Abstract
Background
Granulomatosis with polyangiitis (Wegener’s) is a systemic necrotizing vasculitis. Cardiac involvement in Wegener’s has rarely been reported. In this study the echocardiographic findings of granulomatosis with polyangiitis (GPA) in a patient is described.
Case Presentation
The case was a 45 years old man admitted to the hospital with a 3 months history of persistent fever and sinusitis. Mild left and right ventricular enlargements as well as three small masses in the right ventricular cavity were identified in echocardiography. One mass was attached to the tricuspid valve and the other two masses were attached to the right septum. Pulmonary artery hypertension (45mmHg) was also reported. The masses responded dramatically to plasma exchanges in combination with steroid therapy, followed by oral cyclophosphamid and low-dose steroid therapy. In the kidney biopsy, 8 out of 11 golomeruli contained fibrous crescents along with sclerotic lesions. Spleen has 140 mm diameter with multiple echo-free lesions and coarse parenchyma in abdominal ultrasound. Serum C-ANCA=671, P-ANCA=1.7 (normal= up to 15U/mL). The diagnosis of granulomatosis with polyangiitis (Wegener’s) was established.
Conclusions
Presence of three small masses in right ventricular cavity and pulmonary artery hypertension in association with the spleen lesions were an uncommon presentation of GPA. GPA should be considered in the differential diagnosis of any nonspecific illnesses with cardiac involvement.
Keywords: Granulomatosis, Polyangiitis, Wegener’s, Vasculitis, Ventricular mass
1. Introduction
In 1936, Friedrich Wegener described a granulomatous disease involving the upper and lower respiratory tract along with glomerulonephritis (1). Granulomatosis with polyangiitis (GPA) has been recently accepted as an alternative name for Wegener’s granulomatosis (2-5). Granulomatosis with polyangiitis is an autoimmune necrotizing vasculitis of small vessels which mainly affects the paranasal sinuses, lungs and kidneys. Although rare, cardiac involvement has also been reported (6,7). We describe a rare case of GPA presented with fever and three small right ventricular masses.
2. Case presentation
The case was a 45 years old man, admitted to the hospital because of 3 months history of fever, sinusitis, sore throat, sputum, nasal discharge and congestion. Symptoms were first attributed to the dental infection therefore various kinds of antibiotics were prescribed without any sign of improvement. The patient had also been hospitalized, during which had received different supportive therapeutic agents including intravenous antibiotics. Other chief complaints were dyspnea, sore throat, muscle pain and muscle cramps. The patient had no history of asthma, diabetes, renal disease or cigarette smoking. Physical examination showed tenderness over maxillary sinuses, redness of the pharynx without exudates, and intense nasal discharges. Heart examination and Jugular vein pressure were normal in the examination. Decreased breath sounds were observed at the base of the both lungs. The skin examination was unremarkable. Abdomen was normal in palpation and no organomegaly was detected. Furthermore, there were 2+ pitting edema on both legs. Vital signs were as follow: Blood pressure=130/70 mmHg, pulse rate= 90/ minute, respiratory rate= 18/minute, and temperature= 38 oC (100.4 oF) at the time of admission. Laboratory tests consisted of a serum creatinine= 0.9 mg/dL and serum BUN= 30 mg/dL, which dramatically rose up to 6.8 mg/dL and to 85 mg/dL in the third day of hospitalization, respectively. In CBC, WBC= 15900 (93% neutrophils, 6% lymphocytes), Hb=9.6g/dL (normochrome normocytic), and platelets counts were in normal range. Corrected reticulocyte count= 0.4%. Peripheral blood smear showed severe