Abstract
Growing Right Onto Wellness (GROW) is a randomized controlled trial that tests the efficacy of a family-centered, community-based, behavioral intervention to prevent childhood obesity among preschool-aged children. Focusing on parent-child pairs, GROW utilizes a multi-level framework, which accounts for macro (i.e., built-environment) and micro (i.e., genetics) level systems that contribute to the childhood obesity epidemic.
Six hundred parent-child pairs will be randomized to a 3-year healthy lifestyle intervention or a 3-year school readiness program. Eligible children are enrolled between ages 3 and 5, are from minority communities, and are not obese. The principal site for the GROW intervention is local community recreation centers and libraries.
The primary outcome is childhood Body Mass Index (BMI) trajectory at the end of the three-year study period. In addition to other anthropometric measurements, mediators and moderators of growth are considered, including genetics, accelerometry, and diet recall.
GROW is a staged intensity intervention, consisting of intensive, maintenance, and sustainability phases. Throughout the study, parents build skills in nutrition, physical activity, and parenting, concurrently forming new social networks. Participants are taught goal-setting, self-monitoring, and problem solving techniques to facilitate sustainable behavior change. The GROW curriculum uses low health literacy communication and social media to communicate key health messages. The control arm is administered to both control and intervention participants.
By conducting this trial in public community centers, and by implementing a family-centered approach to sustainable healthy childhood growth, we aim to develop an exportable community-based intervention to address the expanding public health crisis of pediatric obesity.
Keywords: childhood obesity, preschool children, family-centered intervention, underserved, obesity prevention
INTRODUCTION
The childhood obesity epidemic continues to be a public health concern given its increasing prevalence among young children [1, 2], potential reduction in life expectancy [3], and associated adverse health consequences from childhood to adulthood [4, 5]. Among U.S. children aged 2 to 5 years, obesity prevalence (Body Mass Index (BMI) percentile ≥95th) has increased more than two-fold in the past 3 decades from 5.0% to 12.1% [1, 2]. According to recent data from the National Health and Nutrition Examination Survey (NHANES), obesity disproportionately affects African American and Latino children between the ages of 2 to 5 years with prevalence rates of 16.2% and 18.9%, respectively [2]. With obese preschool children more likely to become obese adults [6], the risk of obesity for children, and especially minority children, cannot be overstated. The need for effective interventions targeting this high-risk population is a public health priority.
Obesity prevention aims to maintain healthy BMI trajectories for children who are not overweight (BMI <85th percentile), and to prevent progression to obesity for children who are overweight (BMI ≥85th percentile and <95th percentile). Because rapid weight gain in early childhood is associated with later obesity, the preschool age is a critical window for obesity prevention [4]. Focusing preventive efforts on early childhood growth trajectories provides an opportunity to address the obesity epidemic before children are indelibly predisposed to obesity [7–9].
There is limited evidence documenting successful behavioral interventions to prevent early childhood obesity [10–12], and even less evidence concerning which factors may be crucial to success. Consequently, the Institute of Medicine (IOM) [13] and the Strategic Plan for NIH Obesity Research [14] call for a community-engaged, culturally-relevant, family-centered sustainable approach to obesity prevention.
The Growing Right Onto Wellness (GROW) Trial is a multi-level, family-centered, community-engaged, randomized controlled trial intended to prevent obesity in high-risk preschool-aged children. The multi-level approach integrates a range of strategies to address the problem of childhood obesity in the context of a complex set of societal, family, and individual factors that contribute to the obesity epidemic [15]. This trial builds on successes from the field of pediatric obesity treatment, considering parents as agents of change, and implementing a family-based behavioral intervention that focuses on healthy nutrition, physical activity, and behavioral modifications [5, 10, 16–20]. Finally, GROW is based in local community centers in order to maximize the use of the built environment [21].
The GROW Trial is one of two unique prevention trials that are part of the Childhood Obesity Prevention and Treatment Research (COPTR) National Institutes of Health Consortium. Funded by the National Heart, Lung, and Blood Institute and Eunice Kennedy Shriver National Institute of Child Health and Human Development (U01 HL103620), the COPTR consortium seeks to identify and capture common data elements across four field sites: two focusing on obesity prevention in preschool-aged children, and two focusing on obesity treatment in school-aged children and adolescents. Each field site has a unique research study design. This paper describes the design, methodology, and proposed evaluation of the GROW Trial.
Study Aims
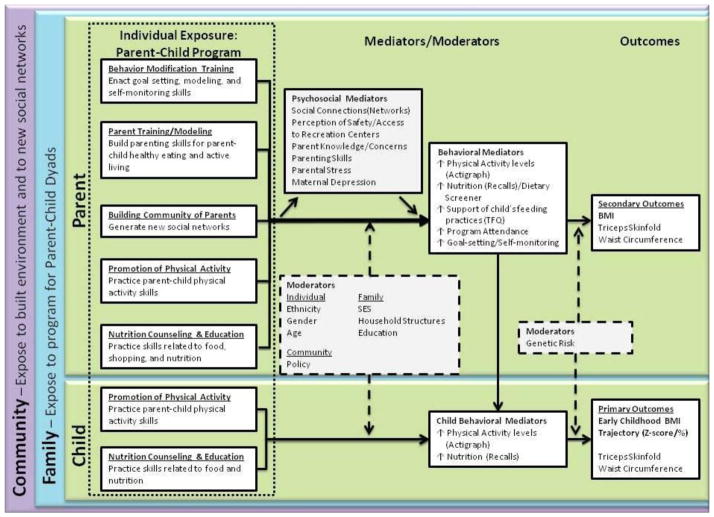
The primary aim of the GROW Trial is to evaluate the efficacy of a family-centered, behavioral intervention to prevent pediatric obesity (i.e., BMI trajectory) among children ages 3–5. There are multiple secondary aims that are designed to evaluate mediators and moderators of early childhood growth and are linked to the GROW Trial’s multi-level approach to preventing pediatric obesity (Figure 1). These include the following:
Figure 1. GROW Conceptual Model.
The primary outcome is early childhood BMI trajectory. This is a multi-level intervention that considers how the community, family, and individual interact with multiple mediators and moderators to affect early childhood growth.
Aim 2: To compare the effect of the intervention in children who made significant changes in their dietary and/or physical activity behaviors to the effect in children who did not.
Aim 3: To evaluate the effect of parents’ physical activity levels and dietary behaviors on children’s levels of the same.
Aim 4: To explore the potential for developing new social networks and their effect on child nutrition and physical activity.
Aim 5: To evaluate the moderating relationship between genetic risk factors and child BMI trajectories over the course of the study.
Aim 6: To assess the degree to which implementation of the GROW program encourages additional lifestyle programming for preschool children and their parents in the Metro Community Centers.
