Abstract
Background
The technique of direct immunoflourescence (IF) is essential in the accurate diagnosis of renal glomerular diseases. The optimal results are obtained when the procedure is done on fresh frozen tissue (IF-F). However, techniques are available for IF study on formalin fixed and paraffin embedded (FFPE) renal biopsy specimens with variable reported success rates.
Objectives
We evaluated three such techniques on FFPE tissue and compared the results with those obtained by IF-F from the same patients.
Materials and Methods
Heat treatment with Tris buffer and citrate buffer, and pronase treatment of the FFPE material was carried out. Direct IF was done for renal panel immunoglobulins and complement components on all biopsies and the results were compared with the historical IF-F study.
Results
When compared to the IF-F, the immunoflourescence staining on the paraffin sections was less sensitive and less intense in all immune complex-mediated renal diseases, but the diagnostic findings were detected in majority of the cases.
Conclusions
In conclusion, it is possible to establish the diagnosis in most cases of immune complex-mediated glomerular diseases with IF on paraffin embedded tissue specimens.
Keywords: Complement, Frozen tissue, Immunoflourescence, Immunoglobulins, Paraffin embedded, Renal biopsies
1. Background
Immunoflourescence (IF) is an essential modality in the investigation of medical renal diseases, particularly the glomerular diseases, by renal biopsy (1-3). IF on the fresh frozen tissue (IF-F) is the gold standard method for the demonstration of immunoglobulins and complement components in the renal biopsy material (4). The major limitation of the currently used IF-F method is the requirement for the fresh frozen tissue; which may not be available in all cases. Sometimes, the frozen tissue may not contain the glomeruli which are essential for the IF study of renal biopsies. Hence, various methods to detect the immune reactants on formalin fixed and paraffin embedded (FFPE) tissue sections have been tried (5-10). However, no reliable and consistent results have been obtained yet, with any single method. The major problem with formaldehyde fixation and paraffin embedding is that tissue antigens are denatured and effective antigen analysis using IF becomes difficult. For this purpose, various antigen retrieval methods have been developed. There are three methods of retrieving antigens from FFPE tissue sections in use (4,11-17).
2. Objectives
In this study, we used both the heat-induced antigen retrieval (HIAT) method utilizing different retrieval buffers and the enzymatic method without heat treatment to recover the antigens in FFPE tissue sections of renal biopsies and perform direct IF using the standard renal panel of immunoglobulins and complement components and compare the results of these methods to the conventional IF-F.
3. Materials and Methods
3.1. Materials
A total of 40 FFPE blocks of renal biopsies received and reported in the histopathology department of Sindh Institute of Urology and Transplantation (SIUT) were studied. The biopsies were selected from the cases showing strong positive results on routine IF-F done during the diagnostic work-up of biopsies in our department. At our centre, two cores of renal biopsies are routinely obtained for complete pathologic evaluation. One core is received in 10% buffered formalin for light microscopy (LM) and the other is divided into two pieces under the dissection microscope; one piece is put in optimal cutting temperature (OCT) compound for IF-F and the other is fixed in glutaraldehyde for electron microscopy (EM). The retrieved blocks were cut at a thickness of 4-5 um as in our previous study and processed for IF using three different antigen retrieval techniques (1). The sections were taken on poly L-lysine coated glass slides. IF staining was performed using one-step or direct method. FFPE renal biopsy sections were treated with three different antigen retrieval methods.
3. 2. Methods
1. Heat-induced antigen retrieval using Tris buffer (TBS):
2.5 L of distilled water (DW) was heated to boil in a 5L stainless steel pressure cooker with operating pressure of 15 psi. Sections were deparaffinized in xylene and brought to alcohol. Slides were placed in coplin jar filled with TBS. Coplin jar lid was tightly sealed. Coplin jar was lowered into the boiling DW, allowed the top to rotate for 7 minutes. The stove was turned off and the pressure cooker allowed cooling down slowly. After 25 minutes, the lid was opened, and the TBS was discarded. Slides were washed in slowly running tap water for 2 minutes.
2. Heat-induced antigen retrieval using Citrate buffer:
The method was similar as pressure cooker antigen retrieval using TBS except that the TBS buffer was replaced by Citrate buffer.
