Abstract
Optineurin is a widely-expressed polyubiquitin (polyUb)-binding protein that has been implicated in regulating cell signaling via its NEMO-homologous C-terminal Ub-binding region. Its functions are controversial, with in vitro studies finding that optineurin suppressed TNF-mediated NF-κB activation and virus-induced activation of IRF3, whereas bone marrow-derived macrophages (BMDM) from mice carrying an optineurin Ub-binding point mutation had normal TLR-mediated NF-κB activation and diminished IRF3 activation. We have generated a mouse model in which the entire Ub-binding C-terminal region is deleted (Optn470T). Akin to C-terminal optineurin mutations found in patients with certain neurodegenerative diseases, Optn470T was expressed at substantially lower levels than the native protein, allowing assessment not only of the lack of Ub-binding but also of protein insufficiency. Embryonic lethality with incomplete penetrance was observed for 129 x C57BL/6 Optn470T/470T mice, but after further backcrossing to C57BL/6, offspring viability was restored. Moreover, the mice that survived were indistinguishable from wild type littermates and had normal immune cell distributions. Activation of NF-κB in Optn470T BMDM and BM-derived dendritic cells (BMDC) with TNF or via TLR4, T cells via the TCR, and B cells with LPS or anti-CD40 was normal. In contrast, optineurin and/or its Ub-binding function was necessary for optimal TBK1 and IRF3 activation, and both Optn470T BMDM and BMDC had diminished IFN-β production upon LPS stimulation. Importantly, Optn470T mice produced less IFN-β upon LPS challenge. Therefore, endogenous optineurin is dispensable for NF-κB activation but necessary for optimal IRF3 activation in immune cells.
Introduction
Activation of the transcription factor NF-κB is essential in signaling induced by pathogen- or damage-associated molecular patterns (PAMPs and DAMPs) as well as cellular stresses such as DNA-damage, hypoxia, and excitotoxicity (1, 2). More than 400 NF-κB-responsive genes have been implicated in regulating stimulus- and cell-type-specific responses (3). Cells of the innate immune system such as macrophages and DC express a variety of NF-κB-coupled receptors (for example Toll-like receptors (TLRs), Rig-like receptors (RLRs), and Nod-like receptors (NLR)) that have a major role in orchestrating immune responses and resolving tissue damage. The importance of NF-κB in immune responses is underscored by the fact that subsequent lines of defense, adaptive T and B cell responses, also require NF-κB for signaling via antigen, costimulatory, and cytokine receptors (4). Another major pathway in immune responses is the activation of the IKK-related kinase, TANK binding kinase 1 (TBK1), which although occurring simultaneously with NF-κB activation, leads to a fundamentally different outcome. TBK1 is the major kinase that phosphorylates the transcription factor IRF3, causing its dimerization, nuclear localization, and initiation of type I IFN production (5, 6).
Ubiquitination is a major mechanism of regulation of both NF-κB and IRF3 pathways. The canonical pathway leading to activation of NF-κB is initiated when the inhibitor of κB kinase (IKK), comprising the kinases IKKα and IKKβ and the regulatory subunit NEMO (NF-κB essential modulator), causes phosphorylation and subsequent lysine 48 (K48)-linked polyubiquitination and proteasomal degradation of the inhibitor of κB (IκB) (7). In addition, non-degradatory ubiquitination with polyUb chains linked via lysine 63 (K63) or linear chains in which the N-terminal methionine of one ubiquitin is linked to the C-terminal glycine of another (M1), is required for assembly of multimeric signaling complexes. K63-and/or M1-ubiquitination of various receptor-associated adaptor molecules such as RIP1, IRAK1, and Bcl10, allows the recruitment of NEMO and IKK activation (8–11). Similarly, TBK1 activation requires recruitment of NEMO to signaling complexes containing polyUb chains (12–14).
NEMO has two ubiquitin-binding domains in its C-terminal half, a ~30 amino acid region termed UBAN (ubiquitin-binding domain of ABIN proteins and NEMO), and a more distal zinc finger (ZF) (15, 16). The presence of both domains confers high-affinity binding to K63- and M1-linked ubiquitin chains. Four other proteins contain a UBAN, optineurin and three A20 interacting proteins (ABIN-1, -2 and -3), whereas only optineurin and ABIN-2 have the ZF domain as well. Notably, replacing NEMO’s UBAN and ZF with the C-terminus of optineurin or ABIN-2 restored ubiquitin binding and NF-κB activation in response to a variety of stimuli (16, 17).
Despite their high level of homology, optineurin was not found in the same TNFR signaling complex as IKKβ and NEMO, and its expression could not complement NEMO-deficiency in a TNF-signaled pre-B cell line (17). To the contrary, several studies have directly implicated optineurin in negative regulation of NF-κB signaling. In one, overexpressed optineurin inhibited TNF-induced NF-κB activation by competing with NEMO for ubiquitinated RIP1 (18). In addition, optineurin recruited the deubiquitinase CYLD to the TNF signaling complex, where CYLD removed polyUb from RIP1 (19). It is notable that like NEMO, optineurin binds TBK1 (20). It has been reported that this interaction is constitutive and occurs via the optineurin N-terminus (20, 21). However, another report has suggested that efficient binding of optineurin to TBK1 requires an intact UBAN (22). As with NF-κB, there is evidence that optineurin is a negative regulator of the IRF3 pathway because overexpressed optineurin inhibited, and silencing enhanced, IRF3 activation in virus-infected cells (22). In contrast, BMDM from mice with a Ub-binding disruptive point mutation in the UBAN (OptnD477N) had diminished TLR-mediated activation of TBK1 and IFN-β secretion, suggesting that ubiquitin-binding by optineurin positively regulates the IRF3 pathway (21). At the same time, LPS-mediated activation of NF-κB and secretion of the proinflammatory cytokines IL-6 and IL-12 was unperturbed. Of possible note is that there was a small amount of residual ubiquitin-binding activity in OptnD477N cells, and that the expression of the mutant protein was substantially higher than that of the wild type (WT).
Due to the existence of these contradictory data on the function of optineurin, we generated a mouse in which both the optineurin UBAN and ZF ubiquitin-binding domains were deleted (Optn470T). As often happens upon introduction of a premature termination codon, the truncated protein was expressed at substantially lower levels than the WT, allowing us to study the effects of defective ubiquitin binding as well as the repercussions of optineurin insufficiency. This is particularly relevant because loss-of-function optineurin mutations due to C-terminal truncations and/or reduced protein levels have been associated with human neurodegenerative diseases such as amyotrophic lateral sclerosis (ALS) and primary open angle glaucoma (23–29). Although understanding of the pathogenesis of both of these diseases is incomplete, it is clear that a non-autonomous neuronal death is a major contributor to disease, with immune cells such as microglia (resident macrophages of the central nervous system), peripheral cells of the monocyte/macrophage lineage, and T cells having a prominent part in lesion progression (30, 31). In this report, we have analyzed all of the major immune cell subsets and an in vivo response in a relevant model of optineurin insufficiency.
Materials and Methods
Mice
β-actin Cre transgenic mice were obtained from the Mouse Cancer Genetics Program in NCI-Frederick. C57BL/6 (B6) mice were obtained from the Frederick Cancer Research Facility (Frederick, MD). Mice harboring a conditional optineurin C-terminal deletion were generated by genetic recombineering (32 and http://frederick.cancer.gov;). The 2.9 kB upstream and 3.9 kB downstream recombination arms surrounding exon 12 were subcloned from the BAC clone RP24-255E22 (BACPAC Resource Center) and placed in the thymidine kinase-containing targeting vector pLMJ235. To introduce the upstream loxP site, a loxP-neoR-loxP cassette was placed upstream of exon 12 and was subsequently excised by Cre induction in EL350 E. Coli cells. The FRT-neoR-FRT-loxP cassette was inserted downstream of exon 12. The vector was linearized with NotI digestion and electroporated into B6 x 129 hybrid mouse embryonic stem cells (C57BL/6J x 129/SvJae). The chimeric mice were screened by Southern blotting and PCR analysis. Southern blotting of HindIII restricted WT DNA detected a 13 kB fragment, whereas introduction of an additional HindIII site by the FRT-neoR-FRT-loxP cassette led to the generation of a 10 kB fragment in chimeras. Whole-body deletion of the loxP-flanked segment was achieved by directly crossing mice that carried the recombinant allele with β-actin Cre. These mice are designated as Optn470T. Optn470T mice were subsequently backcrossed onto the B6 background to generate F3 and F5 offspring, which were used in these studies. The homozygous Optn470T were always compared to WT mice of the same generation. All animal studies were approved by the NCI Animal Care and Use Committee.