Theoretical Framework and Conceptual Model
We base our conceptual model on the Centers for Disease Control and Prevention’s theory that obesity is affected by both micro-level (i.e., genetics) and macro-level systems (i.e., built-environment) over time at critical windows of development, such as early childhood [15]. Recognizing that a child interacts with the environment through the context of family and community [22], the GROW trial utilizes a conceptual model that accounts for multi-level exposures, mediators, and moderators of childhood growth (Figure 1). We assess the interaction between genetics, environment, and behavior over time.
The GROW intervention content is developed around key behavior change techniques and key principles of Social Cognitive Theory (SCT), with a focus on goal setting, self-monitoring, problem solving, and self-efficacy [23–25]. According to SCT, individuals are more likely to engage in behaviors they see modeled or rewarded, as well as those for which their engagement receives direct reinforcement [25]. Thus, a key component of our intervention for young children is to involve parents in modeling health-promoting behaviors to children in the earliest years. Social media/email/print materials provided at regular intervals throughout the study serve as a consistent “cue to action” to utilize skills learned in the participant’s built environment to support healthy lifestyles over the three-year study period.
In addition, the GROW trial focuses on parents as agents of change, which has been previously shown as a more effective method for improving childhood obesity than educational interventions alone [26]. The GROW curriculum provides parents with knowledge in key areas related to preventing obesity, such as nutrition, physical activity, sleep, and parenting styles. It also builds on that foundation by giving parents the tools necessary to make those changes for themselves and their children. Using the above theoretical foundation in social cognitive theory, the intervention teaches parents how to change their parenting behaviors related to the key intervention messages.
Considering a recently developed taxonomy for common behavioral interventions by Michie et al, the behavioral components of the GROW Intervention focus on the two clusters of “Goals and Planning” and “Feedback and Monitoring,” utilizing the following specific measures:1) self-monitoring of outcomes and behaviors, 2) problem solving/coping planning, 3) goal setting (outcomes and behaviors), and 4) review of goals (outcome and behavior) [27].
MATERIALS AND METHODS
Trial Design Overview
The GROW Trial is a parallel-group, randomized controlled trial (RCT) designed to prevent childhood obesity by delivering a multi-level, healthy lifestyle intervention to high-risk parent-child dyads (children ages 3–5) in the built environment.
Parent-child dyads are randomized to either the intervention or control group. The GROW Intervention (“GROW Healthier” Group) is a three-year, family-centered behavioral intervention that focuses on skills-building around healthy lifestyles for children and their parents/primary caregivers. The control condition (“GROW Smarter” Group) is a three-year, family-centered school readiness and school success program (refer to section on Control Group). Both groups are exposed to the control condition in order to create a “true” comparator between treatment groups.
The primary outcome of BMI trajectory considers raw BMI collected at 6 points over the study period, which allows for characterization of the BMI growth curve during this dynamic time of early childhood growth. Other measurements are tied to a multi-level construct of expected mediators and moderators of childhood growth (Figure 1), including: anthropometrics, accelerometry, diet recall, genetics, social network data, built environment data, policy changes, and survey data collected over the course of the study period.
By comparing the BMI trajectories for children in the intervention arm with children in the control arm, we will determine the efficacy of this intervention to prevent the development of childhood obesity.
Study Setting and Population
Six hundred parent-child dyads with Latino or African-American children ages 3–5 years from largely underserved neighborhoods are being enrolled. Three hundred dyads are randomly assigned to each condition. Participants include English and Spanish-speaking parents/caregivers. “Underserved” is defined by self-report of household participation in or qualification for one of the following programs/services: TennCare, CoverKids, WIC, Food Stamps (SNAP), Free and Reduced Price School Lunch and Breakfast, Income-Based Housing, and/or Families First (TANF). For a full list of inclusion/exclusion criteria see respective sections below. Prevention is the focus of this RCT; therefore, eligible children include children in the top tier of normal weight (BMI ≥ 50% and < 85%) and overweight (BMI ≥ 85% and <95%) categories.
The principal site for the GROW intervention is local community recreation centers in Davidson County, TN. The GROW trial is a partnership with Nashville’s Metro Parks and Recreation, Nashville’s Public Library, and the Monroe Carell Jr. Children’s Hospital at Vanderbilt. We have identified public recreation centers that serve the traditional underrepresented minority populations in South and Northeast Nashville. Public community centers are chosen because they provide access to physical activity for those populations at highest risk for obesity. Additionally, eligible participants are recruited from defined zip code regions surrounding each community recreational center to reinforce the principle of utilizing their built-environment to promote and potentially sustain family-centered physical activity and healthy lifestyles [21].
Eligibility and Exclusions
Inclusion Criteria
For the purposes of this study, we define the participating index “parent” as the legal guardian of the child who identifies that they spend the majority of time with that child at home. Classification of participants’ BMI is based on The Center for Disease Control and Prevention’s (CDC) guidelines [28]. For children, normal weight is defined as BMl-for-age between the 50th and 84th percentile. Overweight is defined as BMl-for-age between the 85th and 95th percentile. Obese is defined as BMI-for-age ≥ 95th percentile. Inclusion criteria for participation in this study are as follows:
Three-to-five year old child
English- or Spanish-speaking
Child’s BMI ≥50% and < 95%
Parental commitment to participate in a three year study
Consistent phone access
Parent age ≥ 18 years
Parents and children must be healthy, without medical conditions necessitating limited physical activity
Child completion of baseline data collection on height and weight, a minimum of two diet recall sessions, minimum accelerometry wear time (at least 3 weekdays and at least 1 weekend day; at least 6 hours of valid time between 5 am and 12 pm), and at least 90% of survey items completed by the parent within 30 days of child height and weight collection
Recruitment from one of two Nashville regions: East Nashville/Region 1 (37206, 37207, 37208, 37213, 37216, 37228, 37189, 37115), surrounding the East Community Center; and South Nashville/Region 2 (37013, 37203, 37204, 37210, 37211, 37217, 37220), surrounding the Coleman Recreation Center
Exclusion Criteria
Children who are < 50% BMI or ≥ 95%
Children outside the specified age range
Families who do not speak English or Spanish
Lack of telephone contact
Lack of parental commitment to participate consistently for a three-year period
Parents and/or children who are diagnosed with medical illnesses where regular exercise might be contraindicated
Parents/children who do not otherwise meet the eligibility criteria listed in the study population description
Incomplete baseline data (refer to inclusion criteria above)
Recruitment
Rolling recruitment and enrollment occur for 18 months until a total of 600 parent-child dyads have been enrolled. Recruitment efforts involve a variety of community sites (e.g., daycares, doctors offices, pre-K programs, churches, community service programs) using an on-site eligibility and pre-screening process. Recruitment strategies are multifaceted and include: posters, flyers, mailed brochures/postcards, radio commercials and guest radio spots, community events, primary care doctor offices, churches, community partner sites that serve our target population, and word-of-mouth. Additionally, we employ community liaisons who are well-respected and deeply integrated individuals in the community. They have specific knowledge and resources to easily reach and effectively communicate with our target population. Examples of community liaisons include local pastors, former participants, and directors of public preschool daycare centers. In addition to this, we have established a Community Advisory Board to help guide our recruitment and retention processes. Based on our experience recruiting large numbers of minority families in Salud con La Familia[29] and in the pilot for this intervention, both transportation to and from data collection sessions and childcare are provided to overcome the most frequently cited barriers to study participation [30–34].
