Abstract
Background
Background: There is no information on the frequency and clinicopathological presentation of the variants of primary focal segmental glomerulosclerosis (FSGS) in adults presenting with idiopathic nephrotic syndrome (INS) in Pakistan.
Objectives
The aim of this study was to determine the frequencies of different histologic variants of primary FSGS with INS at our center and to compare our findings with those published in literature.
Patients and Methods
All consecutive adults (≥18 years) with INS, and diagnosis of FSGS on renal biopsies, were included. Their clinicopathological features at the time of presentation were retrieved and compared among the variants.
Results
There were 120 (65.2%) males and 64 (34.8%) females. The mean age was 30.62±12.02 years. The mean 24-hr urinary protein excretion was 4.69±2.36 grams. Microscopic hematuria was found in 30 (16.3%) patients. The mean serum creatinine was 1.58±0.87 mg/dL. At presentation, 128 (69.6%) patients were normotensive, while 56 (30.4%) exhibited hypertension. FSGS, not otherwise specified (NOS) was the predominant variant, comprising 76.6% of all; collapsing variant comprised 12%, tip variant, 9.8%, perihilar, 1.1%, and cellular, 0.5%. The mean number of glomeruli involved by segmental scarring was 3.41±2.87 and there was significant difference among the variants (p= 0.001). Arteriolopathy was found in 23.4 % cases and fibrointimal thickening of arteries in 18.5%. Tubular atrophy and interstitial fibrosis (IF/TA) was noted in 93% of cases. There was no significant difference in vasculopathy and IF/TA among the variants.
Conclusions
Collapsing variant was the second most common variant following NOS and these findings are different from other regional studies.
Keywords: Adults, Focal segmental glomerulosclerosis, Histological variants, Nephrotic syndrome, Pakistan
1. Background
The term idiopathic nephrotic syndrome (INS) refers to a distinct syndrome of clinical and laboratory findings of heavy proteinuria of ≥3.5 g/day, hypoalbuminemia, hyperlipidemia and generalized edema (1). It is one of the most common clinical presentations in both pediatric and adult nephrology practice. Focal segmental glomerulosclerosis (FSGS) has emerged as the predominant cause of primary glomerular diseases in adults presenting with INS during recent years (2).
FSGS is not a single disease, rather it represents a descriptive clinicopathological diagnosis characterized clinically in most cases by nephrotic range proteinuria and histologically by the presence of segmental sclerotic lesions involving some but not all of the glomeruli (3-5).
FSGS may be primary (idiopathic) or secondary to various etiologic agents and pathogenetic mechanisms (6). The prevalence of FSGS has increased significantly in the biopsy series in recent years through out the world (7-11). We have earlier reported a prevalence of 39.87% on native renal biopsies performed on adult nephrotic patients (12). Since the first description by Rich in 1957, several different histological variants of FSGS have been recognized (13-16). Recently, a group of nephropathologists proposed a standardized pathological classification system for FSGS based entirely on light microscopic (LM) examination, popularly known as Columbia classification (17,18). According to this classification, five histologic variants of FSGS are described; FSGS, not otherwise specified (NOS), perihilar variant, cellular variant, tip variant and collapsing variant.
There are several studies published in international literature on the characteristics of different histologic variants of FSGS (19-24). These studies have shown that the variants have different etiologies, pathogenetic mechanisms and diverse clinical behavior in terms of presentation, remission of proteinuria, progression of disease and therapeutic response (19-24). All the studies have reiterated the clinical importance of histologic variants of primary FSGS. It is also evident from above studies that the relative frequency of the variants of primary FSGS differs in different populations. The frequency of FSGS, NOS variant in published literature varies from 32% to 72.5%, perihilar variant from 4% to 26%, cellular variant from 3% to 25.5%, tip variant from 4.8% to 37% and collapsing variant from 2% to 24% (19-24). There is no study on the frequency and the clinicopathological presentation of FSGS variants in adult patients from Pakistan.
2. Objectives
This study was undertaken to determine the frequencies of different histologic variants of primary FSGS in adults presenting with INS at our center and to compare our findings with those published in literature. Moreover, we have also compared clinical parameters recorded at the time of presentation and histological parameters among these variants.
3. Patients and Methods
3.1. Patients
This study was conducted from January 2009 to July 2012 at the department of Histopathology, Sindh Institute of Urology and Transplantation (SIUT). All consecutive adult patients (≥18 years) who presented with INS at adult nephrology OPD, SIUT and in whom the diagnosis of FSGS was made on percutaneous ultrasound guided native renal biopsies, were included in the study.
