Abstract
Background
Isolated or predominant tubulointerstitial lupus nephritis is rare.
Case Presentation
Here we report the case of a thirty eight years old male who was diagnosed with systemic lupus erythematosus (SLE) according to clinical and laboratory criteria and presented with impaired renal function and non nephrotic range proteinuria. Renal biopsy revealed normal glomeruli but interstitial momonuclear cell infiltration. Immunohiostochemistry (IHC) showed immune deposits in the tubular basement membranes (TBMs), and the peritubular capillary basement membranes (PTCBMs). He was started on high dose oral steroids, which were gradually tapered over one month. His renal functions improved over few days and normalized by the end of the first month of treatment. He was continued on low dose steroids and azathioprine with no evidence of relapse.
Conclusions
Predominant tubulointerstitial lupus can occur, although rarely; and it runs a favorable course with good response to treatment.
Keywords: Lupus nephritis, Tubulointerstitial lupus nephritis, Immunohiostochemistry
1. Introduction
ubulointerstitial disease (TID) of the kidneys is a well recognized feature of lupus nephritis (LN), occurring in 66% of kidney biopsy specimens of patients with systemic lupus erythematosus (SLE)(1). In most instances of TID, the associated tubular atrophy, interstitial fibrosis, and inflammation accompany severe glomerular lesions(2).
However, the predominant or isolated presence of tubulointerstitial changes in the setting of minimal or absent glomerular abnormalities in patients with SLE is rare. To the best of our knowledge only twelve cases had been reported(3).
This possibility should be suspected when a patient with SLE presents with a rising plasma creatinine concentration and a urinalysis that is relatively normal or shows only a few red cells and/or white cells. These changes may be accompanied by signs of tubular dysfunction such as metabolic acidosis due to type 1 (distal) renal tubular acidosis (RTA), hyperkalemia due to impaired distal potassium secretion, or hypokalemia due to salt-wasting and secondary hyperaldosteronism. Autoantibodies directed against the acid-secreting intercalated cells in the collecting tubule may be responsible for the acid secretory defect in at least some patients (4).
Here we describe a case of isolated/predominant tubulointerstitial LN in which the diagnosis of SLE was made one year ago on the basis of clinical, and serologic criteria. The patient presented with impaired renal function, sub nephrotic proteinuria and evidence of tubular dysfunction.
2. Case report
Thirty eight years old male who was diagnosed with SLE one year prior to presentation according to clinical presentation of malar rash, oral ulcers, hair loss, arthralgia, fatigue and anemia. He was investigated thoroughly at that time and discovered to have anemia of 10 g/dL hemoglobin, leucopenia, positive antinuclear antibody (ANA) with 1/320 titer and positive anti-double-stranded DNA (anti-dsDNA).
Renal indices, glomerular filtration rate (GFR), electrolytes and urine examination were normal. Other serology for Sjogren’s syndrome and other connective tissue diseases was negative.
He received non steroidal anti-inflammatory drugs (NSAIDs) for arthralgia just for few weeks and 20 mg prednisolone per orally/day for ten months. No history of other medications use. There was no previous history of renal disease, passing stone or hematuria.
On July 2010 he presented to our unit with one month history of increasing fatigue, lower limb edema, loin pain and nocturia.
Clinical examination revealed conscious, oriented male with malar rash, and + lower limb edema, blood pressure of 120/80 mmHg, pulse rate of 78 bpm and he was afebrile. Heart, lung and abdominal examination were unremarkable.
A battery of laboratory tests was ordered and revealed blood urea of 60 mg/dl and serum creatinine of 1.3 mg/dL corresponding to GFR of 68 mL/min/1.73 m2 using four variables MDRD equation. Urinalysis showed a pH of 6, 2+ blood and trace protein; sediment examination revealed 4-5 red blood cells/HPF with no red cells casts , and 2-3 granular casts. Twenty-four-hour urinary protein was done twice and was 0.5-1 g. Serum sodium was 133 mEq/L, potassium, 3.8 mEq/L, chloride, 110 mEq/L, bicarbonate, 16 mEq/L, and albumin, 3.1 g/dL. Hematocrit was 38%, white blood cell count, 6.4x109 /mm, platelet count, 150x109/mm, erythrocyte sedimentation rate, 75 mm/hr, and normal PT and PTT. ANA was positive in a diffuse pattern, positive anti-ds-DNA ,C3 was 31 (84-122 mg/dL), and C4 was 14 (20-40 mg/dl).
Other serology for Sjogren’s syndrome, C-ANCA and P-ANCA were all negative as well as negative HBV and HCV serology. Renal ultrasound showed normal size kidneys with no abnormalities.
Accordingly percutaneous renal biopsy under real time ultrasound guidance was done. Fourteen glomeruli examined, showing normal cellularity, normal basement membrane thickness, with few RBCs in capillary lumen, normal mesangium with no proliferative changes. Interstitium showed mild mononuclear and plasma cell infiltration, and tubular hyaline casts. Examination of renal vessels was unremarkable. Immunoperoxidase protocol revealed negative glomerular immunostaining but there was +3/+4 IgG, +2/+4 IgA, +2/+4 C3, +3/+4 C1q deposition along the TBMs and in the PTCBMs. Immunostaining for IgM was negative.
Electron microscopy examination was not done because of unavailability.
Other causes of tubulointerstitial nephritis like drugs and toxins were excluded from history, clinical examination and laboratory tests.
The patient was started on high dose oral steroids, 60 mg/day with gradual tapering over one month. After few days of treatment, the patient’s general status improved and his renal function started to normalize and on discharge his serum creatinine was 1mg/dL. On follow up, there was no clinical evidence of lupus activity and by the end of the month his serum creatinine was 0.8 mg/dl. He was continued on oral steroids, 10 mg/day and azathioprine, 75 mg/day. On regular bases the patient has been followed and still there is no clinical nor laboratory evidence of relapse and he continued on low dose steroid and azathioprine for two years.
Figure 1.