Informed Consent and Assent
Written informed consent is obtained by all study participants prior to baseline data collection. Since child participants are under the age of 7 when enrolled, parents/legal guardians must grant permission for their child’s participation in the research study. As it is difficult to gauge a preschooler’s assent to participate, children who consistently show dissenting behavior (i.e., unwilling to be measured at baseline data collection) are excluded from the study. Consent forms are provided in English and Spanish, presented, and reviewed with participants in their preferred language. All consent forms are translated by the Tennessee Foreign Language Institute (TFLI). In addition to providing written consent for each participant in the language of their choice, we augment our approach with low literacy populations by using a visual representation of the consent form (Appendix 1). Data are collected by trained bilingual research staff. This study is approved by the Vanderbilt University Review Board (IRB No.120643) and is registered at ClinicalTrials.gov (NCT01316653).
Randomization
Participants are eligible for randomization once the following criteria are met within a 30-day run-in period: child height and weight are collected, a minimum of two diet recall sessions are completed, minimum accelerometry wear time is met (at least 3 weekdays and at least 1 weekend day; at least 6 hours of valid time between 5 am and 12 pm), and at least 90% of survey items are completed.
Assignment to Study Groups
Parent-child dyads are grouped within their respective community center regions and stratified according to parent dominant language preference (English versus Spanish). Then dyads within the strata are randomized to the intervention and control groups using a sequence with randomly permuted blocks of varying size. Block randomization of this type ensures equal representation at intermittent recruitment points while minimizing the probability of correctly guessing subsequent condition assignment. To minimize contamination between the intervention and control groups, once randomized, we separate data collection sessions, communication, and community-based events.
Blinding
The potential for bias is a concern for any research study and can manifest in study conduct, data management, data analysis, and interpretation. All study staff have signed a blinding policy developed by COPTR to address these issues. Key data elements to be kept un-blinded have been determined a priori. Staff who implement the intervention or collect data do not have access to group aggregated data that would allow them to bias implementation or data collection. Also, aggregate level outcome data by group (treatment/control) is blinded to prevent impact on the study’s outcome. Baseline data are not blinded to ensure randomization fidelity.
Retention
Successful retention begins at recruitment. We are implementing a multi-pronged strategy that includes: 1) obtaining multiple methods of contact from participants at study initiation that are frequently updated; 2) developing participant tracking databases with clear protocols for those lost to follow-up; 3) building consistent relationships between study participants and project staff throughout the study; 4) providing systematic frequent contact via texting and phone calls; 5) offering tiered financial incentives for continued study participation; and 6) providing scheduling flexibility around participants’ preferences.
Intervention Group: “GROW Healthier”
Intervention Overview
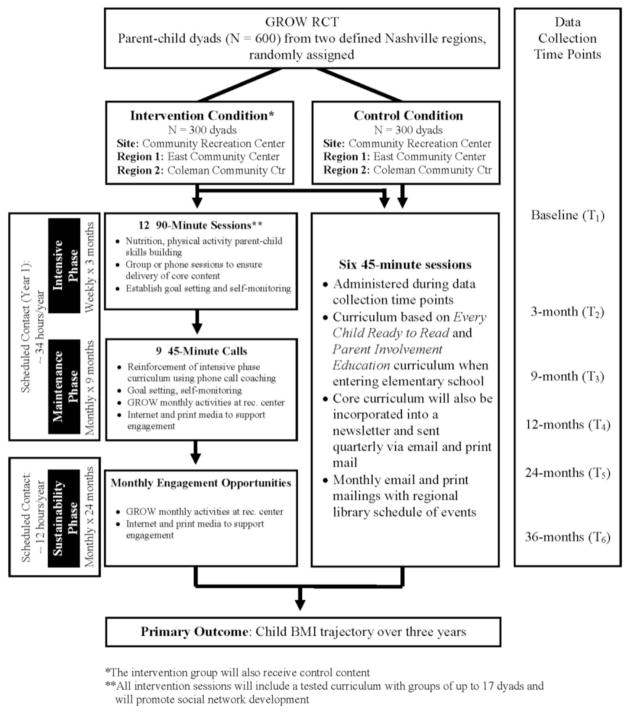
The GROW Trial is a pragmatic clinical trial that matches the study design to the needs of those who will use the results [35]. Therefore, we offer practical options that are sensitive and flexible to participant needs [36]. The intervention is divided into three phases: intensive, maintenance, and sustainability (Figure 2). This tiered intervention approach is based on effective obesity intervention strategies [37–40]. The 3-month intensive phase is conducted at the community recreation center and consists of 12 weekly sessions that build skills in nutrition, physical activity, and parenting. Following the intensive phase, participants are exposed to a 9-month maintenance phase, which consists of monthly phone coaching calls and monthly GROW sessions at the community center to reinforce key messages and skills taught in the intensive phase [41, 42]. The sustainability phase lasts two years and consists of monthly GROW events at recreation centers and continued engagement through social media and print materials. Below we provide further details of each of the three-tiered intervention phases.
Figure 2. GROW Study Design Overview.
*The intervention group will also receive control content
**All intervention sessions will include a tested curriculum with groups of up to 17 dyads and will promote social network development
Interventionists undergo training sessions and certification conducted by the GROW study team and the Vanderbilt Center for Integrative Health, a credentialed organization that provides phone-based health coaching training and certification. Bilingual interventionists lead Spanish-speaking sessions.
Intensive Phase
As part of our pragmatic approach, we offer two modes of intensive phase delivery, face-to-face or phone call coaching sessions. Both modes contain the following components: 1) goal check-in with problem solving, 2) interactive didactic taught by the interventionist, 3) small group/individual discussion, 4) individual goal setting, and 5) provision of a tangible tool that allows for continued use of new skills attained (mailed to those who select phone call coaching). In-person sessions also include a hands-on activity applying information learned with parent and child and also includes a shared meal. Examples of hands-on activities include practicing games that could be used to decrease media-use, and food preparation that is kid-friendly.
Goal check-in is a discussion facilitated by the interventionist, where participants quantitatively assess the success of their goal set at the end of the previous session. The interventionist engages participants in practical problem solving, applying principles of motivational interviewing such as reflective listening and summarization.
