Abstract
Background
There is a limited knowledge about the morphological features of IgA nephropathy (IgAN)in the middle east region.
Objectives
The objective of this study was to evaluate the spectrum of histopathological findings in IgAN patients at our laboratory.
Patients and Methods
At this work, an observational study reported which was conducted on IgAN patients using the Oxford-MEST classification system.
Results
In this survey, of 102 patients 71.6 % were male. The mean age of the patients was 37.7 ± 13.6 years. Morphologic variables of MEST classification was as follows; M1: 90.2 %, E: 32 %, S: 67 % also,T in grads I and II were in 30% and 19% respectively, while 51% were in grade zero. A significant difference was observed in segmental glomerulosclerosis (P=0.003) and interstitial fibrosis/tubular atrophy frequency distribution (P= 0.045), between males and females . Furthermore, it was found that mesangial hypercellularity was more prevalent in yonger patients. Moreover, there was a significant correlation between serum creatinine and crescents (P<0.001). There was also significant correlation of serum creatinine with segmental glomerulosclerosis (P<0.001).
Conclusions
Higher prevalence of segmental glomerulosclerosis and interstitial fibrosis/ tubular atrophy, as the two of, four variables of Oxford-MEST classification of IgAN in male patients further attests that male gender is a risk factor in this disease.In this study the significant correlation between serum creatinine and crescent was in an agreement with previous studies and suggests for the probable accomodation of extracapillary proliferation as a new variable in MEST system.
Keywords: IgA nephropathy, Classification, Proliferation, Tubular atrophy, Interstitial fibrosis, Glomerulosclerosis
1. Background
IgA nephropathy (IgAN) was first described by Berger (1,2) and is the most common type of glomerulonephritis worldwide (2-5). Its prevalence varies across the world, ranging from less than 10% in United States to 30-40% in Asia (2-6) and around 40% of patients progress to end-stage renal disease (ESRD) after 20 years (3-5).
IgAN is a disease of young males and commonly presents itself with episodic macroscopic and microscopic hematuria or with kidney failure and hypertension (3-7). There is no doubt that IgA nephropathy is an extremely common disease (5-8). Indeed, IgAN is the main cause of ESRD in patients of all ages with primary glomerular disease (4-9). Its diagnosis was based on the histopathologic and immunohistochemical evaluation of renal biopsy (4). By light microscopy, the glomeruli may show various morphologic manifestations of immune complex glomerulonephritis from normal to severe crescentic glomerulonephritis through mesangial proliferation, endocapillary proliferation and tuft necrosis (10-15). By immunohistochemistry, IgA is the sole immunoglobulin in 26% of biopsies; around 25% have IgA, IgG and IgM. Also C3 presents in 95% (10-20) of the cases. C1q if presents only in trace amounts in biopsies and if prominent should raise the possibility of systemic lupus erythematosus(10-20). Segmental proliferation and segmental scars are also common (10-17) and usuallyglomerular scars are associated with tubular atrophy and interstitial fibrosis (10-18). The wide variety of clinical and histopathologic presentations of IgAN, indicate the utility of the proposed histologic classification to asses patient’s likelihood of developing ESRD (15-17,20).
Indeed there has been a lack of international consensus on the classification and the predictive value of the histopathology findings in IgAN. Previously Lee and Haas, have published a classifications method for IgAN (15-20). The recent Oxford classification of IgAN assesses the presence of hypercellularity, proliferation in mesangial and endocapillary regions, segmental glomerulosclerosis and tubular atrophy/interstitial (MEST).Also it has been reported as having prognostic value (14,21-24). This classification method looked for reproducible pathological features that were correlated to renal survival independent of clinical features, rather than grouping patients into categories (24).
2. Objectives
The prevalence and clinical picture of IgAN varies from region to region.This study was undertaken, firstly to study the morphologic changes in a group of IgAN patients, to assess the Oxford classification method and other morphologic variables, and secondly to compare pathological findings with published studies.
3. Patients and Methods
After publication of Oxford classification of IgAN on 1 July 2009 (25-27), we applied it in this study for the classification of IgAN.
3.1. Definition of IgAN
The pathologic diagnosis of IgAN requires the demonstration of IgA-dominant mesangial or mesangio-capillary immune deposits through immune fluorescence (IF) microscopy. The immune deposits were semiquantitied from 0 to 3+ positive bright. The definition of IgAN needs the presence of diffuse and global IgA deposits that were graded ≥ 2+ and the absence of C1q deposition (3,27-29). All renal biopsies performed at various medical centers (university hospitals or private centers) from July 2009 to February 2012, were sent to a reference laboratory. Except for two Afghan patients, the rest of cases were Iranian. None of the patients was treated before the biopsy. In one hundred ten (110) biopsies, prominent IgA depositis were observed on IF study. Of 110 biopsies with dominant IgA deposition, one was due to a kidney transplantation (reccurrence of IgAN), one patient with diagnosis of Henoch Schonelin purpura , two of the patients with the dominant IgA deposits in association with concurrent diabetes mellitus and one patient with IgAN, whose biopsy had been taken from medulla for light microscopy (without glomerules), which were excluded from the study.Biopsies with less than 8 glomeroli were also excluded from the study.Two of the patients underwent re-biopsy, due to absence of glomeruli for IF or light microscopic study. None of the patients was diagnosed as IgAN, having history of collagen vascular diseases and liver cirrhosis based on questionaire filled at the time of biopsy admission, laboratory data in patients’ records and a breif history provided by referee physicians at the time of biopsy admission.