3. Pronase method:
FFPE tissue sections were cut as described above, deparaffinized in xylene and brought to alcohol. Slides were marked with their appropriate numbers and circled the sections on slide with Dako pen. Ready to use pronase was applied on the tissue sections for digestion. Slides were kept at 37ºC for 1 hour.
Direct IF technique (1):
After retrieval of antigenic sites, slides were kept in phosphate buffered saline (PBS). Sections were placed on wet tray.
Excess PBS was drained and wiped off around sections. Sections were then incubated with optimally diluted primary flourescein isothyocyanate (FITC)-conjugated antibodies (Dako, Glostrup, Denmark), 1:40 for polyclonal rabbit anti-human IgG and 1:20 for IgA, IgM, C3 and C1q for 45 minutes at room temperature.
Slides were washed in PBS twice for 10 minutes and mounted with fluorescent mounting medium.
This procedure was carried out in darkness to avoid quenching of the fluorescent signal. The slides were examined under epifluorescence microscope in the dark and read as diffuse or focal, granular or linear positivity on an intensity scale of 0-3+ (where 0;absent, 1+; mild, 2+; moderate, 3+; strong) and distribution described as mesangial and membranous, as described in previous studies (1,18-20).
3.3. Statistical analysis
The collected data was entered into SPSS version 10.0 for analysis (SPSS, Chicago, IL, USA). Mean ±SD was used for continuous variables such as age. Numbers (percentages) were used for categorical variables such as positivity and intensity of immunoglobulins and complement components.
4. Results
4.1. Demographics
A total of 40 cases of immune complex-mediated GN were included from July 2010 till December 2010. Of these, 17 were males (42.5%) and 23 females (57.5%) with a male to female ratio of 1:1.35. The study included both adolescents and adult patients and the mean age was 29.70±10.68 years (range: 12-60 years). A breakdown of the final diagnoses of these cases is shown in table I. As is obvious from this table, this disease distribution does not represent a particular pattern of renal diseases, as the cases were purposively selected on the basis of strong positive results on IF-F during their routine work-up.
Table I. A breakdown of the final diagnoses of 40 cases of renal glomerular diseases included in the study.
| Diseases | Number | Percentage |
| LN | 15 | 37.5 |
| MGN | 11 | 27.5 |
| IgMN | 10 | 25 |
| MPGN | 2 | 5 |
| IgAN | 2 | 5 |
| Total | 40 | 100 |
LN; lupus nephritis, IgAN; IgA nephropathy, IgMN; IgM nephropathy, MGN; membranous GN, MPGN; membranoproliferative GN.
4.2. Overall IF results
4.2.1. IF-F method
Overall results of immunoreactants’ positivity and intensity are given in Table II. IgG was positive in 27 (67.5%) cases and negative in 13 (32.5%). Out of 27 positive cases, 12 (44.4%) cases showed 2+ intensity and 15 (55.5%), 3+ intensity (Figure 1). IgA was positive in 20 (50%) cases and negative in 20 (50%). Out of 20 positive cases, one case (5%) showed 1+ intensity, 13 (65%), 2+ intensity and 6 (30%), 3+ intensity. IgM was positive in 32 cases (80%) and negative in 8 (20%). Out of 32 positive cases, 4 (12.5%) cases showed 1+ intensity, 6 (18.7%), 2+ intensity and 22 (68.7%), 3+ intensity. C3 was positive in 31 (77.5%) cases and negative in 9(22.5%). Out of 31 positive cases, 3 (9.7%) cases showed 1+ intensity, 24 (77.4%), 2+ intensity, and 4 (12.9%) 3+ intensity. C1q was positive in 22 (55%) cases and negative in 18 (45%). Out of 22 positive cases, 7 (31.8%) cases showed 1+ intensity, and 10 (45.4%), 2+ intensity, and 5 (22.7%), 3+ intensity (Table II).
