, Mortazavi M. et al. Administration of vitamin E and losartan as prophylaxes in cisplatin-induced nephrotoxicity model in rats. J Nephrol . 2012;25(3):410–7. doi: 10.5301/jn.5000018. [DOI] [PubMed] [Google Scholar]
- 30.ESHRAGHI JF, NEMATBAKHSH M, NASRI HAMID TA, HAGHIGHI M, PEZESHKI Z, SAFARI TAHEREH AF. The protective role of endogenous nitric oxide donor (L-arginine) in cisplatin-induced nephrotoxicity: Gender related differences in rat model. JOURNAL OF RESEARCH IN MEDICAL SCIENCES (JRMS) . 2011;16(11):1389–96. [PMC free article] [PubMed] [Google Scholar]
- 31.Vesey DA, Cheung C, Pat B, Endre Z, Gobe G, Johnson DW. Erythropoietin protects against ischaemic acute renal injury. Nephrol Dial Transplant . 2004;19(2):348–55. doi: 10.1093/ndt/gfg547. [DOI] [PubMed] [Google Scholar]
- 32.Vaziri ND, Zhou XJ, Liao SY. Erythropoietin enhances recovery from cisplatin-induced acute renal failure. Am J Physiol . 1994;266(3 Pt 2):F360–6. doi: 10.1152/ajprenal.1994.266.3.F360. [DOI] [PubMed] [Google Scholar]
- 33.Bagnis C, Beaufils H, Jacquiaud C, Adabra Y, Jouanneau C, Le Nahour G. et al. Erythropoietin enhances recovery after cisplatin-induced acute renal failure in the rat. Nephrol Dial Transplant . 2001;16(5):932–8. doi: 10.1093/ndt/16.5.932. [DOI] [PubMed] [Google Scholar]
- 34.Yang CW, Li C, Jung JY, Shin SJ, Choi BS, Lim SW. et al. Preconditioning with erythropoietin protects against subsequent ischemia-reperfusion injury in rat kidney. FASEB J . 2003;17(12):1754–5. doi: 10.1096/fj.02-1191fje. [DOI] [PubMed] [Google Scholar]
- 35.Gong H, Wang W, Kwon TH, Jonassen T, Li C, Ring T. et al. EPO and alpha-MSH prevent ischemia/reperfusion-induced down-regulation of AQPs and sodium transporters in rat kidney. Kidney Int . 2004;66(2):683–95. doi: 10.1111/j.1523-1755.2004.00791.x. [DOI] [PubMed] [Google Scholar]
- 36.Dang J, Jia R, Tu Y, Xiao S, Ding G. Erythropoietin prevents reactive oxygen species generation and renal tubular cell apoptosis at high glucose level. Biomed Pharmacother . 2010;64(10):681–5. doi: 10.1016/j.biopha.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 37.Chatterjee PK. Novel pharmacological approaches to the treatment of renal ischemia-reperfusion injury: a comprehensive review. Naunyn Schmiedebergs Arch Pharmacol . 2007;376(1-2):1–43. doi: 10.1007/s00210-007-0183-5. [DOI] [PubMed] [Google Scholar]
- 38.Ates E, Yalcin AU, Yilmaz S, Koken T, Tokyol C. Protective effect of erythropoietin on renal ischemia and reperfusion injury. ANZ J Surg . 2005;75(12):1100–5. doi: 10.1111/j.1445-2197.2005.03612.x. [DOI] [PubMed] [Google Scholar]
- 39.Rjiba-Touati K, Boussema IA, Belarbia A, Achour A, Bacha H. Protective effect of recombinant human erythropoietin against cisplatin-induced oxidative stress and nephrotoxicity in rat kidney. Int J Toxicol . 2011;30(5):510–7. doi: 10.1177/1091581810411931. [DOI] [PubMed] [Google Scholar]
- 40.Rjiba-Touati K, Ayed-Boussema I, Bouaziz C, Belarbia A, Azzabi A, Achour A. et al. Protective effect of erythropoietin against cisplatin-induced nephrotoxicity in rats: antigenotoxic and antiapoptotic effect. Drug Chem Toxicol . 2012;35(1):89–95. doi: 10.3109/01480545.2011.589440. [DOI] [PubMed] [Google Scholar]
- 41.Wang W, Zhang J. Protective effect of erythropoietin against aristolochic acid-induced apoptosis in renal tubular epithelial cells. Eur J Pharmacol . 2008;588(2-3):135–40. doi: 10.1016/j.ejphar.2008.03.062. [DOI] [PubMed] [Google Scholar]
- 42.Goldfarb M, Rosenberger C, Ahuva S, Rosen S, Heyman SN. A role for erythropoietin in the attenuation of radiocontrast-induced acute renal failure in rats. Ren Fail . 2006;28(4):345–50. doi: 10.1080/08860220600591420. [DOI] [PubMed] [Google Scholar]
- 43.Ghaznavi R, Faghihi M, Kadkhodaee M, Shams S, Khastar H. Effects of nitric oxide on gentamicin toxicity in isolated perfused rat kidneys. J Nephrol . 2005;18(5):548–52. [PubMed] [Google Scholar]
- 44.Nagai J, Takano M. Molecular aspects of renal handling of aminoglycosides and strategies for preventing the nephrotoxicity. Drug Metab Pharmacokinet . 2004;19(3):159–70. doi: 10.2133/dmpk.19.159. [DOI] [PubMed] [Google Scholar]
- 45.Song YR, Lee T, You SJ, Chin HJ, Chae DW, Lim C. et al. Prevention of acute kidney injury by erythropoietin in patients undergoing coronary artery bypass grafting: a pilot study. Am J Nephrol . 2009;30(3):253–60. doi: 10.1159/000223229. [DOI] [PubMed] [Google Scholar]
- 46.Endre ZH, Walker RJ, Pickering JW, Shaw GM, Frampton CM, Henderson SJ. et al. Early intervention with erythropoietin does not affect the outcome of acute kidney injury (the EARLYARF trial) Kidney Int . 2010;77(11):1020–30. doi: 10.1038/ki.2010.25. [DOI] [PubMed] [Google Scholar]