An interactive didactic session follows, providing a knowledge and skills-building program based on best-practices for childhood health and developed with input from experts in health literacy and childhood obesity [43–48]. Each week has three simple core messages (Table 1) that relate to healthy childhood development. During the interactive didactic, GROW provides 60-minute physical activity sessions for the index child and free sibling childcare as needed by participants (“Kids Club”).
Table 1.
Intervention Modules and Key Messages
| Session No & Theme | Key Messages (from Booklets) |
|---|---|
| 1. Expectations & Set Healthy Goals |
|
| 2. Choose Healthy Foods |
|
| 3. Be an Active Family |
|
| 4. Find a Fitness Home |
|
| 5. Healthy Snacks & Drinks |
|
| 6. Use Media Wisely |
|
| 7. Mindful Eating |
|
| 8. Eat Together |
|
| 9. Engaged Parenting |
|
| 10. Plan Healthy Meals |
|
| 11. Sleep Matters |
|
| 12. Growing Home |
|
Participants are then encouraged to develop SMART goals (Specific, Measureable, Achievable, Realistic, Timely), which are tracked throughout the course of the study and specifically discussed at the subsequent session. For the remaining 30 minutes, index children join their parents in the session for a hands-on activity to reinforce the lesson learned and the core principle for the week. Parents are given one tangible tool per session (e.g., child drinking cup, pedometer, chopsticks, etc), with a total of 12 tangible tools that are consistent with key messages of each module and serve as a cue-to-action towards healthy living. Transportation to and from the community center is provided for families if needed.
Health Literacy
As a part of each weekly session in the intensive phase, parents receive a booklet to reinforce key messages along with tangible tools to help achieve each weekly goal (Appendix 2). According to principles outlined by the Institute of Medicine [47], all materials are designed to reduce health-information complexity: key messages are written using plain language, simple text, maximal white space, and behavior-oriented images/pictograms. All session booklets are written at a 5th grade readability level. Moreover, a panel of experts in health literacy has helped to develop the intervention materials. Cognitive interviews have been performed using the materials developed with families from our target populations. Final written modules have been assessed utilizing the Suitability Assessment of Materials (SAM) instrument [48, 49].
Maintenance Phase
The maintenance phase begins immediately after the intensive phase and lasts for nine months. During this time, parents receive monthly coaching calls that reinforce the core messages from the intensive phase. These calls, conducted by their assigned interventionist (health coach), use motivational interviewing and encourage participants to continue to set SMART goals and facilitate problem solving. This strategy is based on successful adult weight loss intervention program that used phone call coaching [42]. During the maintenance phase, participants will be scheduled to participate in monthly GROW activities at the community center that range from teaching kitchens to sports leagues.
Sustainability Phase
The sustainability phase of the intervention takes place during the second and third year of a dyad’s enrollment, monthly for 24 months. During this time, participants continue to participate in monthly GROW activities that reinforce key messages from the intensive phase and are implemented by Parks and Recreation utilizing their existing infrastructure. These activities occur in neighborhood community centers. Participants also have continued access to existing activities at their assigned community center.
Adaptive Intervention for Non-responders (Intervention Group Only)
We utilize an a priori adaptive intervention approach [50] for children who are not responding to the intervention based on their BMI trajectories between data collection points. For the purposes of this adaptive intervention, a child is considered a non-responder if her/his BMI categorization shifts upward between data collection points (e.g., normal weight shifts to overweight or obese, overweight shifts to obese). Child BMI change is reported using an easily understandable and comprehensive growth feedback report and mailed/e-mailed to all parents after measurements are collected (Appendix 3). The adaptive intervention occurs after each BMI collection point. The interventionist reviews the feedback report with the parent via phone call and solicits successes and barriers faced with incorporating GROW content and associated skills into their everyday lives. Responders receive mailed/e-mailed feedback reports with a brief verbal feedback of encouragement via phone call coaching.
Social Media
Social media platforms are used to reinforce targeted behaviors and engage participants throughout the 3-year intervention. Social media is defined as a means of interaction between people that allows content to be created, shared, and exchanged within virtual communities and networks. Participants have access to a WordPress-based privacy-protected website that is accessible from both mobile devices and computers. WordPress is a social media tool and content management system that integrates regularly updated content, on a secured server. It also allows for sustainable integration of other social media platforms such as Facebook. The website 1) provides frequent updates on key intervention concepts such as nutrition, physical activity, and parenting skills; 2) encourages use of the built environment by promoting both GROW and local events; 3) facilitates interaction among fellow participants and interventionists via a blogging feature; and 4) allows participants to set and track their individual goals throughout the 3-year intervention. A tutorial of the website is integrated throughout the intensive phase. Participants who do not have computer access at home have access immediately before and after intervention sessions at the community center and at their public libraries.
Control Group: “GROW Smarter”
Participants receive 45-minute school readiness/school success sessions during each of the 6 data collection time points (Table 2). To serve as a true control comparator, the program is delivered to dyads in both the intervention and control arm. The materials and curriculum have been newly developed through a partnership with the Nashville Public Library and involves developing parental skills to support school success while also creating a practice-based learning environment for parent-child dyads around school success. The curriculum utilizes key elements of Every Child Ready to Read [51], a project of the Association for Library Service to Children and the Public Library Association. As children age in the study and enter elementary school, the parent-child dyad receives a curriculum that integrates core elements from the Parent Involvement Education curriculum, tested and implemented by the Parent Institute for Quality Education (PIQE) to improve school success [52]. The core curriculum is also incorporated in a quarterly newsletter distributed via email and print mail over a period of 3-years to both study arms. Within the study’s first year, 1 field trip is held to expose families to local public library facilities, encouraging their use of library resources and introducing them to library staff. In addition to the quarterly newsletters, participants receive a calendar of monthly library events in order to continuously engage families with existing infrastructure in their built environment that integrates the core curriculum.
Table 2.
Control Session Modules and Key Messages
| Session Theme | Key Messages |
|---|---|
| 1. Getting Ready to Read |
|
| 2. Home Is a Learning Zone |
|
| 3. Words and Letters in the World Around Us |
|
| 4. Fun with Play |
|
| 5. Work that Body, Feed that Mind! |
|
| 6. Preparing for School Advocacy |
|
Library sessions are coordinated with the data collection time points (T2 through T6) to facilitate attendance and minimize participant burden. Post-randomization library sessions, data collections, and field trip are separated by treatment groups to minimize contamination.
Participant Timeline
All data collection for both treatment groups take place at the dyad’s assigned recreation center, trusted community partner site, or in-home setting (participant preference) at 6 points in time over a 3 year period (T1–T6): baseline (T1), 3-months (T2), 9-months (T3), 12-months (T4), 24-months (T5), and 36-months (T6). See Table 3 for primary and secondary outcomes and Appendix 4 for a full list of measures and timing of data collection.
Table 3.
