Abstract
Background
Background: Idiopathic steroid resistant nephrotic syndrome (ISRNS) represents about 10-20% of children with nephrotic syndrome with variable outcome.
Objectives
To determine the histological patterns of ISRNS in Egyptian children and the histological details of the commonest types which might be the reason for the steroid resistance.
Patients and Methods
The study included 53 cases with ISRNS. Their renal biopsies were retrieved from the archive of Electron microscopy unit and pathology department, Ain Shams University Specialized Hospital (ASUSH) in the duration from 2005-2011. The biopsies were examined histologically, with immunohistochemistry, and by electron microscopy.
Results
They were 36 males (67.9%) and 17 females (32.1%), the age at diagnosis ranged from 1.5- 16 years with a mean of 6.71 years. Lower limb oedema was the commonest presentation (100%), haematuria was revealed in (17%) of cases. Histological examination showed three major patterns; Focal segmental glomerulosclerosis (FSGS) in 30.2%, minimal change glomerulopathy (MCG) in 24.5% and IgA nephropathy in 13.2 %. Mesangial hypercellularity was very common among MCG patients (85.3% ±6.7). Tubulointerstitial inflammation and fibrosis were common among cases with IgA nephropathy (40.4% ±11, 53.7% ±8 respectively).
Conclusions
ISRNS in Egyptian children could be attributed mainly to three major diseases (FSGS, MCG and IgA nephropathy). Mesangial hypercellularity and severe tubulointerstitial disease might be the major causes for steroid resistance in MCG and IgA nephropathy respectively. Renal biopsy with electron microscopy examination should be done for all children with nephrotic syndrome at first time of presentation for proper assignment of treatment protocol.
Keywords: Focal segmental glomerulosclerosis (FSGS), Minimal change glomerulopathy (MCG), IgA nephropathy, Predinsone resistant nephrotic syndrome, Mesangial hypercellularity, Tubulointerstitial disease
1. Background
Idiopathic steroid resistant nephrotic syndrome (ISRNS) of children can be defined as; a child with nephrotic syndrome who fails to show a complete remission of symptoms after using the full prescribed steroid treatment. The usual steroid protocol used in these cases comprises of prednisone 60 mg/m2/day for four weeks, followed by 40 mg/m2/48 hours for another four weeks (1).
The steroid resistance can be grouped into primary resistance in which there is failure of complete remission after treatment during the first time of nephrotic syndrome presentation, while in the secondary resistance the child initially responds well to steroid regimen for a period of time, after which he shows recurrence of symptoms and failure of complete response to steroid treatment (2).
ISRNS accounts for 10-20% of children with nephrotic syndrome (3). The prognosis of the disease is usually unfavorable, progression to end-stage kidney disease (ESKD) is usually the end result after a variable duration; thus different aggressive and even potentially toxic treatment regimens have been tried to delay disease progression (4,5).
Different studies revealed that focal and segmental glomerulosclerosis (FSGS) and minimal change disease (MCD) are the most common morphologic patterns seen in children with ISRNS in western countries (3,4), as well as many other parts of the world (6,7).
However, some times the incidence of the disease can be controlled when the cause is preventable via vaccination; this is obvious in South Africa where membranous nephropathy associated with hepatitis B infection was reported as a main cause of ISRNS (8).
Histological features of the renal biopsy can give a clue to the future response of the patient to therapy; such as mesangial hypercellularity in cases of minimal change glomerulopathy (9).
Although clinical data are stronger predictors for the disease outcome in cases of IgA nephropathy (10), some histological features can be associated with poor prognosis; such as glomerular crescents and/or moderate to severe tubulointerstitial inflammation (11).
2. Objectives
The aim of this study is to determine the histological patterns of ISRNS in Egyptian children and compare it with the findings worldwide. In addition the study will highlight the histological details of the commonest types of ISRNS which might be the reason for their steroid resistance.