poikilocytosis, ovalocytes, tear drops RBCs, neutrophylic leukocytosis with bandemia (#80%) and also reactive lymphocytes (#20%). ESR=85 mm/hr. In urinalysis= 3+ proteinuria, 3+ hematuria (with dimorphic RBCs), leukocyturia and granular casts were identified. Protein on a 24-hour urine collection = 2475mg. Blood cultures were reported negative for three times. Serum albumin; 2.4 g/dL, serum AST; 36 IU/L and serum ALT; 128 IU/L. Serum ANA= 11.4 U/mL (<21: negative). Anti-ds DNA=92 IU/mL (up to 120). Lupus anticoagulants were negative. Serum C3, C4, CH50 and ASO titer were within normal limits. Serum C-ANCA=671, P-ANCA=1.7 (normal= up to 15U/mL). Chest radiography showed blunting of left plural angle. No cavity or mass was reported. In the paranasal sinuses x-ray, septal deviation, opacity of the maxillary sinuses, right ethmoid and frontal sinusitis, also opacity of the right nasal cavity as well as thickening of the mucosa were identified (fig.1). In abdominal ultrasonography, spleen had 140 mm diameter with multiple echo-free lesions and a coarse parenchyma. Enlarged kidneys (right kidney= 142 mm, left kidney= 150 mm) were reported too. The liver, para-aortic area and pelvic region were normal. Electrocardiography was unremarkable. In echocardiography, mild left and right ventricular enlargement along with three small masses, one attached to the tricuspid valve and the other two masses attached to the right septum, were reported (M-mode, two-dimensional, Doppler and color-doppler trans-thoracic echocardiogram). Moreover mild tricuspid regurgitation was detected. No pericardial effusion was found. Pulmonary artery pressure (PAH) was 45 mmHg (fig. 2A, 2B). Considering masses as vegetation or clot, patient was first treated with Cloxacillin IV and heparin infusion (1000 IU/hr). Urinary output was 1425 cc/24hr. and the intake was 1280 cc/24 hr. Granulomatosis with polyangiitis (Wegener’s) was diagnosed and 1 g pulse of methylprednisolon succinate was administered daily for three days, followed by 1 mg/kg prednisolone daily. Three days later, antibiotic therapy was discontinued. In addition, patient underwent six sessions of plasmapheresis. Fever subsided three days after admission. After 5 days heparin infusion was stopped and was switched to 5000 IU every 12 hour. Cyclophosphamid 50 mg/d as tablet was added at the same time. Patient was discharged 20 days after admission. Serum creatinine was 2.5 mg/dL at the time of discharge. Kidney biopsy was performed one month after patient discharge. Biopsy fragment contained 14 glomeruli, out of which 3 were totally sclerotic. of 11 glomeruli, 8 contained fibrous crescents which healed to sclerotic lesions (fig. 3). Cellular crescent was absent. Periglomerular inflammation by monocyte-macrophages “periglomerular granulomatose” reaction was also observed. Furthermore, moderate interstitial fibrosis and mild tubular cell flattening were detected. A mild arteriolosclerosis was noted too. There were no sign of inflammation around the vessels. In immunofluorescence study, IgA, IgG, IgM, C3 and C1q depositions were negative although there was a 1+ deposit of fibrin in two of the glomeruli. Using recent ANCA associated vasculitis classification (8), lesions were in “Mixed Class“. Prednisolon was continued and gradually was tapered to a maintenance dose of 2.5 mg per day. Enalapril tablet at a 10 mg per doses was also administered. Serum creatinine dropped to 1mg/dL one month after discharge from the hospital and remained at this level during regular follow up for 20 months later. A control echocardiography was performed by the same cardiologist one month after the discharge date and disappearance of masses in right ventricular cavity was found. In addition, PAH was normalized. The lesions in the spleen became undetectable too.
Figure1.