Reagents
Antibodies to the C-terminus of optineurin were purchased from Cayman (#100000), to the central region (ab101592) from Abcam, and to the N-terminus (C-2) from Santa Cruz. Antibodies to phospho-Ser473 Akt, P-ERK, phospho-Ser172 TBK1, TBK1, IRF3, and phospho-Ser396 IRF3 were from Cell Signaling, to IFN-β for ELISA (7F-D3) from Abcam, and to IκBα from Santa Cruz. Secondary antibodies conjugated with HRP were purchased from GE Healthcare, and antibodies conjugated to IR670 or IR800 were purchased from Rockland. Nitrocellulose membranes were from Biorad. Protease and phosphatase inhibitor cocktails and SuperScript® First-Strand Synthesis System were obtained from Roche, and enhanced chemiluminiscence reagent from Pierce. β-ME and antibodies to β-actin and Flag, and LPS were from Thermo Scientific. Purified anti-mouse CD3 (145-2C11), anti-mouse CD28, anti-mouse CD40 and flow cytometry antibodies to CD3ε, CD4, CD8, CD25, CD44, CD62L, CD69, TCRβ, B220, CD21/35, CD23, CD11b, NK1.1, CD19, CD11b, 2.4G2 (Fc block) were from BD Pharmingen. Antibodies against CD8, CD11c, IFN-γ, and MHC Class II, ELISA kits for IL-12p70, TNF and IL-6, TMB solution, and FlowCytomix Simplex kits for the following cytokines: IL-12p70, TNF, IL-1β, IFN-γ, IL-6 and CXCL1/KC, were purchased from eBioscience. Power SYBR Green and luciferase/β-galactosidase reagents were obtained from Applied Bioscience. High-molecular weight poly I:C and CpGA were from Invivogen, Fixable Dead Cell Stain, NuPAGE®SDS-PAGE, CFSE and Lipofectamine 2000 were from Invitrogen/Life Technologies, and luciferase reagents from Applied Biosystems. Recombinant TNF and IFN-β and secondary rabbit polyclonal anti-IFN-β antibodies for ELISA were purchased from R&D and recombinant GM-CSF from Prospec. GP33-41 H-2Db (GP33), GP276-286 H-2Db (GP276), and NP396-404 H-2Db tetramers (NP396) were obtained from the NIH Tetramer facility at Emory University, Atlanta, GA. GP33-41 peptide was purchased from Peptide 2.0 Inc.
Cell lines and plasmids
Human embryonic kidney 293 cells (293) were obtained from the American Type Culture Collection (ATCC) and 293 cells expressing TLR3 (hTLR3-293) from InvivoGen. Cell lines were maintained in DMEM, and primary cells in RPMI, supplemented with 10% fetal calf serum, 5 mM glutamine, 5 μM β-mercaptoethanol, and 100 μg/ml gentamicin. Human full length optineurin (WT), the C-terminal truncation (1–467), and the previously described D474N mutation (18) were placed in N-terminal Flag-tagged mammalian expression vector Gateway®pDEST™26 (Invitrogen). The p-55D1BLuc construct that expresses luciferase under the control of repeated PRDI domains recognized by IRF3 was a kind gift of Dr. Takashi Fujita (33). pcDNA3.1/His/LacZ containing the gene encoding β-galactosidase was obtained from Invitrogen, and pNF-κB luc encoding luciferase from Clontech.
Cell transfection and luciferase reporter assays
Plasmids encoding luciferase under the control of the NF-κB or IRF3 promoters, β-galactosidase, and optineurin variants or control vector were co-transfected in 293 cells using Lipofectamine 2000, and were left unstimulated or stimulated with 20 ng/ml TNF or 10 μg/ml poly I:C 24 hr later. Cells were lysed 5 hr after stimulation and luciferase activity measured using a 96-well luminometer and Veritas software and normalized to β-galactosidase.
Cell preparation and purification
Mouse embryonic fibroblasts were prepared from E11.5 and E13.5 embryos as described (34). BMDM and BMDC were generated from bone marrow cultured for 5 days with 30% L929 supernatant (BMDM) or 8 days with recombinant GM-CSF (BMDC). BMDM and BMDC were stimulated with 100–500 ng/ml LPS, or 10–20 ng/ml TNF. T and B cells were purified from lymph nodes or spleen, respectively, using the specific Easy Sep enrichment kit (StemCell Technologies) following the manufacturer’s protocol and the number of live cells was assessed by trypan blue exclusion. Purity was determined by flow cytometry, and for all experiments was > 90%.
T and B cell proliferation assay
Proliferation assays were performed in 96-well flat-bottomed plates in a final volume of 200 μl. For T cells, wells were coated with anti-CD3 and anti-CD28 overnight at 4°C in PBS. Cells were cultured for 48 hr, pulsed with 1 μCi [3H]-thymidine, and harvested 18 hr later. [3H]-thymidine uptake was determined with a Wallac 1450 MicroBeta Liquid Scintillation Counter. All experimental points were performed in triplicate and the error bars represent the standard error of the mean. CFSE was used at a concentration of 500 nM and cells were stained following the manufacturer’s instructions.
Flow cytometry
After Fc blockade, cells were stained with the indicated antibodies and flow cytometry was performed with a BD LSRFortessa cytometer using BD FACSDiva software (BD Biosciences). The same cytometer was used to analyze Flowcytomix beads after cytokine capture. All flow cytometry data analysis was performed with FlowJo software (Tree Star).
Immunoblotting
Cells were normalized by number and lysed either in IP lysis Buffer (Pierce) or in sample buffer (50 mM Tris pH 6.8, 10% glycerol, 2% SDS, 2% β-mercaptoethanol, and 0.04% bromophenol blue), resolved with NuPAGE®SDS-PAGE gels, transferred to nitrocellulose membranes, and immunoblotted with the indicated antibodies. To detect P-Akt and P-IRF3, samples were lysed in RIPA buffer (Sigma-Aldrich) and normalized to protein concentration. Immunoblots were developed with either HRP-conjugated secondary antibodies and enhanced chemiluminiscence or fluorescently-labeled secondary antibodies and an Odyssey imaging system (Li-COR Biosciences). Densitometry was performed using ImageJ software.
Immunodetection assays
ELISA for IL-12, TNF, and IL-6 were done according to manufacturer’s instructions (eBioscience). IFN-β ELISA was performed as described (35). In brief, rat monoclonal anti-mouse IFN-β was used for plate coating, rabbit polyclonal anti-mouse IFN-β in solution, and HRP-conjugated goat anti-rabbit IgG was used for detection. Flowcytomix kit was used according to manufacturer’s protocol.
PCR and quantitative RT-PCR (qRT-PCR)
The following primers were used to generate the Southern blot probe, forward: TATTCCGATTCACTCCTGCCCC; reverse: ACAGATTATCCCTCCTGAGACGC. The primers used to distinguish floxed and WT optineurin alleles were, forward: GCTACCATGCTCAGCCAGAGTTTC; reverse: GGCTTCAGGGATGCATGAATC. The Optn470T allele was detected with the following primers, forward: GCAACACAGACCTGAACAGACG; reverse: ACTCCACCCATAAGTCATCAAAGC. Real-time PCR was performed with SYBR Green using a 7500 Real Time PCR System by Applied Bioscience. Housekeeping ribosomal 18S RNA was amplified to normalize RNA content of the lysate and obtain a ΔCT value. The primers used were, Optn-Forward: GCTCCGAAATCAAGATGGAG; Optn-Reverse: GCAGAGTGGCTAACCTGGAC; Ifnb-Forward: CTGGCTTCCATCATGAACAA; Ifnb- Reverse: AGAGGGCTGTGGTGGAGAA; 18S-Forward: AAATCAGTTATGGTTCCTTTGGTC; 18S-Reverse: GCTCTAGAATTACCACAGTTATCCAA.
In vivo studies
LPS (3 mg/kg) was injected intraperitoneally (i.p.) and blood was drawn 6 hr later. For LCMV infection, Armstrong strain 53b was grown in baby hamster kidney cells, and viral titers were determined as reported (36). WT and Optn470T mice were injected i.p. with 2 x105 plaque forming units (PFU) of virus, and spleens were collected 8 days after infection. For intracellular cytokine detection, 3 × 106 splenocytes were either stimulated with medium alone or 0.3 μg/ml GP33–41 for 4 hr in the presence of BD GolgiStop. Cells were stained with Abs to surface markers and fixed. The 2.4G2 Ab was added to the permeabilization buffer to block Fc-receptor binding prior to the addition of anti-cytokine Abs.
Statistical analysis
Statistical analysis was done using Student’s t test with GraphPad Prism software.
Results
In vitro suppression of NF-κB and IRF3 by overexpressed optineurin requires the C-terminal region
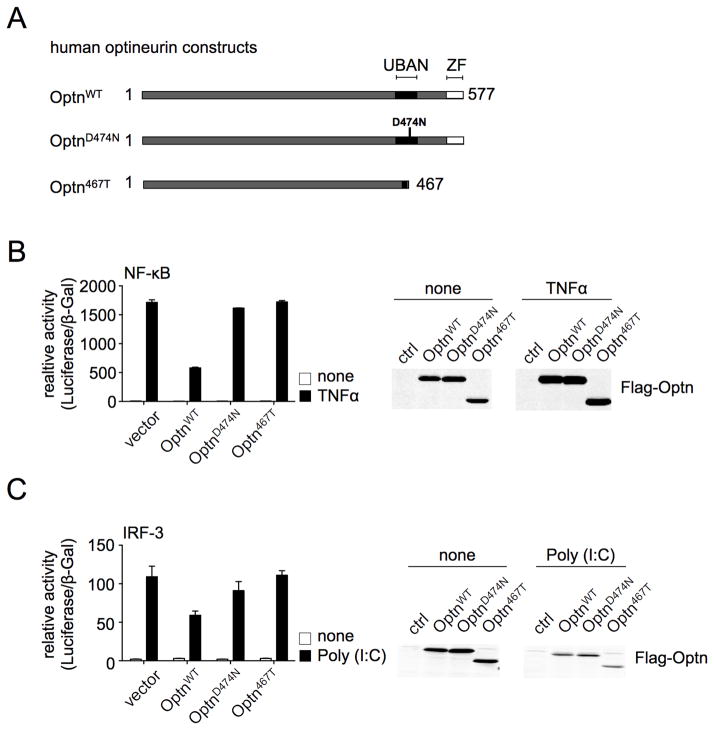
The importance of the C-terminal Ub-binding region of optineurin to NF-κB- and IRF3-dependent transcription was assessed in 293 cells transiently expressing WT or mutated human optineurin (Fig. 1). Stimulation with TNF highly induced NF-κB reporter activity, which was substantially inhibited by overexpression of full-length optineurin but not the Ub-binding mutant optineurinD474N, as described (18). Importantly, the C-terminal deletion, Optn467T (corresponding to mouse Optn470T) was incapable of mitigating NF-κB activation (Fig 1B). Stimulation with the TLR3 agonist polyinosinic:polycytidylic acid (poly I:C) increased IRF3-reporter activity, which was reduced by co-transfection of full length but not OptnD474N, as reported (22), or Optn467T (Fig 1C). Thus, overexpression of optineurin inhibits both NF-κB and IRF3 pathways in a manner dependent upon its Ub-binding domain.
