Abstract
Context
Vasculitis is a clinicopathological entity characterized by inflammation and necrosis of blood vessels.
Evidence Acquisitions
Directory of Open Access Journals (DOAJ), Google Scholar, Pubmed (NLM), LISTA (EBSCO) and Web of Science have been searched.
Results
Two major autoantigens for ANCA are myeloperoxidase (MPO) and proteinase 3 (PR3), which are proteins in the primary granules of neutrophils and in the lysosomes of monocytes. They are expressed in mature neutrophils of patients with ANCA, while absent in healthy subjects.
Conclusions
The kidney is the most commonly affected vital organ in ANCA-associated vasculitis, and patient outcomes are largely determined by the severity of renal disease at diagnosis and by its response to treatment.
Keywords: Vasculitis, Antineutrophil cytoplasmatic autoantibody, Crescentic glomerulonephritis, Chapel Hill Consensus Conference classification, ANCA-associated small-vessel vasculitis
1. Context
Vasculitis is a clinicopathological entity characterized by inflammation and necrosis of blood vessels. Antineutrophil cytoplasmatic autoantibodies (ANCA) are associated with a group of necrotizing small vessel vasculitides that have a paucity (pauci = few/little) of vascular deposition of immunoglobulin and complement (1,2). Medium vessels may occasionally also be involved.
2. Evidence Acquisition
Directory of Open Access Journals (DOAJ) Google Scholar, Pubmed (NLM), LISTA (EBSCO) and Web of Science were searched with key words relevant to Vasculitis, Antineutrophil cytoplasmatic autoantibody, Crescentic glomerulonephritis, Chapel Hill Consensus Conference classification and ANCA-associated small-vessel vasculitis.
3. Results
42 research and review articles relevant to this topic directly or indirectly have been found. From the information given in these papers, the following aspects were drawn out.
Two major autoantigens for ANCA are myeloperoxidase (MPO) and proteinase 3 (PR3), which are proteins in the primary granules of neutrophils and in the lysosomes of monocytes (2,3). They are expressed in mature neutrophils of patients with ANCA, while absent in healthy subjects. In spite of the fact that pauci-immune ANCA diseases have scanty immunoglobulin deposits in blood vessel walls, there is evidence supporting a pathogenic role for ANCA (2,3). When immunofluorescence on renal biopsy material demonstrates absence of or scanty immune deposition in the glomerulus, and when there are circulating ANCA, a diagnosis of pauci-immune ANCA-associated renal vasculitis is made. In these cases, necrotising crescentic glomerulonephritis (GN) is usually found on light microscopy. Clinical symptoms of systemic vasculitis could also be present. According to The Chapel Hill Consensus Conference classification (Table 1), the group of systemic small vessel vasculitides associated with ANCA include: microscopic polyangiitis (MPA), Wegener’s granulomatosis (WG), and Churg–Strauss syndrome (4).
Table 1. The Chapel Hill Consensus Conference classification of vasculitis (4).
|
Large blood vessels vasculitis Takayasu arteritis Temporal arteritis |
|
Medium-sized blood vessels vasculitis Polyarteritis nodosa Kawasaki disease Central nervous system isolated angiitis |
|
Small blood vessels vasculitis ANCA-associated small-vessel vasculitis: Microscopic polyangiitis, Wegener’s granulomatosis, Churg–Strauss syndrome, Drug-induced ANCA-associated vasculitis Immune-complex small-vessel vasculitis Henoch–Schönlein purpura Cryoglobulinemic vasculitis Lupus vasculitis Rheumatoid vasculitis Sjögren’s syndrome vasculitis Hypocomplementemic urticarial vasculitis Behçet’s disease Goodpasture’s syndrome Serum-sickness vasculitis Drug-induced immune-complex vasculitis Infection-induced immune-complex vasculitis Paraneoplastic small-vessel vasculitis Lymphoproliferative neoplasm-induced vasculitis Myeloproliferative neoplasm-induced vasculitis Carcinoma-induced vasculitis Inflammatory bowel disease vasculitis |
3.1. Pathogenesis of ANCA-associated vasculitis
ANCA directed to proteinase 3 (PR3-ANCA) or myeloperoxidase (MPO-ANCA) are strongly associated with the small blood vessels vasculitides: MPA, WG and Churg-Strauss syndrome (Table 2).
Table 2. The prevalence of antineutrophil cytoplasmatic autoantibodies (ANCA) in small blood vessels vasculitis (5).
| VASCULITIS |
PR3-ANCAa
(C-ANCA , % ) |
MPO-ANCAb
(P-ANCA, %) |
NEGATIVE
ANCA |
| Wegener’s granulomatosis | 75 | 20 | 5 |
| Microscopic polyangiitis | 40 | 50 | 10 |
| Churg-Strauss syndrome | 10 | 60 | 30 |
| Microscopic polyangiitis limited to kidney | 20 | 70 | 10 |
a PR3 = proteinase 3; b MPO=myeloperoxidase
Most patients (70–80%) with WG are positive for PR3-ANCA, and few (10%) are positive for MPO-ANCA (Table 2). Conversely, the majority of patients with MPA (60%) are positive for MPO-ANCA, and fewer (30%) are positive for PR3-ANCA (5). Clinical relapses coincide in many cases with the increase in levels of these autoantibodies. Despite a relative predominance of antigenic specificity for each of the diseases, neither ANCA subtype differentiates between the three most common phenotypes of small blood vessels necrotizing vasculitis. A few patients (10%) with either WG or MPA are ANCA-negative (6).