3.2. Definition of FSGS
Standard definitions of FSGS and its variants were used (17,18). Briefly, FSGS was diagnosed when there was segmental collapse/scarring associated with hyalinosis involving part of/whole glomerulus with/without associated podocyte changes. Columbia classification of FSGS was used for the categorization of FSGS lesions, as illustrated in Figures 1 to 5 (18).
Figure 1.
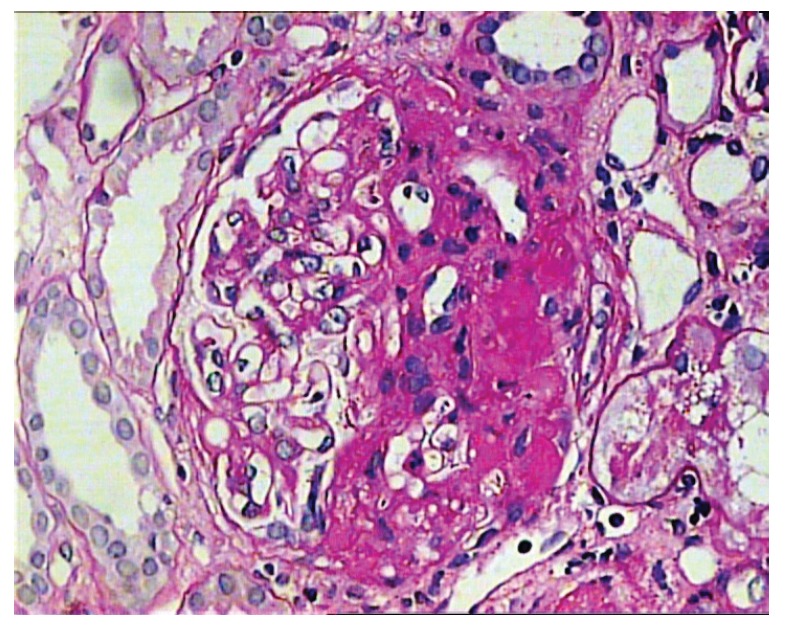
Glomerulus showing segmental sclerosis/hyalinosis involving almost half of the whole glomerulus. The lesion extends from the hilum to the tubular pole. The other half the glomerulus is unremarkable. (PAS, ×400).
Figure 2 .
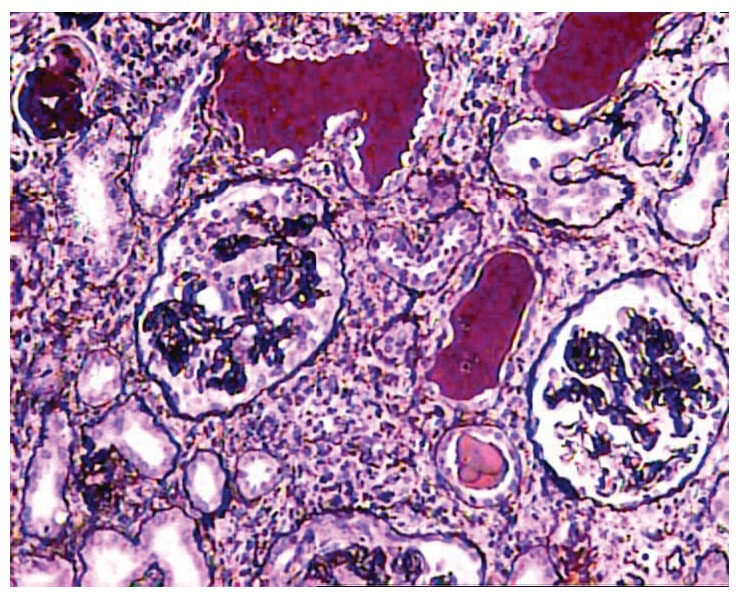
One of the glomeruli at the center of the field shows global collapse of capillary tufts associated with marked hypertrophy and hyperplasia of visceral epithelial cells in a case of collapsing FSGS. Surrounding parenchyma reveals moderate interstitial inflammation, tubular atrophy, and typical hyaline casts with scalloped edges in some of the tubular lumena (JMS, ×200).
Figure 3 .