3.2. Histologic data
All renal biopsies were prepared for light and direct immunofluorescence microscopy. Tissue was fixed in 10% formalin for histologic sectioning. Each kidney biopsy was prepared by cutting paraffin blocks into 3-μm sections and staining 2 slides with periodic acid Schiff, 2 slides for hematoxylin and eosin, 1 slide for Jones methenamine silver and 1 slide for trichrome. Each slide contained 2-3 sections. Materials usedfor IF was snap-frozen in liquid nitrogen. Sections (Six-micron in thickness) were stained for immunofluorescence study with fluorescein isothiocyanate-conjugated antibodies specific for human IgG, IgM, IgA, C1q, C3 and fibrin (DAKO, Produktionsvej 42, DK-2600 Glostrup, Denmark). IF slides were reported in a scale of 0, to 3+ positive bright. IF slides were reported by a nephropathologist (HN) and a general pathologist (SB). IF study was performed unaware of patients’ data and before reviewing the slides for light microscopy. After IF diagnosis of IgAN, histopathology glass slides were reviewed to assess the morphologic variables, which were applied in Oxford-MEST classification method. Other important pathology findings were also assessed including glomerular fibrinoid necrosis, extracapillary proliferation (cellular, fibro-cellular or fibrous crescents), mesansial interposition and mesangiolysis. Also, the presence of foamy cells and tubular thyroidization in interestitium were assessed. Vascular lesions were scored and attention was made toward the presence of thrombotic microangiopathy in cases. After selection of biopsies having dominant deposition IgA by IF study and in the absence of exclusion criteria, glass slides were independently reported for the classification of Oxford-MEST. Each reviewer completed a score sheet for every biopsy and these were used to compile the pathological data set. If there were any differences among the score sheets, the histologic sections would be reviewed concurrently by them at a two headed microscope and the discrepancy reconciled. Therefore, a consensus was established to confirm each parameter.
3.3. Definitions of pathological variables
Total glomeruli and the number of glomeruli with global sclerosis were recorded for each biopsy. The presence of (i) mesangialhypercellularity, (ii) endocapillary proliferation, (iii) segmental glomerulosclerosis and (iv) the proportion of tubular atrophy and interstitial fibrosis were estimated as follows (25-27);
Mesangial hypercellularity
Present in ≤ 50% of the glomeruli: M0
Present in > 50% of the glomeruli: M1
Segmental glomerulosclerosis
Absent: S0
Present: S1
Endocapillary hypercellularity
Absent: E0
Present: E1
Tubular atrophy/interstitial fibrosis
0 – 25% of cortical area: T0
26 – 50% of cortical area: T1
> 50% of cortical area: T2
3.4. Vascular definitions
Arterial lesions are scored based on the most severe lesions. Intimal thickening is scored by comparing the thickness of the intima to that of the media in the same segment of vessel. Intima is scored variously as as normal (score: 0), and thickened to more or less than the thickness of the media (score: 1). Arteriolar hyaline is noted as the proportion of arterioles affected and is scored according to the following categorization; normal arterioles (score: 0), 1–25% affected arterioles (score: 1), 26–50% affected arterioles (score: 2) and>50% affected arterioles (score: 3) (25).
3.5. Clinical Studies and Laboratory Data
The medical records of patients were reviewed to obtain various demographic, clinical and laboratory information at the time of biopsy and for follow-up activities. Data gathered at the time of biopsy included race, gender, age, serum creatinine(Cr) and proteinuria (based on a 24hour urine collection).
3.6. Statistical analysis
Mean values and standard deviations were calculated, and statistical significance of the differences between groups were calculated using T, Chi-square and Likelihood Ratio tests. Because of high positive skewness of data, the Spearman’s coefficient of correlation was used to check the correlation. A computer program (SPSS version 16.0, Chicago, IL, USA) was used for statistical analysis. P < 0.05 was considered statistically significant.
4. Results
4.1. Population Characteristics
This is an observational study, conducted on IgAN patients. A total of 102 biopsies were enrolled to the study.
4.2. Prevalence
In this survey, of 102 patients 71.6 % were male, 2 % were below 20 years, 60.8 % were between 20-40 years and 37.2 % were more than 40 years of age. The age of patients was from 18 to 73 years with mean of 37.7 ± 13.6 years (medain: 35 years). Mean±SD of proteinuria and serum creatinine were 1795 ± 1369 mg/day and 1.651 ± 1.61 mg/dL, respectively. The medain level of proteinuria and serum creatinine were 1500 mg/day and 1.2 mg/dL respectively. The mean serum creatinine in females was 1.14±0.73 mg/dL and in males was 1.85±1.80 mg/dL. Nephrotic range proteinuria was in 8.8% of patients, all of which were male. Also, the mean of proteinuria were 1323±735 mg/day and 1982±1515 mg/day,in females and males repectively.