3. Patients and Methods
This is a retrospective study. The material was retrieved from archival files for children with ISRNS who underwent ultrasound guided renal needle biopsy, in the duration from 2005-2011, and referred to the electron microscopy unit and pathology department at ASUSH, which is a tertiary referral hospital. The electron microscopy unit is one of the very few diagnostic units in Egypt that serves a huge percentage of population and is the referral centre for nearly all the nephrology centers all over the country. This study was approved by the local ethical institutional review board.
The study included all cases of nephrotic syndrome with steroid resistance, either primary or secondary, that started at age of >1 year and ≤16 years.
We excluded cases of steroid resistance in nephrotic syndrome with congenital or syndromic forms, as well as those cases with incomplete clinical and/or laboratory investigations.
The patients were evaluated according to standardized values:
-Nephrotic range proteinuria ≥3.5 g /24 h.
-Urine protein or spot urine protein/creatinine ratio >3.
-Hypoalbuminemia, serum albumin ≤3.5 g/dl in adults and ≤2.5 g/dl in children.
-Renal insufficiency, serum creatinine >1.2 mg/dl in adults and more than the age-related normal values in children.
-Hematuria, >5 red blood cells per high power field on microscopic examination of the urinary sediment or positive blood by dipstick (5).
3.1. The pathological review
The light microscopic and immunohistochemistry slides, as well as the electron microscopy photographs were reviewed for confirmation of the diagnosis. Certain glomerular and tubulointerstitial changes were interestingly focused for the three commonest histological patterns.
Glomeruli were counted and examined for:
-The number with global and/or segmental sclerosis.
-The number of crescents.
-The presence or absence of hypercellularity and the type of the infiltrating cells and/or endocapillary proliferation.
The results were expressed quantitatively by counting the total number of glomeruli and calculate the percentage of the affected ones.
Tubulointerstitial changes were examined for the presence of:
-Tubular atrophy and/or tubulitis.
-Interstitial fibrosis and/or interstitial inflammation.
The ratio of the affected tubulointerstitium was calculated and presented.
3.2. Statistical analyses
Different variables are reported as the mean ± SEM using SPSS for Windows, version 15.0 (SPSS, Chicago, Illinois, USA).
4. Results
4.1. Demographic data
The ISRNS cases included 62 cases; four cases were excluded due to inadequate clinical data, and further five cases with their diagnosis titled ‘’ suggestive of IgA nephrpathy’’ were excluded; they had microscopic or macroscopic haematuria, their electron microscopy showed mesangial and paramesangial deposits, but they lacked confirmatory immunohistochemical staining. Thus we obtained finally 53 cases; 36 males (67.9%), and 17 females (32.1%) with a female: male ratio 2.1:1, the age of the patients at diagnosis of steroid resistance ranged from 1.5- 16 years with a mean age of 6.7±2.4 years.
4.2. Clinical results
48 patients (90.6%) had primary ISRNS, while five patients with focal and segmental glomerulosclerosis (9.4%) had secondary ISRNS; the first diagnosis of those patients was minimal change glomerulopathy, the duration between the first time of diagnosis and the steroid resistance ranged from 1.5-10 years with mean duration of 5.1 years.
The commonest presenting symptom was lower limb edema; it was presented in all cases; followed by facial oedema in (18.9%) of patients. Haematuria was evident in 9 cases (17%); 8 were microscopic haematuria (four cases with IgA nephropathy and single case for each of (focal segmental glomerulosclerosis FSGS, membranoproliferative glomerulonephritis MPGN, resolving post infectious glomerulonephritis rPIGN, and diffuse mesangial proliferative glomerulonephritis MesPGN), one case with gross haematuria showed IgA nephropathy. Nephrotic/nephritic syndrome was presented in one case with MesPGN. Four cases were hypertensive with arterial blood pressure 140/90 (7.5%); the rest of patients showed the age-related normal values. First degree consanguity was recorded for the parents in tow cases, one with Minimal change disease MCD, and the other with FSGS. Positive family history with an affected older brother was evident in one case with MCD.
Past clinical history of the children involved in the study revealed; one male child 15 years old with HCV virus diagnosed as MCG three years ago; he experienced secondary steroid resistance afterward; thus second biopsy was done; and diagnosed as FSGS.