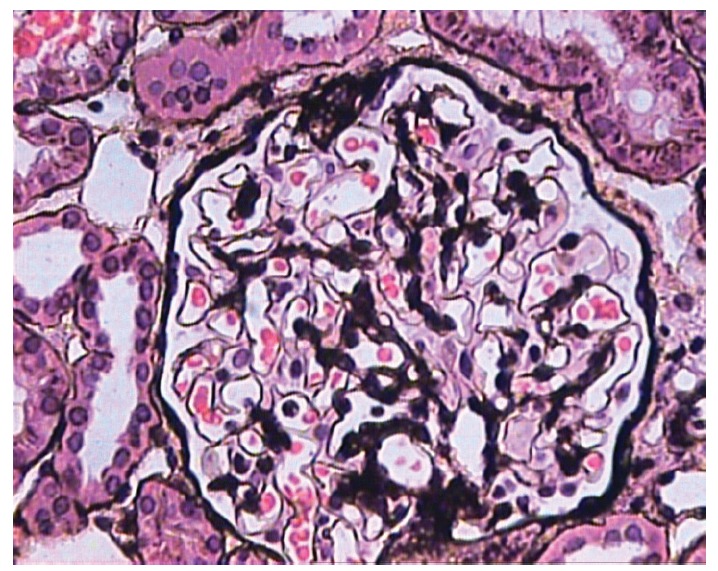
Glomerulus showing a small segmental scar involving the tubular pole of the glomerulus with adhesion formation with Bowman’s capsule. The lesion is quite subtle and can be easily missed if not diligently looked for. Rest of the glomerulus shows no significant morphological change. (JMS, ×400).
Figure 4 .
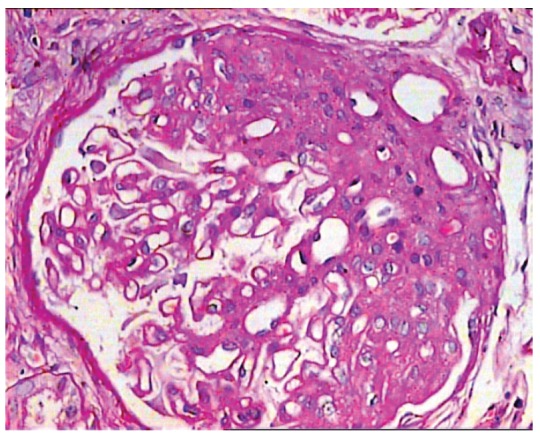
Glomerulus showing segmental sclerosis/hyalinosis involving the hilar pole of the glomerulus in a case of perihilar variant. This topographic involvement must be present in more than 50% of glomeruli to qualify for this designation. There is no podocyte hyperplasia or hypertrophy. There is mild arteriolar hyalinosis in one of the cross sections of the wall of the arterioles. (PAS, ×400).
Figure 5 .
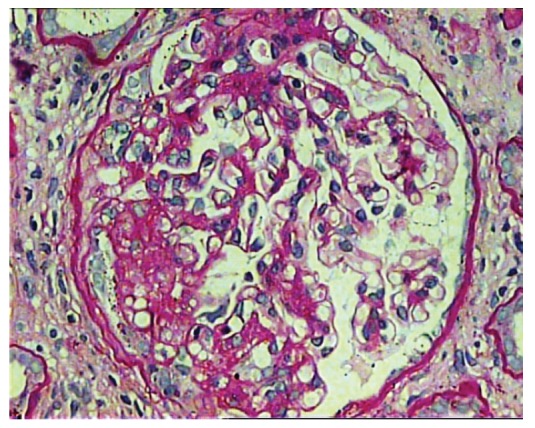
Glomerulus showing segmental occlusion of the capillary tufts by endocapillary hypercellularity. There is also mild podocyte hyperplasia and hypertrophy overlying area of endocapillary expansion. The lesion involves almost one third of the whole glomerulus. The lesion extends from the hilum to the tubular pole. The other half the glomerulus is unremarkable. (PAS, ×400).
The biopsies were studied by light microscopy (LM), immunoflourescence (IF) and electron microscopy (EM) as described in detail in our previous report (12). Cases of secondary FSGS, like lupus nephritis or IgA nephropathy (IgAN) were excluded. Written informed consent was taken from patients for the biopsy procedure and for inclusion in the study. Their clinical and laboratory parameters at the time of presentation were recorded from the case files. The histological features, IF findings and final diagnosis were recorded from the original biopsy forms.
