Abstract
Background
Antiphospholipid syndrome (APS) is a systemic disorder characterizes by recurrent arterial and venous thrombosis and/or pregnancy miscarriages and positive test for antiphospholipid antibodies.
Case
Here we report a 31 year-old female whose main complain was symmetrical motor polyneuropathy and hand muscular weakness over a long period of time. Her clinical picture became more complex by Mitral valve regurgitation and renal dysfunction. Finally with the diagnosis of APS glucocorticoid therapy was started and her renal dysfunction improved profoundly, however her cardiac valvular involvement and peripheral neuropathy remained.
Conclusions
APS is a disease with wide clinical presentations and it continues to confound the clinicians.
Keywords: Antiphospholipid antibody syndrome, Motor polyneuropathy, Venous thrombosis, Nephropathy
1. Introduction
Antiphospholipid syndrome (APS) is characterized by recurrent arterial and venous thrombosis that affecting all segments of vascular beds. Its main laboratory picture is the prescience of antiphospholipid antibodies (APLA) which includes: anticardiolipin, Lupus anticoagulant and anti-β2 glycoprotein 1 (anti-β2GP1). Generally, the diagnosis of APS is made when one clinical (i.e.; vascular thrombosis or pregnancy morbidity) and at least one antiphospholipid antibodies does exist (1,9) The APS is called primary when it is without other autoimmune condition and secondary when it happens as a part of other autoimmunity disorder.(9) Here we report a case of APS who presented with peripheral neuropathy. Longley thereafter, renal and cardiac problems complicated her clinical picture.
2. Case
A 31 yr-old female was referred to nephrology clinic for further evaluation of her recently discovered renal dysfunction. She was complaining from progressive hand griping weakness and muscular atrophy since three years ago. Echocardiography that was done two months ago had discovered mitral valve thickening and regurgitation. She hadn’t history of venous thrombosis or pregnancy miscarriage. Physical examination on admission revealed: pulse rate; 100/min, blood pressure 140/100 mmHg, respiration rate 20/min, and body temperature 37°C.Laboratory tests were as the followings; serum creatinine: 3.2 mg/dL. Blood urea nitrogen: 63 mg/dL, white blood cell count: 9200/µL, hemoglobin: 9.2 g/dL, platelet count: 92000/µL, erythrocyte sedimentation rate 122 mm/h, serum albumin 32 g/L (normal 34-50 g/L), alkaline phosphate (ALP) 187 U/L, and total bilirubin 10 mg/dL. Levels of aspartate aminotransferase(AST); 23 U/L, alanin aminotransferase (ALT): 20 U/L, prothrombine time(PT): 12 second, parial thromboplastine time(PTT):35 second. Lactic dehydrogenase (LDH): 850 U/L, Serologic tests for HCV, HBV, HIV, all were negative. Serology activity for systemic lupus erythromatosis and serum complements were within normal levels. Urine analysis showed proteinuria and glomerular hematuria. 24-hours urine collection revealed 1.4 g/day protein excretion.
Conventional nerve conduction velocity (NCV) study of right and left median, ulnar, superficial radial nerves at wrist and elbow were compatible with motor polyneuropathy of upper extremity. Brain MRI showed multiple hypersignal lesions at anterior and posterior watershed areas, at corpous calleosum, thalami, basal ganglia and paraventricular areas (Figure 1). The primary impression of radiologist was hypertensive encephalopathy or vasculitis. Transeosophageal echocardiography showed mixomatous changes of the mitral valve with sever posterior oriented mitral regurgitation.
Figure 1.
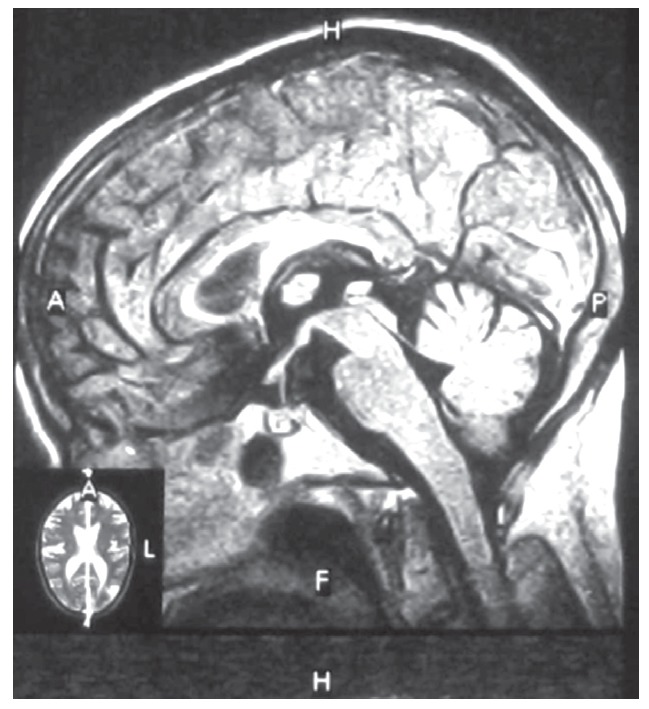
Brain MRI shows multiple hyper-signal lesions at anterior and posterior watershed areas in both white and gray matters.