Measurements of Primary and Secondary Outcomes
| Primary Outcomes - CHILD | ||||
|---|---|---|---|---|
| Item | Measurement Tool | Description | Method | Collection Time |
| Early Childhood BMI Trajectory | Scale, stadiometer | Change in BMI% over time | Weight (kg)/height (m2), CDC calculator | T1 – T6 |
| Body Fat % (Triceps Skin Fold) | Caliper | Change in % body fat over time | Caliper | T1, T4, T5, T6 |
| Waist Circumference | Measuring tape | Change in waist circumference | Measuring tape | T1, T4, T5, T6 |
| Secondary Outcomes – PARENT | ||||
| BMI | Scale, stadiometer | Change in BMI over time | Weight (kg)/height (m2) | T1 – T6 |
| Body Fat % (Triceps Skin Fold) | Caliper | Change in % body fat over time | Staff measured | T1, T4, T5, T6 |
| Waist Circumference | Measuring tape | Change in waist circumference | Staff measured | T1, T4, T5, T6 |
Incentives
Monetary compensation is provided for participation in data collection sessions. In addition, all participants from both intervention and control groups receive family memberships to their respective community recreation center for one year. Families in the “GROW Healthier” group (intervention condition) are given their recreation center memberships at the beginning of the intervention and have the opportunity to extend their memberships for up to three years if they use the facility at least once per month, tracked with an electronic membership card. For the “GROW Smarter” group (control condition), this membership begins at the end of the study and lasts for one year.
Even though this study is designed as a pragmatic clinical trial [53, 54], an incentive structure is used to encourage participant recruitment and retention. As in all trials, we are balancing the competing pressures of designing an immediately exportable intervention with using incentives to facilitate long-term retention necessary to evaluate the intervention’s efficacy.
Assessment and Measures
Primary outcome measures – common measures
Body mass index (BMI) is the primary outcome variable of all four COPTR trials. BMI assesses body weight adjusted for height and in previous studies has been correlated with percent body fat as assessed by dual energy x-ray absorptiometry [55–58]. When calculated using measured anthropometrics BMI is highly reliable. BMI demonstrates clinical validity in its associations with type 2 diabetes mellitus [59, 60], hyperinsulinemia [61], blood pressure and hypertension [55, 61, 62], adverse lipoprotein profiles[61–63] and early atherosclerotic lesions among children and adolescents [64, 65]. Importantly, BMI can be assessed easily in clinical and public health settings and is generally accepted and well understood. The objective of the BMI measures is to provide a precise and accurate measure of the impact of the intervention on relevant aspects of body size in the children studied in COPTR. All consented index children in the COPTR studies have weight and height measured at the beginning and end of the intervention (36 months) and at two common interim time points (12 and 24 months). All baseline anthropometric data will be collected prior to randomization.
Weight and height are measured with the participant in light clothing without shoes. Weight is measured to the nearest 0.1 kg using research precision grade, calibrated, digital scales. Height is measured to the nearest 0.1 cm using a free-standing or wall mounted stadiometer. BMI is calculated as weight in kilograms divided by the square of height in meters.
Secondary Outcomes, Moderators and Mediators
Anthropometric Measurements
In addition to collecting weight and height, waist circumference, and triceps skinfolds are collected for all index children and index parents. Waist circumference is measured to the nearest 0.1 cm just above the uppermost lateral border of the right ilium using a Gulick II tape measure, model 67020. The triceps skinfold is measured using a Lange skinfold caliper (or a Harpenden caliper if the measurement exceeds capacity of the Lange skinfold caliper). Measurements are taken in the midline of the posterior aspect (back) of the arm, over the triceps muscle, at a point midway between the lateral projection of the acromion process of the scapula (shoulder blade) and the inferior margin (bottom) of the olecranon process of the ulna (elbow). Triceps skinfolds are measured to the nearest 0.1 mm. For quality control, 10% of the anthropometric measurements are measured by two different data collectors.
Physical Activity
Accelerometry data are collected on all index children and index parents using the GT3X+ monitor. The child and parent each wear a GT3X+ monitor on the right hip for seven complete days, including sleeping and during water activity (e.g., bathing, swimming, showering). The ActiGraph GT3X+ assesses acceleration in three individual orthogonal planes using a vertical axis, horizontal axis, and a perpendicular axis. The GT3X+ is set to a 40-hertz frequency. The valid wear time criteria (minimum) are 4 days (3 weekdays and 1 weekend day) of at least 6 hours of activity between 5:00 a.m. and 11:59 p.m.
Dietary Assessment
Dietary intake is measured using 24-hour recall conducted on two weekdays and one weekend day using NDS-R software. Dietary recalls are collected over the telephone in English or Spanish. The “Food Amounts Booklet” is used by the respondent to assist in identifying portion sizes. To avoid collecting days with similar foods, recalls are not conducted on consecutive days. In addition, in order to capture variability of food supplies in the home, all three recalls do not occur within a seven-day period. The third recall is collected more than one week after the first recall. All three recalls are collected within 30 days. The adult responsible for child feeding (e.g. parents, daycare providers) reports the child’s intake on a food record form. Full quality assurance checks are conducted on at least 10% of the dietary recalls according to NDS-R standard protocols.
Social Network Measurement
Recent research has shown that new social networks can emerge as a result of an obesity prevention intervention with parents and preschool aged children [66]. Social networking data is collected from participant survey, and analyzed to determine the following social networking constructs: isolates, density, centrality, subgroups, transitivity, and cohesion [67]. These are obtained at study start, mid-way, and at the end of the intensive phase. In addition to measuring new social network features and how they change over time, we also conduct social network diagnostics by session 6 to guide interventionists in enhancing group cohesion during face-to-face sessions [68].
Genetic Data
Saliva is collected from interested parent-child dyads participating in the study [69]. This process is completely voluntary and requires a separate consent process. For adults and children, saliva is obtained utilizing the Oragene saliva kit and assayed using a modified version of the Puregene DNA (Gentra, Inc). All biologic data are bio-banked to assess genetic and epigenetics as a potential moderating variable.
Study Questionnaire
The study questionnaire is administered to the index parent and measures a variety of domains related to the intervention (see Appendix 4 for measurement details). The survey is administered by a GROW data collector, with answers recorded electronically. The survey is administered in either English or Spanish, according to parent preference. The survey takes about 60 minutes to complete at baseline and about 30–45 minutes at follow-up data collections. COPTR has developed 57 common survey questions, listed in Appendix 5.
Barriers to Physical Activity Questionnaire
This component of the study survey is based on the Environmental Supports for Physical Activity Questionnaire [70] to assess individual perceptions of physical activity supports in the environment, use of the built environment, current physical activity behavior, and recreation center use. This survey takes about 15–20 minutes to complete and has been validated in previous literature [71].