After stratification of patients according to their proteinuria, we found that, 23.5% of the patients had proteinuria below 1 g/day, 65.7% had proteinuria between 1-3 g/day and 10.8% of the patients had proteinuria more than 3 g/day.
In this study, of total 15 ± 7.3 glomeruli (median=14 glomeruli) in all biopsies, 2.5 ± 3 were totally sclerotic (median=2 glomeruli) and 0.46±1.11 of them were crescentic. In general, extracapillary proliferation occurred in 23 % of the patients and types of crescents were as follows; cellular crescent in 10.8%, fibro-cellular crescent in 7.8% and fibrous crescents in 8.8% of biopsies.
4.3. Oxford classification (MEST)
Four variables of Oxford classification were analyzed in our patients;
M1; we found , 66% of the patients with mesangial hypercellularity in >50% of glomeruli. However, mesangial hypercellularity occurred in 90.2% of cases, similarly mesangial widening in 92.2% of patient was observed. Also The mean (SD) age of all patients whohad M0 and M1 was 42.27 ± 14.09 and 35.10 ± 12.69 years respectively.
E1; endocapillary lesions; were found in 32 % of patients.
S1; segmental glomerulosclerosis whichincludes mesangial sclerosis with capsular adhesions occurred in 67 % of the patients. However simple tuft-capsule adhesion, without mesangial sclerosis, was observedin 61% of patients. The percentage of segmental glomerulosclerosis were 41.1% and 72.6 %, in females and males respectively.
T; tubular atrophy/interstitial fibrosis (scores I and II) were 30% and 19% respectively, while 51% showed zero score. Furthermore, mean (SD) percent of interstitial fibrosis was 14.6 ± 21.1% with a median of 5%. T1 and T2 in females were as follows; 17.2% and 13.8% , while these frequencies were 37% and 20.5% for males, respectively.
4.4. Vascular lesions
Score I of intimal thickening of interlobular arteries was 55.9%, while zero score was observed in 44.1% of the patients. Scores of arteriolar hyaline were as follows; score I and II in 31.4 % and 24.5%, respectively. Also, score 0 was observed in 44.1 % of the patients. We also found thrombotic microangiopathy in 2% of our patients.
4.5. Other lesions
Glomerular fibrinoid necrosis were present in 6.9% of the patients. Mesangial interposition and mesangiolysis each occurred in 1% of the patients, respectively. Foamy cells and tubular thyroidization of the interstitial area were found in 3.9% and 11.8% of patients, respectively.
4.6. Gender and group differences
The independent samples t-test showed a higher serum creatinine (P=0.044) and proteinuria (P=0.028) in males. The Chi-square test showed significant difference in segmental glomerulosclerosis between males and females with higher values in male geneder (P= 0.003). The Likelihood Ratio test showed significant differences in frequency distribution of interstitial fibrosis/tubular atrophy in males and females, with higher values in male geneder (P= 0.045). Also, M1 was more prevalent in younger patients (P= 0.01).
4.7. Correlations
In this study, there was a significant correlation between serum Cr and total crescents (Spearman’s rho = 0.386, P0.001) (fig.1).
Figure 1.
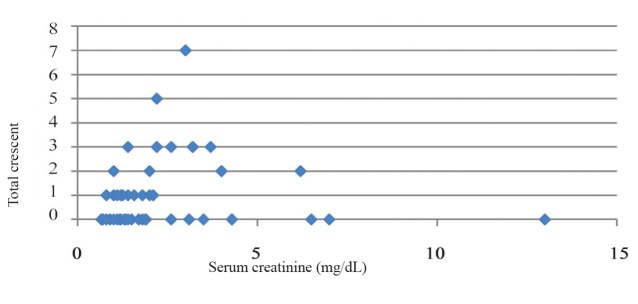
Scatter plot of total crescent based on
In this study there was also a significant correlation between serum Cr and segmental glomerulosclerosis (Spearmans rho = 0.521, P0.001) (fig.2).
Figure 2.