Figure 1. WT but not Optn470T expression inhibits activation of NF-κB and IRF3.
Human optineurin constructs, homologous to murine WT (1–584), the Ub-binding point-mutant D477N, and the C-terminal truncation (1–470) are depicted (A). Human embryonic kidney 293 cells were transiently transfected with the indicated constructs and stimulated or not with TNF. Reporter luciferase activity was normalized to β-galactosidase activity (B, left panel), and anti-Flag blotting for Flag-optineurin variants is shown (B, right panel). hTLR3-293 cells, which stably express TLR3, were transiently transfected as indicated and stimulated or not with poly I:C. Luciferase activity (C, left panel), and anti-Flag blotting (C, right panel) were performed as in panel B. An average of duplicate samples of one representative experiment out of three is shown.
The generation of Optn470T knock-in mice
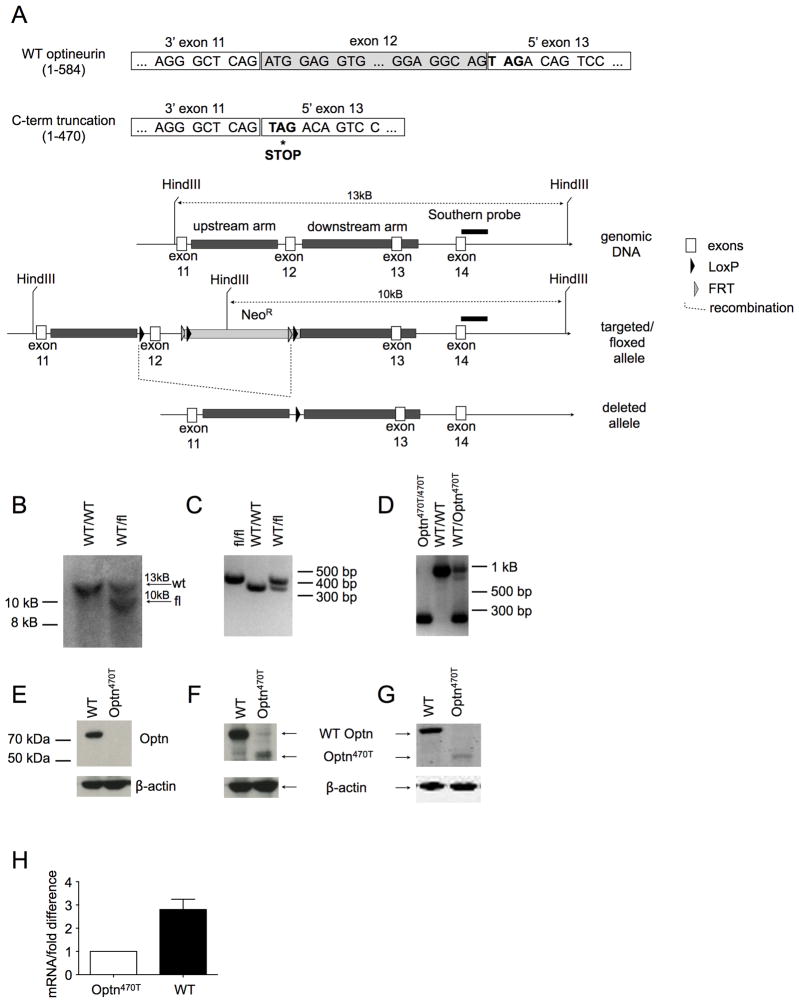
To generate a mouse with a conditional optineurin C-terminal deletion, exon 12 was flanked with loxP recombination sites (Fig 2A). Upon the predicted Cre-mediated deletion, a stop codon would be exposed immediately at the beginning of exon 13, resulting in translation of a truncated protein of 470 amino acids. Mice carrying the recombinant floxed allele were verified by Southern blot (Fig 2B) and PCR (2C), and were crossed to mice expressing Cre-recombinase under the control of the β-actin promoter to achieve exon 12 deletion. The excision of the exon 12 was verified by PCR of tail DNA (Fig 2D), and the lysates of BMDM from homozygous WT and Optn470T mice were blotted for optineurin. An antibody that binds the C-terminus detected the WT 75 kDa protein, but no band was found in homozygous Optn470T cells (Fig 2E). Antibodies against central (Fig 2F) and N-terminal regions of optineurin (Fig 2G) showed the expected smaller band of approximately 55 kDa in Optn470T cells. Notably, the level of the truncated protein was approximately 7-fold lower than that of the WT. Attempts to rescue protein expression with inhibitors of common proteases (aprotinin, leupeptin, pepstatin), caspases (zVAD), lysosomal proteases (chloroquine), and the proteasome (MG-132) had no effect, suggesting that the lower levels were not the result of proteolysis (our unpublished observation). On the other hand, as often happens after introduction of a premature termination signal, Optn470T mRNA expression was decreased (~3-fold) in Optn470T mice (Fig. 2H). The likely cause was a conserved eukaryotic quality-control mechanism known as nonsense-mediated mRNA decay (NMD), which is often initiated when a premature stop codon is detected (37, 38). In our case, the introduced stop signal was located ~80 nucleotides downstream of the exon 13–14 boundary, which is a greater distance than the minimum 50–55 nucleotide separation from an exon-exon boundary that activates NMD. Thus, the substantial reduction of Optn470 protein (Fig. 2F and G) could be largely accounted for by mRNA instability and translational repression associated with NMD (37).
Figure 2. Generation of Optn470T mice.
(A) To generate mice with C-terminal truncation of optineurin (Optn470T), a targeting construct with LoxP sites flanking exon 12 was inserted into the endogenous locus by homologous recombination. Upon Cre-mediated deletion, a stop codon was exposed at the beginning of exon 13, resulting in a truncated protein of 470 amino acids. Neo cassette, Southern probe, and recombination sites are indicated. (B) Southern blot distinguishing WT and chimeric mice (WT/fl) is shown. The introduced HindIII site in the Neo cassette leads to generation 10 kB band in the fl allele. The PCR of the indicated mice distinguishing WT and floxed (C) and WT and deleted alleles is shown (D). Blotting BMDM from the indicated mice with optineurin antibodies raised against C-terminal (E), central (F) and N-terminal epitopes (G). Optineurin mRNA was detected in BMDM by qRT-PCR, ΔCT of Optn470T was designated as 1, and the difference between ΔCT of WT and Optn470T is depicted as mean ± SEM for 3 independent experiments (H).
Homozygous Optn470 knock-in is embyonic lethal with incomplete penetrance
Interbreeding of heterozygous (het) WT/ Optn470T F3 mice resulted in very few homozygous Optn470T offspring. Of the first >100 mice from such het x het breedings, instead of the expected 25% homozygous Optn470T mice, only 3% were found (Table I). Similarly, only 1 out of 20 embryos taken between embryonic day 11.5 and 13.5 was homozygous Optn470T. Embryonic death occurred early, as the inspection of embryos on day E11.5 found that ~25% of the placental sacs had completely resorbed embryos, arguing that the critical period when optineurin is required for development occurs well prior to embryonic day 11.5. The few live homozygous Optn470T mice were physically indistinguishable from WT littermates, and were fertile when intercrossed, indicating incomplete phenotypic penetrance. The initial ES cells used to create the knock-ins were 129 x B6. Further breeding of heterozygous Optn470T mice onto the B6 background for the total of five backcrosses resulted in viable Optn470T offspring that were born at the expected Mendelian ratios (Table II). The mice studied in this report are from a colony generated from the survivor animals of independent heterozygous breeding couples. In conclusion, our results suggest that embryos of the 129 background are particularly prone to death when there are suboptimal levels of optineurin and/or optineurin lacks the C-terminus.
Table I. Optn470T mice on the 129 x B6 genetic background die embryonically.
Heterozygous OptnWT/470T F3 mice were intercrossed and the genotype of the offspring was analyzed by PCR from tail DNA of adult mice or mouse embryonic fibroblasts generated from E11.5–E13.5 day old embryos. The expected and observed frequencies from 135 adult mice and 20 embryos are shown.
| WT/Optn470T x WT/Optn470T | WT/WT | WT/Optn470T | Optn470T/Optn470T |
|---|---|---|---|
| expected frequency | 25% | 50% | 25% |
| adult mice (number) | 48 | 83 | 4 |
| observed frequency of adult mice | 35% | 61% | 3% |
| developed embryos (number) | 5 | 14 | 1 |
| observed frequency of developed embryos | 25% | 70% | 5% |
Table II. Optn470T mice further backcrossed to B6 background were born at the expected Mendelian ratios.