Another two and half years old female with FSGS gave a history of preeclampsia of her mother during pregnancy; and the child had delayed mental and motor milestones.
Another four years old female with MCG had an associated idiopathic thrombocytopenia.
None of the children showed chronic renal failure; while a single child with MesPGN showed renal function impairment with elevated serum creatinine (4 mg/dl); the rest of the children serum creatinine ranged from 0.1-0.6 mg/dl; with mean value of 0.34 ± 0.1mg/dl. Serum albumin ranged from 2- 4g/dl with a mean value of 2.82 ± 0.61. Serum cholesterol ranged from 360-395 mg/dl; with a mean value of 380 ± 8.5 mg/dl. Table (1).
Table 1. Clinical features of the patients with ISRNS.
| Clinical feature | Number of patients (%) |
| Lower limb oedema | 53 (100%) |
| Facial oedema | 10 (18.9%) |
| Ascitis | 2 (3.8%) |
| Microscopic haematuria * | 8 (15.1%) |
| Gross haematuria** | 1 (1.9%) |
| Hypertension*** | 4 (7.5%) |
| Nephrotic/nephritic° | 1 (1.9%) |
| Renal impairment°° | 1 (1.9%) |
| Consangiuty°°° | 2 (3.8%) |
| Positive family history°°°° | 1 (1.9%) |
*Due to IgA nephropathy (n=4), FSGS (n=1), MPGN (n=1), rPIGN (n=1), and MesPGN (n=1).
**Due to IgA nephropathy (n=1).
***Due to Membranous glomerulonephritis (MGN) stage III (n=1), IgA nephropathy (n=1) MCD (n=1), and MesPGN (n=1).
°Due to MesPGN (n=1).
°°Due to MesPGN (n=1).
°°°Due to FSGS (n=1), MCD (n=1).
°°°°Due to MCD (n=1).
4.3. Histopathological findings
The mean number of glomeruli studied was 14±3 (range 6–38). FSGS was the most common histopathological subtype, occurring in 16 of 53 children (30.2%). MCD was the second common histological pattern which was found in 13 patients (24.5%). IgA nephropathy was the third; it was diagnosed in 7 cases (13.2%). MGN was presented in five children (9.4%). MPGN was reported in four children (7.5%), Dense Deposit Disease, and rPIGN were diagnosed in three cases for each (5.7%), diffuse mesangial proliferation (MesPGN) and IgM nephropathy were encountered in only one patients for each (1.9%). Table (2).
Table 2. Histopathological patterns of patients with ISRNS.
| Histological pattern | Number of patients (%) |
| Focal and segmental glomerulosclerosis (FSGS) | 16 (30.2%) |
| Minimal change disease (MCG) | 13 (24.5%) |
| IgA nephropathy | 7 (13.2%) |
| Membranous glomerulonephritis (MGN) | 5 (9.4%) |
| Membranoproliferative glomerulonephritis (MPGN) | 4 (7.5%) |
| Dense deposit disease (DDD) | 3 (5.7%) |
| Resolving post infectious glomerulonephritis (rPIGN) | 3 (5.7%) |
| Diffuse mesangial proliferative glomerulonephritis (MesPGN) | 1 (1.9%) |
| IgM nephropathy | 1 (1.9%) |
| Total | 53 (100%) |
Special interest was given to certain glomerular and tubulointerstitial findings concerning the three commonest histological patterns. (Table 3) and figure (1&2).
Table 3. Glomerular and tubulointerstial findings for the three commonest histological patterns.
| Tubulo-interstitium | Glomeruli | The histological pattern | ||||
| fibrosis (%) | inflammation (%) | Mesangial hypercellularity (%) | Crescent (%) | Global sclerosis (%) | Segmental sclerosis (%) | |
| 33±11 | 25± 8 | 43± 8 | 2.8±0.7 | 7.1±2 | 35±7 | FSGS |
| 0±0 | 0±0 | 85.3±6.7 | 0±0 | 0±0 | 0±0 | MCG |
| 53.7±8 | 40.4±11 | 30±4 | 2.1±0.2 | 0±0 | 1.8±0.6 | IgA |
Figure 1.