3.3. Statistical analysis
Data were analyzed using the IBM compatible SPSS for Windows version 10 (SPSS, Chicago, IL, USA). Descriptive statistics such as mean ± standard deviation (SD) were used for continuous variables such as age and laboratory data. Numbers (%) were used to describe the proportion of categorical variables such as sex and the frequency of morphological variants. Statistical analysis was done using appropriate statistical tests, such as the chi-Square test, ANOVA tests, Mann-Whitney U tests, and Kruskal-Wallis tests. A p-value of ≤0.05 was considered to be significant.
4. Results
4.1. Patient characteristics
The demographic, clinical and laboratory characteristics are shown in Table 1. A total of 184 patients satisfied study criteria and were entered into the study. Of these, 120 (65.2%) were males and 64 (34.8%) were females, with a male to female ratio of 1.9:1. Their mean age was 30.62±12.02 years with a range of 18 to 70 years.
Table 1. Patient and clinical characteristics at the time of presentation in all patients and in different variants of FSGS.
| All patients (n=184) | NOS (n=141) | Collapsing (n=22) | Tip (n=18) | Perihilar (n=2) | Cellular (n=1) | P-value | ||
| Sex (M/F) | 120/64 | 92/49 | 13/9 | 13/5 | 1/1 | 1/0 | 0.82 | |
| Age at biopsy (years) | 30.6±12.02 | 30.3±11.73 | 32.3±13.8 | 31.0±12.7 | 21.5±3.5 | 43.0 | 0.61 | |
| Serum creatinine (mg/dl) | 1.58±0.87 | 1.52±0.87 | 2.3±1.39 | 1.20±0.5 | 1.4±0.14 | 2.60 | 0.002 | |
| Raised serum creatinine (%) | 47.8 | |||||||
| Proteinuria (g/24 h) | 4.69±2.36 | 4.77±2.53 | 4.42±1.95 | 4.31±1.38 | 5.05±1.06 | 5.60 | 0.89 | |
| Mean arterial blood pressure (mm/Hg) | 97.58±12.14 | |||||||
| Hypertension (%) | 30.4 | 28.4 | 50 | 11.1 | 0 | 0 | 0.006 | |
| Duration to biopsy (months) | ||||||||
| Mean±SD | 6.99±11.90 | 6.96±11.30 | 3.64±4.91 | 11.72±20.1 | 6.0 | 3.0 | 0.31 | |
| Range | (1-96) | (1-96) | (1-24) | (2-72) | (6-6) | 3 |
4.2. Clinical and laboratory findings on presentation
All 184 adult patients by inclusion criteria had nephrotic range proteinuria with mean 24-hr urinary protein excretion of 4.69±2.36 (range: 3-15) grams. Microscopic hematuria was found in 30 (16.3%) patients. The mean serum creatinine was 1.58±0.87 mg/dL (range: 0.43- 5.7 mg/dL). Among all patients, 128 (6.99%) were normotensive at the time of presentation, while 56 (30.4%) exhibited variable degrees of hypertension. The mean arterial pressure was 97.58±12.14 (range: 73.3-130) mmHg. Raised serum creatinine was found in 47.8% of the patients at the time of presentation. Duration of symptoms before biopsy was recorded in months with a median of 3 (mean: 6.99±11.9, range: 1-96) months. The clinical and laboratory parameters of all cases and different histologic variants are also shown in Table 1.
Table 1.
4.3. Pathological findings
The main histopathologic findings on renal biopsies from 184 patients are given in Table 2. Our results show that FSGS, NOS was the predominant variant, comprising 76.6% of all cases. Collapsing variant comprised 12%, tip variant 9.8%, perihilar 1.1%, and cellular 0.5%. The mean number of glomeruli included in all biopsies was 15.5±8.2. Global glomerulosclerosis was found in 45.5% of all cases with no statistically significant difference among the different variants of FSGS (p= 0.55). The mean number of glomeruli involved by segmental scarring was 3.41±2.87 and there was a statistically significant difference among the different variants (p= 0.001). Non-sclerotic glomeruli showed variable degrees of mesangial proliferation in 35.9% of cases, while 62.5 % showed minor changes. Arteriolopathy was found in 23.4 % cases and fibrointimal thickening of arteries (FITC) was noted in 18.5% of total cases. Tubular atrophy and interstitial fibrosis (IF/TA) paralleled each other and variable degrees of IF/TA were noted in 93% of all cases but there was no significant difference in the vasculopathy and IF/TA among the variants of FSGS in this cohort. IF study showed focal to diffuse mesangial positivity of IgM in 37.5% cases, associated with C3 deposition in 19% and C1q in 9% of cases, while IgG and IgA were negative in all cases. The detailed results of histopathological findings of each variant are given in table 2. Table 3 provides a comparison of the prevalence of different histological variants of FSGS in studies from different parts of the world. As is evident from this table, FSGS, NOS is the predominant type all over the world. We also noted a higher prevalence of collapsing variant as compared with other regional studies.