Heterozygous OptnWT/470T mice backcrossed onto the B6 background for five generations (F5) were intercrossed and the genotype of the offspring was analyzed by PCR of tail DNA. The expected and observed frequencies of 67 adult mice are shown.
| WT/Optn470T x WT/Optn470T | WT/WT | WT/Optn470T | Optn470T/Optn470T |
|---|---|---|---|
| expected frequency | 25% | 50% | 25% |
| adult mice (number) | 14 | 38 | 15 |
| observed frequency of adult mice | 21% | 57% | 22% |
The C-terminus of optineurin is dispensable for immune cell development and T and B cell activation
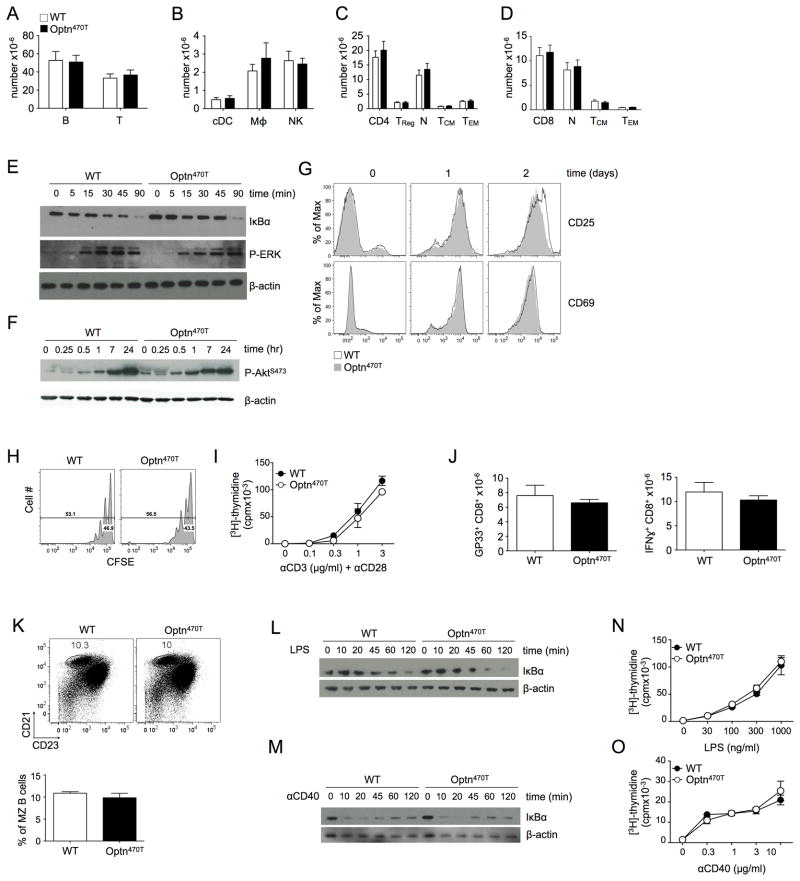
Because many molecules in the NF-κB signaling pathway influence the development of cells of the immune system (39, 40), we analyzed the peripheral lymphoid tissues of homozygous Optn470T animals. Homozygous Optn470T F3 survivors and F5 mice were indistinguishable, so the data from all homozygous mice were pooled. Splenocyte numbers were comparable (data not shown) and all major innate and adaptive immune subsets were present in normal frequencies and numbers (Fig 3A and 3B). Moreover, no signs of excessive activation were detected in the T cell compartment, as the number of regulatory CD4 T cells (TReg, Fig 3C) and ratios of naïve to memory cell subsets within both CD4 (Fig 3C) and CD8 T cell (Fig 3D) compartments were comparable between WT and Optn470T mice.
Figure 3. T and B cell development and activation are unimpaired in Optn470T mice.
T and B (A), conventional DC (cDC), macrophage, and NK cell numbers (B) in spleens of the indicated mice are shown. CD4 (C) and CD8 T cell (D) numbers and their respective compartments are shown. Naïve cells were gated as CD44loCD62Lhi, TCM as CD44hiCD62Lhi, and TEM as CD44hiCD62Llo. The data from 8 mice is shown with mean ± SEM. T cells freshly purified or stimulated with anti-CD3 (3 μg/ml) and anti-CD28 (2 μg/ml) were lysed at the indicated times, and IκBα degradation and ERK1/2 phosphorylation (E), and Akt phosphorylation (F) were detected by immunoblotting. Expression of the indicated activation markers was monitored on WT and Optn470T purified T cells at the indicated times after stimulation with plate-bound anti-CD3 (3 μg/ml) and anti-CD28 (2 μg/ml) (G). CFSE-labeled T cells were stimulated as in C, and CFSE dilution was monitored after 60 hr (H). Purified T cells were cultured with 2 μg/ml of anti-CD28 and the indicated concentrations of anti-CD3 for 48 hr, pulsed with [3H]-thymidine, and harvested 18 hr later (I). WT or Optn470T mice were infected with LCMV Armstrong and the absolute numbers of GP33-tetramer+ CD8 T cells analyzed on the peak of the response (day 8; J, left panel). Splenocytes were restimulated in vitro with GP33 peptide and evaluated for cytokine production by intracellular staining. Absolute numbers of IFN-γ producing CD8 T cells are shown (J, right panel). The data represent mean ± SEM of 6 individual mice per strain from two independent experiments. Distribution of CD21hiCD23int MZ B cells (gated in B220+TCRβ- population) in splenocytes of WT and Optn470T mice was determined by flow cytometry. Numbers represent the percentage of cells in the gated region and the bar graph below represents the average of 3 mice per group ± SEM (K). B cells freshly purified or stimulated with LPS (100 ng/ml) (L) or anti-CD40 (1 μg/ml) (M) were lysed at the indicated times and IκBα was detected by immunoblotting. Purified B cells were cultured with the indicated concentration of LPS (N) or anti-CD40 (O) for 48 hr, pulsed with [3H]-thymidine, and harvested 18 hr later. Each panel is representative of two or three independent experiments.
TCR-mediated signaling in Optn470T T cells was analyzed. Anti-CD3/CD28 induced rapid degradation of IκBα and phosphorylation of ERK1/2 and Akt, with no obvious differences between WT and Optn470T T cells (Fig 3E and F). Flow cytometric analysis of the activation markers CD25 and CD69 also found no difference between WT and Optn470T CD4 and CD8T cells at rest or over the course of two days of in vitro activation (Fig 3G and data not shown). T cell proliferation, analyzed by CFSE dilution and 3[H]-thymidine incorporation, was similar between WT and Optn470T T cells (Fig 3H and 3I). To test in vivo responses, Optn470T mice were infected with lymphocytic choriomeningitis virus (LCMV) Armstrong strain. LCMV infection causes extensive CD8 T cell proliferation that is crucial in clearing the virus (41). Infection of Optn470T mice with LCMV Armstrong induced expansion of GP33+ (Figure 3J), NP396+, and GP276+ (data not shown) antigen-specific CD8 T cells, and IFN-γ production upon restimulation with peptide comparable to WT mice. Analysis of the viral load at day 8 showed clearance of LCMV by both WT and Optn470T mice (data not shown). Therefore, optineurin insufficiency has no apparent effect on T cell activation and function.
Dysregulation of NF-κB signaling is associated with impaired generation of B cells with a marginal zone (MZ) phenotype (42, 43). Notably, the percentage of CD21hiCD23int splenic MZ B cells was indistinguishable between WT and Optn470T mice (Fig 3K). Intact NF-κB signaling is also required for optimal B cell function (40). Engagement of TLR4 and CD40 induced IκBα degradation with comparable kinetics in WT and Optn470T B cells (Fig 3L and 3M) and triggered proliferation to a similar extent (Fig 3N and 3O). Therefore, the Optn470T mutation does not perturb B cell NF-κB signaling or activation.