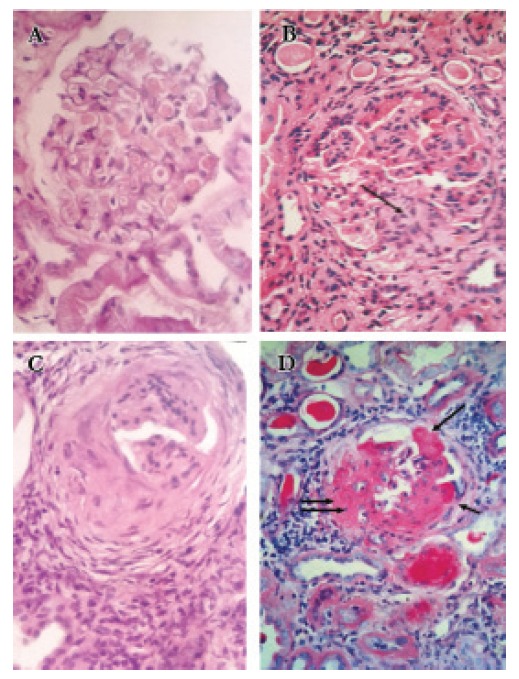
[A] a case of MCG; the se section shows one glomerulus with mild mesangial hypercellularity, H & E x 400, [B] a case of FSGS; it shows a lobulated glomerulus with segmental glomerulosclerosis(arrow) and moderate global hypercellularity, H & E x 400. [C] A glomerulus with collapsed tuft, fibrocellular crescent, periglomerular fibrosis, and interstitial inflammation, H & E x 400. [D] A glomerulus with global sclerosis, adhesions (single arrow), fibrous crescent (double arrow) with interstitial inflammation and tubular atrophy, PAS x 400.
Figure 2.
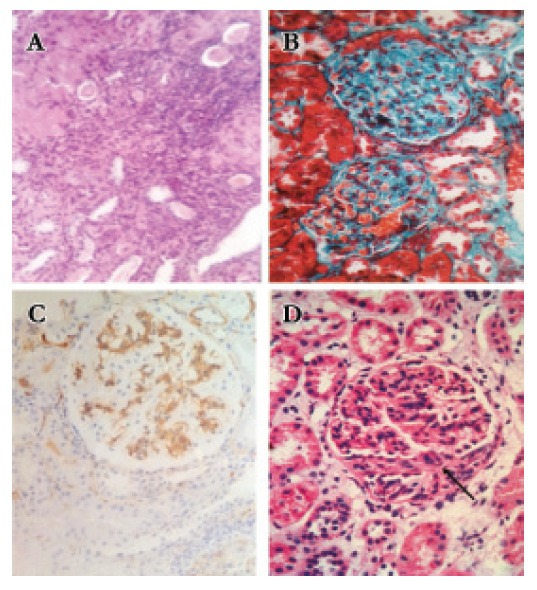
[A] the section shows prominent tubular atrophy and interstitial fibrosis, H & E x 100. [B] Global sclerosis, tubular atrophy, and interstitial fibrosis, MT x 400. [C] IgA nephropathy showing strong +3 mesangial staining for IgA, x 400. [D] Global hypercellularity with fibrocellular crescent (arrow), H & E x 400.
Mesangial hypercellularity was very common among children with MCG (85.3% ± 6.7), while tubulointerstitial inflammation and fibrosis was remarkably high among children with IgA nephropathy (40.4% ± 11, 53.7% ± 8 respectively)
5. Discussion
Idiopathic nephrotic syndrome is one of the commonly glomerular diseases in children. Most patients are steroid sensitive and respond to the therapy with complete remission of proteinuria. Approximately 10-20% of children with nephrotic syndrome who do not completely respond to corticosteroids are qualified as steroid resistant (2).