Table 2. Comparison of histological parameters of different morphological variants of FSGS.
| NOS | Collapsing | Tip | Perihilar | Cellular | Total | P value | |
| No of glomeruli (%) | 15.4±8.2 | 15.4±8.7 | 16.5±9.0 | 17±1.4 | 16.0 | 15.5±8.2 | 0.98 |
| Glomeruli sclerosed (%) | |||||||
| Globally sclerosed | 45.4 | 59.1 | 22.3 | 50 | 100 | 45.5 | 0.55 |
| Segmentally sclerosed | 3.42±2.91 | 4.09±2.43 | 1.78±0.94 | 10.5±4.95 | 3.0 | 3.41±2.87 | 0.001 |
| Mesangial proliferation (%) (in rest of the glomeruli) | |||||||
| Absent | 63.1 | 77.3 | 61.1 | 0 | 100 | 64.1 | 0.45 |
| Mild | 32.6 | 18.2 | 38.9 | 100 | 0 | 32.1 | |
| Moderate | 4.3 | 4.5 | 0 | 0 | 0 | 3.8 | |
| Severe | 0 | 0 | 0 | 0 | 0 | 0 | |
| Minor changes (%) (in rest of the glomeruli) | |||||||
| Absent | 38.3 | 27.3 | 38.9 | 100 | 0 | 37.5 | 0.29 |
| Present | 61.7 | 72.7 | 61.1 | 0 | 100 | 62.5 | |
| Vasculopathy in arterioles (%) | |||||||
| Absent | 76.6 | 68.2 | 88.9 | 100 | 0 | 76.6 | 0.42 |
| Arteriolar hyalinosis (%) | 17.7 | 27.3 | 11.1 | 0 | 100 | 17.7 | |
| Arteriolosclerosis (%) | 5.7 | 4.5 | 0 | 0 | 0 | 5.7 | |
| Fibrointimal thickening of arteries (%) | |||||||
| Absent | 81.6 | 72.7 | 94.4 | 50 | 100 | 81.6 | 0.45 |
| Mild | 14.2 | 27.3 | 5.6 | 50 | 0 | 14.2 | |
| Moderate | 4.3 | 0 | 0 | 0 | 0 | 4.3 | |
| Severe | 0 | 0 | 0 | 0 | 0 | 0 | |
| Tubular atrophy/interstitial fibrosis (%) | |||||||
| Absent | 6.4 | 9.1 | 11.1 | 0 | 0 | 7.1 | 0.50 |
| Mild | 58.9 | 45.5 | 77.8 | 0 | 100 | 58.7 | |
| Moderate | 31.2 | 40.9 | 11.1 | 100 | 0 | 31 | |
| Severe | 3.5 | 4.5 | 0 | 0 | 0 | 3.3 |
Table 3. Comparison of the prevalence of different histological variants of FSGS in different studies. All figures are in percentages (%).
| Present (Pakistani) | Nada et al. (Indian) | Deegens et al. (Dutch) | Shi et al. (Chinese) | Thomas et al. (Multiethnic) | Testagrossa et al. (Brazilian) | |
| FSGS-NOS | 76.6 | 72.5 | 55.9 | 32 | 42 | 38.2 |
| Collapsing | 12 | 2 | 6.9 | 5 | 11 | 36.6 |
| Tip | 9.8 | 13.5 | 4.8 | 37 | 17 | 14.5 |
| Perihilar | 1.1 | 4 | 6.9 | 26 | 26 | 6.9 |
| Cellular | 0.5 | 8 | 25.5 | 0 | 3 | 3.8 |
5. Discussion
This study is an important contribution to the literature on the histological variants of FSGS from this region of the world. We are cognizant that there are certain limitations in the study, such as its origin from a single center, and a cross-sectional analysis with no information on the treatment and the clinical outcome. Despite above shortcomings, we believe that this study provides important insights into the presenting clinicopathological features and prevalence of the different variants of FSGS in our population.