In WG, these ANCA-negative patients mostly have localized disease, although some of them might develop ANCA once the disease progresses into a generalized form. In Churg–Strauss syndrome, only 50-65% of patients are MPO-ANCA-positive. In vitro, ANCA can further activate neutrophils to release reactive oxygen species and lytic enzymes, and, in conjunction with neutrophils, can damage and lyse endothelial cells. Patients with Wegener’s granulomatosis or microscopic polyangitis have an increased percentage of neutrophils that constitutively express PR3 on their membranes (7). These neutrophils can be stimulated by ANCA, without priming. Together, clinical, in vitro and in vivo experimental data support a pathogenic role for ANCA in WG and MPA, although this role is more evident for MPO-ANCA than for PR3-ANCA (8,9). Clinical observations, including the efficacy of B-cell depletion via rituximab treatment, support, but do not prove, a pathogenic role for ANCA in the small blood vessels vasculitides. In vitro experimental studies show that the interplay of ANCA, neutrophils, the alternative pathway of the complement system, and endothelial cells could result in lysis of the endothelium (9). Several animal models of ANCA disease have been described that are pathologically identical to human ANCA disease (10,11). Finally, microbial factors, in particular Staphylococcus aureus and gram-negative bacteria, seem to be involved in disease induction and expression, but further studies are needed to define their precise role in disease development (11).
Pauci-immune necrotizing and crescentic GN is a frequent component of ANCA disease. The pathological and immunological features of GN are similar in different clinicopathologic variants of ANCA diseases, including granulomatosis with polyangiitis (WG), MPA, Churg-Strauss syndrome and renal-limited pauci-immune necrotising crescenting glomerulonephritis (Table 3, ref 12,13).
Table 3. The renal changes in patients with vasculitis.
| VASCULITIS | PATIENTS WITH RENAL DISEASE (%) | GLOMERULONEPHRITIS | RENAL VESSELS LESIONS (%) |
| Microscopic polyangiitis | 80-90 | present | 15-35 |
| Wegener’s granulomatosis | 75-85 | present | 8 |
| Churg-Strauss syndrome | 25-40 | present | rare |
3.2. Renal features
The kidney is the most commonly affected vital organ in ANCA-associated vasculitis, and patient outcomes are largely determined by the severity of renal disease at diagnosis and by its response to treatment. In an elderly patient, ANCA-associated renal vasculitis is the most common cause of acute renal failure with normal sized kidneys without an obvious precipitant (1,2). The manifestations of ANCA disease can be limited to the kidney alone, or may involve upper respiratory tract, the lungs, the skin, or a number of other organs in various combinations (12,13).
ANCA-associated pauciimmune glomerulonephritis is more common cause of rapidly progressive glomerulonephritis and death as compared with immune complex rapidly progressive glomerulonephritis or anti-glomerular basement membrane (GBM) disease (14,15). Rapidly progressive glomerulonephritis is the most frequent clinical manifestation of ANCA-associated glomerulonephritis, although the syndromes of asymptomatic hematuria with minimal amounts of proteinuria or acute nephritis are possible as well. Renal disease is manifested by hematuria with dysmorphic red blood cells and red blood cells casts in urine sediment, while proteinuria is usually moderate (2–3 g/day). At least 50% of patients with ANCA-associated glomerulonephritis have pulmonary disease. Massive pulmonary hemorrhage affects about 10% of patients with ANCA glomerulonephritis, and is associated with an elevated risk of death (16). Constitutional signs and symptoms, such as fever, myalgias, arthralgias, and malaise, often accompany small-vessel vasculitis. Many patients describe a “flulike” syndrome early in the course of their disease. Arthralgias are migratory and affect both small and large joints, with evidence of synovitis in small number of patients. Vessels in the skin, respiratory tract, kidneys, gut, peripheral nerves, and skeletal muscle are often involved, but the frequencies vary among different phenotypes of small blood vessel vasculitis (2,7).
The typical renal biopsy feature in ANCA-associated renal vasculitis is pauci-immune necrotizing glomerulonephritis with crescents formation. The lesions are focal or diffuse, with scanty or absent glomerular staining for immunoglobulin by immunofluorescence microscopy (17). The extensive histologic analysis of MPO-ANCA positive, PR3-ANCA positive, and ANCA-negative kidney biopsies showed that there was no significant difference in the main glomerular lesions, tubular as well as interstitial histologic features between these three groups (1,2,3). By definition, in ANCA-associated renal vasculitis, immune deposits are absent or scanty and scattered, hence the term pauci-immune glomerulonephritis. Instead, the fibrinogen antiserum strongly stains areas of necrosis of the tuft and fibrin deposits into the crescents (Figure 1). A focal form of crescentic GN is reported more frequent than diffuse in ANCA-associated renal vasculitis. On electron microscopy examination absence of or a few electron-dense immune deposits is the main finding. Some 5% of ANCA-associated renal vasculitis patients present with simultaneous renal vasculitis and anti-GBM disease. These patients are older, have more severe renal disease and are more likely to have pulmonary involvement than just ANCA-associated renal vasculitis.
Figure 1.
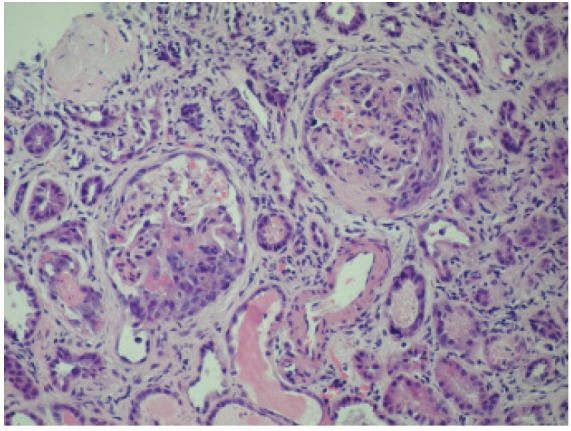
Necrotizing ANCA glomerulonephritis with cellular and fibrocellular crescents (microscopic polyangiitis).
A glomerulus shows segmental necrosis with small cellular and fibrocellular crescent formation. The non-necrotic segments are histologically unremarkable. There is interstitial inflammation, including tubulitis (PAS, x400).
In vasculitis, the Birmingham Vasculitis Activity Score (BVAS) (18) and other similar scores have been applied to assess disease activity, whereas the Vasculitis Damage Index (DVI, 19) provides information on disease damage. The BVAS has also been shown to have prognostic value, at least in short to medium term mortality, which makes clinical sense (18).
3.2.1. Microscopic polyangiitis (MPA)
Microscopic polyangiitis (MPA) is a necrotizing angiitis involving capillaries, venules, and arterioles of one or more organs either simultaneously or at different times, including the kidneys, lungs, skin, spleen, liver, heart, and muscle (2,7,15).