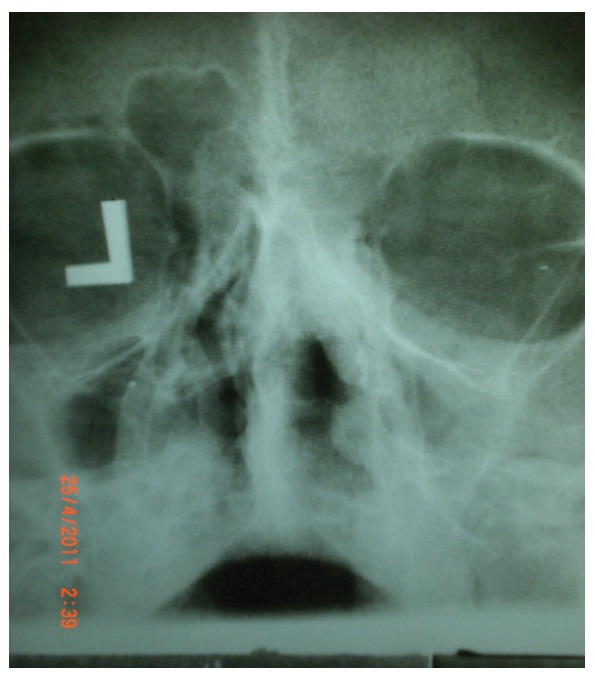
Paranasal sinuses x-ray, septal deviation, opacity of the maxillary sinuses, right ethmoid and frontal sinusitis, also opacity of the right nasal cavity with thickening of mucosa was evident.
Figure 2A, 2B.
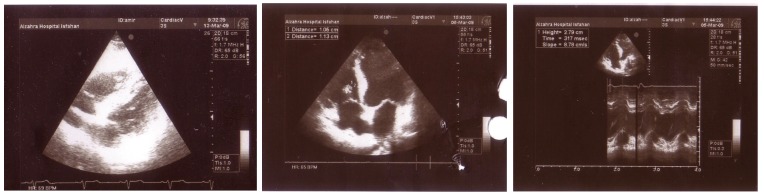
Mild left and right ventricular enlargement with three small masses in right ventricular cavity.
Figure3A, 3B.
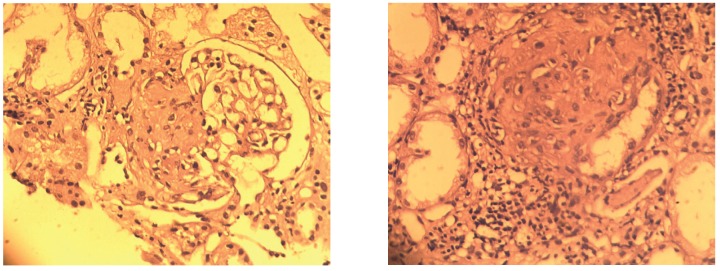
Fibrous crescents which healed to sclerotic lesions.
3.Discussion
Wegener’s granulomatosis which has been recently named as granulomatosis with polyangiitis (GPA) was first described over 70 years ago. GPA is an uncommon multi-organ vasculitis, affecting a wide age range, however, most commonly presents in the 5th decade. Males are involved more commonly than females. Upper-respiratory tract involvement including sinusitis is a common presentation. More than 90% of patients with GPA will have pulmonary involvement at various stages of their disease which manifests as cough, pleurisy, dyspnea and hemoptysis (6,7). Granulomatosis with polyangiitis is classified as ANCA-associated small vessel vasculitis (8-10). The major diagnostic criteria are nasal or oral inflammation, abnormal chest radiography, active urinary sediment and typical histology. A patient is considered to have GPA with 88.2% sensitivity and 92% specificity if two of these criteria are present (6,7). In our patient, the diagnosis of ANCA-associated small vessel vasculitis, mostly granulomatosis with polyangiitis (Wegener’s), was based on its association with sinusitis and high serum level of C-ANCA. Organ involvement in ANCA-associated systemic vasculitides syndromes was detected by focal necrotizing lesions that affect different vessels and organs. Also, GPA has additional granulomatous lesions. Cardiac valvular involvement has been rarely reported (11,12). The heart may be involved in many different ways by the systemic vasculitides and presents in 6% to 44% of GPA cases (11,12). In a study on 50 patients with GPA conducted by Kosovsky et al., 82% of patients presented with ENT involvement, which is the most common site of inflammation. Lungs in 72%, kidneys in 36% and eyes in 24%, while heart involvement was found in only 8% of cases (13). Cardiac involvement may be subclinical or the principal source of symptoms (14,15). It usually occurs late in the course of the disease but it may also be the first manifestation (15-20). Studies revealed that around 50% of the cases present with pericarditis or coronary arteritis, 25% with myocarditis, 21% endocarditis or valvulitis, 17% with conduction system abnormality and 11% with myocardial infarction (16-20). Valvular disease commonly involves the aortic valve, resulting in regurgitation (21-23) and may present as a systemic illness resembling infective endocarditis or with classical cardiac symptoms. However, incidental finding of subclinical valvular involvement has also been reported. The patient studied here, had a rare cardiac manifestation of GPA resulting in three masses in right ventricle (24-29). Hence, GPA should be considered in the differential diagnosis of any nonspecific illness with cardiac involvement. This could be culture-negative endocarditis with mass lesions and vasculitis (25-29). Valvular