FSGS is one of the major causes of chronic renal diseases in both pediatric and adult patients presenting with INS world wide (11). Its treatment is still empirical, largely because the precise pathogenesis of the lesion is still unclear in majority of cases. Improved knowledge of the pathogenetic mechanisms will certainly be of help in the development of individualized and more efficient therapeutic approaches. The recognition of the histologic variants of FSGS, defined in Columbia classification, can be the first step in this direction in the case of primary FSGS (17,18). Several studies have been published in the literature, emphasizing the wide variation in the prevalence of these variants among different populations (19-24). It is also well established that certain histologic variants of FSGS are associated with variable presenting clinical features, poor clinical outcome, including rapid deterioration of renal functions and marked proteinuria (20).
The frequency of variants in our population is compared with Indian, Chinese, Dutch, multiethnic and Brazilian cohorts in table 3. It is obvious from our results that FSGS, NOS was the predominant variant among all in our population, and these results are compatible with most of the published studies, including Indian, Chinese, multiethnic and Brazilian groups. Collapsing was the second most common variant in our study, and there is wide variation in the reported prevalence of this variant among different studies, varying from 2% to 36.6% (20). The highest frequency was reported from Brazilian study, where it was the second most common variant after NOS variant (24). It is well known that there is a higher prevalence of collapsing variant in studies which have included African Americans and HIV patients. However, all patients included in our study did not belong to both of the above categories. Hence, these studies in different ethnic groups, including observations in the present study, reiterate the wide variations in the reported frequency of this variant. These results raise the possibility of various environmental, infectious and socioeconomic etiologic factors behind its pathogenesis (20).
The third most common variant in this study was the tip variant and these findings are almost similar to those observed in multiethnic study by Thomas et al. and Brazilian study (21,24). However, it was the predominant lesion among Dutch population and second most common variant among Indian population (19,20). Least reported frequency of tip variant is from Chinese population, where it comprised only 4.8% of cases (22). Tip variant is followed by perihilar and cellular variants in this study, accounting for 1.1% and 0.5% of all cases, respectively. There is also low prevalence of perihilar variant in studies from India, China and Brazil (20,22,24), whereas it comprised 26% of cases in the series by Deegan et al. (19) and Thomas et al. (21). These findings re-emphasize the fact that the study population affects the prevalence of various variants.
Cellular variant was the least common variant among our patients, comprising 0.5% of all cases. These findings are in concordance with those reported in multiethnic group and Brazilian studies (21,24). Deegan et al. have not reported this variant in Dutch population (19). Our findings on this variant are in disagreement with results from Indian and Chinese studies, where it comprised for 8 % and 25.5%, respectively (19,22). We have also compared the clinical parameters recorded at the time of presentation among all variants, and our results indicate that males were predominant in all groups. Among all the demographic and clinical parameters, serum creatinine and hypertension were significantly different among these variants (p= 0.002 and 0.006 respectively). Deegan et al. (19) also reported significant difference in serum creatinine among all variants, however no significant difference was noted in Indian cohort (20). Highest frequency of hematuria and hypertension were found in collapsing variant in 31.8% and 50% of cases respectively. These findings are similar to those observed in Indian cohort by Nada et al. (20), who have reported hypertension in 100% of cases of collapsing variant; however, highest frequency of hematuria was found in cellular variant. Although there are very few numbers of patients in cellular and perihilar variants in our study, but hypertension and hematuria was not observed in these variants.
In the present study, the mean duration of symptoms in months before biopsy was 6.99±11.90 months. There is variation in duration of presenting symptoms among these variants. The cellular and collapsing variants were biopsied the earliest, at 3.0 and 3.64±4.91 months, respectively, followed by perihilar and NOS variants, at 6.0 and 6.96±11.30, while the tip variant presented at a later stage, at 11.72±20.1 months, but this difference was not statistically significant (p=0.31). These findings are similar to those observed in Indian cohort, which showed that the cellular variant was biopsied the earliest, at 4.38±5.57 months, followed by collapsing variant at 10.75±16.88 months (20). In the series by Deegan et al. (19), collapsing lesion was biopsied the earliest followed by tip and NOS variants, however perihilar variant was found to present at later stage in Dutch and Chinese studies (19,22). Some studies did not find significant difference in pre-biopsy duration among these variants (21).