Table II. Overall positivity and intensity of renal panel immunoreactants according to different methods of immunoflourescence.
| Overall Positive Results with Intensity | |||||
| Total | 3+ | 2+ | 1+ | Method | Immunoreactants |
| 27 (67.5%) 26 (65%) 18 (45%) 15 (37.5%) |
15 1 - - |
12 16 5 - |
- 9 13 15 |
IF-F TBS-HIAT Citrate-HIAT Pronase |
IgG |
| 20 (50%) 19 (47.5%) 18 (45%) 17 (42.5%) |
6 1 - - |
13 12 2 - |
1 6 16 17 |
IF-F TBS-HIAT Citrate-HIAT Pronase |
IgA |
| 32 (80%) 32 (80%) 32 (80%) 30 (75%) |
22 - - - |
6 28 - - |
4 4 32 30 |
IF-F TBS-HIAT Citrate-HIAT Pronase |
IgM |
| 31 (77.5%) 31 (77.5%) 19 (47.5%) 18 (45%) |
4 - - - |
24 17 - - |
3 14 19 18 |
IF-F TBS-HIAT Citrate-HIAT Pronase |
C3 |
| 22 (55%) 11 (27.5%) 10 (25%) 10 (25%) |
5 - - - |
10 - - - |
7 11 10 10 |
IF-F TBS-HIAT Citrate-HIAT Pronase |
C1q |
IF-F; immunoflourescence on frozen tissue, TBS-HIAT; Heat-induced antigen retrieval using tris buffer
Figure1.
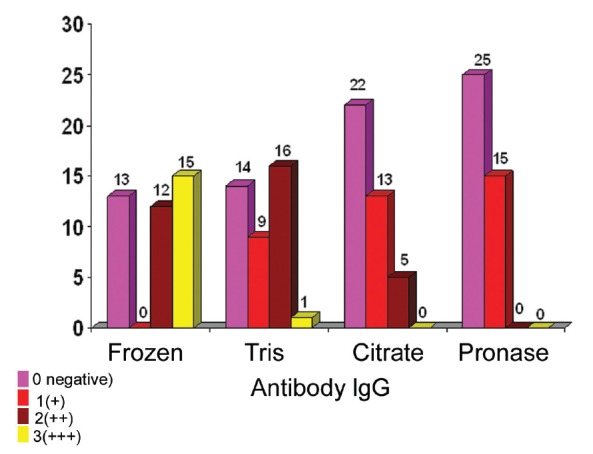
The overall results of immunoflourescence for IgG using all the four methods.
4.2.2. Tris buffer based HIAT method
IgG was positive in 26 (65%) cases and negative in 14 (35%) cases. Out of 26 positive cases, 9 (34.6%) cases showed 1+ intensity, 16 (61.5%), 2+ intensity, and 1 (3.8%) case, 3+ intensity. IgA was positive in 19 (47.5%) cases and negative in 21 (52.5%) cases. Out of 19 positive cases, 6 (31.5%) cases showed 1+ intensity, 12 (63.1%), 2+ intensity, and 1 (5.2%) case, 3+ intensity. IgM was positive in 32 (80%) cases and negative in 8 (20%) cases. Out of 32 positive cases, 4 (12.5%) cases showed 1+ intensity, and 28 (87.5%), 2+ intensity. C3 was positive in 31 (77.5%) cases and negative in 9 (22.5%) cases. Out of 31 positive cases, 14 (45.1%) cases showed 1+ intensity, and 17 (54.8%), 2+ intensity. C1q was positive in 11 (27.5%) cases and negative in 29 (72.5%) cases. All cases showed 1+ positivity.
4.2.3. Citrate buffer based HIAT method
IgG was positive in 18 (45%) cases and negative in 22 (55%) cases. Among the positive cases, 13 (72.2%) cases showed 1+ intensity, and 5 (27.8%), 2+ intensity. IgA was positive in 18 (45%) cases and negative in 22 (55%) cases. Out of the positive cases, 16 (88.8%) cases showed 1+ intensity, and 2 (11.2%), showed 2+ intensity. IgM was positive in 32 (80%) cases and negative in 8 (20%) of cases. All cases showed 1+ intensity. C3 was positive in 19 (47.5%) cases and negative in 21 (52.5%) cases. All cases showed 1+ intensity. C1q was positive in 10 (25%) cases and negative in 30 (75%) cases. All cases showed 1+ intensity.
4.2.4. Pronase enzyme method
IgG was positive in 15 (37.5%) cases and negative in 25 (62.5%) cases. All the positive cases showed 1+ intensity. IgA was positive in 17 (42.5%) cases and negative in 23 (57.5%) cases. All the positive cases showed 1+ intensity. IgM was positive in 30 (75%) cases and negative in 10 (25%) cases. All the positive cases showed 1+ intensity.C3 was positive in 18 (45%) cases and negative in 22 (55%) cases. All cases showed 1+ intensity. C1q was positive in 10 (25%) cases and negative in 30 (75%) cases. All cases showed 1+ intensity.