Typically, there are MPO-ANCA (Table 1) and it shows similar organ involvement as WG, but the symptoms of the upper respiratory tract are usually milder in MPA. In MPA there is no granulomatous inflammation, which is usually present in WG. The recurrence is rare in patients with MPA.
3.2. 2.Renal limited MPA
The presence of only general systemic symptoms such as fever, with renal features, is highly suggestive of renal limited vasculitis. In this disease there is no respiratory disease or any other organ involvement.
3.2.3. Granulomatosis with polyangiitis (WG)
Granulomatosis with polyangiitis has been recently accepted as an alternative name for WG (5). WG is a necrotizing vasculitis of the small and medium-sized vessels, associated with granulomatous inflammation of upper and lower respiratory tract and the frequent finding of glomerulonephritis. In active disease in about 90% of cases PR3-ANCA are found (Table 1). The risk of relapse is not uniform among all patients with ANCA vasculitis. An increased risk of relapse has been associated with a diagnosis of WG (as opposed to MPA). Relapses in the kidney are heralded by the recurrence of microscopic hematuria, red blood cell casts, and worsening renal function. Fluctuations in the amount of proteinuria are not good indicators of active disease, and are related to glomerulosclerosis.
3.2.4. Churg-Strauss syndrome
Rarely presenting as rapidly progressive glomerulonephritis, Churg-Strauss syndrome is diagnosed when asthma, allergic rhinitis and increased circulating eosinophils are present. MPO-ANCA can be detected in up to 60% of cases (Table 1) Renal involvement is seen in approximately 25% of patients. (5,7). Depending on the timing of renal biopsy, glomeruli can be affected by active lesions or by more sclerotic alterations. It is not infrequent to observe both types of lesions in the same renal biopsy and even in the same glomerulus.
3.3. Treatment
Treatment for vasculitis requires induction of remission and remission maintenance therapy (Table 4).
Table 4. Induction of remission therapy in ANCA-associates vasculitis.
| INDUCTION OF REMISSION |
| 1. Glucorticoids – 3 intravenous pulses (0.5-1g/day) followed by 1mg/kg/day orally for 3-6 months |
| 2. Cyclophosphamide – 1-2mg/kg/day orally for 3-6 months |
| 3. Plasmapheresis – in patients presenting with pulmonary hemorrhage (A) and/or severe renal disfunction (B) |
When the natural history of WG was described in 1958, it was usually a fatal disease without effective treatment and patient survival after diagnosis averaged 5 months. The introduction of glucocorticosteroids (GC) in the 1960s extended average survival only by 8 months (5). This changed radically when Fauci and Wolff pioneered the use of cyclophosphamide in the early 1970s (20). The administration of daily oral cyclophosphamide (1-2 mg/kg) and prednisone (1mg/kg) resulted in a dramatic clinical benefit. Prednisone was tapered and discontinued within 6 to 9 months, while cyclophosphamide was maintained at least for a year.
3.4.1. Induction therapy
The treatment of renal vasculitis involves the use of high dose GC in combination with cyclophosphamide to induce remission of disease (21,22). This treatment is the “standard of care“for renal vasculitis. The duration of induction therapy is 3-6 months (figure 2). In the presence of kidney failure, plasma exchange (plasmapheresis) is often used in addition to pharmacological treatment (16,21,22). Once remission is achieved, treatment is scaled back to maintenance therapy with lower doses of GC, while cyclophosphamide is replaced by a less toxic immunosuppressant, such as azathioprine.
Figure 2.
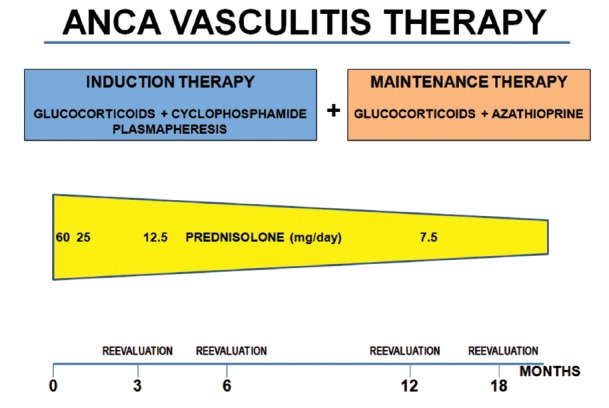
Treatment of ANCA-associated vasculitis.
Daily oral cyclophosphamide plus GC substantially advanced the treatment of generalized WG and remains the gold standard therapy. Cyclophosphamide can be given as a daily oral dose or as intermittent oral or intravenous pulses and is continued for 3-6 months. Intravenous regimens tend to give a lower total dose and have fewer side effects, but may later give a higher rate of relapse (23). In patients receiving intravenous cyclophosphamide pulse therapy, antiemetic drugs must be given immediately before and some hours after treatment. At least 3 liters of fluid must be administered on the day of cyclophosphamide treatment (21,22). The cyclophosphamide dose has to be adjusted to the peripheral leukocyte count, which is at a minimum of 3,000/μl. Studies have found remission rates between 70% and 100% and early mortality rates of less than 10% with increased treatment-related morbidity (21,22). Therefore, research centered on searching for equally effective but safer treatments, including changing the route of administration and dosage of cyclophosphamide and testing a monthly intravenous regimen. A few nonrandomized studies demonstrated similar rates of remission induction with intermittent intravenous cyclophosphamide as those using the daily oral drug (21). The advantage of the intravenous route is the smaller cumulative dosage and, therefore, fewer adverse events. Three randomized trials have compared the effectiveness and security profile of pulsed cyclophosphamide with daily oral administration for remission induction (20,23,24). A meta-analysis that summarized the results from these trials concluded that pulsed cyclophosphamide is as effective as daily oral cyclophosphamide with much less severe toxic effects, yet possibly with a higher relapse rate (21).