The detailed morphologic features of these variants are given in table 2. Among all the histologic features, number of glomeruli involved by segmental scarring were significantly different among all variants (p= 0.001). The lowest number of glomeruli was involved in the tip variant (1.78±0.94) and the highest number of glomeruli involved by segmental scarring was found in the perihilar variant (10.5±4.95). On the other hand, in the Indian cohort, no significant difference was found in the number of glomeruli involved by segmental scarring (20). In this study no significant difference was noted in the frequency of vasculopathy and IF /TA among these variants and these results are in concordance with the Indian cohort (20).
6. Conclusions
Columbia classification of idiopathic FSGS into five histologic variants provides insights into the distinct clinicopathologic characteristics of these variants among different populations and it should be further elaborated with detailed long term follow up studies in future. There is remarkable variation in the prevalence of these variants among different studies. In this cohort, collapsing variant was the second most common variant following NOS and these findings are different from other regional studies.
Conflict of interest
SS and MM designed and performed the research. SS and MM analyzed the data. JIK, NJ and EA wrote some parts of the manuscript. All authors contributed to the final preparation of the manuscript.
Conflict of interest
The authors declared no competing interests.
Funding/Support
None declared.
Acknowledgments
The authors would like to thank staffs of Histopathology Department of Sindh Institute of Urology and Transplantation.
Implication for health policy/practice/research/medical education:
Focal segmental glomerulosclerosis (FSGS) is a heterogeneous lesion with different etiologies, pathogenesis and clinical outcome. Recent classification of FSGS on histological grounds into five variants reflects this heterogeneity of the lesion. A study of these variants in different populations is of utmost importance in understanding the varied etiopathogenetic mechanisms underlying this common lesion in nephrology practice.
Please cite this paper as: Shakeel Sh, Mubarak M, Kazi JI, Jafry N, Ahmed E. Frequency and clinicopathological characteristics of variants of primary focal segmental glomerulosclerosis in adults presenting with nephrotic syndrome. J Nephropathology. 2013; 2(1): 28-35. DOI: 10.5812/nephropathol.8959
References
- 1.Orth SR, Ritz E. The nephrotic syndrome. N Engl J Med . 1998;338:1202–11. doi: 10.1056/NEJM199804233381707. [DOI] [PubMed] [Google Scholar]
- 2.Deegens JKJ, Steenbegen EJ, Wetzels JFM. Review on diagnosis and treatment of focal segmental glomerulosclerosis. Neth J Med . 2008;66:3–12. [PubMed] [Google Scholar]
- 3.D’Agati V. The many masks of focal segmental glomerulosclerosis. Kidney Int . 1994;46:1223–41. doi: 10.1038/ki.1994.388. [DOI] [PubMed] [Google Scholar]
- 4.Cameron JS. The enigma of focal segmental glomerulosclerosis. Kidney Int . 1996;50:S119–S131. [PubMed] [Google Scholar]
- 5.Korbet SM. Primary focal segmental glomerulosclerosis. J Am Soc Nephrol . 1998;9:1333. doi: 10.1681/ASN.V971333. [DOI] [PubMed] [Google Scholar]
- 6.Meyrier A. E pluribus unum: the riddle of focal-segmental glomerulosclerosis. Semin Nephrol . 2003;23:135–40. doi: 10.1053/snep.2003.50013. [DOI] [PubMed] [Google Scholar]
- 7.Haas M, Spargo BH, Coventry S. Increasing incidence of focal segmental glomerulosclerosis among adult nephropathies: a 20-years renal biopsy study. Am J Kidney Dis . 1995;26:740–50. doi: 10.1016/0272-6386(95)90437-9. [DOI] [PubMed] [Google Scholar]