Optineurin does not influence the NF-κB activation in innate immune cells
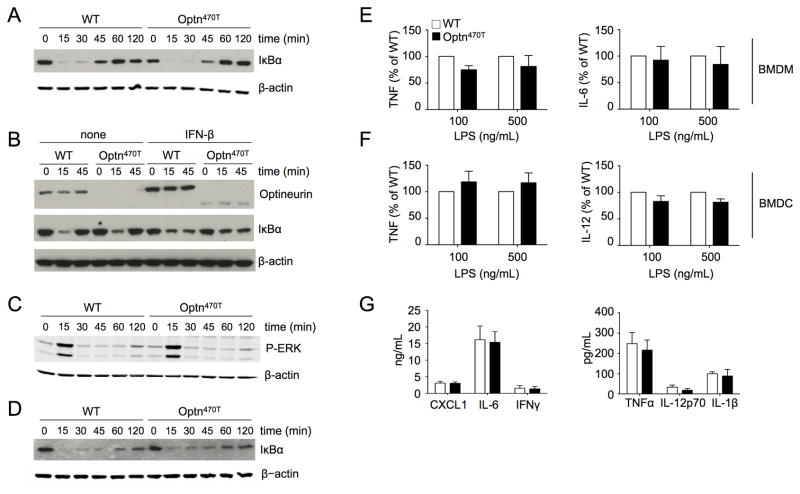
Experiments in cell lines have suggested a role for optineurin in the negative regulation of TNF-induced NF-κB (Fig 1 and (18, 19)). To assess TNF-induced NF-κB activation in primary cells, we generated BMDM from bone marrows cultured with L929 supernatants containing macrophage colony-stimulating factor (M-CSF). Of note, BMDM differentiation was unperturbed in Optn470T mice (data not shown). Comparable kinetics of IκBα degradation and resynthesis in TNF-treated WT and Optn470T cells was observed (Fig 4A). Because optineurin is itself upregulated by various cytokines, including TNF and IFN-β (17), it is possible that it regulates later phases of NF-κB activation. To test this, BMDM were pretreated with IFN-β for 16 hr and then treated with TNF (Fig 4B). Both WT and Optn470T protein were upregulated by IFN-β. However, WT and Optn470T BMDM showed similar pattern of TNF-induced IκB degradation, arguing against a role for optineurin in late NF-κB activation. TNF treatment also activates mitogen-activated protein kinases (MAPK) such as ERK1/2. The degree of ERK1/2 activation, as measured by phosphorylation of its activation loop, was indistinguishable between WT and Optn470T BMDM (Fig 4C). Analysis of IκB degradation in response to the TLR4 ligand LPS also showed no difference between WT and Optn470T BMDM (Fig 4D). Similar results were obtained in another innate immune cell type, BMDC (data not shown). Thus, no obvious function of optineurin was found in innate immune cells activated via TNFR or TLR4.
Figure 4. BMDM and BMDC from Optn470T mice have unimpaired NF-κB responses.
BMDM from WT and Optn470T mice were stimulated with TNF for the indicated times and degradation of IκBα was detected by immunoblotting (A). BMDM pretreated overnight with 300 U/ml of IFN-β or not were stimulated with TNF as indicated, and lysates were blotted for optineurin and IκBα (B). Cell lysates of TNF-treated BMDM were blotted for P-ERK (C). Cell lysates of LPS-stimulated BMDM were blotted for IκBα (D). A representative example for two to three experiments is shown for all blots, with β-actin staining as a loading control. The indicated cytokines secreted upon LPS stimulation of Optn470T BMDM (E) and BMDC (F) are depicted as the percent of WT values. The data from for two to three independent experiments are shown as mean ± SEM. Cytokines detected in the sera of LPS-injected WT and Optn470T BMDM are shown (G). The mean ± SEM from 4 mice from two independent experiments is shown.
LPS induces a large number of signaling events, and it is possible that pathways that were not examined are regulated by optineurin. To examine more global readouts of activation with LPS, we measured cytokine secretion by BMDM and BMDC. No differences were found in LPS-induced TNF and IL-6 production by BMDM, or TNF and IL-12 production by BMDC (Fig 4E and 4F). A broader set of cytokines and chemokines was analyzed upon LPS injection in vivo, a model for septic shock. Notably, CXCL1, IL-6, IFN-γ, TNF, IL-12p70, and IL-1β all had similar levels in the sera of WT and Optn470T mice (Fig 4G). Together, these results strongly argue that the Ub-binding domain of optineurin is not required for TNF- or LPS-mediated activation of cells of the innate immune system.
Optineurin positively regulates TBK1 and IRF3
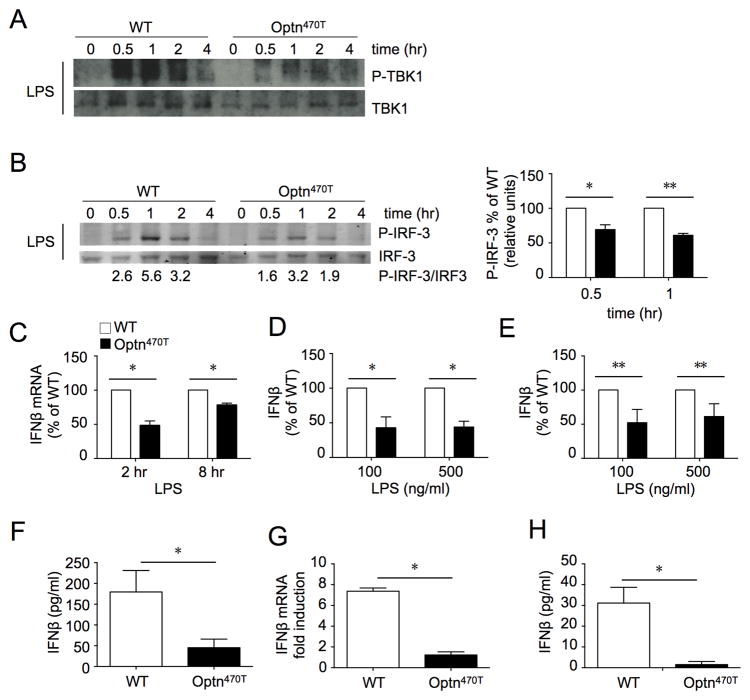
BMDM from OptnD474 mice had impaired TBK1 and IRF3 activation and reduced IFN-β secretion in response to TLR3/4 stimulation (21). To determine the effect of deletion of the entire C-terminus as well as very low optineurin expression, BMDM were treated with LPS and TBK1 activation was assessed. Phosphorylated TBK1 is active and required for phosphorylation of IRF3. Whereas stimulation of WT BMDM with LPS induced strong TBK1 phosphorylation that peaked at 30 minutes, Optn470T BMDM showed much weaker phosphorylation at all time points (Fig 5A). The subsequent phosphorylation of IRF3 peaked 1–2 hr after stimulation in WT BMDM (Fig 5B). Although the time course of activation was similar in WT and Optn470T BMDM, phosphorylation of IRF3 in the latter was diminished by 30–40% at the peak of activation (Fig 5B, right panel). Consequently, upregulation of mRNA for IFN-β (Fig 5C) and IFN-β secretion were lower in Optn470T than WT BMDM (Fig 5D). Similar results were found in BMDC, for which LPS stimulation resulted in lower IFN-β secretion in Optn470T cells (Fig 5E). To assess the biological relevance of these findings, WT and Optn470T mice were injected with LPS and IFN-β levels were measured 6 hr later. Substantially lower levels of IFN-β were detected in the sera of Optn470T mice (Fig 5F). Until recently, the TIR-domain-containing adapter-inducing IFNβ (TRIF) was the sole adapter thought to convey TLR3/4-mediated signals that lead to TBK1-mediated type I IFN secretion. However, it was recently shown that BMDM MyD88-coupled TLRs also activate the TBK1 pathway (44). To determine if optineurin is capable of mediating TBK1 activation without TRIF, we tested assessed IFN-β responses upon BMDM stimulation with the TLR9 ligand CpGA, a potent agent that mimics viral and bacterial (unmethylated) DNA and whose signaling requires MyD88 but is TRIF-independent. CpGA stimulation of Optn470T BMDM resulted in substantially lower levels of IFN-β mRNA and protein than in WT cells (Figure 5G and 5H), providing evidence that optineurin is required for MyD88-mediated TBK1 activation. Together, these results establish optineurin as a positive regulator of IRF3 and Type I IFN production.
Figure 5. BMDM and BMDC from Optn470T mice have diminished IRF3 responses.
Phospho-TBK1, total TBK1 (A), and phospho-IRF3 and total IRF3 (B) were detected by blotting lysates of BMDM from WT and Optn470T mice at the indicated times after LPS treatment. The lanes were rearranged for clarity. A representative blot of 5 experiments is shown. The densitometry was done on two phospho-IRF3 and total IRF3 blots that were developed with fluorescently-labeled secondary antibodies; numbers below the blot represent the quantitation, and an average of two independent experiments ± SD is displayed (right panel). mRNA for IFN-β was amplified from BMDM at 2 and 8 hr (C), and IFN-β was measured in supernatants at 24 hr (D) after LPS treatment. The mean ± SEM of Optn470T is depicted as percent of WT. IFN-β in supernatants of WT and Optn470T BMDC treated with LPS (E). The data are representative of 2–5 experiments each. IFN-β was measured in the sera of WT and Optn470T mice 6 hr after LPS injection (F). An average of seven mice ± SEM is shown per each genotype. BMDM from WT and Optn470T mice were stimulated with CpGA (2 μM). mRNA for IFN-β was amplified after 8 hr (G), and IFN-β was measured in supernatants at 24 hr (H). The data represent mean ± SD measured from two independent experiments. * p < 0.05; ** p <0.001.
Discussion
Controlled activation of NF-κB is vital to the health of the organism. Whereas suboptimal NF-κB activation can lead to immunodeficiencies, excessive activation is linked to autoimmunity, neurodegeneration, and cancer (45–47). Based upon experiments performed in cell lines, optineurin has been proposed to be a negative regulator of TNF-induced NF-κB activation by preventing the binding of NEMO and recruitment of CYLD to ubiquitinated RIP1 (18, 19). Another clue that optineurin inhibits excessive NF-κB activation came from studies of two human neurodegenerative diseases, amyotrophic lateral sclerosis (ALS) and primary open angle glaucoma (23, 24), diseases in which microglia-derived TNF is thought to potentiate the activation of glutamate receptors and excitotoxic neuronal death (48). Many of the optineurin mutations associated with neurodegeneration have either large C-terminal deletions or point mutations in the Ub-binding region, suggesting that the lack of Ub-binding is a major pathogenic mechanism. Fittingly, mutations found in ALS patients were incapable of inhibiting TNF-induced NF-κB activation in the NSC-34 neuroblastoma/spinal-cord cell line (24). Our study reproduced some of these findings showing that an intact optineurin Ub-binding domain confers the ability to suppress NF-κB activation in vitro. However, the results obtained with primary immune cells from Optn470T mouse, similar to previously published OptnD477N BMDM, suggest that the in vitro data were an overexpression artifact rather than the demonstration that optineurin is a negative regulator of NF-κB. Off-target activity is not uncommon for Ub-binding proteins (49). For example, even though NEMO is an essential positive regulator of the NF-κB, its overexpression leads to reduced NF-κB activation due to off-target effects (49).