Two glomeruli, one of them with narrowed Bowman’s space, with focal interstitial infiltration and hyaline cast (H & E 4X).
Figure 2.

The normal looking glomeruli. (H& E 40X).
Figure 3.

Interstitial infiltrate with mononuclear and plasma cells (H & E 40X).
Figure 4.

A Glomerulus with NO immune deposits (Immunoperoxidase IHC).
Figure 5.
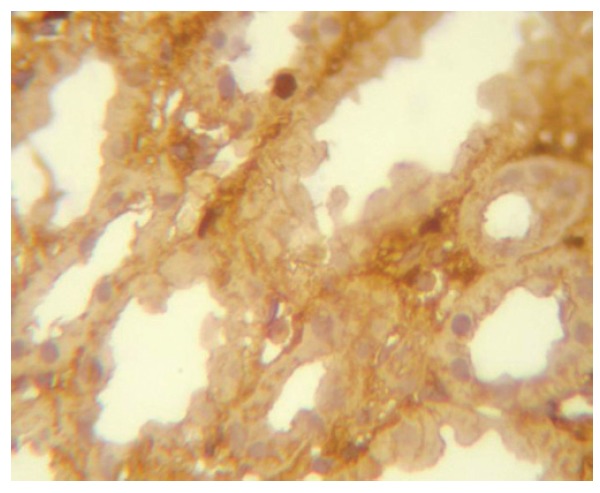
Tubulointerstitial C3 deposits (Immunoperoxidase IHC).
Figure 6.