Renal histology provides important information in those patients before initiating treatment with potentially nephrotoxic agents, although that there is no evidence based recommendations regarding the role of renal biopsy on outcome. (12)
In our study the three major causes of ISRNS were FSGS (30.2%), MCG (24.5%), and IgA nephropathy (13.2%). The prevelance of FSGS was comparable to studies done in South Africa (8), India (13), France, and USA (14); while was lower than what reported in Nigeria (3), Grmany/Austria (15), KSA (16), and Tunis (2). FSGS was more common than that reported in Kuwait (17).
MCG was reported in 24.5% of cases. It was a higher figure compared to KSA (2) and Nigeria (3), and it was less common than what was reported in India (13), Kuwait (17), and InternationI study of kidney diseases in children ISKDC (18).
The predominance of FSGS in many studies could be explained on the bases that FSGS could be a primary disease, or secondary complicating other glomerular lesion and it is known to be resistant for steroid therapy with poor afterward prognosis (19).
The reasons for disparities in the prevalence of MCD and FSGS are not entirely clear; they are probably explained by demographic and environmental factors (20-23), as well as the difficulty of histologic distinction between FSGS and MCG depending on the adequacy of the biopsy taken.
In our study five patients (9.4%) revealed secondary resistance to steroid therapy. They were responsive at the first time of clinical presentation, and were diagnosed as MCG. After a mean duration of 5.1 years they showed steroid resistance with renal biopsy revealed FSGS. Several studies documented patients with ISRNS whose initial kidney biopsy specimens demonstrated MCD and subsequently had a follow-up biopsy specimen that showed FSGS. This has led to the conclusion that MCNS and FSGS may represent a natural progression of the same process (12).
The most striking finding was the high percentage of IgA among Egyptian children with ISRNS (13.2%) in comparison with reported cases in the studied experiences. KSA reported only 3% for IgA (2). This could be attributed to the treatment protocol for the children with ISRNS among nephrologists in Egypt; when the clinician received a child with nephrotic syndrome, he started corticosteroid therapy without proceeding renal biopsy, if the child showed no response; the clinician would proceed to renal biopsy with a title of “steroid resistant nephrotic syndrome” written in the pathology request.
Some of IgA nephropathy cases do not respond well to steroid therapy alone according to the degree of glomerulosclerosis and tubulointerstitial reaction (23).
Mesangial hypercellularity was revealed in 85.3%±6 of patients with MCG, which might account as a major reason for steroid resistance in those patients (9).
Remarkable tubulointerstitial inflammation and fibrosis were observed in 40.4%±11, 53.7%±8 respectively in between patients with IgA nephropathy, which could be attributed as a reason for steroid resistance. It was reported that patients with significant tubulointerstitial fibrosis and/or glomerulosclerosis would not get any benefit from steroid therapy and they were more likely to be harmed from various side effects (11).
Thus we recommend that renal biopsy should be done for all children with nephrotic syndrome at the first time of presentation for proper diagnosis. In cases of MCG, if mesangial hypercellularity is detected in the renal biopsy, the nephropathologist must title it in the diagnosis as ‘’minimal change disease with mesangial hypercellularity’’ , Also in cases of IgA nephropathy, the diagnosis must be titled with the degree of the severity of tubulointerstial involvement, this can give a clue to the clinician about the prognostic steroid response, so as to choose the correct treatment protocol and to save patients from unnecessary drugs side effects.
6. Conclusions
FSGS, MCG, and IgA nephropathy were the major histological patterns of childhood idiopathic steroid resistant nephrotic syndrome in Egypt. Renal biopsy with electron microscopy examination should be done at the first time of presentation to choose the correct treatment protocol. Nephrotic syndrome with a fair steroid response might turn resistant after certain duration of follow up due to change of the renal histological pattern.
Authors’ contributions
EIS designed and performed the final review of the manuscript. EASI, NGE and MIS performed the research, analyzed data and wrote the manuscript.
Conflict of interest
The author declared no competing interests.
Funding/Support
None declared.
Acknowledgments
We acknowledge the help of all the staff members of Electron Microscopy Unit in performance of our research.