involvement in GPA is rare; however, the review of the literatures has mainly addressed the involvement of the aortic valve (24-34). Herbst et al., reported the first case of GPA with severe mitral valve involvement. Their patient was a 56-year-old woman who had a mass with 1 cm thickness on the anterior leaflet of the mitral valve which had been detected during echocardiographic evaluations. After histological assessment of the mass lesion, she was diagnosed to have GPA (20). To describe the spectrum and clinical implications of echocardiographic findings associated with GPA, a retrospective study during 1976 to1997 was conducted by Oliveira et al., on 85 patients in their study, 86% were found to have echocardiographic abnormalities. They found regional wall motion abnormalities, left ventricular systolic dysfunction with decreased ejection fraction and pericardial effusion. Other findings included valvulitis, left ventricular aneurysm and a large intracardiac mass (34,35). In another study conducted by Morelli et al., of nine GPA patients with echocardiography, aortic valve thickening in eight patients was detected (14). Likewise, Kosovsky et al., reported a 16-year-old boy who was diagnosed as GPA after a cardiac mass had been detected and the pathological examination had revealed granulomatous vasculitis (13). In our case three masses in the right ventricular cavity was detected without a previous history of any valvular disease or any bacterial growth in blood cultures. After one month of corticosteroid therapy and six session of plasma exchange, full resolutions of masses were seen by control enchocardiography. We believe that the presence of three small masses in right ventricular cavity and the presence of PAH in association with lesions in the spleen with normal chest roentrogram at patient admission was an uncommon presentation of GPA (20). In our case plasmaphersis was a safe choice due to the concern of accompanied bacterial endocarditis. Launay et al., described 4 cases of anti-neutrophil cytoplasmic antibodies (ANCA)-related systemic vasculitis (three with GPA and one with microscopic polyangiitis) associated with pulmonary arterial hypertension. PAH was diagnosed after the onset of systemic vasculitis in three cases. In one case, systemic vasculitis was active at the diagnosis of PAH and treatment of the vasculitis led to a significant improvement of PAH. They concluded that systemic vasculitides have to be added to the conditions associated with PAH. It is possible that in some cases PAH may be due to direct pulmonary arteries involvement by the vasculitic process (35). Our patient has also a PAP of 45 mmHg at admission which reduced to normal pressure after treatment. Involvement of both valves was also reported by Koyalakonda et al., in a 52-year-old patient with aortic regurgitation and mitral stenosis due to GPA requiring replacement of both valves (21).
4.Conclusions
Cardiac events in Wegener’s granulomatosis are probably underestimated due to their subclinical manifestations with vague and atypical symptoms. Cardiac involvement may be associated with a poor prognosis. Although cardiac manifestations are rare presentation of GPA, systematic and regular cardiac assessment in the follow-up of patients with GPA should be considered.