Tubulointerstitial IgA deposits (Immunoperoxidase IHC).
3. Discussion
Tubulointerstitial lesions may be encountered in all classes of lupus nephritis, but most commonly encountered in class IV lupus nephritis. The lesions also occur frequently in class III and to a lesser degree in class V, with lowest frequency found in classes I and II. The types of lesions that affect the tubulointerstitial compartment can be divided into acute and chronic subgroups, those related to an interstitial inflammatory process or resulting largely from prolonged nephrotic proteinuria. There are rare cases in which severe tubulointerstitial damage occurs in the presence of only trivial glomerular lesions and the presence of abundant tubulointerstitial deposits in most of these patients attests to their pathogenetic relation to lupus rather than representing a superimposed, unrelated tubulointerstitial process (2,5).
Here we reported a patient diagnosed with SLE, with no history of proteinuria and only short history of using NSAIDs who presented with renal impairment, evidence of RTA and non nephrotic range proteinuria. Urine analysis did not provide any evidence of acute glomerulonephritis. Percutaneous renal biopsy revealed no glomerular pathology by light microscopy (LM) and Immunohistochemistry (IHC) technique but on the contrary, there was momonuclear cell infiltration of the interstitium and immune deposits along the TBMs and the peritubular capillaries. These clinical, laboratory and biopsy data suggested that the primary target of the lesion was the tubulointerstitium. Moreover, the absence of eosinophils in the interstitial infiltrate nearly excluded the allergic bases of the interstitial inflammatory process.
Proteinuria of less than 1 gm/24 hr may be of tubular origin but also may indicate a glomerular injury which may be missed by biopsy although this seems to be extremely unlikely in the absence of clinical and laboratory evidence.
The pathogenesis of tubulointerstitial LN is still mysterious but it seems to be the result of circulating immune complexes specifically interacting with one or more tubulointerstitial autoantigens, which are either not present or not expressed in glomeruli or by formation of in situ immune complexes (6). Others have found correlation between interstitial immune complexes and serological activity(7). This can be seen in the present case with positive ANA, anti-ds-DNA and hypocomplementemia. From another point of view, it is possible that the tubular damage was not caused by deposits of immune complexes, but rather by cell mediated injury. Although the mononuclear cell infiltrates have not been functionally characterized in SLE, morphologically similar cell populations in other types of interstitial nephritis have been identified as activated T cells(8). In another case report from University School of Medicine, Akita, Japan, immunophenotyping of the interstitial infiltrating cells disclosed a predominance of T cells (9).
In the literature reviews 1,3 most of the cases were treated with high dose steroids, namely methyleprednisolone, probably because the presentation was more fulminate with higher serum creatinine and acute kidney injury (AKI) while in our case and that from Akita, Japan (9) oral corticosteroids were enough to normalize mildly impaired renal function within two months of treatment.
It is important to explain the logic behind initiation of Azathioprine and it was the serological activity of lupus and our internal fear from relapse with a more aggressive presentation.
The long term renal outcome in the majority of the previously reported patients was favorable and the disease followed a relatively benign, steroid-responsive course (3) as in this case.
4. Conclusions
Predominant TID is a rare presentation of LN that fortunately runs a benign steroid responsive course, that should be kept in mind and the decision to add immunosuppression should be individualized depending on clinical and serological data plus the experience of the treating nephrologist.
Acknowledgments
The authors would like to express their deepest gratitude to Professor Mahasin Suad Salim and Professor Ban A. Abdulmajid for their kind help, support and advice.
Authors’ contributions
AA prepared the primary draft. SAW wrote some parts of the manuscript. All authors read and approved the final draft.
Funding/Support
None declared.
Conflict of interest
Nothing to disclose.
Implication for health policy/practice/research/medical education:
Renal tubulointerstitium can be involved in all classes of lupus nephritis but predominant tubulointerstitial lupus without evidence of glomerular lesion is rare. In this study we described a pure tubulointerstitial lupus nephritis.
Please cite this paper as: Ali A, Al-Windawi S. Tubulointerstitial Lupus nephritis. J Nephropathology. 2013; 2(1): 75-80. DOI: 10.5812/nephropathol.9000
References
- 1.Gur H, Kopolovic Y, Gross DJ. Chronic predominant interstitial nephritis in a patient with systemic lupus erythematosus: A follow up of three years and review of the literature. Ann Rheum Dis . 1987;46:617–23. doi: 10.1136/ard.46.8.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vivette D, D’Agati . Renal Disease in Systemic Lupus Erythematosus, Mixed Connective Tissue Disease, Sjögren’s Syndrome, and Rheumatoid Arthritis. In: Jennette J, Charles Olson, Jean L, Schwartz , Melvin M, Silva , Fred G, . 6 ed. Lippincott Williams & Wilkins: Hepinstall’s Pathology of the kidney; 2007. 518-614. http://kidneypathology.com.ar/...
- 3. Singh AK, Ucci A, Madias NE. Predominant tubulointerstitial lupus nephritis. Am J Kidney Dis 1996;27(2):273-78. http://cat.inist.fr/?aModele=a... [DOI] [PubMed]
- 4.Mori Y, Kishimoto N, Yamahara H. et al. Predominant tubulointerstitial nephritis in a patient with systemic lupus nephritis. Clin Exp Nephrol . 2005;9:79–84. doi: 10.1007/s10157-004-0338-3. [DOI] [PubMed] [Google Scholar]
- 5.Case records of. Case records of the Massachusetts General HospitalWeekly clinicopathological exercisesCase 2-1976. N Engl J Med . 1976;294(2):100–5. doi: 10.1056/NEJM197601082940208. [DOI] [PubMed] [Google Scholar]
- 6.Couser WJ, Salant DJ, Madaio MP, Adler A, Groggel GC. Factors influencing glomerular and tubulointerstitial patterns of injury in SLE. Am J Kidney Dis . 1982;12(2):126–34. [PubMed] [Google Scholar]
- 7.Hunter MG, Hurwitz S, Bellamy CO, Duffield JS. Quantitative morphometry of lupus nephritis: the significance of collagen, tubular space, and inflammatory infiltrate. Kidney Int . 2005;67(1):94–102. doi: 10.1111/j.1523-1755.2005.00059.x. [DOI] [PubMed] [Google Scholar]
- 8.Husby G, Tung KS, Williams RC Jr. Characterization of renal tissue lymphocytes in patients with interstitial nephritis. Am J Med . 1981;70:31–8. doi: 10.1016/0002-9343(81)90408-3. [DOI] [PubMed] [Google Scholar]
- 9.Omokawa A, Wakui H, Okuyama Okuyama. et al. Predominant tubulointerstitial nephritis in a patient with systemic lupus erythematosus: phenotype of infiltrating cells. Clin Nephrol . 2008;69(9):436–44. doi: 10.5414/cnp69436. [DOI] [PubMed] [Google Scholar]