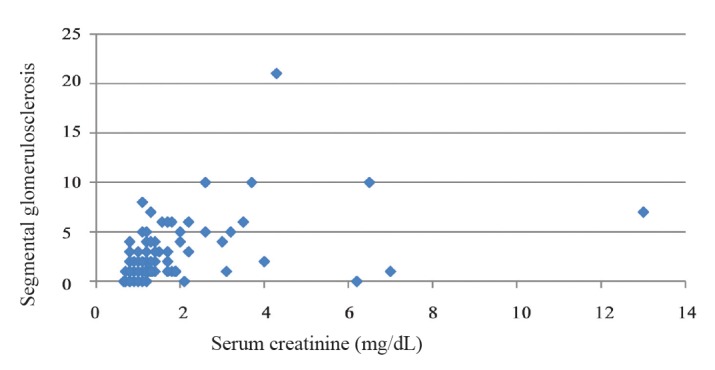
Scatter plot of segmental glomerulosclerosis based on serum creatinine
5. Discussion
Oxford classification of IgA nephropathy is identified by four histological features, mesangial cellularity, endocapillary proliferation, segmental sclerosis and tubular atrophy/interstitial fibrosis, which are the independent predictors of clinical outcome (25-27,30-34).
In the present study, mesangial proliferation and mesangial widening were observed in 90.2% and 92.2%, respectively. However only 66% of biopsies showed score M1, while M1 was more prevalent in younger patients. Furthermore, variables of E1 and S1were found in 32% and 67% of patients, respectively. In our survey, of 102 patients, 71.6 % were male, and mean age of patients was 37.7 ± 13.6 years. This finding is comparable with findings in a study conducted by Mittal et al. in India in which 66 patients with IgAN were identified. The mean age of their patients was 29.9 years with a male: female ratio of 4.4:1. They found that, most patients presented with renal failure and a significant percentage (23%) also had nephrotic range proteinuria (35). This finding is in contrast to our results because in our study, the mean of proteinuria and serum creatinine were 1795 ± 1369 mg/day and 1.651 ± 1.61 mg/dL, respectively. Also, nephrotic range proteinuria was observed in 8.8% of patients. Study of Mittal et al., showed advanced disease in much younger individuals (35), which was not supported by our study. Khawajah et al. studied a total of 42 patients, who were diagnosed to have primary IgAN in Saudi Arabia. Majority of the study cases were males, in the second, third and fourth decades of life. There were varying degrees of mesangial proliferation (36). To analyze the findings of 343 patients with IgAN, Maixnerova et al. have assessed the main demographic, clinical and histological data (37). Similarly, they found that, male gender is predominated over female gender. The median age of IgAN patients was 32.3 years, and the median level of proteinuria was 1.8 g/day. These findings were in agreement with our study, in which patients had a median age of 35 years and median proteinuria of 1.5g/day. To clarify the prognostic factors and the renal survival rates of 223 patients with IgAN, Kang et al. found that glomerular sclerosis, is a risk factor for developing ESRD. We also found a significant correlation between serum Cr and segmental glomerulosclerosis, a finding which correlates with Kang’s study. To find the relationship of four variables applied in the MEST classification with the outcome of the disease, Grcevska et al., conducted a study in 40 adult patients with IgAN. They found that mesangial hypercellularity, glomerular sclerosis, endocapillary hypercellularity and IFTA were associated with the renal outcome likelihood ratio (22). To evaluate the predictive value of the Oxford classification on renal survival defined by doubling creatinine or end-stage renal disease in patients with IgA nephropathy, 183 patients with IgA nephropathy were investigeted by Alamartine et al., in France. They found that tubular atrophy/interstitial fibrosis, segmental glomerulosclerosis, and endocapillary hypercellularity strongly impacted doubling creatinine or end-stage renal disease. On the contrary, mesangial hypercellularity was not associated with renal outcome. They concluded that the baseline renal function seems to be of a greater importance than pathologic lesions (38). Shi et al., studiedthe Likelihood Ratio of 410 patients with IgAN, from China. They evaluated the predictive value of the Oxford classification and found that tubular atrophy and interstitial fibrosis are the independent predictive factors of ESRD. Patients who had >25% of glomeruli with endocapillary hypercellularity showed higher proteinuria, lower glomerular filtration rate and higher mean arterial pressure (MAP) than patients with less endocapillary hypercellularity (39). Similarly data obtained from 146 cases with biopsy proven IgAN were analyzed by Walsh et al., from Canada. Their data revealed that >40% glomerular sclerosis and a systolic blood pressure>150 mmHg were risk thresholds. Moreover, they found that baseline creatinine, proteinuria, systolic blood pressure, glomerular sclerosis, interstitial fibrosis and crescentic disease were the predictors of primary outcome (40). Furthermore, they showed that interstitial fibrosis, glomerular sclerosis and crescents remained as the independent predictors of primary outcome and significantly improved model fit compared to clinical characteristics alone. They concluded that baseline histopathologic parameters are the predictors of adverse outcomes in IgAN even after taking into consideration clinical characteristics. Also, relatively small degrees of interstitial fibrosis confer an increased risk for progressive IgAN (40). In a study conducted by El Karoui et al., on 128 IgAN cases with mean age of 38.7 years (range18–78) in France , they found that mean proteinuria was 2.43 g/day(33), while we found 1795 ± 1369 mg/day proteinuria in our cases.The medain level of proteinuria in our patients was 1.5g/day, implies that patients studied by El Karoui et al., were more proteinuric. We also found that nephrotic range proteinuria was observed in 8.8% of our patients, where all were male. Similarly, the age of our patients was ranging from 18 to 73 years with a mean of 37.7 ± 13.6 years.