Implication for health policy/practice/research/medical education:
Idiopathic steroid resistant nephrotic syndrome in Egyptian children could be attributed mainly to three major diseases (focal segmental glomerulosclerosis, minimal change glomerulopathy and IgA nephropathy). Mesangial hypercellularity and severe tubulointerstitial disease might be the major causes for steroid resistance in minimal change glomerulopathy and IgA nephropathy respectively. Renal biopsy with electron microscopy examination should be done for all children with nephrotic syndrome at first time of presentation for proper assignment of treatment protocol.
Please cite this paper as: Seif EI, Ibrahim EA, Elhefnawy NG, Salman MI. Histological patterns of idiopathic steroid resistant nephrotic syndrome in Egyptian children: A single centre study. J Nephropathology. 2013; 2(1): 53-60. DOI: 10.58.12/nephropathol.8997
References
- 1. International Study of Kidney Disease in Children. International Study of Kidney Disease in ChildrenThe primary nephrotic syndrome in childrenIdentification of patients with minimal change nephrotic syndrome from initial response to prednisoneA report of the International Study of Kidney Disease in Children. J Pediatr . 1981;98(4):561–4. doi: 10.1016/s0022-3476(81)80760-3. [DOI] [PubMed] [Google Scholar]
- 2.Gargah T, Labessi A, Goucha- Louzir R, Ben Moussa F, Lakhoua MR. Histopathological spectrum of childhood idiopathic steroid-resistant nephrotic syndrome in Tunisia. Tunis Med . 2011;89(3):258–61. [PubMed] [Google Scholar]
- 3.Olowu WA, Adelusola KA, Adefehiniti O. Childhood idiopathic steroid resistant nephrotic syndrome in Souwestern Nigeria. Saudi J kidney Dis Transpl . 2010;21(5):979–90. [PubMed] [Google Scholar]
- 4.Bagga A, Sinha A, Moudgil A. Rituximab in patients with the steroid-resistant nephrotic syndrome. N Eng J Med . 2007;356:2751–2. doi: 10.1056/NEJMc063706. [DOI] [PubMed] [Google Scholar]
- 5.Ehrich JH, Geerlings C, Zivicnjak M, Franke D, Geerlings H, Gellermann J. Steroid-resistant idiopathic childhood nephrosis: overdiagnosed and undertreated. Nephrol Dial Transplant . 2007;22:2183–93. doi: 10.1093/ndt/gfm092. [DOI] [PubMed] [Google Scholar]
- 6.Ali A, Ali D, Mehran H, Ali Z. Idiopathic nephrotic syndrome in Iranian children. Indian Pediatr . 2008;45(1):52–3. [PubMed] [Google Scholar]
- 7.Doe JY, Funk M, Mengel M, Doehring E, Ehrich JH. Nephrotic syndrome in African children: lack of evidence for ‘tropical nephrotic syndrome’? Nephrol Dial Transplant . 2006;21(3):672–6. doi: 10.1093/ndt/gfi297. [DOI] [PubMed] [Google Scholar]
- 8.Bhimma R, Coovadia HM, Adhikari M. Nephrotic syndrome in South African children: changing perspectives over 20 years. Pediatr Nephrol . 1997;11(4):429–34. doi: 10.1007/s004670050310. [DOI] [PubMed] [Google Scholar]
- 9.Niaudet P. [Lipoid nephrosis in childhood] Rev Prat . 2003;53(18):2027–32. [PubMed] [Google Scholar]
- 10.Bartosik LP, Lajoie G, Sugar L, Cottran DC. Predicting progression in IgA nephropathy. Am J Kidney Dis . 2001;38(4):728–35. doi: 10.1053/ajkd.2001.27689. [DOI] [PubMed] [Google Scholar]
- 11.Viviante A, Afek A, Frenkel-Nir Y, Tzur D, Farfel A, Golan E. et al. Persistent asymptomatic isolated microscopic hematuria in Israeli adoescets and young adults ad risk for end stage renal disease. JAMA . 2011;306(7):729–36. doi: 10.1001/jama.2011.1141. [DOI] [PubMed] [Google Scholar]