The European Vasculitis Study Group (EUVAS) devised a randomized trial comparing the efficacy of oral cyclophosphamide (2 mg/kg per day) with intravenous pulsed cyclophosphamide (15 mg/kg every 2 weeks for the first 3 pulses and every 3 weeks thereafter) with the same GC regimen in both groups, (CYCLOPS study). The study included 149 patients with generalized ANCA-associated vasculitis with renal involvement with vital organ manifestations. Preliminary results show that pulsed cyclophosphamide is equally effective as oral cyclophosphamide for remission induction (25). Unexpectedly, there were no differences in severe adverse events or deaths between groups.
Patients receiving pulse cyclophosphamide should also be given oral or intravenous 2-mercaptoethanesulfonate sodium (mesna) which binds to acrolein, a toxic metabolite of cyclophosphamide, making it less toxic for urothelial epitelium. Mesna may also be beneficial in patients receiving continuous oral cyclophosphamide. Determining the optimum mode (oral versus intravenous) and duration of treatment with cyclophosphamide depends on several factors and considerations. The short and long-term complications associated with the use of cyclophosphamide are commensurate with the cumulative dose received. In general, the intravenous regimen allows for a two to three times smaller total dose of cyclophosphamide than the oral regimen. In prospective and retrospective analyses, intravenous therapy was associated with a significant decrease in the rate of clinically significant neutropenia and other complications. The optimum length of therapy with cyclophosphamide has not been determined and is the subject of ongoing discussion. GC and cyclophosphamide based treatements have a long-term remission rate of between 60% and 92%. In patients achieving complete remission within 6 months of therapy, treatment can be stopped with the institution of close patient follow-up. In those individuals with persistently active disease at 6 months, it is reasonable to continue cyclophosphamide therapy for a full 12 months. An alternative regimen consists of switching cyclophosphamide to oral azathioprine at the end of 3 months if the patient is in remission. Azathioprine is then continued for 18 months. This regimen offers the advantage of a limited use of cyclophosphamide and results in rates of remission and relapses similar to the cyclophosphamide only-based therapies.
Patients presenting with pulmonary hemorrhage benefit also from the institution of plasmapheresis in a regimen similar to that used for patients with Goodpasture’s disease or anti-GBM disease (16). Plasmapheresis is typically performed daily until the pulmonary hemorrhage ceases and then every other day for a total of seven to ten treatments. The addition of plasma exchange to cyclophosphamide and GC also improves the chances of renal function recovery in patients with severe renal dysfunction (serum creatinine≥500μmol/L) or needing dialysis at presentation. For patients without severe renal failure or without pulmonary hemorrhage, controlled and observational trials have not demonstrated a beneficial role of the addition of plasmapheresis to cyclophosphamide and GC (16,26,27,28).
Other therapies, like the inhibitors of lymphocyte proliferation mycophenolatic acid salts (mycophenolate mofetil or sodium mycophenolate) and leflunomide, the B cell-depleting chimeric antibody rituximab, methotrexate, anti-CD52 therapy and intravenous immunoglobulin have been suggested but their place is not yet clear.
Methotrexate has been evaluated as an alternative to cyclophosphamide for the induction of remission in a randomized, controlled trial that revealed a remission rate at 6 months of 89.8% in patients receiving methotrexate, which was not inferior to that in patients treated with cyclophosphamide, which was 93.5% (29). Relapse rates at 18 months were significantly more frequent in the methotrexate group (69.5%) than in the cyclophosphamide group (46.5%, p=0.023). The dose of methotrexate should be decreased by 50% in patients with renal insufficiency and its use is contraindicated if the creatinine clearence is < 10mL/minute. Trimethoprim-sulfamethoxazole (TMP-SMX) for prophylaxis against Pneumocystis jiroveci (carinii) pneumonia cannot be used concomitantly with methotrexate.
Two randomized controlled trials examined rituximab as a first-line induction therapy for ANCA vasculitis (RITUXIVAS and RAVE trial; 30,31). Rituximab showed equivalent efficacy to cyclophosphamide in induction therapy and the evidence didn’t show any difference in rates of adverse effects. Rituximab can be recommanded for remission induction when cyclophosphamide is contraindicated (for example, in infectioous disease, intolerance or strong desire to avoid its use).
3.3.3.Practical recommendations
Pulse cyclophosphamide with oral GC can be used to induce remission in patients with generalized organ-threatening disease. Patients should be started with 1mg/kg per day of oral prednisone, and tapered progressively over 3–4 months plus intravenous pulse cyclophosphamide (every 3 weeks for 6 months). Intravenous methylprednisolone (0.5 to 1.0 g/day for 3 days) can be considered in the beginning of the treatement. Prednisone should be tapered to 10 mg at 6 months and maintained at this dose until month 15, when it should be tapered to 7.5mg and maintained for at least 3 more months followed by local practice. Cyclophosphamide doses should be adjusted by age, renal function, and leukocyte count.
Patients presenting with pulmonary hemorrhage and severe renal failure also benefit from the institution of plasmapheresis.
TMP-SMX has been used in WG mainly to prevent the occurrence of Pneumocystis jiroveci (carinii) infection, upper respiratory tract infection and subsequent relapse of disease (21,22). Rituximab shows equivalent efficacy to cyclophosphamide in induction therapy and may be superior in relapsing disease.
3.3.2.Maintenance therapy
The goal of the maintenance therapy is the prevention of disease relapses. Disease relapses occur in 75% of WG and in 50% of MPA cases by 5 years (Table 5). The combination of azathioprine and daily prednisone effectively maintains remission. Azathioprine has equivalent efficacy as a maintenance agent to cyclophosphamide with fewer episodes of leukopenia. Remission maintenance therapy should be continued for at least 2 years especially in WG (21,22). Two milligrams per kilogram of azathioprine should be started when cyclophosphamide is discontinued and after 6 months it should be reduced to 1.5 mg/kg per day and maintained for at least 6 more months. The role of azathioprine in the maintenance therapy in patients with ANCA vasculitis was examined as an alternative to a prolonged course of cyclophosphamide (32). Patients were treated with standard induction therapy of cyclophosphamide 2mg/kg/day and prednisolone 1mg/kg/day. At remission, 144 patients were randomised to either azathioprine 2mg/kg/day and prednisolone 10mg/day or continued cyclophosphamide 1.5mg/kg/day until 12 months, after which all patients were treated with azathioprine 1.5mg/kg/day and prednisolone 7.5mg/day. At the end of 12 months of follow-up, the risk of relapse was not significantly different between the two groups and there was not a significant difference in the rate of adverse events, except that leukopenia was significantly less likely with azathioprine. This was not reflected in an increase in infectious complications in patients on cyclophosphamide. Azathioprine allergy or intolerance occurs in 5-10% and testing for thiopurine s-methyltransferase activity identifies rare patients at risk of severe myelosupression. Hypersensitivity reaction occurs within 2-3 weeks of commencing azathioprine therapy.