4.2.5. IF results according to disease categories
The results of IF-P with the three different antigen retrieval techniques are briefly described below according to specific disease categories. Only the results of main immunoreactants are given and these are shown for IF-F in Table III.
Table III. The positivity and intensity of renal panel immunoreactants on snap-frozen immunoflourescence in different glomerular diseases.
| Diseases | |||||||
| Total | LN | MPGN | MGN | IgMN | IgAN | Intensity | Immunoreactants |
| - 12 15 |
- 7 8 |
- - 1 |
- 5 6 |
- - - |
- - - |
1+ 2+ 3+ |
IgG |
| 1 13 6 |
- 9 5 |
1 1 - |
- - - |
- 2 - |
- 1 1 |
1+ 2+ 3+ |
IgA |
| 4 6 22 |
1 2 12 |
- 1 1 |
2 - 1 |
- 2 8 |
1 1 - |
1+ 2+ 3+ |
IgM |
| 3 24 4 |
- 12 3 |
- 2 - |
2 3 1 |
- 6 - |
1 1 - |
1+ 2+ 3+ |
C3 |
| 7 10 5 |
1 9 5 |
1 1 - |
1 - - |
2 - - |
2 - - |
1+ 2+ 3+ |
C1q |
LN; lupus nephritis, IgAN; IgA nephropathy, IgMN; IgM nephropathy, MGN; membranous GN, MPGN; membranoproliferative GN.
4.3. IgA nephropathy (IgAN) (n=2)
4.3.1. IF-F method
Both cases of IgAN were positive for IgA and C3. The intensity of IgA positivity was 2+ and 3+ in one case each. The intensity of C3 positivity was 1+ and 2+ in one case each.
4.3.2. Tris buffer based HIAT method
The results of IF for IgA and C3 by this method showed similar results as with the IF-F method.
4.3.3. Citrate buffer based HIAT method
Both cases of IgAN were positive for IgA and C3 with intensity of 1+ each in each case. 4.3.4. 4.3.4.Pronase enzyme method
Pronase technique showed same results as by the citrate buffer based technique.
4.4. IgM nephropathy (IgMN) (n=10)
4.4.1. IF-F method
All cases of IgMN exhibited diffuse granular mesangial positivity (3+ in eight cases and 2+ in two cases) of IgM. C3 was also positive in all the cases with 2+ (four cases) and 1+ (six cases) positivity.
4.4.2. Tris buffer based HIAT method
All cases exhibited diffuse granular mesangial positivity of IgM (2+ in each case) and C3 (1+) was seen.
4.4.3. Citrate buffer based HIAT method
All cases exhibited diffuse granular mesangial positivity (1+ in each case) of IgM in all cases and C3 (trace to 1+) in 7 cases.
4.4.4. Pronase enzyme method
Pronase technique showed same results as by the citrate buffer based method.
4.5. Membranous GN (MGN) (n=11)
4.5.1. IF-F method
All cases of MGN showed diffuse granular membranous positivity of IgG (2+ to 3+ in each case) (Figure 2).
Figure2.
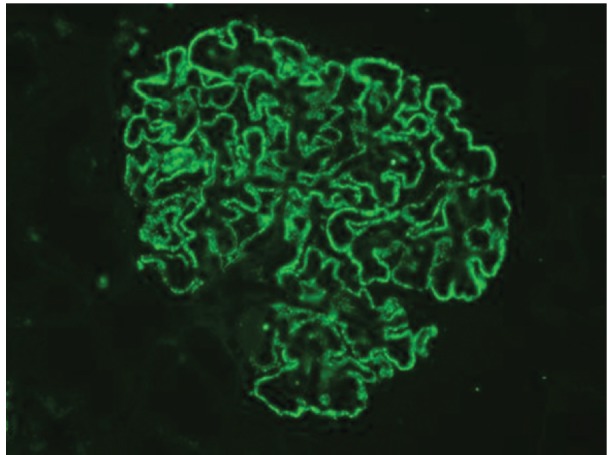
Global, granular, membranous positivity of IgG (3+ positivity) on snap-frozen sections in a case of membranous GN (IF, ×400)
4.5.2. Tris buffer based HIAT method
IgG was negative in 1 case, while 4 cases showed 1+ intensity, 6 showed 2+ intensity (Figure 3 and 4). C3 was negative in 3 cases, while 3 cases showed 1+ intensity, and 5 cases 2+ intensity. All cases showed negativity for C1q.
