Abstract
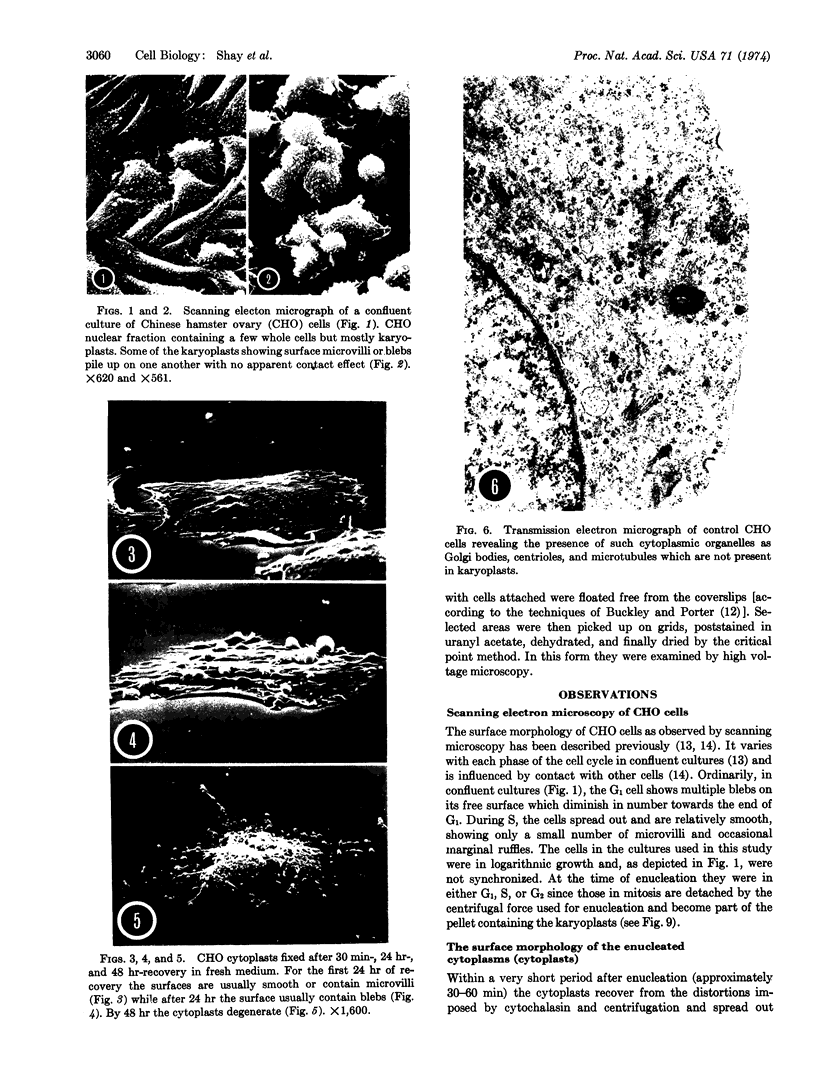
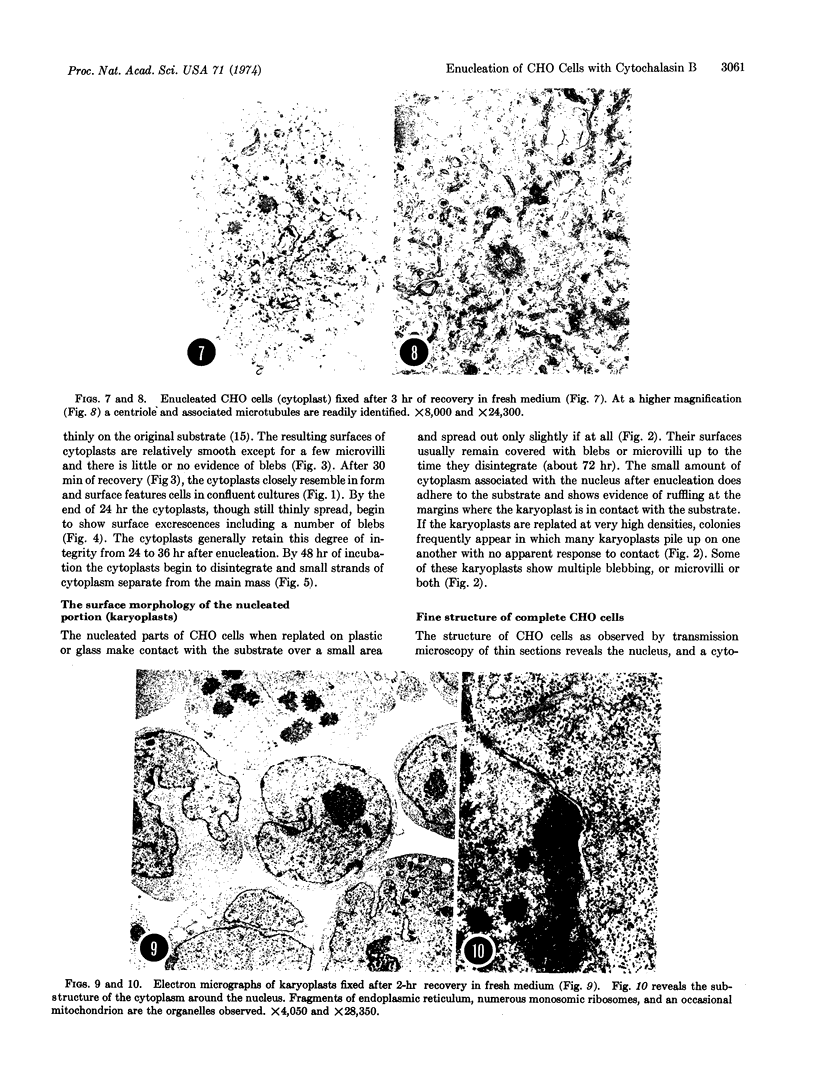
Chinese hamster ovary cells grown in monolayer culture and exposed to cytochalasin B were enucleated by centrifugation. Thereafter, the karyoplasts (the nucleated parts obtained from the bottoms of the centrifuge tubes) and the cytoplasts (the enucleated cytoplasmic parts attached to the coverslips) were allowed to recover and subsequently were examined by scanning and transmission electron microscopy. Microscopy of thin sections revealed that the karyoplasts, limited by an intact plasma membrane, contain an intact nucleus surrounded by a layer of cytoplasm that includes ribosomes, mitochondria, and fragments of the endoplasmic reticulum, but no centrioles or microtubules. The cytoplasts, similarly examined, appear to contain all cytoplasmic organelles and systems, including centrioles and microtubules. The karyoplasts, when replated in fresh medium adhere to the substrate but remain essentially spherical and are incapable of motility. They disintegrate in about 72 hr. The cytoplasts, under identical conditions, recover a shape similar to that of the whole Chinese hamster ovary cell and display some motility. They generally survive not more than 48 hr. It appears that this enucleation procedure consistently separates the nucleus and limited cytoplasm from the centrosphere and microtubule-containing cytoplasts and, furthermore, that the formdetermining and motility mechanisms reside in the cytoplast and function without nuclear participation for the short period of viability.