Except of four variables of MEST system, there was a concern, whether other pathology variables in the glomeruli, interstitial or vessels may have prognostic outcome. Among them, more importantly, however, is extracapillary proliferation.Indeed, this classification is entirely limited to four above mentioned morphological features (19) and did not include crescent in the analysis (25-27,29,31).Likewise, there are other pathology variables, which may be associated with the renal outcome and need further attention. These are IF and electron microscopic findings, which were not included in this classification (19). Various studies, however, showed that crescentic disease is a predictor of the primary outcome and had been reported as one of the most important prognosticators in IgAN (15,28,41). In our study, extracapillary proliferation occurred in 23% of patients, which was lower than what had been observed in the Oxford study (45%). This lower percentage of extracapillary proliferation was also found in the study conducted by El Karoui et al., in pares. They found that extracapillary proliferation occurs in 31 (24.2%) out of 128 IgAN cases, while mean age of their patients was similar to ours (33).Likewise, Bitencourt-Dias et al., analyzed 144 patients with IgAN. Crescents were found in 18% of their patients. They concluded that the presence of crescents was associated with higher levels of initial serum creatinine and urinary protein excretion and a higher frequency of hypertension and progression to end-stage renal disease(42). Simiraly we found a significant correlation between serum Cr and total crescents. Edström Halling et al., investigeted ninety-nine children with IgAN and showed that mesangial hypercellullarity score >0.5, presence of endocapillary hypercellularity or IFTA of >25% were each associated with a poor outcome. They found that three of the four histology lesions identified in the Oxford classification, as well as the presence of crescents, were valid in predicting a poor outcome in their patient (10,43,44). Similarly, Katafuchi et al., conducted a study on the likelihood ratio of 702 patients with IgAN. They suggested extracapillary proliferation to be included in the next version of the Oxford classification of IgAN to widen the scope of classification (31). Hence, it seems that data on prognostic significance of extracapillary proliferation,as a variable which need to accommodate on MEST, is increasing.
Thrombotic microangiopathy (TMA) occurs in IgA nephropathy, but is uncommon in the setting of IgA nephropathy and its significance, as a concomitant histologic finding, is unclear (45). In a study on 10 patients with an established diagnosis of IgA nephropathy and concurrent findings of TMA, based on their renal biopsies, Chang et al., found six patients presented with malignant hypertension, while three others had severe hypertension and five patients had nephrotic-range proteinuria (45). They also found that seven patients had occasional arteriolar thrombi identified by light microscopy and prominent glomerular subendothelial space widening by electron microscopy, while three patients demonstrated only ultrastructural features of thrombotic microangiopathy. They suggested that a thrombotic microangiopathy injury, when present, is usually found in advanced stages of IgA nephropathy and can be associated with severe proteinuria (45). In our study, of 102 patients with IgAN, 2% of them were found to have TMA, while all of them had malignant hypertension. In contrast, a high frequency of thrombotic microangiopathy (TMA) was observed by some investigators in the cases of IgAN. El Karoui et al., retrospectively examined a series of 128 patients diagnosed with IgA nephropathy in France. In their series, 53% presented with lesions of TMA, acute or organized, in arteries and/or arterioles. Among patients with TMA, 4% were normotensive, 25% had controlled hypertension, and 71% had uncontrolled hypertension. Of those with uncontrolled hypertension, 26% had malignant hypertension (46). Histologically, the group with TMA had a significantly greater percentage of sclerotic glomeruli and worse tubulointerstitial fibrosis than those of the group without TMA. However, a significant minority of patients had near-normal histology, with minimal tubular atrophy (20%) and/or <20% interstitial fibrosis (24%). Their study showed that, TMA rarely occurs in the absence of significant proteinuria. They concluded that lesions of TMA are frequent in IgA nephropathy and may occur in normotensive patients with near-normal renal histology. However, this needs further investigation regarding its association with IgAN and its prognostic value. Their studies rule out severe hypertension or advanced renal disease as the sole causes of TMA (46).
6. Conclusions
Higher prevalence of segmental glomerulosclerosis and interstitial fibrosis/tubular atrophy, as the two of four variables of Oxford-MEST classification in male patients with IgAN, further attests that male gender is a risk factor in this disease. Significant correlation between serum creatinine and crescent in this study, was in an agreement with previous studies and suggests the probable accomodation of crescent as a new variable in the MEST system.
Acknowledgments
The authors express their sincere appreciation to the physicians who participated in this study. Also we thank the staffs of division of renal immunopathology of Dr. Baradaran Laboratory for performing and preparing all biopsies.
Author’s contributions
HN, AG, SB, HS, AB, SM, MS, AEN and MRA designed and performed the research. SK analyzed data and wrote some parts of paper.PH, SJ, YM, AM, MBG, MRK, NA and MRT and SMAS provided extensive intellectual contribution.MBG and AB reviewed the manuscript. SMAS edited the draft. HN prepared the final draft.