Figure3.
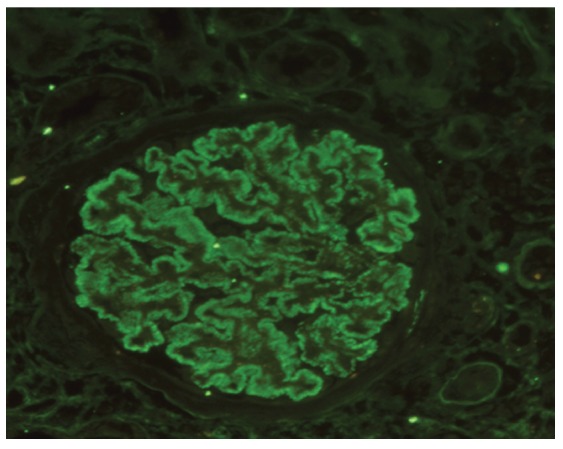
Global, granular, membranous positivity of IgG (3+ positivity) on TBS-HIAT treated paraffin sections in a case of membranous GN (IF, ×400).
Figure4.
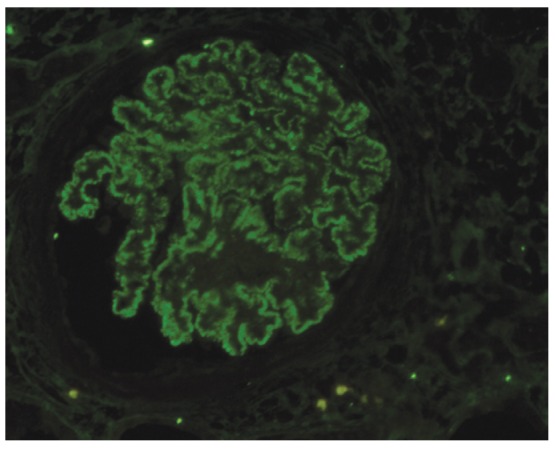
Global, granular, membranous positivity of IgG (1+ positivity) on TBS-HIAT treated paraffin sections in a case of membranous GN (IF, ×400).
4.5.3. Citrate buffer based HIAT method
IgG was negative in 5 cases, while 4 cases showed 1+ intensity, 2 cases showed 2+ intensity. C3 was negative in 4 cases, while 7 cases showed 1+ intensity.
4.5.4. Pronase enzyme method
IgG was negative in 6 cases, while 5 cases showed 1+ intensity. C3 was negative in 5 cases, while 6 cases showed 1+ intensity.
4.6. Membranoproliferative GN (MPGN) (n=2)
4.6.1. IF-F method
IgG was negative in one case and positive in the other case with 3+ intensity. IgA was negative in one case, while other one was positive with 1+ intensity. IgM was positive in both cases with 3+ intensity. C3 was positive in both cases with 2+ intensity. C1q was positive in both cases with 1+ intensity in one case, and 2+ in the other.
4.6.2. Tris buffer based HIAT method
IgG was negative in one case, and positive in the other case with 3+ intensity. IgA was negative in 1 case other 1 was positive with 1+ intensity. IgM was positive in both cases with 2+ intensity in each case.C3 was positive in both cases with 1+ intensity in each case. C1q was negative in both cases.
4.6.3. Citrate buffer based HIAT method
IgG was negative in one case, while the other one was positive with 2+ intensity. IgA was negative in one case, while the other one was positive with 1+ intensity. IgM was positive in both cases with 1+ intensity in each case.C3 was negative in both cases. C1q was negative in 1 case, while the other one was positive with 1+ intensity.
4.6.4. Pronase enzyme method
IgG was negative in one case, while the other one was positive with 1+ intensity. IgA negative in1 case, while other 1 was positive with 1+ intensity. IgM was positive in both cases with 1+ intensity in each case. C3 was negative in both cases. C1q was negative in one case, while the other one was positive with 1+ intensity.