Geographic Information Systems (GIS)
In addition to assessing individual perceptions of physical activity supports in their environment, Geographic Information Systems (GIS), ArcMap 10.1 is used to objectively measure how key active living indicators change in Nashville over time and how these measures relate to the above perceptions. Specifically, six primary built environment measures demonstrated in our formative phase to affect the use of one’s built environment to support healthy behaviors, are mapped throughout GROW: (1) crime; (2) sidewalk and bicycle infrastructure; (3) bus route access; (4) park, recreation center, and open space access; (5) unattended dogs; and (6) joint use requests. Objectively measured built environment data are analyzed at the regional level and compared to the policy environment as well as at the individual level surrounding each participant’s home. These are assessed annually over the study period.
Cognitive Assessment Measures
We assess developing executive functioning and IQ [72].. To measure intelligence, we use the Woodcock-Johnson III Tests of Cognitive Abilities – Brief Battery. This tool involves a battery of tasks where children expressively respond to an assortment of pictures and words in a flipbook. To measure executive functioning, we use Carlson’s Executive Function Scale for Preschoolers (based upon the Dimensional Change Card Sort Task) [73]. The battery of hands-on tasks (e.g., card sorting) takes approximately 10 minutes. This is assessed in year one and three.. Trained and certified cognitive assessors administer these tests individually with each child in their primary language spoken at home.
Process Measures
The GROW trial process measures include: participation rates collected via attendance logs; session fidelity checks to ensure consistency and accuracy of content administration; logs to assess use of recreation center and library outside of mandatory GROW-related sessions; user log-in to social media; and parent-child satisfaction with study participation.
For quality assurance, the GROW trial uses a series of range checks and branching logic to ensure accuracy. All instruments are verified for completeness. During data processing, the GROW trial conducts 10% quality checks for all anthropometric measurements with predetermined ranges. Failure to meet these ranges leads to data collector retraining. A treatment fidelity plan that is applied to both the intervention and control conditions has been developed following the methodological practices recommended by the Treatment Fidelity Workgroup of the NIH Behavior Change Consortium [74]. The plan includes interventionist training and supervision, identification of essential treatment components for verification, sampling to ensure treatment consistency, control for differences between interventionists, and use of fidelity measures (e.g., length, number, frequency of session, participation rates) with the goal of monitoring intervention delivery as intended. Phone calls undergo the same fidelity assessment, utilizing a software audio program that captures the utterances of the phone call coach to assess content and process delivery.
Statistical Analyses
Primary analyses
Although childhood obesity is a well-documented public health concern, most studies have assessed the obesity outcome (e.g., BMI) using only a single time point or incorporating a pre-post design, leaving us with little knowledge about the actual shape or growth rate of trajectories of BMI during this critical period of development. By measuring BMI at multiple time points, we are examining growth trajectories in early childhood. This allows us to examine the effect of a prevention program on these varying trajectories [75, 76]. As Barker et al. demonstrated, it is the change in BMI over time in early childhood, rather than BMI at any single time point, that is linked with health consequences in adulthood [9]. Moreover, an earlier childhood adiposity rebound is associated with an increased risk of later obesity [7, 8]. For our primary analysis, which is an intention-to-treat analysis, we model BMI longitudinally using a quadratic mixed model. Time is represented by child’s age, centered on age at study enrollment. Gender and ethnicity are among other variables entered as covariates as well as an interaction between gender and age to account for differences in trajectories over time by gender. Our hypothesis is that the change in the quadratic term for the intervention group as compared to the control group is significantly different from zero at the 0.05 level.
Moreover, in order to determine if the mode of dose delivery (i.e., face-to-face, phone call coaching) may differentially affect the outcome, we will conduct a sensitivity analysis. In addition, we plan to conduct other post-hoc tests as appropriate to explore potential differences in this regard.
Detectable difference, sample size and power
Refer to Table 4 for the power analysis of our primary outcome measure. Our sample size estimation, used the Optimal Design (OD) [77] software consistent with our planned analysis. This software allows us to examine two-group repeated-measures trials with quadratic change, the same model being used for the analysis.
Table 4.
Power Calculations
| Power/Effect Size | Sample size for Standardized Effect size = 0.3 | Sample size for Standardized Effect size = 0.4 | Sample size for Standardized Effect size = 0.5 |
|---|---|---|---|
| 70% | 500 | 285 | 186 |
| 80% | 640 | 360 | 232 |
| 90% | 860 | 480 | 308 |
This software uses a standardized effect size as defined in Raudenbush and Liu, namely, the group difference on the polynomial trend divided by the “population standard deviation of the polynomial trend of interest” [78]. This specification, particularly the denominator, is quite different from cross-sectional standardized effect sizes such as Cohen’s D, given that, with a polynomial model (i.e., quadratic), the difference between groups depends on the point in time examined. In particular, given our hypothesis, we expect that, after adiposity rebound is reached, the BMI of children in the intervention group will grow more slowly than that of children in the control group, such that the differences between their mean BMIs will increase over time. Our expectation implies that we are interested in the significance of the quadratic term in the model, and expect that the difference between the control and treatment group quadratic effect will be significantly different from zero.
Currently, the design for the GROW trial includes 600 children, and, though we would expect to be adequately powered at a smaller number of subjects (i.e., 480), we plan to recruit 600 subjects to allow for potential attrition. Based on previous data from a similar study (Salud Con La Familia), we chose a conservative standardized effect size estimate of 0.40 (the default within the Optimal Design program). Our sample size goal of 600 participants would yield 95% power with this effect size. We have multiple measures in place to keep attrition to a minimum, but even with 20% attrition, a sample size of 480 would yield a power of 90%. Table 4 illustrates how things would change with different standardized effect sizes.
Secondary analyses: Social Network Analysis
Social network analysis is conducted using the software packages UCINET and In-Flow. UCINET is used for entering and analyzing network data and, along with In-flow, for generating network measures and graphical displays. This data set will thus contain both network and attribute variables at the individual level of analysis. Applying standard statistical techniques (e.g., linear regression, logistic regression, etc.) these independent variables are modeled with selected dependent variables. The analysis examines the change in these social networks over time and their impact on the main outcomes of interest including: growth trajectories (children’s BMI); body composition (child and adult), parenting practices (child feeding); physical activity (child and adult), and total energy intake.
The social network hypothesis suggests that members of a given network group will share health behavior characteristics more than members of other groups. Variance shared by individuals who join clusters are measured by an intra-class correlation in a multilevel model. Using PASS, we made power estimates for a range of ICCs. We have assumed 234 parent-child dyads in network groups of 15–20. As cluster size becomes larger than 20, the design effect reduces power, and as clusters become smaller, power improves. The 80% power point was ICC = 0.085. According to Raudenbush, small/medium/large ICCs are .05/. 10/. 15. The proposed study, having 80% power at ICC = .085, can detect small -to-medium size ICCs with adequate power [79].