TNF-mediated NF-κB activation was not assessed in the recently reported OptnD477N mice, although NF-κB activation by TLR-ligands resulted in normal IκB degradation and IL-6 and IL-12 secretion in BMDM (21). As this particular mutation has not been described in human disease, the possibility remains that it does not completely reproduce findings in patients and/or that the C-terminal region harbors other sites relevant for optineurin function. Moreover, the previously reported OptnD477N mice had levels of optineurin that were higher than in WT mice, whereas many of the optineurin C-terminal truncations found in human disease result in protein insufficiency (24, 25, 27, 28, 50, 51). Here we have addressed the role of optineurin in TNFR-signaling and other stimulation settings in a genetic mouse model in which WT optineurin was replaced by a mutant lacking both ubiquitin binding domains (UBAN and ZF) and the linker region between them that harbors a putative binding site for the CYLD deubiquitinase (16). Of note, Optn470T was also expressed at a substantially lower level than WT, allowing us to address the effect of optineurin insufficiency. Although the development of the major immune cell subsets depends on NF-κB (39, 40), no evidence of impaired cellularity or autoimmunity was found in these mice. Moreover, activation of T cells via the TCR, macrophages and DC via TNFR or TLR4, and B cells via TLR4 or CD40, showed no signs of impaired NF-κB activation, strongly arguing against the role of optineurin in these pathways. Whereas it is formally possible that low levels of residual Optn470T, even if devoid of Ub-binding, are sufficient for regulating NF-κB, we think this is unlikely because overexpressed Optn470T was incapable of mitigating NF-κB activation. An alternative possibility is that although expressed in almost all cell types tested, optineurin has cell-specific functions that are perhaps conspicuous only in specific neurons or glial cells. A recent report demonstrated a slight upregulation of TNF-mediated NF-κB activation upon optineurin silencing in a neuroblastoma cell line that resulted in increased cell death (52). It is of note that the phenotype in that cell line was not very prominent, and that further neurological analysis of the aged Optn470T mice will allow us to address those issues in primary neuronal cells and in vivo.
A prominent finding in Optn470T mice with a B6 x 129 background was early embryonic lethality. It is of note that mice with impaired NF-κB activation due to deficiency of either NEMO, IKKβ, or p65 die at a similar or later stage of embryonic development as the Optn470T mice (53–56). Because crossing all of these strains to either TNF- or TNFR1-deficient animals led to rescue from embryonic lethality, it is thought that the death occurs due to hypersensitivity to TNF caused by inadequate NF-κB activation. Curiously, although TBK1 is not directly implicated in IKK activation and TNF responses in primary cells, embryonic lethality of TBK1-deficient animals was also rescued by disruption of TNFR1 expression (57). We found that simply backcrossing of the Optn470T mice further onto the B6 background led to normal litters, making it unlikely that the cause of lethality was TNF sensitivity. Notably, a similar phenomenon was observed in ABIN1-deficient mice, which died embryonically on a mixed B6 x 129 background but for which postnatal survival was found when backcrossed further onto B6 (58). The pro-survival role of optineurin in a mixed B6 x 129 background is unclear. Because homozygous Optn470T mice on the mixed background died at an earlier embryonic stage than most deaths due to NF-κB deficiency (the most severe defect observed was in NEMO−/− embryos, but they were not yet resorbed on E11.5) (53–56), it seems likely that the requisite role of optineurin is unrelated to this pathway. A prominent role of optineurin in neuronal degeneration could perhaps argue for lethality due to impaired organogenesis/neurogenesis (59, 60).
TBK1−/− cells have a major defect in mounting an IFN-β response to TLR3 and TLR4 stimulation (61). As a TBK1 interacting protein, optineurin has been implicated as either enhancing or impeding signaling via these receptors. Optineurin overexpression inhibited and silencing enhanced IRF3 activation in virus-infected cells (22). On the other hand, studies in OptnD477N BMDM implicated optineurin as a positive regulator the IRF3 pathway via TLR3/4 stimulation (21). In this study we observed that C-terminal optineurin deletion and/or the substantial loss of the protein in Optn470T mice diminished TLR4-induced IFN-β responses in BMDM and BMDC and in an in vivo sepsis model, further establishing the physiological role of optineurin as a positive regulator of the IRF3 pathway. It is notable that Optn470T IFN-β responses were also diminished in response to a TLR9-agonist, arguing for the role of optineurin in mediating not only TRIF- but also MyD88-dependent stimuli. Type I interferons are also major costimulators in CD8 T cell expansion in response to LCMV virus, with IFNAR-deficient cells being almost incapable of expansion (62). However, in vivo CD8 T cell expansion upon LCMV infection was not diminished in Optn470T mice, which is consistent with the data in the literature arguing that only complete IFNAR-unresponsiveness hampers this process (62). The fact that the IFN-β response was diminished but not absent suggests that either factors other than optineurin contribute to TBK1 activity or that the remaining response depends on the activity of IKKε (63), an inducible TBK1-related kinase expressed in myeloid cells that, unlike TBK1, does not interact with optineurin (17). NF-κB and IRF3 activation was focused on because these pathways have been reported to be regulated by optineurin in a variety of in vitro and in vivo studies. However, it is possible that optineurin has important roles in other cellular events. Indeed, the fact that the Optn470T mice underwent embryonic death tells us that this protein has vital roles that appear, in this instance, to be strain-dependent. Whether this is due to speculated roles in Golgi integrity (64), vesicle trafficking (65, 66), autophagy (67, 68), or cell division (69), remains to be determined.
Acknowledgments
We are grateful to Lino Tessarollo and Eileen Southon (Mouse Cancer Genetics Program, NCI Frederick) for their assistance during generation of Optn470T mice, Mirela Kuka and Paul Mittelstadt for helpful discussions, Bei Dong, Ehydel Castro, Steven Leung and Neda Savic for technical assistance, Dr. Takashi Fujita for allowing the use of p-55D1BLuc construct, Dr. Patricia Johnson for the IFN-β ELISA protocol, Dr. Rafi Ahmed for LCMV Armstrong, and the NIH Tetramer facility at Emory University for supply of tetramers.