4.7. Lupus nephritis (LN) (n=15)
4.7.1. IF-F method
IgG was positive in all cases, seven cases showed 2+ intensity, while eight cases showed 3+ intensity. IgA was negative in one case, while nine cases were positive with 2+ intensity and five cases with 3+ intensity. IgM was positive in all cases, with one case showing 1+ positivity, two cases 2+ intensity and 12 cases 3+ intensity. C3 was positive in all cases, 12 cases showed 2+ intensity and three cases 3+ intensity. C1q was positive in all cases, with 1+ positivity in one case, while nine cases showed 2+ intensity and five showed 3+ intensity.
4.7.2. Tris buffer based HIAT method
IgG was positive in all cases, 5 cases showed 1+ intensity , and 10 cases, 2+ intensity. IgA was negative in one case, while three cases showed 1+ intensity, 9 cases, 2+ intensity and 2 cases, 3+ intensity. IgM was negative in one case, while14 cases showed 2+ intensity. C3 was positive in all 15 cases, six cases showed 1+ intensity, and 9 cases, 2+ intensity. C1q was positive in 10 cases, all showing 1+ positivity.
4.7.3. Citrate buffer based HIAT method
IgG was negative in four cases, while nine cases showed 1+ intensity, and 2 cases 2+ intensity. IgA was negative in two cases; while10 cases showed 1+ intensity, and three cases showed 2+ intensity. IgM was negative in one case, while 14 cases showed 1+ intensity. C3 was negative in six cases, while nine cases showed 1+ intensity. C1q was negative in seven cases, while eight cases showed 1+ intensity.
4.7.4. Pronase enzyme method
IgG was negative in six cases, while nine cases showed 1+ intensity. IgA was negative in three cases, while12 cases showed 1+ intensity. IgM was negative in two cases, while 13 cases showed 1+ intensity. C3 was negative in six cases, while nine cases showed 1+ intensity. C1q was negative in seven cases, while eight cases showed 1+ intensity.
5. Discussion
To the best of our knowledge, this is the first study from Pakistan on the direct IF examination of FFPE tissue sections of native renal biopsies in a number of immune complex-mediated glomerular diseases. Although, IF-F is the method of choice to detect and localize antigens in the different compartments of the glomerulus, the facilities for procuring fresh frozen tissue are not available in all hospitals and sometimes frozen tissue does not contain glomeruli (4). In such situations, the only alternative that remains is that of the use of FFPE tissue for IF study (4). However, formalin fixation leads to marked changes in the quaternary structure of protein antigens, resulting in the denaturation and loss of antigenic epitopes, which are recognized by antibodies for antigen-antibody binding (2-4). These antigenic epitopes can be recovered by various treatments of FFPE tissue including HIAT and enzymatic methods (4-17). Many researchers have tried different recipes for antigen recovery and IF on FFPE tissue specimens. However, these methods are still not well standardized. Some studies have shown acceptable results with these various methods of antigen retrieval, whereas other studies have not found antigen retrieval methods very successful (4,7,9,11).
Antigen retrieval has been proved effective with HIAT using TBS and citrate buffers and the enzyme pronase (4). In this study, we used all the above three methods to retrieve the antigens and perform the IF subsequently. We found TBS based HIAT most useful among the three methods. It was found to retrieve antigens successfully in most of the cases of immune complex-mediated GN. IF staining yield with this method was much better than either citrate buffer based HIAT or pronase methodologies.
While comparing the IF done on FFPE tissue to the IF-F in cases of IgAN, we found compatible results with IF-F and the Tris buffer method. Two cases of IgAN were included in our study, showing 2+ and 3+ positivity for IgA on IF-F and the Tris buffer method. IF staining on citrate buffer and pronase treated FFPE sections exhibited 1+ positivity for IgA by either method. Nasr et al. (4) compared the IF findings on frozen sections and pronase digested paraffin embedded tissue. They found 88% positivity of IgA by the pronase digestion method. Our findings are slightly inferior to those of Nasr et al. (4) when pronase treatment of the tissue sections is considered. However, TBS based HIAT yielded equivalent results to those of IF-F. Fogazzi et al. (8) also found a high concordance rate between the positive and negative cases in IgAN.