- 8.Haas M, Meehan SM, Karrison TG, Spargo BH. Changing etiologies of unexplained adult nephrotic syndrome: a comparison of renal biopsy findings from 1976-1979 and 1995-1997. Am J Kidney Dis . 1997;30:621–31. doi: 10.1016/s0272-6386(97)90485-6. [DOI] [PubMed] [Google Scholar]
- 9.Korbet SM, Schwartz MM, Lewis EJ. Primary focal segmental glomerulosclerosis: clinical course and response to therapy. Am J Kidney Dis . 1994;23:773–83. doi: 10.1016/s0272-6386(12)80128-4. [DOI] [PubMed] [Google Scholar]
- 10.Rydel JJ, Korbet SM, Borok RZ, Schwartz MM. Focal segmental glomerular sclerosis in adults: presentation, course, and response to treatment. Am J Kidney Dis . 1995;25:534–42. doi: 10.1016/0272-6386(95)90120-5. [DOI] [PubMed] [Google Scholar]
- 11.Kitiyakara C, Kopp JB, Eggers P. Trends in the epidemiology of focal segmental glomerulosclerosis. Sem Nephrol . 2003;23:172–82. doi: 10.1053/snep.2003.50025. [DOI] [PubMed] [Google Scholar]
- 12.Kazi JI, Mubarak M, Ahmed E, Akhter F, Naqvi SA, Rizvi SA. Spectrum of glomerulonephritides in adults with nephrotic syndrome in Pakistan. Clin Exp Nephrol . 2009;13(1):38–43. doi: 10.1007/s10157-008-0075-0. [DOI] [PubMed] [Google Scholar]
- 13.Chun MJ, Korbet SM, Schwartz MM, Lewis EJ. Focal segmental glomerulosclerosis in nephrotic adults: presentation, prognosis, and response to therapy of the histologic variants. J Am Soc Nephrol . 2004;15:2169–77. doi: 10.1097/01.ASN.0000135051.62500.97. [DOI] [PubMed] [Google Scholar]
- 14.Korbet SM. Clinical picture and outcome of primary focal segmental glomerulosclerosis . Nephrol Dial Transplant. 1999;14:68–73. doi: 10.1093/ndt/14.suppl_3.68. [DOI] [PubMed] [Google Scholar]
- 15.Cameron JS. Focal segmental glomerulosclerosis in adults. Nephrol Dial Transplant . 2003;18:vi45–vi51. doi: 10.1093/ndt/gfg1058. [DOI] [PubMed] [Google Scholar]
- 16.Meyrier A. Nephrotic focal-segmental glomerulosclerosis in 2004: an update. Nephrol Dial Transplant . 2004;19:2437–44. doi: 10.1093/ndt/gfh320. [DOI] [PubMed] [Google Scholar]
- 17.D’Agati V. Pathologic classification of focal segmental glomerulosclerosis. Sem Nephrol . 2003;23:117–34. doi: 10.1053/snep.2003.50012. [DOI] [PubMed] [Google Scholar]
- 18.D’Agati V, Fogo AB, Bruijin JA, Jennette JC. Pathologic classification of focal segmental glomerulosclerosis: a working proposal. Am J Kidney Dis . 2004;43:368–82. doi: 10.1053/j.ajkd.2003.10.024. [DOI] [PubMed] [Google Scholar]
- 19.Deegens JK, Steenbergen EJ, Borm EF, Wetzels JFM. Pathological variants of focal segmental glomerulosclerosis in an adult Dutch population-epidemiology and outcome. Nephrol Dial Transplant . 2008;23:186–92. doi: 10.1093/ndt/gfm523. [DOI] [PubMed] [Google Scholar]
- 20.Nada R, Kharbanda JK, Bhatti A, Minz RW, Sakhuja V, Joshi K. Primary focal segmental glomerulosclerosis in adults: is the Indian cohort different? Nephrol Dial Transplant . 2009;24:3701–7. doi: 10.1093/ndt/gfp328. [DOI] [PubMed] [Google Scholar]
- 21.Thomas DB, Franceschini N, Hogan SL, Ten Holder S, Jennette CE, Falk RJ. et al. Clinical and pathologic characteristics of focal segmental glomerulosclerosis pathologic variants. Kidney Int . 2006;69:920–6. doi: 10.1038/sj.ki.5000160. [DOI] [PubMed] [Google Scholar]
- 22.Shi SF, Wang SX, Zhang YK, Zhao MH, Zou WZ. Clinicopathologic study of different variants of focal segmental glomerulosclerosis. Zhonghua Bing Li Xue Za Zhi . 2007;36:11–4. [PubMed] [Google Scholar]
- 23.Taneda S, Honda K, Uchida K, Nitta K, Yumura W, Oda H. et al. Histological heterogeneity of glomerular segmental lesions in focal segmental glomerulosclerosis. Int Urol Nephrol . 2012;44:183–96. doi: 10.1007/s11255-011-9932-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Testagrossa LA, Malheiros DM. Study of the morphologic variants of focal segmental glomerulosclerosis: a Brazilian report. J Bras Pathol Med Lab . 2012;48:211–5. [Google Scholar]