References
- 1.Ghosh S, Hayden MS. Celebrating 25 years of NF-kappaB research. Immunol Rev. 2012;246:5–13. doi: 10.1111/j.1600-065X.2012.01111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lebrun-Julien F, Duplan L, Pernet V, Osswald I, Sapieha P, Bourgeois P, Dickson K, Bowie D, Barker PA, Di Polo A. Excitotoxic death of retinal neurons in vivo occurs via a non-cell-autonomous mechanism. J Neurosci. 2009;29:5536–5545. doi: 10.1523/JNEUROSCI.0831-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sen R, Smale ST. Selectivity of the NF-{kappa}B response. Cold Spring Harb Perspect Biol. 2010;2:a000257. doi: 10.1101/cshperspect.a000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerondakis S, Siebenlist U. Roles of the NF-kappaB pathway in lymphocyte development and function. Cold Spring Harb Perspect Biol. 2010;2:a000182. doi: 10.1101/cshperspect.a000182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fitzgerald KA, McWhirter SM, Faia KL, Rowe DC, Latz E, Golenbock DT, Coyle AJ, Liao SM, Maniatis T. IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat Immunol. 2003;4:491–496. doi: 10.1038/ni921. [DOI] [PubMed] [Google Scholar]
- 6.Hemmi H, Takeuchi O, Sato S, Yamamoto M, Kaisho T, Sanjo H, Kawai T, Hoshino K, Takeda K, Akira S. The roles of two IkappaB kinase-related kinases in lipopolysaccharide and double stranded RNA signaling and viral infection. J Exp Med. 2004;199:1641–1650. doi: 10.1084/jem.20040520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Israel A. The IKK complex, a central regulator of NF-kappaB activation. Cold Spring Harb Perspect Biol. 2010;2:a000158. doi: 10.1101/cshperspect.a000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu CJ, Conze DB, Li T, Srinivasula SM, Ashwell JD. Sensing of Lys 63-linked polyubiquitination by NEMO is a key event in NF-kappaB activation [corrected] Nat Cell Biol. 2006;8:398–406. doi: 10.1038/ncb1384. [DOI] [PubMed] [Google Scholar]
- 9.Ea CK, Deng L, Xia ZP, Pineda G, Chen ZJ. Activation of IKK by TNFalpha requires site-specific ubiquitination of RIP1 and polyubiquitin binding by NEMO. Mol Cell. 2006;22:245–257. doi: 10.1016/j.molcel.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 10.Wu CJ, Ashwell JD. NEMO recognition of ubiquitinated Bcl10 is required for T cell receptor-mediated NF-kappaB activation. Proc Natl Acad Sci U S A. 2008;105:3023–3028. doi: 10.1073/pnas.0712313105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conze DB, Wu CJ, Thomas JA, Landstrom A, Ashwell JD. Lys63-linked polyubiquitination of IRAK-1 is required for interleukin-1 receptor- and toll-like receptor-mediated NF-kappaB activation. Mol Cell Biol. 2008;28:3538–3547. doi: 10.1128/MCB.02098-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao T, Yang L, Sun Q, Arguello M, Ballard DW, Hiscott J, Lin R. The NEMO adaptor bridges the nuclear factor-kappaB and interferon regulatory factor signaling pathways. Nat Immunol. 2007;8:592–600. doi: 10.1038/ni1465. [DOI] [PubMed] [Google Scholar]
- 13.Zeng W, Xu M, Liu S, Sun L, Chen ZJ. Key role of Ubc5 and lysine-63 polyubiquitination in viral activation of IRF3. Mol Cell. 2009;36:315–325. doi: 10.1016/j.molcel.2009.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang L, Li S, Dorf ME. NEMO Binds Ubiquitinated TANK-Binding Kinase 1 (TBK1) to Regulate Innate Immune Responses to RNA Viruses. PLoS One. 2012;7:e43756. doi: 10.1371/journal.pone.0043756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wagner S, Carpentier I, Rogov V, Kreike M, Ikeda F, Lohr F, Wu CJ, Ashwell JD, Dotsch V, Dikic I, Beyaert R. Ubiquitin binding mediates the NF-kappaB inhibitory potential of ABIN proteins. Oncogene. 2008;27:3739–3745. doi: 10.1038/sj.onc.1211042. [DOI] [PubMed] [Google Scholar]
- 16.Laplantine E, Fontan E, Chiaravalli J, Lopez T, Lakisic G, Veron M, Agou F, Israel A. NEMO specifically recognizes K63-linked poly-ubiquitin chains through a new bipartite ubiquitin-binding domain. EMBO J. 2009 doi: 10.1038/emboj.2009.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwamborn K, Weil R, Courtois G, Whiteside ST, Israel A. Phorbol esters and cytokines regulate the expression of the NEMO-related protein, a molecule involved in a NF-kappa B-independent pathway. J Biol Chem. 2000;275:22780–22789. doi: 10.1074/jbc.M001500200. [DOI] [PubMed] [Google Scholar]
- 18.Zhu G, Wu CJ, Zhao Y, Ashwell JD. Optineurin negatively regulates TNFalpha-induced NF-kappaB activation by competing with NEMO for ubiquitinated RIP. Curr Biol. 2007;17:1438–1443. doi: 10.1016/j.cub.2007.07.041. [DOI] [PubMed] [Google Scholar]
- 19.Nagabhushana A, Bansal M, Swarup G. Optineurin Is Required for CYLD-Dependent Inhibition of TNFalpha-Induced NF-kappaB Activation. PLoS One. 2011;6:e17477. doi: 10.1371/journal.pone.0017477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morton S, Hesson L, Peggie M, Cohen P. Enhanced binding of TBK1 by an optineurin mutant that causes a familial form of primary open angle glaucoma. FEBS Lett. 2008;582:997–1002. doi: 10.1016/j.febslet.2008.02.047. [DOI] [PubMed] [Google Scholar]
- 21.Gleason CE, Ordureau A, Gourlay R, Arthur JS, Cohen P. Polyubiquitin Binding to Optineurin Is Required for Optimal Activation of TANK-binding Kinase 1 and Production of Interferon {beta} J Biol Chem. 2011;286:35663–35674. doi: 10.1074/jbc.M111.267567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mankouri J, Fragkoudis R, Richards KH, Wetherill LF, Harris M, Kohl A, Elliott RM, Macdonald A. Optineurin negatively regulates the induction of IFNbeta in response to RNA virus infection. PLoS Pathog. 2010;6:e1000778. doi: 10.1371/journal.ppat.1000778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rezaie T, Child A, Hitchings R, Brice G, Miller L, Coca-Prados M, Heon E, Krupin T, Ritch R, Kreutzer D, Crick RP, Sarfarazi M. Adult-onset primary open-angle glaucoma caused by mutations in optineurin. Science. 2002;295:1077–1079. doi: 10.1126/science.1066901. [DOI] [PubMed] [Google Scholar]
- 24.Maruyama H, Morino H, Ito H, Izumi Y, Kato H, Watanabe Y, Kinoshita Y, Kamada M, Nodera H, Suzuki H, Komure O, Matsuura S, Kobatake K, Morimoto N, Abe K, Suzuki N, Aoki M, Kawata A, Hirai T, Kato T, Ogasawara K, Hirano A, Takumi T, Kusaka H, Hagiwara K, Kaji R, Kawakami H. Mutations of optineurin in amyotrophic lateral sclerosis. Nature. 2010;465:223–226. doi: 10.1038/nature08971. [DOI] [PubMed] [Google Scholar]
- 25.Millecamps S, Boillee S, Chabrol E, Camu W, Cazeneuve C, Salachas F, Pradat PF, Danel-Brunaud V, Vandenberghe N, Corcia P, Le Forestier N, Lacomblez L, Bruneteau G, Seilhean D, Brice A, Feingold J, Meininger V, Leguern E. Screening of OPTN in French familial amyotrophic lateral sclerosis. Neurobiol Aging. 2011;32:557.e11–557.e13. doi: 10.1016/j.neurobiolaging.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 26.Belzil VV, Daoud H, Desjarlais A, Bouchard JP, Dupre N, Camu W, Dion PA, Rouleau GA. Analysis of OPTN as a causative gene for amyotrophic lateral sclerosis. Neurobiol Aging. 2011;32:555.e13–555.e14. doi: 10.1016/j.neurobiolaging.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 27.Weishaupt JH, Waibel S, Birve A, Volk AE, Mayer B, Meyer T, Ludolph AC, Andersen PM. A novel optineurin truncating mutation and three glaucoma-associated missense variants in patients with familial amyotrophic lateral sclerosis in Germany. Neurobiol Aging. 2012 doi: 10.1016/j.neurobiolaging.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 28.Tumer Z, Bertelsen B, Gredal O, Magyari M, Nielsen KC, Lucamp, Gronskov K, Brondum-Nielsen K. Novel heterozygous nonsense mutation of the OPTN gene segregating in a Danish family with ALS. Neurobiol Aging. 2012;33:208.e1–208.e5. doi: 10.1016/j.neurobiolaging.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 29.van Blitterswijk M, van Vught PW, van Es MA, Schelhaas HJ, van der Kooi AJ, de Visser M, Veldink JH, van den Berg LH. Novel optineurin mutations in sporadic amyotrophic lateral sclerosis patients. Neurobiol Aging. 2012;33:1016.e1–1016.e7. doi: 10.1016/j.neurobiolaging.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 30.Lobsiger CS, Cleveland DW. Glial cells as intrinsic components of non-cell-autonomous neurodegenerative disease. Nat Neurosci. 2007;10:1355–1360. doi: 10.1038/nn1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lasiene J, Yamanaka K. Glial cells in amyotrophic lateral sclerosis. Neurol Res Int. 2011;2011:718987. doi: 10.1155/2011/718987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu P, Jenkins NA, Copeland NG. A highly efficient recombineering-based method for generating conditional knockout mutations. Genome Res. 2003;13:476–484. doi: 10.1101/gr.749203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoneyama M, Suhara W, Fukuhara Y, Sato M, Ozato K, Fujita T. Autocrine amplification of type I interferon gene expression mediated by interferon stimulated gene factor 3 (ISGF3) J Biochem. 1996;120:160–169. doi: 10.1093/oxfordjournals.jbchem.a021379. [DOI] [PubMed] [Google Scholar]
- 34.Abbondanzo SJ, Gadi I, Stewart CL. Derivation of embryonic stem cell lines. Methods Enzymol. 1993;225:803–823. doi: 10.1016/0076-6879(93)25052-4. [DOI] [PubMed] [Google Scholar]
- 35.Cross JL, Kott K, Miletic T, Johnson P. CD45 regulates TLR-induced proinflammatory cytokine and IFN-beta secretion in dendritic cells. J Immunol. 2008;180:8020–8029. doi: 10.4049/jimmunol.180.12.8020. [DOI] [PubMed] [Google Scholar]