Conflict of interest
The authors declared no competing interests.
Financial disclosure
None of the authors declared a conflict of interest.
Funding/Support
A part of this study was funded by Dr. Baradaran Laboratory, Isfahan, Iran.
Implication for health policy/practice/research/medical education:
The prevalence and clinical picture of IgA nephropathy (IgAN) varies from region to region. There is a limited knowledge about the morphological features of this disease according to recent Oxford-MEST classification in Iran and Middle East.In this study higher prevalence of segmental glomerulosclerosis and interstitial fibrosis/tubular atrophy as the two of four variables of this classification in male patients further attests that male gender is a risk factor in this kind of disease. Significant correlation between serum creatinine and extracapillary proliferation was in an agreement with previous studies and was suggested for the probable accommodation of crescent as a new variable in the MEST system.
Please cite this paper as: Nasri H, Mortazavi M, Ghorbani A, Shahbazian H, Kheiri S, Baradaran A, Emami-Naieni A, Saffari M, Mardani S, Momeni A, Madihi Y, Baradaran-Ghahfarokhi M, Rafien-Kopaie M, Hedayati P, Baradaran S, Ardalan MR , Sajjadieh S, Assarzadegan N, Ahmadi Soleimani SM, Tamadon MR. Oxford-MEST classification in IgA nephropathy patients: A report from Iran. J Nephropathology. 2012; 1(1): 31-42. DOI: 10.5812/jnp.7
References
- 1.Berger J, Hinglais N. Intercapillary deposits of IgA-IgG] Journal d’urologie et de néphrologie . 1968;74(9):694. [PubMed] [Google Scholar]
- 2.Julian BA, Waldo FB, Rifai A, Mestecky J. IgA nephropathy, the most common glomerulonephritis worldwide. A neglected disease in the United States? The American journal of medicine . 1988;84(1):129. doi: 10.1016/0002-9343(88)90019-8. [DOI] [PubMed] [Google Scholar]
- 3.Tipu HN, Ahmed TA, Bashir MM. Clinical, Histopathological and Immunofluorescent Findings of IgA Nephropathy. IJI . 2011;8(2): IJI 2011;8(2). [PubMed] [Google Scholar]
- 4.Mubarak M. The prevalence of IgA nephropathy in Pakistan: only a tip of the iceberg. JPMA The journal of the Pakistan Medical Association . 2009;59(10):733. [PubMed] [Google Scholar]
- 5.Mubarak M. IgA nephropathy: an update on pathogenesis and classification. JCPSP . 2011;21(4) [PubMed] [Google Scholar]
- 6.Bartosik LP, Lajoie G, Sugar L, Cattran DC. Predicting progression in IgA nephropathy. American journal of kidney diseases . 2001;38(4):728–35. doi: 10.1053/ajkd.2001.27689. [DOI] [PubMed] [Google Scholar]
- 7.Woo K, Edmondson R, Wu A, Chiang G, Pwee H, Lim C. The natural history of IgA nephritis in Singapore. Clinical nephrology . 1986;25(1):15. [PubMed] [Google Scholar]
- 8.Droz D, Kramar A, Nawar T, Noel L. Primary IgA nephropathy: prognostic factors. Contributions to nephrology . 1984;40:202. doi: 10.1159/000409751. [DOI] [PubMed] [Google Scholar]
- 9.Geddes CC, Rauta V, Gronhagen-Riska C, Bartosik LP, Jardine AG, Ibels LS. et al. A tricontinental view of IgA nephropathy. Nephrology Dialysis Transplantation . 2003;18(8):1541–8. doi: 10.1093/ndt/gfg207. [DOI] [PubMed] [Google Scholar]
- 10.Weber CL, Rose CL, Magil AB. Focal segmental glomerulosclerosis in mild IgA nephropathy: a clinical-pathologic study. Nephrology Dialysis Transplantation . 2009;24(2):483–8. doi: 10.1093/ndt/gfn513. [DOI] [PubMed] [Google Scholar]
- 11.Berg U, Bohman S, Widstam-Attorps U. Renal histological changes in relation to renal function and urinary protein excretion in IgA nephropathy. Archives of disease in childhood . 1991;66(5):593–7. doi: 10.1136/adc.66.5.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kazi J, Mubarak M. IgA nephropathy. J Coll Physicians Surg Pak . 2010;20(12):779–80. [PubMed] [Google Scholar]
- 13.Goto M, Wakai K, Kawamura T, Ando M, Endoh M, Tomino Y. A scoring system to predict renal outcome in IgA nephropathy: a nationwide 10-year prospective cohort study. Nephrology Dialysis Transplantation . 2009;24(10):3068–74. doi: 10.1093/ndt/gfp273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mubarak M. Nomenclature of the Oxford classification of IgA nephropathy: do we need to be careful? Kidney Int . 2010;77(1):74. doi: 10.1038/ki.2009.370. author reply -5. [DOI] [PubMed] [Google Scholar]