Indeed cardiologists should be aware of the potential cardiac involvement of GPA. Therefore, every patient suffering from this disease should be considerably examined by a cardiologist. Regular electrocardiogram and echocardiographic examinations should be performed in GPA because of subclinical cardiac involvement in these patients.
Acknowledgments
The authors would like to express their gratitude to the staffs of Nephrology ward of Al-Zahra Hospital, and the Division of Renal Immunopathology, Baradaran Laboratory for their kind technical support and advice.
Financial Disclosure
The author declared no competing interests.
Funding/Support
None declared.
Implication for health policy/practice/research/medical education:
Granulomatosis with polyangiitis (Wegener’s) is a systemic necrotizing vasculitis, rarely presented with ventricular masses and also multiple echo-free lesions in the spleen. Cardiac masses and spleen lesions completely resolved after corticosteroid pulse therapy. GPA should be considered in the differential diagnosis of any nonspecific illnesses with cardiac involvement.
Please cite this paper as: Mortazavi M. Nasri H. Granulomatosis with polyangiitis (Wegener’s) presenting as the right ventricular masses: A case report and review of the literature. J Nephropathology. 2012; 1(1): 49-56. DOI: 10.5812/jnp.9
References
- 1.Wegener F. Über generalisierte, septische Gefässerkrankungen. Verh Dtsch Ges Pathol . 1936;29:202–10. [Google Scholar]
- 2.Falk RJ, Gross WL, Guillevin L, Hoffman GS, Jayne DR, Jennette JC. et al. Granulomatosis with polyangiitis (Wegener’s): an alternative name for Wegener’s granulomatosis. Arthritis Rheum . 2011;63(4):863–4. doi: 10.1002/art.30286. [DOI] [PubMed] [Google Scholar]
- 3.Jennette JC. Nomenclature and classification of vasculitis: lessons learned from granulomatosis with polyangiitis (Wegener’s granulomatosis) Clin Exp Immunol . 2011;164 Suppl 1:7–10. doi: 10.1111/j.1365-2249.2011.04357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Falk RJ, Gross WL, Guillevin L, Hoffman G, Jayne DR, Jennette JC. et al. Granulomatosis with polyangiitis (Wegener’s): an alternative name for Wegener’s granulomatosis. J Am Soc Nephrol . 2011;22(4):587–8. doi: 10.1681/ASN.2011010081. [DOI] [PubMed] [Google Scholar]
- 5.Falk RJ, Gross WL, Guillevin L, Hoffman G, Jayne DR, Jennette JC. et al. Granulomatosis with polyangiitis (Wegener’s): an alternative name for Wegener’s granulomatosis. Ann Rheum Dis . 2011;70(4):704. doi: 10.1136/ard.2011.150714. [DOI] [PubMed] [Google Scholar]
- 6.Duna GF, Galperin C, Hoffman GS. Wegener’s granulomatosis. Rheum Dis Clin North Am . 1995;21(4):949–86. [PubMed] [Google Scholar]
- 7.Leavitt RY, Fauci AS, Bloch DA, Michel BA, Hunder GG, Arend WP. et al. The American College of Rheumatology 1990 criteria for the classification of Wegener’s granulomatosis. Arthritis Rheum . 1990;33(8):1101–7. doi: 10.1002/art.1780330807. [DOI] [PubMed] [Google Scholar]
- 8.Berden AE, Ferrario F, Hagen EC, Jayne DR, Jennette JC, Joh K. et al. Histopathologic classification of ANCA-associated glomerulonephritis. J Am Soc Nephrol . 2010;21(10):1628–36. doi: 10.1681/ASN.2010050477. [DOI] [PubMed] [Google Scholar]