- 36.Dutko FJ, Oldstone MB. Genomic and biological variation among commonly used lymphocytic choriomeningitis virus strains. J Gen Virol. 1983;64:1689–1698. doi: 10.1099/0022-1317-64-8-1689. [DOI] [PubMed] [Google Scholar]
- 37.Baker KE, Parker R. Nonsense-mediated mRNA decay: terminating erroneous gene expression. Curr Opin Cell Biol. 2004;16:293–299. doi: 10.1016/j.ceb.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 38.Thermann R, Neu-Yilik G, Deters A, Frede U, Wehr K, Hagemeier C, Hentze MW, Kulozik AE. Binary specification of nonsense codons by splicing and cytoplasmic translation. EMBO J. 1998;17:3484–3494. doi: 10.1093/emboj/17.12.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pasparakis M, Luedde T, Schmidt-Supprian M. Dissection of the NF-kappaB signalling cascade in transgenic and knockout mice. Cell Death Differ. 2006;13:861–872. doi: 10.1038/sj.cdd.4401870. [DOI] [PubMed] [Google Scholar]
- 40.Hayden MS, Ghosh S. NF-kappaB in immunobiology. Cell Res. 2011;21:223–244. doi: 10.1038/cr.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003;77:4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sasaki Y, Derudder E, Hobeika E, Pelanda R, Reth M, Rajewsky K, Schmidt-Supprian M. Canonical NF-kappaB activity, dispensable for B cell development, replaces BAFF-receptor signals and promotes B cell proliferation upon activation. Immunity. 2006;24:729–739. doi: 10.1016/j.immuni.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 43.Wang G, Gao Y, Li L, Jin G, Cai Z, Chao JI, Lin HK. K63-linked ubiquitination in kinase activation and cancer. Front Oncol. 2012;2:5. doi: 10.3389/fonc.2012.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clark K, Takeuchi O, Akira S, Cohen P. The TRAF-associated protein TANK facilitates cross-talk within the I{kappa}B kinase family during Toll-like receptor signaling. Proc Natl Acad Sci U S A. 2011;108:17093–17098. doi: 10.1073/pnas.1114194108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Courtois G, Gilmore TD. Mutations in the NF-kappaB signaling pathway: implications for human disease. Oncogene. 2006;25:6831–6843. doi: 10.1038/sj.onc.1209939. [DOI] [PubMed] [Google Scholar]
- 46.Baldwin AS. Regulation of cell death and autophagy by IKK and NF-kappaB: critical mechanisms in immune function and cancer. Immunol Rev. 2012;246:327–345. doi: 10.1111/j.1600-065X.2012.01095.x. [DOI] [PubMed] [Google Scholar]
- 47.DiDonato JA, Mercurio F, Karin M. NF-kappaB and the link between inflammation and cancer. Immunol Rev. 2012;246:379–400. doi: 10.1111/j.1600-065X.2012.01099.x. [DOI] [PubMed] [Google Scholar]
- 48.Tolosa L, Caraballo-Miralles V, Olmos G, Llado J. TNF-alpha potentiates glutamate-induced spinal cord motoneuron death via NF-kappaB. Mol Cell Neurosci. 2011;46:176–186. doi: 10.1016/j.mcn.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 49.Krappmann D, Hatada EN, Tegethoff S, Li J, Klippel A, Giese K, Baeuerle PA, Scheidereit C. The I kappa B kinase (IKK) complex is tripartite and contains IKK gamma but not IKAP as a regular component. J Biol Chem. 2000;275:29779–29787. doi: 10.1074/jbc.M003902200. [DOI] [PubMed] [Google Scholar]
- 50.Ito H, Nakamura M, Komure O, Ayaki T, Wate R, Maruyama H, Nakamura Y, Fujita K, Kaneko S, Okamoto Y, Ihara M, Konishi T, Ogasawara K, Hirano A, Kusaka H, Kaji R, Takahashi R, Kawakami H. Clinicopathologic study on an ALS family with a heterozygous E478G optineurin mutation. Acta Neuropathol. 2011;122:223–229. doi: 10.1007/s00401-011-0842-y. [DOI] [PubMed] [Google Scholar]
- 51.Kamada M, Izumi Y, Ayaki T, Nakamura M, Kagawa S, Kudo E, Sako W, Maruyama H, Nishida Y, Kawakami H, Ito H, Kaji R. Clinicopathologic features of autosomal recessive amyotrophic lateral sclerosis associated with optineurin mutation. Neuropathology. 2013 doi: 10.1111/neup.12051. [DOI] [PubMed] [Google Scholar]
- 52.Akizuki M, Yamashita H, Uemura K, Maruyama H, Kawakami H, Ito H, Takahashi R. Optineurin suppression causes neuronal cell death via NF-kappaB pathway. J Neurochem. 2013 doi: 10.1111/jnc.12326. [DOI] [PubMed] [Google Scholar]
- 53.Beg AA, Sha WC, Bronson RT, Ghosh S, Baltimore D. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-kappa B. Nature. 1995;376:167–170. doi: 10.1038/376167a0. [DOI] [PubMed] [Google Scholar]
- 54.Li Q, Van Antwerp D, Mercurio F, Lee KF, Verma IM. Severe liver degeneration in mice lacking the IkappaB kinase 2 gene. Science. 1999;284:321–325. doi: 10.1126/science.284.5412.321. [DOI] [PubMed] [Google Scholar]
- 55.Rudolph D, Yeh WC, Wakeham A, Rudolph B, Nallainathan D, Potter J, Elia AJ, Mak TW. Severe liver degeneration and lack of NF-kappaB activation in NEMO/IKKgamma-deficient mice. Genes Dev. 2000;14:854–862. [PMC free article] [PubMed] [Google Scholar]
- 56.Bonnard M, Mirtsos C, Suzuki S, Graham K, Huang J, Ng M, Itie A, Wakeham A, Shahinian A, Henzel WJ, Elia AJ, Shillinglaw W, Mak TW, Cao Z, Yeh WC. Deficiency of T2K leads to apoptotic liver degeneration and impaired NF-kappaB-dependent gene transcription. EMBO J. 2000;19:4976–4985. doi: 10.1093/emboj/19.18.4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Perry AK, Chow EK, Goodnough JB, Yeh WC, Cheng G. Differential requirement for TANK-binding kinase-1 in type I interferon responses to toll-like receptor activation and viral infection. J Exp Med. 2004;199:1651–1658. doi: 10.1084/jem.20040528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhou J, Wu R, High AA, Slaughter CA, Finkelstein D, Rehg JE, Redecke V, Hacker H. A20-binding inhibitor of NF-kappaB (ABIN1) controls Toll-like receptor-mediated CCAAT/enhancer-binding protein beta activation and protects from inflammatory disease. Proc Natl Acad Sci U S A. 2011;108:E998–1006. doi: 10.1073/pnas.1106232108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Juriloff DM, Harris MJ. Mouse models for neural tube closure defects. Hum Mol Genet. 2000;9:993–1000. doi: 10.1093/hmg/9.6.993. [DOI] [PubMed] [Google Scholar]
- 60.Sakamaki K, Inoue T, Asano M, Sudo K, Kazama H, Sakagami J, Sakata S, Ozaki M, Nakamura S, Toyokuni S, Osumi N, Iwakura Y, Yonehara S. Ex vivo whole-embryo culture of caspase-8-deficient embryos normalize their aberrant phenotypes in the developing neural tube and heart. Cell Death Differ. 2002;9:1196–1206. doi: 10.1038/sj.cdd.4401090. [DOI] [PubMed] [Google Scholar]
- 61.McWhirter SM, Fitzgerald KA, Rosains J, Rowe DC, Golenbock DT, Maniatis T. IFN-regulatory factor 3-dependent gene expression is defective in Tbk1-deficient mouse embryonic fibroblasts. Proc Natl Acad Sci U S A. 2004;101:233–238. doi: 10.1073/pnas.2237236100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aichele P, Unsoeld H, Koschella M, Schweier O, Kalinke U, Vucikuja S. CD8 T cells specific for lymphocytic choriomeningitis virus require type I IFN receptor for clonal expansion. J Immunol. 2006;176:4525–4529. doi: 10.4049/jimmunol.176.8.4525. [DOI] [PubMed] [Google Scholar]
- 63.tenOever BR, Sharma S, Zou W, Sun Q, Grandvaux N, Julkunen I, Hemmi H, Yamamoto M, Akira S, Yeh WC, Lin R, Hiscott J. Activation of TBK1 and IKKvarepsilon kinases by vesicular stomatitis virus infection and the role of viral ribonucleoprotein in the development of interferon antiviral immunity. J Virol. 2004;78:10636–10649. doi: 10.1128/JVI.78.19.10636-10649.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sahlender DA, Roberts RC, Arden SD, Spudich G, Taylor MJ, Luzio JP, Kendrick-Jones J, Buss F. Optineurin links myosin VI to the Golgi complex and is involved in Golgi organization and exocytosis. J Cell Biol. 2005;169:285–295. doi: 10.1083/jcb.200501162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.del Toro D, Alberch J, Lazaro-Dieguez F, Martin-Ibanez R, Xifro X, Egea G, Canals JM. Mutant huntingtin impairs post-Golgi trafficking to lysosomes by delocalizing optineurin/Rab8 complex from the Golgi apparatus. Mol Biol Cell. 2009;20:1478–1492. doi: 10.1091/mbc.E08-07-0726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nagabhushana A, Chalasani ML, Jain N, Radha V, Rangaraj N, Balasubramanian D, Swarup G. Regulation of endocytic trafficking of transferrin receptor by optineurin and its impairment by a glaucoma-associated mutant. BMC Cell Biol. 2010;11:4. doi: 10.1186/1471-2121-11-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wild P, Farhan H, McEwan DG, Wagner S, Rogov VV, Brady NR, Richter B, Korac J, Waidmann O, Choudhary C, Dotsch V, Bumann D, Dikic I. Phosphorylation of the Autophagy Receptor Optineurin Restricts Salmonella Growth. Science. 2011 doi: 10.1126/science.1205405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tumbarello DA, Waxse BJ, Arden SD, Bright NA, Kendrick-Jones J, Buss F. Autophagy receptors link myosin VI to autophagosomes to mediate Tom1-dependent autophagosome maturation and fusion with the lysosome. Nat Cell Biol. 2012;14:1024–1035. doi: 10.1038/ncb2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kachaner D, Filipe J, Laplantine E, Bauch A, Bennett KL, Superti-Furga G, Israel A, Weil R. Plk1-dependent phosphorylation of optineurin provides a negative feedback mechanism for mitotic progression. Mol Cell. 2012;45:553–566. doi: 10.1016/j.molcel.2011.12.030. [DOI] [PubMed] [Google Scholar]