- 15.Haas M. IgA nephropathy histologically resembling focal-segmental glomerulosclerosis: A clinicopathologic study of 18 cases. American journal of kidney diseases . 1996;28(3):365–71. doi: 10.1016/s0272-6386(96)90493-x. [DOI] [PubMed] [Google Scholar]
- 16.Katafuchi R, Kiyoshi Y, Oh Y, Uesugi N, Ikeda K, Yanase T. et al. Glomerular score as a prognosticator in IgA nephropathy: its usefulness and limitation. Clinical nephrology . 1998;49(1):1–8. [PubMed] [Google Scholar]
- 17.Yamamoto R, Imai E. A novel classification for IgA nephropathy. Kidney international . 2009;76(5):477–80. doi: 10.1038/ki.2009.206. [DOI] [PubMed] [Google Scholar]
- 18.Fogo AB, Alpers CE, D D’Agati V. FSGS lesions in IgA nephropathy. Kidney international . 2011;80(3):319. doi: 10.1038/ki.2011.137. [DOI] [PubMed] [Google Scholar]
- 19.Mubarak M. The oxford classification of IgA nephropathy: A role model for classifying other renal diseases. Saudi Journal of Kidney Diseases and Transplantation . 2011;22(5):897. [PubMed] [Google Scholar]
- 20.Ikezumi Y, Suzuki T, Imai N, Ueno M, Narita I, Kawachi H. et al. Histological differences in new-onset IgA nephropathy between children and adults. Nephrology Dialysis Transplantation . 2006;21(12):3466–74. doi: 10.1093/ndt/gfl455. [DOI] [PubMed] [Google Scholar]
- 21.Coppo R, Troyanov S, Camilla R, Hogg RJ, Cattran DC, Cook HT. et al. The Oxford IgA nephropathy clinicopathological classification is valid for children as well as adults. Kidney international . 2010;77(10):921–7. doi: 10.1038/ki.2010.43. [DOI] [PubMed] [Google Scholar]
- 22.Grcevska L, Ristovska V, Nikolov V, Petrusevska G, Milovanceva-Popovska M, Polenakovic M. The Oxford classification of IgA nephropathy: single centre experience. Prilozi . 2010;31(2):7–16. [PubMed] [Google Scholar]
- 23.Kang SH, Choi SR, Park HS, Lee JY, Sun IO, Hwang HS. et al. The Oxford classification as a predictor of prognosis in patients with IgA nephropathy. Nephrology Dialysis Transplantation . 2012;27(1):252–8. doi: 10.1093/ndt/gfr295. [DOI] [PubMed] [Google Scholar]
- 24.Yau T, Korbet SM, Schwartz MM, Cimbaluk D. The Oxford Classification of IgA Nephropathy: A Retrospective Analysis. American Journal of Nephrology . 2011;34(5):435–44. doi: 10.1159/000332223. [DOI] [PubMed] [Google Scholar]
- 25.Roberts ISD, Cook HT, Troyanov S, Alpers CE, Amore A, Barratt J. et al. The Oxford classification of IgA nephropathy: pathology definitions, correlations, and reproducibility. Kidney international . 2009;76(5):546–56. doi: 10.1038/ki.2009.168. [DOI] [PubMed] [Google Scholar]
- 26.Cattran DC, Coppo R, Cook HT, Feehally J, Roberts ISD, Troyanov S. et al. The Oxford classification of IgA nephropathy: rationale, clinicopathological correlations, and classification. Kidney international . 2009;76(5):534–45. doi: 10.1038/ki.2009.243. [DOI] [PubMed] [Google Scholar]
- 27.Kawamura T, Joh K, Okonogi H, Koike K, Utsunomiya Y, Miyazaki Y, et al. A histologic classification of IgA nephropathy for predicting long-term prognosis: emphasis on end-stage renal disease. J Nephrol. 2012:0. [DOI] [PubMed] [Google Scholar]
- 28.Haas M. Histologic subclassification of IgA nephropathy: a clinicopathologic study of 244 cases. American journal of kidney diseases . 1997;29(6):829–42. doi: 10.1016/s0272-6386(97)90456-x. [DOI] [PubMed] [Google Scholar]
- 29.Bellur SS, Troyanov S, Cook HT, Roberts IS. Immunostaining findings in IgA nephropathy: correlation with histology and clinical outcome in the Oxford classification patient cohort. Nephrol Dial Transplant . 2011;26(8):2533–6. doi: 10.1093/ndt/gfq812. [DOI] [PubMed] [Google Scholar]
- 30.Moriyama T, Nakayama K, Iwasaki C, Ochi A, Tsuruta Y, Itabashi M. et al. Severity of nephrotic IgA nephropathy according to the Oxford classification. Int Urol Nephrol . 2012;44(4):1177–84. doi: 10.1007/s11255-011-0109-5. [DOI] [PubMed] [Google Scholar]