Secondary analyses: Genetic Analysis
Genotyping is performed using the Sequenom MALDI-TOF (Matrix Assisted Laser Desorption-ionization Time-of-Flight) system, with individualized assay designs created by automated Spectro design software (Sequenom, San Diego, CA), and assay methods that have previously been described. Next, we are examining observed genotype distributions for significant deviation from Hardy- Weinberg equilibrium frequencies (p < 0.01). Given that this field is advancing rapidly, we are bio-banking all specimens and re-evaluating our genetic analysis approach after study completion.
Monitoring
Data monitoring
At all major data collection time points, data are entered directly into a REDCap database [80]. REDCap is a secure, web-based data management system that allows direct entry of participant data (e.g., measurements, responses to survey questions, etc.) into an electronic format. This direct entry system facilitates the process of downloading and transferring data to the research coordinating unit (RCU). Other REDCap features include builtin data validity checks as well as automated export procedures for downloads to Excel and common statistical packages (SPSS, SAS, Stata and R).
Participant Safety
Participants are assessed for adverse events at the time of enrollment and when the data is collected at each time-point. The Principal Investigator, co-investigators, study coordinator, interventionists, and all members of the research staff are responsible for the assessment and reporting of adverse events. All spontaneous reports by subjects, observations by clinical research staff, and reports to research staff by family or health care providers are investigated. The investigators assess the relationship of the adverse event as not related, possibly related or definitely related using standard criteria for clinical trials.
DISCUSSION
The current study is a multi-level, family-centered, community-engaged healthy lifestyle randomized controlled trial designed to prevent childhood obesity in high-risk preschool-aged children over a 3-year period of time. Culturally-tailored, theory-driven, and extensively refined through its formative phase (e.g., cognitive interviews, focus groups, and pilots), this study investigates BMI trajectories of children during this critical window of child development.
This intervention has several distinguishing features: 1) the primary outcome is BMI trajectory during early childhood [9, 81, 82]; 2) the study is conducted in the built environment, using public community recreational centers that are routinely available to all populations, including minority populations, thereby considering sustainability and future translation as part of the study design; 3) an adaptive intervention is used to account for children who do not respond to the intervention 4) the intervention utilizes a multi-level theoretical model, addressing macro-system level components, such as built environment, mezzo-level components such as parent-child objective physical activity behavior, and micro-system level components, such as genetics; 5) a low-health literacy approach is used to design all components of the curriculum to be easily accessible to low literacy populations; and 6) the intervention actively builds social networks and utilizes social media to develop and sustain positive healthy behavior, allowing intervention participants to interact between study contacts guided by their interest and needs.
CONCLUSION
In conclusion, behavioral interventions that address obesity during the early years of life have enormous potential to prevent childhood obesity and improve lifelong health outcomes. Given that 60% of overweight preschoolers will go on to become overweight adolescents, prevention is essential [83]. Moreover, by utilizing the built infrastructure of parks and recreation that reaches more than 200 million Americans, pragmatic trials such as GROW, have the potential to improve the public’s health and prevent childhood obesity.
Supplementary Material
Acknowledgments
This trial is supported by Award Number U01 HL103620 from the National Heart, Lung, And Blood Institute, the Eunice Kennedy Shriver National Institute of Child Health and Development and the Office of Behavioral and Social Sciences Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, And Blood Institute or the National Institutes of Health. The REDCap Database is supported by NCATS/NIH, grant number: UL1 TR000445.
Footnotes
Conflict of Interest
None of the authors have any conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Prevalence of Obesity Among Children and Adolescents: United States, Trends 1963–1965 through 2007–2008. [ http://www.cdc.gov/nchs/data/hestat/obesity_child_07_08/obesity_child_07_08.htm]
- 2.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. Jama. 2012;307(5):483–490. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olshansky SJ, Passaro DJ, Hershow RC, Layden J, Carnes BA, Brody J, Hayflick L, Butler RN, Allison DB, Ludwig DS. A potential decline in life expectancy in the United States in the 21st century. N Engl J Med. 2005;352(11):1138–1145. doi: 10.1056/NEJMsr043743. [DOI] [PubMed] [Google Scholar]
- 4.Baker JL, Olsen LW, Sorensen TI. Childhood body-mass index and the risk of coronary heart disease in adulthood. N Engl J Med. 2007;357(23):2329–2337. doi: 10.1056/NEJMoa072515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pena MM, Dixon B, Taveras EM. Are you talking to ME? The importance of ethnicity and culture in childhood obesity prevention and management. Child Obes. 2012;8(1):23–27. doi: 10.1089/chi.2011.0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh AS, Mulder C, Twisk JW, van Mechelen W, Chinapaw MJ. Tracking of childhood overweight into adulthood: a systematic review of the literature. Obes Rev. 2008;9(5):474–488. doi: 10.1111/j.1467-789X.2008.00475.x. [DOI] [PubMed] [Google Scholar]
- 7.Rolland-Cachera MF, Deheeger M, Bellisle F, Sempe M, Guilloud-Bataille M, Patois E. Adiposity rebound in children: a simple indicator for predicting obesity. Am J Clin Nutr. 1984;39(1):129–135. doi: 10.1093/ajcn/39.1.129. [DOI] [PubMed] [Google Scholar]
- 8.Cole TJ. Children grow and horses race: is the adiposity rebound a critical period for later obesity? BMC Pediatr. 2004;4:6. doi: 10.1186/1471-2431-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barker DJ, Osmond C, Forsen TJ, Kajantie E, Eriksson JG. Trajectories of growth among children who have coronary events as adults. N Engl J Med. 2005;353(17):1802–1809. doi: 10.1056/NEJMoa044160. [DOI] [PubMed] [Google Scholar]
- 10.Birch L, Ventura AK. Preventing childhood obesity: what works? Int J Obes (Lond) 2009;33:74–81. doi: 10.1038/ijo.2009.22. [DOI] [PubMed] [Google Scholar]