- 9.Bajema IM. Pathological classification of anti-neutrophil cytoplasmic antibody (ANCA)-associated glomerulonephritis. Clin Exp Immunol . 2011;164 Suppl 1:14–6. doi: 10.1111/j.1365-2249.2011.04359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luqmani RA, Suppiah R, Grayson PC, Merkel PA, Watts R. Nomenclature and classification of vasculitis - update on the ACR/EULAR diagnosis and classification of vasculitis study (DCVAS) Clin Exp Immunol . 2011;164 Suppl 1:11–3. doi: 10.1111/j.1365-2249.2011.04358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goodfield NE, Bhandari S, Plant WD, Morley-Davies A, Sutherland GR. Cardiac involvement in Wegener’s granulomatosis. Br Heart J . 1995;73(2):110–5. doi: 10.1136/hrt.73.2.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forstot JZ, Overlie PA, Neufeld GK, Harmon CE, Forstot SL. Cardiac complications of Wegener granulomatosis: a case report of complete heart block and review of the literature. Semin Arthritis Rheum . 1980;10(2):148–54. doi: 10.1016/0049-0172(80)90005-0. [DOI] [PubMed] [Google Scholar]
- 13.Kosovsky PA, Ehlers KH, Rafal RB, Williams WM, O’Loughlin JE, Markisz JA. MR imaging of cardiac mass in Wegener granulomatosis. J Comput Assist Tomogr . 1991;15(6):1028–30. doi: 10.1097/00004728-199111000-00024. [DOI] [PubMed] [Google Scholar]
- 14.Morelli S, Gurgo Di Castelmenardo, Conti F, Sgreccia A, Alessandri C, Bernardo ML. et al. Cardiac involvement in patients with Wegener’s granulomatosis. Rheumatol Int . 2000;19(6):209–12. doi: 10.1007/s002960000059. [DOI] [PubMed] [Google Scholar]
- 15.Gerbracht DD, Savage RW, Scharff N. Reversible valvulitis in Wegener’s granulomatosis. Chest . 1987;92(1):182–3. doi: 10.1378/chest.92.1.182. [DOI] [PubMed] [Google Scholar]
- 16.Davenport A, Goodfellow J, Goel S, Maciver AG, Walker P. Aortic valve disease in patients with Wegener’s granulomatosis. Am J Kidney Dis . 1994;24(2):205–8. doi: 10.1016/s0272-6386(12)80182-x. [DOI] [PubMed] [Google Scholar]
- 17.Fox AD, Robbins SE. Aortic valvulitis complicating Wegener’s granulomatosis. Thorax . 1994;49(11):1176–7. doi: 10.1136/thx.49.11.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Timoshenko VS, Polushin OG, Fisenko AI. [A case of Wegener’s granulomatosis with involvement of the aortic valve] Arkh Patol . 1989;51(12):55–8. [PubMed] [Google Scholar]
- 19.Bruno P, Le Hello C, Massetti M, Babatasi G, Saloux E, Galateau F. et al. Necrotizing granulomata of the aortic valve in Wegener’s disease. J Heart Valve Dis . 2000;9(5):633–5. [PubMed] [Google Scholar]
- 20.Herbst A, Padilla MT, Prasad AR, Morales MC, Copeland JG. Cardiac Wegener’s granulomatosis masquerading as left atrial myxoma. Ann Thorac Surg . 2003;75(4):1321–3. doi: 10.1016/s0003-4975(02)04662-3. [DOI] [PubMed] [Google Scholar]
- 21.Koyalakonda SP, Krishnan U, Hobbs WJ. A rare instance of multiple valvular lesions in a patient with Wegener’s granulomatosis. Cardiology . 2010;117(1):28–30. doi: 10.1159/000319603. [DOI] [PubMed] [Google Scholar]