Table 5. Maintenance therapy of ANCA-associated vasculitis.
| MAINTENANCE THERAPY |
| 1. Glucocorticoids – prednisolone 40-60mg/day orally, with dose tapering for at least 12 (usually 24) months |
| 2. Azathioprine – 2mg/kg/day bid; should be started when cyclophosphamide is discontinued; after 6 months reduced to 1.5mg/kg/day bid and maintained for 6 months more |
Several other agents have been evaluated in a limited way as adjunctive or maintenance therapy for ANCA vasculitis. These include the inhibitors of lymphocyte proliferation mycophenolate acid salts and leflunomide, the B cell-depleting chimeric antibody rituximab, blockers of the tumor necrosis factor (TNF)-α pathway infliximab and etanercept. The possible role of etanercept for the maintenance of remission was evaluated in a randomized, placebo-controlled trial of 180 patients in the Wegener’s Granulomatosis Etanercept Trial (WGET) (33). In addition to etanercept or placebo, patients received standard therapy with glucocorticoids plus cyclophosphamide or methotrexate. After 3–6 months of cyclophosphamide, patients in remission discontinued cyclophosphamide and were treated with etanercept and either methotrexate or azathioprine (depending on their degree of renal insufficiency). Over a mean follow up of 27 months, 72% of patients attained a sustained remission, but only 50% of patients remained in remission for the remainder of the trial. There were no significant differences between the etanercept and control groups in the rates of sustained remission, in the relative risk of disease flares per 100 person-years of follow-up, or in the severity of relapses. During the study, 56.2% of patients in the etanercept group and 57.1% of those in the control group had at least one severe or life threatening adverse event or died. Solid cancers developed in six patients in the etanercept group, as compared with none in the control group (P=0.01). It was concluded that etanercept was not effective for the maintenance of remission in patients with WG (33).
Whether methotrexate is effective in preventing relapses after induction therapy with cyclophosphamide has also been evaluated in open-label, multicenter trial (34). 159 eligible patients who entered remission received azathioprine or methotrexate. The two agents appear to be similar alternatives for maintenance thrapy in patients with WG and MPA after initial remission (34). Leflunomide has been shown to be superior to methotrexate for the prevention of relapse in WG (35) and mycophenolate mofetil was less effective than azathioprine for the prevention of relapses in ANCA vasculitis after cyclophosphamide induction therapy (36).
3.3.4.Practical recommendations
The combination of azathioprine and daily prednisone effectively maintains remission. Two milligrams per kilogram of azathioprine should be started when cyclophosphamide is discontinued. At 6 months azathioprine should be reduced to 1.5 mg/kg per day and maintained for at least 6 more months. Azathioprine is the preferred maintenance immunosupressive agent. Methotrexate is indicated for maintenance therapy in patients intolerant of azathioprine.
3.3.5.Treatment of relapse
Relapse is initially manifested by a return or increase in haematuria and proteinuria with deterioration of renal function and is usually associated with ANCA appearance and raised inflammatory markers. Repetead renal biopsy is indicated if there is uncertainity as to whether or not renal relapse is occurring. Such relapses are treated by a return to an induction protocol with cyclophosphamide or rituximab. Severe relapses should be treated with cyclophoshamide, GC and plasmapheresis. When renal vasculitis has caused renal impairement a response to therapy is indicated by an improvemenet in serum creatinine, usually within the first 2 weeks. Renal function then usually continues to improve for 6-12 months. For dialysis-dependent patients at presentation, recovery is usually seen within 2-3 weeks, but sometimes it can occur later (37,38).
3.3.6.Response to treatment
Patients treated with either intravenous or oral cyclophosphamide have a long-term remission rate of between 60% and 92%. Based on a large observational ‘’cohorts’’ of patients with ANCA vasculitis, female gender, black race, and potentially older age were associated with a higher likelihood of treatment resistance. Patients with PR3-ANCA may have a better chance of remission than patients with MPO-ANCA (39,40).
3.3.7. Refractory disease
Cyclophosphamide and GC are generally considered standard induction therapy for ANCA-associated vasculitis (Table 6). However, a subset of patients are refractory or intolerant to this treatment (37). New therapeutic approaches ‘’include’’ rituximab, a chimeric anti-CD20 antibody, which has emerged as a main therapy for refractory vasvulitis (38,39,40). Cumulative data from several open studies on the treatment of ANCA-associated MPA suggest that in the vast majority of cases rituximab has a beneficial effect. Two randomized controlled trials confirmed these promising results, suggesting that rituximab might be considered even as an option for the first-line induction therapy in ANCA-associated vasculitis, and providing an additional tool for treating patients with disease relapse after previous therapy. While rituximab is very effective in the depletion of B cells, current research suggests it could also influence other immune system cells and reestablish immune homeostasis and tolerance. The safety profile of rituximab reveals that most reactions are infusion-related and that the incidence of serious side effects is low. Systemic infection remains a major concern and may result in death. A small number of cases of progressive multifocal leukoencephalopathy reported in patients receiving rituximab in off-label use (albeit none with ANCA-associated vasculitis) highlights the importance of pharmacovigilance.