- 12.Indian Society of, Gulati A, Bagga A, Gulati S, Mehta KP, Vijayakumar M. Management of steroid resistant nephrotic syndrome. Indian Pediatr . 2009;46(1):35–47. [PubMed] [Google Scholar]
- 13.Mantan M, Sriram CS, Hari P, Dinda A, Bagga A. Efficacy of intravenous pulse cyclophosphamide treatment versus combination of intravenous dexamethasone and oral cyclophosphamide treatment in steroid-resistant nephrotic syndrome. Pediatr Nephrol . 2008;23(9):1495–502. doi: 10.1007/s00467-008-0860-8. [DOI] [PubMed] [Google Scholar]
- 14.Niaudet P. Niaudet PTreatment of childhood steroid-resistant idiopathic nephrosis with a combination of cyclosporine and prednisoneFrench Society of Pediatric Nephrology. J Pediatr . 1994;125(6 Pt 1):981–6. doi: 10.1016/s0022-3476(05)82020-7. [DOI] [PubMed] [Google Scholar]
- 15.Plank C, Kalb V, Hinkes B, Hildebrandt F, Gefeller O, Rascher W. et al. Cyclosporin A is superior to cyclophosphamide in children with steroid-resistant nephrotic syndrome: a randomized controlled multicentre trial by the Ar-beitsgemeinschaft fur Padiatrische Nephrologie. Pediatr Nephrol . 2008;23(9):1483–93. doi: 10.1007/s00467-008-0794-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kari JA, Halawani M, Mokhtar G, Jalalah SM, Anshasi W. Pattern of steroid resistant nephrotic syndrome in children living in Kingdom of Saudi Arabia: A single centre study. Saudi J kidney Dis Transpl . 2009;20(5):854–57. [PubMed] [Google Scholar]
- 17.El-Reshaid K, Kapoor M, Nampoory N, Madda J, Jawad N, Johny K. Treatment of children with steroid refractory idiopathic nephrotic syndrome: the Kuwaiti experience. Ren Fail . 1999;21(5):487–94. doi: 10.3109/08860229909045188. [DOI] [PubMed] [Google Scholar]
- 18.A Report of. Nephrotic syndrome in children: Prediction of histopathology from clinical and laboratory characteristics at time of diagnosis. Kidney Int . 1978;13(2):159–65. doi: 10.1038/ki.1978.23. [DOI] [PubMed] [Google Scholar]
- 19.D’Agati V. Pathologic classification of focal segmental glomerulosclerosis. Nephrol Semin . 2003;23(2):117–34. doi: 10.1053/snep.2003.50012. [DOI] [PubMed] [Google Scholar]
- 20.Kari JA, Halawani M, Mokhtar G, Jalalah SM, Anshasi W. Histopathology of steroid-resistant nephrotic syndrome in children living in the Kingdom of Saudi Arabia. Pediatr Nephrol . 2009;24(7):1429–30. doi: 10.1007/s00467-008-1106-5. [DOI] [PubMed] [Google Scholar]
- 21.Hamasaki Y, Yoshikawa N, Hattori S, Sasaki S, Iijima K, Nakanishi K. et al. Cyclosporine and steroid therapy in children with steroid-resistant nephrotic syndrome. Pediatr Nephrol . 2009;24(11):2177–85. doi: 10.1007/s00467-009-1264-0. [DOI] [PubMed] [Google Scholar]
- 22.Mekahli D, Liutkus A, Ranchin B, Yu A, Bassenay L, Girardin E. et al. Long-term outcome of idiopathic steroid-resistant nephrotic syndrome: a multicenter study. Pediatr Nephrol . 2009;24(8):1525–32. doi: 10.1007/s00467-009-1138-5. [DOI] [PubMed] [Google Scholar]
- 23.Izzi C, Ravani P, Torres D, Prati E, Viola BF, Guerini S. et al. IgA nephropathy: The presence of familial disease does not confer an increased risk for progression. Am J Kidney Dis . 2006;47(5):761–9. doi: 10.1053/j.ajkd.2006.01.010. [DOI] [PubMed] [Google Scholar]