- 22.Strizhakov LA, Kogan DN, Fedorov DN, Krivosheev OG, Semenkova EN, Sorokin Iu D. [Myocarditis and fibroplastic endocarditis in Wegener’s granulomatosis] Arkh Patol . 2010;72(1):42–4. [PubMed] [Google Scholar]
- 23.Dupuy M, Marcheix B, Grunenwald E, Dickson Z, Cron C, Chauveau D. [Mitral regurgitation associated with Wegener’s granulomatosis] Ann Cardiol Angeiol (Paris) . 2009;58(3):180–2. doi: 10.1016/j.ancard.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 24.Dropinski J, Szczeklik W, Rubis P. [Cardiac involvement in systemic autoimmune disease] Pol Arch Med Wewn . 2003;109(4):375–81. [PubMed] [Google Scholar]
- 25.Mishell JM. Cases from the Osler Medical Service at Johns Hopkins University. The American journal of medicine . 2002;113(7):607–9. doi: 10.1016/s0002-9343(02)01349-9. [DOI] [PubMed] [Google Scholar]
- 26.Anthony DD, Askari AD, Wolpaw T, McComsey G. Wegener granulomatosis simulating bacterial endocarditis. Arch Intern Med . 1999;159(15):1807–10. doi: 10.1001/archinte.159.15.1807. [DOI] [PubMed] [Google Scholar]
- 27.Inoue K, Oda Y, Tomiyasu K, Kondo M. Hypertrophic cranial pachymeningitis with inflammatory valvular disease. Intern Med . 1999;38(1):74. doi: 10.2169/internalmedicine.38.74. [DOI] [PubMed] [Google Scholar]
- 28.Kane GC, Keogh KA. Involvement of the heart by small and medium vessel vasculitis. Curr Opin Rheumatol . 2009;21(1):29–34. doi: 10.1097/BOR.0b013e32831cb94d. [DOI] [PubMed] [Google Scholar]
- 29.Onal IK, Ozcakar L, Temirel K, Aran R, Kurt M. Fatal endocarditis in Wegener’s granulomatosis: mitral valve involvement and an intracardiac mass. Joint Bone Spine . 2005;72(6):585–7. doi: 10.1016/j.jbspin.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 30.Lacoste C, Mansencal N, Ben M’rad M, Goulon-Goeau C, Cohen P, Guillevin L. et al. Valvular involvement in ANCA-associated systemic vasculitis: a case report and literature review. BMC Musculoskelet Disord . 2011;12:50. doi: 10.1186/1471-2474-12-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lotek L, Szturmowicz M, Wiatr E, Kober J, Wiechecka A, Jezierska-Anczukow A. et al. [Sepsis with staphylococcal vegetation on tricuspid valveDifferential diagnosis with Wegener’s granulomatosis] Pneumonol Alergol Pol . 2003;71(5-6):253–60. [PubMed] [Google Scholar]
- 32.Mirsadraee S, Fraser A, Kerr MA, James TE, van Doorn C. Inflammatory response in an immunosuppressed patient with Wegener’s granulomatosis. Perfusion . 2004;19(2):127–31. doi: 10.1191/0267659104pf726oa. [DOI] [PubMed] [Google Scholar]
- 33.Chirinos JA, Corrales-Medina VF, Garcia S, Lichtstein DM, Bisno AL, Chakko S. Endocarditis associated with antineutrophil cytoplasmic antibodies: a case report and review of the literature. Clin Rheumatol . 2007;26(4):590–5. doi: 10.1007/s10067-005-0176-z. [DOI] [PubMed] [Google Scholar]
- 34.Oliveira GH, Seward JB, Tsang TS, Specks U. Echocardiographic findings in patients with Wegener granulomatosis. Mayo Clin Proc . 2005;80(11):1435–40. doi: 10.4065/80.11.1435. [DOI] [PubMed] [Google Scholar]
- 35.Launay D, Souza R, Guillevin L, Hachulla E, Pouchot J, Simonneau G. et al. Pulmonary arterial hypertension in ANCA-associated vasculitis. Sarcoidosis Vasc Diffuse Lung Dis . 2006;23(3):223–8. [PubMed] [Google Scholar]