Table 6. Criteria for treatment response.
|
A) Complete remission The absence of symptoms or signs attributable to active vasculitis, with a Birmingham Vasculitis Activity Score of 0-1 (18). The absence of renal disease activity was indicated by stable or falling creatinine levels and the absence of erythrocyte cell casts. The results of ANCA testing were not used as criteria for remission or partial remission. Persistence proteinuria was not considered indicative of persistence of disease activity. |
|
B) Partial remission Clear-cut suppression of the disease, with improvement or stopping in disease progresion and stabilization of renal function |
|
C) Treatment resistance (1) progressive decline in renal function with the persistence of an active urine sediment, or (2) persistence or new appearance of any extrarenal manifestation of vasculitis despite immunosuppressive therapy |
|
D) Relapse The re-emergence of clinical symptoms attributable to vasculitis or worsening of original manifestations after 4 weeks of complete clinical remission had been achieved or occurrence of at least one of the following: (1) rapid rise in serum creatinine accompanied by an active urine sediment, (2) a renal biopsy demonstrating active necrosis or crescent formation, (3) active vasculitis of respiratory tract (hemoptysis), pulmonary nodules without evidence for infection, (4) active vasculitis of gastrointestinal ‘’tract’’, (5) iritis or uveitis, (6) new mononeuritis multiplex, (7) necrotizing vasculitis identified by biopsy in any tissue |
3.3.8. Supportive therapy
Special effort must be exercised to minimize the short- and long-term complications of treatment with GC and cyclophosphamide of ANCA-associated vasculitis. When GC are used, the development of osteoporosis can be minimized with the early institution of calcium and vitamin D supplementation, while in patients with established osteoporosis, calcitonin or bisphosphonates (if not contraindicated by azotemia or esophagitis) are considered.
Rigorous control of blood pressure with sodium restriction and antihypertensive therapy is essential to minimize the additive effect of hypertension in loss of renal function, following active nephritis. Hormonal manipulation during cytotoxic therapy may allow the preservation of gonadal function.
3.3.9. Prognosis and prognostic factors
Untreated, systemic vasculitis is associated with an year mortality. The introduction of GC, cyclophosphamide and azathioprine led to a marked improvement in survival to 84% and 76% at 1 and 5 years, respectively. Predictors of death include increased age and creatinine at presentation, disease extent and severity at diagnosis, pulmonary haemorrhage, and treatment-related infection (5,7,41,42).
Several studies have examined the question of prognostic factors in ANCA associated vasculitis. The presence of pulmonary hemorrhage was the most important determinant of patient survival, whereas other pulmonary findings (eg, infiltrates, nodules, or cavities) did not increase the risk of death. The risk of end stage renal disease is largely determined by the degree of renal dysfunction at the time of diagnosis. Serum creatinine is the single most important prognostic marker for long-term renal outcome as exemplified by a 1.24-fold increased risk for end stage renal disease for each 1 mg/dL increase in serum creatinine at baseline. Nevertheless, there is no threshold of renal dysfunction below which treatment is futile, as remission occurs in 57% of individuals with an estimated GFR<10mL/minute. Histopathologic features of chronic renal scarring (glomerulosclerosis, interstitial fibrosis and tubular atrophy) have consistently been associated with poor renal outcomes. A high proportion of normal glomeruli, glomeruli with active crescents and acute tubular necrosis in the renal biopsy are predictors of a good renal outcome. The impact of renal damage as a predictor of resistance emphasizes the importance of early diagnosis and prompt institution of therapy (41,42).
Recurrence of ANCA vasculitis after renal transplantation occurs in about 20% of patients. Time to recurrence varies widely, from a few days to several years post transplantation. Patients with WG appear more likely to relapse than patients with MPA or necrotizing crescentic glomerulonephritis alone, whereas the transplantation should be delayed in patients with active disease. In the majority of reported cases, recurrent disease after transplantation responded well to treatment with cyclophosphamide and GC pulses.
4. Conclusions
Vasculitis is a clinicopathological entity characterized by inflammation and necrosis of blood vessels. Antineutrophil cytoplasmatic autoantibodies (ANCA) are associated with a group of necrotizing small vessel vasculitides that have a paucity of vascular deposition of immunoglobulin and complement. The kidney is the most commonly affected vital organ in ANCA-associated vasculitis, and patient outcomes are largely determined by the severity of renal disease at diagnosis and by its response to treatment.
Conflict of interest
Main draft write up and editing by KG. Important intellectual content and critical revision by DL and IH.
Conflict of interest
The authors declared no competing interests.
Funding/Support
No funding from any source.
Acknowledgments
None declared.
Implication for health policy/practice/research/medical education:
Vasculitis is a clinicopathological entity characterized by inflammation and necrosis of blood vessels. Antineutrophil cytoplasmatic autoantibodies (ANCA) are associated with a group of necrotizing small vessel vasculitides that have a paucity of vascular deposition of immunoglobulin and complement. The kidney is the most commonly affected vital organ in ANCA-associated vasculitis, and patient outcomes are largely determined by the severity of renal disease at diagnosis and by its response to treatment.
Please cite this paper as: Galesic K, Ljubanovic D, Horvatic I. Treatment of renal manifestations of ANCA-associated Vasculitis. J Nephropathology. 2013; 2(1): 6-19. DOI: 10.5812/nephropathol.8971
References
- 1. Mansi IA, Opran A, Rosner F . ANCA-Associated Small-Vessels Vasculitis. Am J Physician 2002;60:1615-20. Available at:http://www.ncbi.nlm.nih.gov/pm...
- 2.Ozaki S. ANCA-associated vasculitis: diagnostic and therapeutic strategy. Allergol Int . 2007;56:87–96. doi: 10.2332/allergolint.R-07-141. [DOI] [PubMed] [Google Scholar]
- 3.Passen P, Tervaert JW, Heeringa P. Mechanisms of vasculitis: How Pauci-Immune is ANCA-Associated renal Vasculitis. Nephron Exp Nephrol . 2007;105:e10–e16. doi: 10.1159/000096960. [DOI] [PubMed] [Google Scholar]
- 4.Jennette JC, Falk RJ, Andrassy K, Bacon PA, Churg J, Gross WL. et al. Nomenclature of systemic vasculitidesProposal of an international consensus conference. Arthritis Rheum . 1994;37(2):187–92. doi: 10.1002/art.1780370206. [DOI] [PubMed] [Google Scholar]
- 5. Nachman PH, Jennette JC, Falk RJ. Vasculitic diseases of the Kidney in Schrier W. R., Gottoschalk W.C.. Diseases of the Kidney&Urinary tract. 8. 2. Philadelphia: Lippincott Williams&Wilkins; 2007. p. 1748-75.