Renal ultrasound and color Doppler study revealed right and left kidney length: 108 mm and 108 mm, without renal vein thrombosis or renal artery stenosis. Intra-renal arterial spectral wave study revealed an elevated resistive index (RI), right RI: % 66 and left RI: %68. Ultrasound guided Kidney biopsy was performed. Light microscopic study disclosed fibro-cellular intrarenal arteriolar narrowing and occlusion. Areas of tubular atrophy and dilatation and glomerular endothelial edema and obstruction were visible (figure 2).
Figure 2.
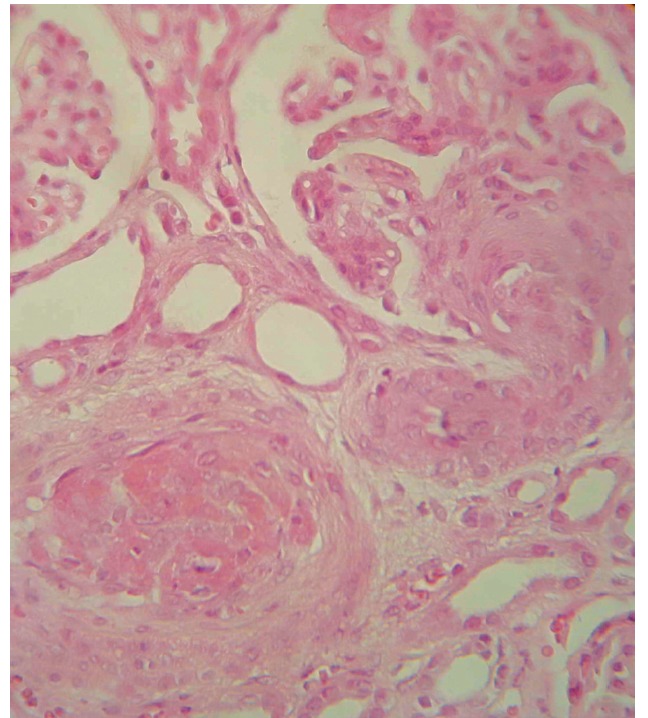
fibroelastic intimal thickening with almost complete obliteration of the lumen of interlobular arteries (haematoxylin and eosin stain ×400).
Further laboratory studies revealed; anticardiolipine IgG: 51.2U/mL (>12 positive), IgM: 9.1 (>12 positive), antiphospholid antibody IgG: 37.1 U/mL (>12 positive), and IgM; 9.2 (>12 positive). With the diagnosis of primary APS, treatment with methylprednisolon IV pulse; 500 mg/day for three consecutive days was started and followed by tapering dose of oral Prednisolon. Within a few weeks patient general condition improved, hemoglobin level elevated to 11 mg /dL, LDH level returned to baseline and serum creatinine level reached to 1.6 mg/dL. Peripheral neuropathy and cardiac problems continued and she was referred to cardiac surgery unit for future management.
3. Discussion
In this patient motor neuropathy that is a rare complication of APS was its presenting figure. Additionally, the absence of common manifestations such as vascular thrombosis and pregnancy miscarriage made the situation more difficult.
The spectrum of neurologic syndromes of APS includes ischemic stroke, seizures, chorea, peripheral neuropathy, and Guillain–Barre syndrome. In our patient multiple high density lesions in brain MRI was compatible with micro infarctions (3,4). Peripheral neuropathy is a rare presenting manifestation of APS. Phospholipids contained in the nerves including sphingomyelin and cephalin could be a potential target of APLA. Vasa nervorum thrombosis and vasculopathy are another explanation for peripheral nerve involvement in APS (3-5). Different cardiopulmonary involvements could happen in APS. These including; pulmonary embolism, pulmonary hypertension, myocardial infarction, cardiomyopathy and valvular involvement (6). Mitral valve thickening and insufficiency is the most common form of cardiac involvement in APS. Systemic Lupus erythromatous (SLE) patients who have a positive serology for anti-phospholipid antibodies are also at increased risk of cardiac valvular involvement (7). It seems that APLA directly damaging the cardiac valves. Infective endocarditis is a very uncommon complication of APS (6,7).