While comparing the IF done on FFPE to the IF done on frozen sections in cases of MGN, we found compatible results on IF-F and IF on TBS based HIAT method. Eleven cases of MGN were included in our study, showing 1+ and 2+ positivity for IgG and C3 in all cases by frozen method and TBS-HIAT treated FFPE sections. The IF staining with citrate buffer and pronase digested FFPE sections exhibited 1+ positivity in all cases of MGN.
Nasr et al. (4) also compared the IF findings on frozen section and pronase digested FFPE sections in eight cases of MGN. They found 50% positivity of IF in MGN by pronase method. In this context, our findings are superior to those of Nasr et al. (4). However, the intensity of staining was lower with enzymatic method as compared with TBS-HIAT method. The experience of Fogazzi et al. (8) was also more or less similar in this disease.
While comparing the IF done on formalin fixed paraffin embedded tissue to the IF done on frozen sections in cases of IgM nephropathy, we found concordant results of frozen and IF on Tris buffer treated paraffin tissue. It may be mentioned here that the studies by Nasr et al. (4), and Fogazzi et al. (8) did not include cases of IgMN.
While comparing the IF done on FFPE sections to the IF-F in cases of MPGN type 1, we found compatible results of frozen and IF-P on Tris buffer. Nasr et al. (4) compared the IF-F and IF-P. They found 60% positivity of IF in five cases of MPGN type 1 by the pronase digestion. Our findings are superior to those of Nasr et al. (4), although the intensity of staining was less with citrate and pronase methods.
While comparing the IF done on FFPE tissue to the IF-F in cases of LN, we found highly concordant results with frozen and the Tris buffer based HIAT method. IF staining on citrate buffer based HIAT and pronase treated FFPE sections exhibited less intense positivity for IgG, IgA, IgM, C3, and C1q. In LN, our results are completely in agreement with those of Nasr et al. (4). They found 100% positivity of LN cases by the pronase digestion method. Fogazzi et al. (8) also found similar high rate of concordance between the IF-F and IF-P methods in this disease.
6. Conclusions
In conclusion, the findings from this study show that the heat treatment and proteolytic digestion of FFPE renal tissue sections are valuable salvage techniques for IF study if frozen tissue is not available. These methods can be utilized in the laboratories which lack the facilities for procurement of fresh frozen renal biopsies.
Conflict of interest
The authors declared no competing interests.
Funding/Support
None declared.
Acknowledgments
None declared.
Implication for health policy/practice/research/medical education:
Heat treatment and proteolytic digestion of formalin fixed and paraffin embedded renal tissue sections are valuable salvage techniques for immunoflourescence study, if frozen tissue is not available. These methods can be utilized in the laboratories which lack the facilities for procurement of fresh frozen renal biopsies.
Please cite this paper as: Mubarak M, Kazi JI, Kulsoom U, Ishaque M. Detection of immunoglobulins and complement components in formalin fixed and paraffin embedded renal biopsy material by immunoflourescence technique. J Nephropathology. 2012; 1(2): 91-100. DOI: 10.5812/nephropathol.7518
References
- 1.Mubarak M, Kazi JI. Role of immunofluorescence and electron microscopy in the evaluation of renal biopsies in nephrotic syndrome in a developing country. Ultrastruct Pathol . 2009;33(6):260–4. doi: 10.3109/01913120903296952. [DOI] [PubMed] [Google Scholar]
- 2.Furness P, Kazi J. Laboratory investigation of renal biopsy specimen. J Nephrol Urol Transplant . 1998;1:19–26. [Google Scholar]
- 3.Furness PN, Boyd S. Electron microscopy and immunocytochemistry in the assessment of renal biopsy specimens: actual and optimal practice. J Clin Pathol . 1996;49(3):233–7. doi: 10.1136/jcp.49.3.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nasr SH, Galgano SJ, Markowitz GS, Stokes MB, D’Agati VD. Immunofluorescence on pronase-digested paraffin sections: a valuable salvage technique for renal biopsies. Kidney Int . 2006;70(12):2148–51. doi: 10.1038/sj.ki.5001990. [DOI] [PubMed] [Google Scholar]
- 5.Chowdhury AR, Ehara T, Higuchi M, Hora K, Shigematsu H. Immunohistochemical detection of immunoglobulins and complements in formaldehyde-fixed and paraffin-embedded renal biopsy tissues; an adjunct for diagnosis of glomerulonephritis. Nephrology (Carlton) . 2005;10(3):298–304. doi: 10.1111/j.1440-1797.2005.00396.x. [DOI] [PubMed] [Google Scholar]
- 6.Sinclair RA, Burns J, Dunnill MS. Immunoperoxidase staining of formalin-fixed, paraffin-embedded, human renal biopsies with a comparison of the peroxidase-antiperoxidase (PAP) and indirect methods. J Clin Pathol . 1981;34(8):859–65. doi: 10.1136/jcp.34.8.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carlton S. Theory and practice of histological techniques. 5 ed.: Churchill Livingstone; 2005.