- 6.Chen M, Yu F, Wang SX, Zou WZ, Zhao MH, Wang HY. Antineutrophil Cytoplasmic Autoantibodies-Negative Pauci-immune Crescentic Glomerulonephritis. J Am Soc Nephrol . 2007;18:599–605. doi: 10.1681/ASN.2006091021. [DOI] [PubMed] [Google Scholar]
- 7.Samarkos M, Loizou S, Vaiopoulos G, Davies KD. The Clinical Spectrum of Primary Vasculitis. Seminars in Artritis and Rheumatism . 2005;35:95–111. doi: 10.1016/j.semarthrit.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Jennette JC, Falk RJ, Gasim AH. Pathogenesis of antineutrofil cytoplasmatic autoantibody vasculitis. Curr Opin Nephrol Hypertens . 2011;20:263–70. doi: 10.1097/MNH.0b013e3283456731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jennette JC, Xiao H, Falk R, Gasim AM. Experimental models of vasculitis and glomerulonephritis induced by antineutrophil cytoplasmic autoantibodies. Contrib Nephrol . 2011;169:211–20. doi: 10.1159/000314776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiao H, Heeringa P, Hu P, Liu Z, Zhao M, Aratani Y. et al. Antineutrophil cytoplasmic autoantibodies specific for myeloperoxidase cause glomerulonephritis and vasculitis in mice. J Clin Invest . 2002;110(7):955–63. doi: 10.1172/JCI15918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pendergraft WF 3rd, Preston GA, Shah RR. et al. Autoimmunity is triggered by cPR-3(105-201), a protein complementary to human autoantigen proteinase-3. Nat Med . 2004;10:72–9. doi: 10.1038/nm968. [DOI] [PubMed] [Google Scholar]
- 12.Bajema IM, Hagen EC, Hermans J. Kidney biopsy as a predictor for renal outcome in ANCA-associated necrotizing glomerulonephritis. Kidney Int . 1999;56:1751–58. doi: 10.1046/j.1523-1755.1999.00758.x. [DOI] [PubMed] [Google Scholar]
- 13.Bajema IM, Hagen EC, Hansen BE, Hermans J, Noël LH, Waldherr R. et al. The renal histopathology in systemic vasculitis: an international survey study of inter- and intra-observer agreement. Nephrol Dial Transplant . 1996;11(10):1989–95. doi: 10.1093/oxfordjournals.ndt.a027086. [DOI] [PubMed] [Google Scholar]
- 14.Hauer HA, Bajema IM, van Houwelingen HC, Ferrario F, Noël LH, Waldherr R. et al. Renal histology in ANCA-associated vasculitis: differences between diagnostic and serologic subgroups. Kidney Int . 2002;61(1):80–9. doi: 10.1046/j.1523-1755.2002.00089.x. [DOI] [PubMed] [Google Scholar]
- 15.Hauer HA, Bajema IM, Hagen EC, Noël LH, Ferrario F, Waldherr R. et al. Long-term renal injury in ANCA-associated vasculitis: an analysis of 31 patients with follow-up biopsies. Nephrol Dial Transplant . 2002;17(4):587–96. doi: 10.1093/ndt/17.4.587. [DOI] [PubMed] [Google Scholar]
- 16.Sugimoto T, Deji N, Kume S, Osawa N, Sakaguchi M, Isshiki K. et al. Pulmonary-renal syndrome, diffuse pulmonary hemorrhage and glomerulonephritis, associated with Wegener’s granulomatosis effectively treated with early plasma exchange therapy. Intern Med . 2007;46(1):49–53. doi: 10.2169/internalmedicine.46.6070. [DOI] [PubMed] [Google Scholar]
- 17.Aasarod K, Bostad L, Hammerstrom J, Jorstad S, Iversen BM. Renal histopathology and clinical course in 94 patients with Wegener’s granulomatosis. Nephrol Dial Tranplant . 2001;16:953–60. doi: 10.1093/ndt/16.5.953. [DOI] [PubMed] [Google Scholar]
- 18.Luqmani RA, Bacon PA, Moots RJ, Janssen BA, Pall A, Emery P. et al. Birmingham Vasculitis Activity Score (BVAS) in systemic necrotizing vasculitis. QJM . 1994;87(11):671–8. [PubMed] [Google Scholar]
- 19.Exley AR, Bacon PA, Luqmani RA, Kitas GD, Gordon C, Savage CO. et al. Development and initial validation of the Vasculitis Damage Index for the standardized clinical assessment of damage in the systemic vasculitides. Arthritis Rheum . 1997;40(2):371–80. doi: 10.1002/art.1780400222. [DOI] [PubMed] [Google Scholar]
- 20.Fauci AS, Wolff SM. Wegener’s granulomathosis: studies in eighteen patients and review of the literature. Medicine . 1973;52:535–61. doi: 10.1097/00005792-197311000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Walters GD, Willis NS, Craig JC. Interventions for renal vasculitis in adultsA systematic review. BMC Nephrol . 2010;24:11–12. doi: 10.1186/1471-2369-11-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mukhtyar C, Guillevin L, Cid MC, Dasgupta B, de Groot K, Gross W. et al. EULAR recommendations for the management of primary small and medium vessel vasculitis. Ann Rheum Dis . 2009;68(3):310–7. doi: 10.1136/ard.2008.088096. [DOI] [PubMed] [Google Scholar]
- 23.Guillevin L, Cordier JF, Lhote F, Cohen P, Jarrousse B, Royer I. et al. A prospective, multicenter, randomized trial comparing steroids and pulse cyclophosphamide versus steroids and oral cyclophosphamide in the treatment of generalized Wegener’s granulomatosis. Arthritis Rheum . 1997;40(12):2187–98. doi: 10.1002/art.1780401213. [DOI] [PubMed] [Google Scholar]