Kidney is a major target organ and hypertension is an important sign of renal vascular involvement (8,9). APS-nephropathy (APSN) refers to kidney damage caused by glomerular and/or renal arterial lesions (8,9). Vascular lesions may be acute and/or chronic. Acute APSN often represents as thrombotic microangiopathy (TMA) that is determined by fibrin thrombi in glomerular and intra-renal vasculature. In our patient, low platelet count, elevated LDH levels and closed capillary loops were compatible with TMA, meanwhile she also had the chronic pathologic pictures of APSN (figure 2) . In chronic form of APSN, arterial fibrocellular hyperplasia and occlusion lead to ischemic interstitial nephritis and tubular atrophy (2,9). Complement activation is necessary for APS-associated thrombosis. Our patient hadn’t any obvious history of venous thrombosis and it could explain the normal complement levels during its frequent measurements (10,11). APS is capable of masquerading as a myriad of entities. It is necessary to be familiar with rare presentations of APS because appropriate treatment could halt the disease progression when it is not in advanced stages.
Authors’ contributions
MRA prepared the primary draft. AV wrote some parts of the manuscript. MRA prepared the final manuscript.
Conflict of interest
The author declared no competing interests.
Funding/Support
None declared.
Acknowledgments
The authors would like to thank all staffs of the Chronic Kidney Disease Research Center, Tabriz University of Medical Sciences.
Implication for health policy/practice/research/medical education:
Antiphospholipid syndrome (APS) is a systemic disorder characterizes by recurrent arterial and venous thrombosis and/or pregnancy miscarriages and positive test for antiphospholipid antibodies.APS is a disease with wide clinical presentations and it continues to confound the clinicians.
Please cite this paper as: Ardalan MR, Vahedi A. Antiphospholipid syndrome: A disease of protean face. J Nephropathology. 2013; 2(1): 81-84. DOI: 10.5812/nephropathol.9001
References
- 1.Cervera R, Boffa M-C, Khamastha MA, Hughes GRU. The Euro-Phospholipid Project epidemiology of the antiphospholipid syndrome in Europe. Lupus . 2009;18:889–93. doi: 10.1177/0961203309106832. [DOI] [PubMed] [Google Scholar]
- 2.Gigante A, Gasperini ML, Cianci R, Barbano B, Giannakakis K, Di Donato D, Fuiano G, Amoroso A. Antiphospholipid Antibodies and Renal Involvement. Am J Nephrol . 2009;30(5):405–12. doi: 10.1159/000235941. [DOI] [PubMed] [Google Scholar]
- 3.Hughes GR. Migraine, memory loss, and ‘’multiple sclerosis.’’ Neurological features of the antiphospholipid (Hughes’) syndrome. Postgrad Med J. 2003;79(928):81–3. doi: 10.1136/pmj.79.928.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodrigues CE, Carvalho JF, Shoenfeld Y. Neurological manifestations of antiphospholipid syndrome. Eur J Clin Invest . 2010;40(4):350–9. doi: 10.1111/j.1365-2362.2010.02263.x. [DOI] [PubMed] [Google Scholar]
- 5.Santos MS, de Carvalho JF, Brotto M, Bonfa E, Rocha FA. Peripheral neuropathy in patients with primary antiphospholipid (Hughes’) syndrome. Lupus . 2010;19(5):583–90. doi: 10.1177/0961203309354541. [DOI] [PubMed] [Google Scholar]
- 6.Turiel M, Muzzupappa S, Gottardi B, Crema C, Sarzi-Puttini P, Rossi E. Evaluation of cardiac abnormalities and embolic sources in primary antiphospholipid syndrome by transesophageal echocardiography. Lupus . 2000;9(6):406–12. doi: 10.1191/096120300678828532. [DOI] [PubMed] [Google Scholar]
- 7.Tenedios F, Erkan D, Lockshin MD. Cardiac manifestations in the antiphospholipid syndrome. Rheum Dis Clin North Am . 2006;32(3):491–507. doi: 10.1016/j.rdc.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 8.Alchi B, Griffiths M, Jayne D. What nephrologists need to know about antiphospholipid syndrome. Nephrol Dial Transplant . 2010;25:3147–54. doi: 10.1093/ndt/gfq356. [DOI] [PubMed] [Google Scholar]
- 9.Amigo MC. Kidney disease in antiphospholipid syndrome. Rheum Dis Clin North Am . 2006;32(3):509–22. doi: 10.1016/j.rdc.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 10.Salmon JE, Girardi G. The role of complement in the antiphospholipid syndrome. Curr Dir Autoimmun . 2004;7:133–48. doi: 10.1159/000075690. [DOI] [PubMed] [Google Scholar]
- 11.Rahgozar S. Revisiting Beta 2 glycoprotein I, the major autoantigen in the antiphospholipid syndrome. Iran J Immunol . 2012;9(2):73–85. [PubMed] [Google Scholar]