- 8.Fogazzi GB, Bajetta M, Banfi G, Mihatsch M. Comparison of immunofluorescent findings in kidney after snap-freezing and formalin fixation. Pathol Res Pract . 1989;185(2):225–30. doi: 10.1016/S0344-0338(89)80256-0. [DOI] [PubMed] [Google Scholar]
- 9.Molne J, Breimer ME, Svalander CT. Immunoperoxidase versus immunofluorescence in the assessment of human renal biopsies. Am J Kidney Dis . 2005;45(4):674–83. doi: 10.1053/j.ajkd.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 10.Choi YJ, Reiner L. Immunofluorescence of renal lesions in paraffin-embedded and fresh-frozen sections. Am J Clin Pathol . 1980;73(1):116–9. doi: 10.1093/ajcp/73.1.116. [DOI] [PubMed] [Google Scholar]
- 11.Dorsett BH, Ioachim HL. A method for the use of immunofluorescence on paraffin-embedded tissues. Am J Clin Pathol . 1978;69(1):66–72. doi: 10.1093/ajcp/69.1.66. [DOI] [PubMed] [Google Scholar]
- 12.Huang SN, Minassian H, More JD. Application of immunofluorescent staining on paraffin sections improved by trypsin digestion. Lab Invest . 1976;35(4):383–90. [PubMed] [Google Scholar]
- 13.Qualman SJ, Keren DF. Immunofluorescence of deparaffinized, trypsin-treated renal tissuesPreservation of antigens as an adjunct to diagnosis of disease. Lab Invest . 1979;41(6):483–9. [PubMed] [Google Scholar]
- 14.Svalander C, Eggertsen G, Olding LB. Conditions for the immunohistochemical demonstration of complement factor C3 in formaldehyde-fixed and paraffin-embedded renal tissues. Histochemistry . 1986;84(1):81–5. doi: 10.1007/BF00493425. [DOI] [PubMed] [Google Scholar]
- 15.Yamashina M, Takami T, Kanemura T, Orii T, Ojima A. Immunohistochemical demonstration of complement components in formalin-fixed and paraffin-embedded renal tissues. Lab Invest . 1989;60(2):311–6. [PubMed] [Google Scholar]
- 16.Turner DR. Advances in understanding the morphology of glomerular disease. J Clin Pathol . 1981;34(11):1207–13. doi: 10.1136/jcp.34.11.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Madaio MP, Harrington JT. The diagnosis of glomerular diseases: acute glomerulonephritis and the nephrotic syndrome. Arch Intern Med . 2001;161(1):25–34. doi: 10.1001/archinte.161.1.25. [DOI] [PubMed] [Google Scholar]
- 18.Kazi JI, Mubarak M, Ahmed E, Akhter F, Naqvi SA, Rizvi SA. Spectrum of glomerulonephritides in adults with nephrotic syndrome in Pakistan. Clin Exp Nephrol . 2009;13(1):38–43. doi: 10.1007/s10157-008-0075-0. [DOI] [PubMed] [Google Scholar]
- 19.Mubarak M, Lanewala A, Kazi JI, Akhter F, Sher A, Fayyaz A. et al. Histopathological spectrum of childhood nephrotic syndrome in Pakistan. Clin Exp Nephrol . 2009;13(6):589–93. doi: 10.1007/s10157-009-0216-0. [DOI] [PubMed] [Google Scholar]
- 20.Wagrowska-Danilewicz M, Zeromski J. Immunofluorescent evaluation of renal biopsy: current point of view. Pol J Pathol . 2010;61(2):83–8. [PubMed] [Google Scholar]