- 24.Haubitz M, Schellong S, Göbel U, Schurek HJ, Schaumann D, Koch KM. et al. Intravenous pulse administration of cyclophosphamide versus daily oral treatment in patients with antineutrophil cytoplasmic antibody-associated vasculitis and renal involvement: a prospective, randomized study. Arthritis Rheum . 1998;41(10):1835–44. doi: 10.1002/1529-0131(199810)41:10<1835::AID-ART16>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 25.de Groot K, Harper L, Jayne DR, Flores Suarez LF, Gregorini G, Gross WL. et al. Pulse versus daily oral cyclophosphamide for induction of remission in antineutrophil cytoplasmic antibody-associated vasculitis: a randomized trial. Ann Intern Med . 2009;150(10):670–80. doi: 10.7326/0003-4819-150-10-200905190-00004. [DOI] [PubMed] [Google Scholar]
- 26.Jayne DR, Gaskin G, Rasmussen N, Abramowicz D, Ferrario F, Guillevin L. et al. Randomized trial of plasma exchange or high-dosage methylprednisolone as adjunctive therapy for severe renal vasculitis. J Am Soc Nephrol . 2007;18(7):2180–8. doi: 10.1681/ASN.2007010090. [DOI] [PubMed] [Google Scholar]
- 27.Klemmer PJ, Chalermskulrat W, Reif MS, Hogan SL, Henke DC, Falk RJ. Plasmapheresis Therapy for Diffuse Alveolar Hemorrhage in Patients With Small-Vessel Vasculitis . Am J Kidney Dis. 2003;42:1149–53. doi: 10.1053/j.ajkd.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 28.Walsh M, Catapano F, Szpirt W, Thorlund K, Bruchfeld A, Guillevin L. et al. Plasma exchange for renal vasculitis and idiopathic rapidly progressive glomerulonephritis: a meta-analysis. Am J Kidney Dis . 2011;57(4):566–74. doi: 10.1053/j.ajkd.2010.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Groot K, Rasmussen N, Bacon PA, Tervaert JW, Feighery C, Gregorini G. et al. Randomized trial of cyclophosphamide versus methotrexate for induction of remission in early systemic antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum . 2005;52(8):2461–9. doi: 10.1002/art.21142. [DOI] [PubMed] [Google Scholar]
- 30.Jones RB, Tervaert JW, Hauser T, Luqmani R, Morgan MD, Peh CA. et al. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis. N Engl J Med . 2010;363(3):211–20. doi: 10.1056/NEJMoa0909169. [DOI] [PubMed] [Google Scholar]
- 31.Stone JH, Merkel PA, Spiera R, Seo P, Langford CA, Hoffman GS. et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med . 2010;363(3):221–32. doi: 10.1056/NEJMoa0909905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jayne D, Rasmussen N, Andrassy K, Bacon P, Tervaert JW, Dadoniené J. et al. A randomized trial of maintenance therapy for vasculitis associated with antineutrophil cytoplasmic autoantibodies. N Engl J Med . 2003;349(1):36–44. doi: 10.1056/NEJMoa020286. [DOI] [PubMed] [Google Scholar]
- 33.Wegener’s Granulomatosis Etanercept. Etanercept plus standard therapy for Wegener’s granulomatosis. N Engl J Med . 2005;352:351–61. doi: 10.1056/NEJMoa041884. [DOI] [PubMed] [Google Scholar]
- 34.Pagnoux C, Mahr A, Hamidou MA, Boffa JJ, Ruivard M, Ducroix JP. et al. Azathioprine or methotrexate maintenance for ANCA-associated vasculitis. N Engl J Med . 2008;359(26):2790–803. doi: 10.1056/NEJMoa0802311. [DOI] [PubMed] [Google Scholar]
- 35.Metzler C, Miehle N, Manger K, Iking-Konert C, de Groot K, Hellmich B. et al. Elevated relapse rate under oral methotrexate versus leflunomide for maintenance of remission in Wegener’s granulomatosis. Rheumatology (Oxford) . 2007;46(7):1087–91. doi: 10.1093/rheumatology/kem029. [DOI] [PubMed] [Google Scholar]
- 36.Hiemstra TF, Walsh M, Mahr A, Savage CO, de Groot K, Harper L. et al. Mycophenolate mofetil vs azathioprine for remission maintenance in antineutrophil cytoplasmic antibody-associated vasculitis: a randomized controlled trial. JAMA . 2010;304(21):2381–8. doi: 10.1001/jama.2010.1658. [DOI] [PubMed] [Google Scholar]
- 37.Rhee EP, Laliberte KA, Niles JL. Rituximab as Maintenace Therapy for Anti-Neutrophil Cytoplasmaitc Antibody-Associated Vasculitis. Clin J Am Soc Nephrol . 2010;5:1394–1400. doi: 10.2215/CJN.08821209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferraro AJ, Day CJ, Drayson MT, Savage CO. Effective therapeutic use of rituximab in refractory Wegener’s granulomatosis. Nephrol Dial Transplant . 2005;20(3):622–5. doi: 10.1093/ndt/gfh599. [DOI] [PubMed] [Google Scholar]
- 39.KDIGO Board Members. Pauci-immune focal and segmental necrotizing glomerulonephritis. Kidney Int. 2012;0:233–239. doi: 10.1038/kisup.2012.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nachman PH, Hogan SL, Jennette JC, Falk RJ. Treatment response and relapse in antineutrophil cytoplasmic autoantibody-associated microscopic polyangiitis and glomerulonephritis. J Am Soc Nephrol . 1996;7:33–9. doi: 10.1681/ASN.V7133. [DOI] [PubMed] [Google Scholar]
- 41.Kaplan-Pavlovcic S, Cerk K, Kveder R, Lindic J, Vizjak A. Clinical prognostic factors of renal outcome in anti-neutrophil cytoplasmatic autoantibody (ANCA)-associated glomerulonephritis in elderly patients. Nephrol Dial Tranplant . 2003;18:v5–v7. doi: 10.1093/ndt/gfg1033. [DOI] [PubMed] [Google Scholar]
- 42.Alexopoulos E, Gionanlis L, Papayianni E, Kokolina E, Leontsini M, Memmos D. Predictors of outcome in idiopathic rapidly progressive glomerulonephritis (IRPGN) BMC Nephrol . 2006;7:16. doi: 10.1186/1471-2369-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]