- 31.Katafuchi R, Ninomiya T, Nagata M, Mitsuiki K, Hirakata H. Validation study of oxford classification of IgA nephropathy: the significance of extracapillary proliferation. Clin J Am Soc Nephrol . 2011;6(12):2806–13. doi: 10.2215/CJN.02890311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cook HT. Focal segmental glomerulosclerosis in IgA nephropathy: a result of primary podocyte injury? Kidney Int . 2011;79(6):581–3. doi: 10.1038/ki.2010.521. [DOI] [PubMed] [Google Scholar]
- 33.El Karoui K, Hill GS, Karras A, Moulonguet L, Caudwell V, Loupy A. et al. Focal segmental glomerulosclerosis plays a major role in the progression of IgA nephropathyIILight microscopic and clinical studies. Kidney Int . 2011;79(6):643–54. doi: 10.1038/ki.2010.460. [DOI] [PubMed] [Google Scholar]
- 34.Hill GS, Karoui KE, Karras A, Mandet C, Duong Van Huyen, Nochy D. et al. Focal segmental glomerulosclerosis plays a major role in the progression of IgA nephropathyIImmunohistochemical studies. Kidney Int . 2011;79(6):635–42. doi: 10.1038/ki.2010.466. [DOI] [PubMed] [Google Scholar]
- 35.Mittal N, Joshi K, Rane S, Nada R, Sakhuja V. Primary IgA nephropathy in north India: is it different? Postgrad Med J . 2012;88(1035):15–20. doi: 10.1136/postgradmedj-2011-130077. [DOI] [PubMed] [Google Scholar]
- 36.Khawajah AQ, Al-Maghrabi J, Kanaan HD, Al-Ghamdi S. IgA nephropathy: a clinicopathologic study from two centers in Saudi Arabia. Saudi J Kidney Dis Transpl . 2010;21(2):269–75. [PubMed] [Google Scholar]
- 37.Maixnerova D, Bauerova L, Skibova J, Rysava R, Reiterova J, Merta M. et al. The retrospective analysis of 343 Czech patients with IgA nephropathy--one centre experience. Nephrol Dial Transplant . 2012;27(4):1492–8. doi: 10.1093/ndt/gfr482. [DOI] [PubMed] [Google Scholar]
- 38.Alamartine E, Sauron C, Laurent B, Sury A, Seffert A, Mariat C. The use of the Oxford classification of IgA nephropathy to predict renal survival. Clin J Am Soc Nephrol . 2011;6(10):2384–8. doi: 10.2215/CJN.01170211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shi SF, Wang SX, Jiang L, Lv JC, Liu LJ, Chen YQ. et al. Pathologic predictors of renal outcome and therapeutic efficacy in IgA nephropathy: validation of the oxford classification. Clin J Am Soc Nephrol . 2011;6(9):2175–84. doi: 10.2215/CJN.11521210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walsh M, Sar A, Lee D, Yilmaz S, Benediktsson H, Manns B. et al. Histopathologic features aid in predicting risk for progression of IgA nephropathy. Clin J Am Soc Nephrol . 2010;5(3):425–30. doi: 10.2215/CJN.06530909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jia Z, Shan L, Jianqing J, Li W. Clinical Pathological Analysis and Treatment of IgA Nephropathy with a Few Quantity of Renal Crescent Formation. J Nephrol Therapeutic.1:107. [Google Scholar]
- 42.Bitencourt-Dias C, Bahiense-Oliveira M, Saldanha LB, Barros RT, Woronik V. Comparative study of IgA nephropathy with and without crescents. Braz J Med Biol Res . 2004;37(9):1373–7. doi: 10.1590/s0100-879x2004000900012. [DOI] [PubMed] [Google Scholar]
- 43.Edstrom Halling S, Soderberg MP, Berg UB. Predictors of outcome in paediatric IgA nephropathy with regard to clinical and histopathological variables (Oxford classification) Nephrol Dial Transplant . 2012;27(2):715–22. doi: 10.1093/ndt/gfr339. [DOI] [PubMed] [Google Scholar]
- 44.Weber CL, Rose CL, Magil AB. Focal segmental glomerulosclerosis in mild IgA nephropathy: a clinical-pathologic study. Nephrol Dial Transplant . 2009;24(2):483–8. doi: 10.1093/ndt/gfn513. [DOI] [PubMed] [Google Scholar]
- 45.Chang A, Kowalewska J, Smith KD, Nicosia RF, Alpers CE. A clinicopathologic study of thrombotic microangiopathy in the setting of IgA nephropathy. Clin Nephrol . 2006;66(6):397–404. doi: 10.5414/cnp66397. [DOI] [PubMed] [Google Scholar]
- 46.El Karoui K, Hill GS, Karras A, Jacquot C, Moulonguet L, Kourilsky O. et al. A clinicopathologic study of thrombotic microangiopathy in IgA nephropathy. J Am Soc Nephrol . 2012;23(1):137–48. doi: 10.1681/ASN.2010111130. [DOI] [PMC free article] [PubMed] [Google Scholar]