Keywords: cytochalasin B, karyoplasts, cytoplasts
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carter S. B. Effects of cytochalasins on mammalian cells. Nature. 1967 Jan 21;213(5073):261–264. doi: 10.1038/213261a0. [DOI] [PubMed] [Google Scholar]
- Flickinger C. J. The development of Golgi complexes and their dependence upon the nucleus inmebae. J Cell Biol. 1969 Nov;43(2):250–262. doi: 10.1083/jcb.43.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flickinger C. J. The effects of enucleation on the cytoplasmic membranes of Amoeba proteus. J Cell Biol. 1968 May;37(2):300–315. doi: 10.1083/jcb.37.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freed J. J., Lebowitz M. M. The association of a class of saltatory movements with microtubules in cultured cells. J Cell Biol. 1970 May;45(2):334–354. doi: 10.1083/jcb.45.2.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDSTEIN L., CAILLEAU R., CROCKER T. T. Nuclear-cytoplasmic relationships in human cells in tissue culture. II. The microscopic behavior of enucleate human cell iragments. Exp Cell Res. 1960 Mar;19:332–342. doi: 10.1016/0014-4827(60)90012-4. [DOI] [PubMed] [Google Scholar]
- GOLDSTEIN L., MICO U. J., CROCKER T. T. Nuclear-cytoplasmic relationships in human cells in tissue culture. IV. A study of some aspects of nucleic acid and protein metabolism in enucleate cells. Biochim Biophys Acta. 1960 Dec 4;45:82–86. doi: 10.1016/0006-3002(60)91428-1. [DOI] [PubMed] [Google Scholar]
- Goldman R. D., Pollack R., Hopkins N. H. Preservation of normal behavior by enucleated cells in culture. Proc Natl Acad Sci U S A. 1973 Mar;70(3):750–754. doi: 10.1073/pnas.70.3.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladda R. L., Estensen R. D. Introduction of a heterologous nucleus into enucleated cytoplasms of cultured mouse L-cells. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1528–1533. doi: 10.1073/pnas.67.3.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack R., Goldman R. Synthesis of infective poliovirus in BSC-1 monkey cells enucleated with cytochalasin B. Science. 1973 Mar 2;179(4076):915–916. doi: 10.1126/science.179.4076.915. [DOI] [PubMed] [Google Scholar]
- Porter K. R., Puck T. T., Hsie A. W., Kelley D. An electron microscopy study of the effects on dibutyryl cyclic AMP on Chinese hamster ovary cells. Cell. 1974 Jul;2(3):145–162. doi: 10.1016/0092-8674(74)90089-0. [DOI] [PubMed] [Google Scholar]
- Porter K., Prescott D., Frye J. Changes in surface morphology of Chinese hamster ovary cells during the cell cycle. J Cell Biol. 1973 Jun;57(3):815–836. doi: 10.1083/jcb.57.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poste G., Reeve P. Inhibition of cell fusion by local anaesthetics and tranquillizers. Exp Cell Res. 1972 Jun;72(2):556–560. doi: 10.1016/0014-4827(72)90029-8. [DOI] [PubMed] [Google Scholar]
- Prescott D. M., Kates J., Kirkpatrick J. B. Replication of vaccinia virus DNA in enucleated L-cells. J Mol Biol. 1971 Aug 14;59(3):505–508. doi: 10.1016/0022-2836(71)90313-5. [DOI] [PubMed] [Google Scholar]
- Prescott D. M., Myerson D., Wallace J. Enucleation of mammalian cells with cytochalasin B. Exp Cell Res. 1972;71(2):480–485. doi: 10.1016/0014-4827(72)90322-9. [DOI] [PubMed] [Google Scholar]
- ROBBINS E., GONATAS N. K. THE ULTRASTRUCTURE OF A MAMMALIAN CELL DURING THE MITOTIC CYCLE. J Cell Biol. 1964 Jun;21:429–463. doi: 10.1083/jcb.21.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise G. E., Prescott D. M. Ultrastructure of enucleated mammalian cells in culture. Exp Cell Res. 1973 Sep;81(1):63–72. doi: 10.1016/0014-4827(73)90111-0. [DOI] [PubMed] [Google Scholar]
- Wright W. E., Hayflick L. Formation of anucleate and multinucleate cells in normal and SV 40 transformed WI-38 by cytochalasin B. Exp Cell Res. 1972 Sep;74(1):187–194. doi: 10.1016/0014-4827(72)90496-x. [DOI] [PubMed] [Google Scholar]