- 11.Kropski JA, Keckley PH, Jensen GL. School-based obesity prevention programs: an evidence-based review. Obesity (Silver Spring) 2008;16(5):1009–1018. doi: 10.1038/oby.2008.29. [DOI] [PubMed] [Google Scholar]
- 12.Summerbell CD, Waters E, Edmunds LD, Kelly S, Brown T, Campbell KJ. Interventions for preventing obesity in children. Cochrane Database Syst Revs. 2005;(3) doi: 10.1002/14651858.CD001871.pub2. [DOI] [PubMed] [Google Scholar]
- 13.Institute of Medicine; Obesity CoPiPC, editor. Committee on Progress in Preventing Childhood Obesity: Progress in Preventing Childhood Obesity: How do we measure up. The National Academies Press; Sep, 2006. [Google Scholar]
- 14.U.S. Department of Health and Human Services. Strategic plan for NIH obesity research: a report of the NIH Obesity Research Task Force. National Institutes of Health; Rockville, MD: 2004. [Google Scholar]
- 15.Huang TT, Drewnosksi A, Kumanyika S, Glass TA. A systems-oriented multilevel framework for addressing obesity in the 21st century. Prev Chronic Dis. 2009;6(3):A82. [PMC free article] [PubMed] [Google Scholar]
- 16.Bottino CJ, Rifas-Shiman SL, Kleinman KP, Oken E, Redline S, Gold D, Schwartz J, Melly SJ, Koutrakis P, Gillman MW, et al. The association of urbanicity with infant sleep duration. Health & place. 2012;18(5):1000–1005. doi: 10.1016/j.healthplace.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wrotniak BH, Epstein LH, Paluch RA, Roemmich JN. The relationship between parent and child self-reported adherence and weight loss. Obes Res. 2005;13(6):1089–1096. doi: 10.1038/oby.2005.127. [DOI] [PubMed] [Google Scholar]
- 18.Epstein LH, Valoski A, Wing RR, McCurley J. Ten-year follow-up of behavioral, family-based treatment for obese children. Jama. 1990;264(19):2519–2523. [PubMed] [Google Scholar]
- 19.Epstein LH, Myers MD, Raynor HA, Saelens BE. Treatment of pediatric obesity. Pediatrics. 1998;101(3 Pt 2):554–570. [PubMed] [Google Scholar]
- 20.Savoye M, Shaw M, Dziura J, Tamborlane WV, Rose P, Guandalini C, Goldberg-Gell R, Burgert TS, Cali AM, Weiss R, et al. Effects of a weight management program on body composition and metabolic parameters in overweight children: a randomized controlled trial. Jama. 2007;297(24):2697–2704. doi: 10.1001/jama.297.24.2697. [DOI] [PubMed] [Google Scholar]
- 21.Dattilo AM, Birch L, Krebs NF, Lake A, Taveras EM, Saavedra JM. Need for early interventions in the prevention of pediatric overweight: a review and upcoming directions. J Obes. 2012;2012:123023. doi: 10.1155/2012/123023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bronfenbrenner U. Ecology of the family as a context for human development: Research prespectives. Developmental Psychology. 1986;22:723–742. [Google Scholar]
- 23.Ryan DH, Espeland MA, Foster GD, Haffner SM, Hubbard VS, Johnson KC, Kahn SE, Knowler WC, Yanovski SZ. Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials. 2003;24(5):610–628. doi: 10.1016/s0197-2456(03)00064-3. [DOI] [PubMed] [Google Scholar]
- 24.Wadden TA, Foster GD. Behavioral treatment of obesity. Med Clin North Am. 2000;84(2):441–461. vii. doi: 10.1016/s0025-7125(05)70230-3. [DOI] [PubMed] [Google Scholar]
- 25.Le Brazidec JY, Pasis A, Tam B, Boykin C, Wang D, Marcotte DJ, Claassen G, Chong JH, Chao J, Fan J, et al. Structure-based design of 2,6,7-trisubstituted-7H-pyrrolo[2,3-d]pyrimidines as Aurora kinases inhibitors. Bioorganic & medicinal chemistry letters. 2012;22(12):4033–4037. doi: 10.1016/j.bmcl.2012.04.085. [DOI] [PubMed] [Google Scholar]
- 26.Faith MS, Van Horn L, Appel LJ, Burke LE, Carson JA, Franch HA, Jakicic JM, Kral TV, Odoms-Young A, Wansink B, et al. Evaluating parents and adult caregivers as “agents of change” for treating obese children: evidence for parent behavior change strategies and research gaps: a scientific statement from the American Heart Association. Circulation. 2012;125(9):1186–1207. doi: 10.1161/CIR.0b013e31824607ee. [DOI] [PubMed] [Google Scholar]
- 27.Michie S, Richardson M, Johnston M, Abraham C, Francis J, Hardeman W, Eccles MP, Cane J, Wood CE. The Behavior Change Technique Taxonomy (v1) of 93 Hierarchically Clustered Techniques: Building an International Consensus for the Reporting of Behavior Change Interventions. Ann Behav Med. 2013;46(1):81–95. doi: 10.1007/s12160-013-9486-6. [DOI] [PubMed] [Google Scholar]
- 28.BMI - Body Mass Index. About BMI for Children and Teens. [ http://www.cdc.gov/nccdphp/dnpa/bmi/childrens_BMI/about_childrens_BMI.htm]
- 29.Barkin SL, Gesell SB, Po’e EK, Escarfuller J, Tempesti T. Culturally Tailored, Family-Centered, Behavioral Obesity Intervention for Latino-American Preschool-aged Children. Pediatrics. 2012;130(3):445–456. doi: 10.1542/peds.2011-3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alvarez RA, Vasquez E, Mayorga CC, Feaster DJ, Mitrani VB. Increasing minority research participation through community organization outreach. West J Nurs Res. 2006;28(5):541–560. doi: 10.1177/0193945906287215. discussion 561–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eakin EG, Bull SS, Riley K, Reeves MM, Gutierrez S, McLaughlin P. Recruitment and retention of Latinos in a primary care-based physical activity and diet trial: The Resources for Health study. Health Educ Res. 2007;22(3):361–371. doi: 10.1093/her/cyl095. [DOI] [PubMed] [Google Scholar]
- 32.Keller CS, Gonzales A, Fleuriet KJ. Retention of minority participants in clinical research studies. West J Nurs Res. 2005;27(3):292–306. doi: 10.1177/0193945904270301. [DOI] [PubMed] [Google Scholar]
- 33.McQuiston C, Uribe L. Latino recruitment and retention strategies: community-based HIV prevention. J Immigr Health. 2001;3(2):97–105. doi: 10.1023/A:1009565900783. [DOI] [PubMed] [Google Scholar]
- 34.Hare ME, Coday M, Williams NA, Richey PA, Tylavsky FA, Bush AJ. Methods and baseline characteristics of a randomized trial treating early childhood obesity: the Positive Lifestyles for Active Youngsters (Team PLAY) trial. Contemp Clin Trials. 2012;33(3):534–549. doi: 10.1016/j.cct.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dryden EM, Hardin J, McDonald J, Taveras EM, Hacker K. Provider perspectives on electronic decision supports for obesity prevention. Clinical pediatrics. 2012;51(5):490–497. doi: 10.1177/0009922812436549. [DOI] [PubMed] [Google Scholar]
- 36.Le Brazidec JY, Pasis A, Tam B, Boykin C, Black C, Wang D, Claassen G, Chong JH, Chao J, Fan J, et al. Synthesis, SAR and biological evaluation of 1,6-disubstituted-1H-pyrazolo[3,4-d]pyrimidines as dual inhibitors of Aurora kinases and CDK1. Bioorganic & medicinal chemistry letters. 2012;22(5):2070–2074. doi: 10.1016/j.bmcl.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 37.Jakicic JM, Marcus BH, Lang W, Janney C. Effect of exercise on 24-month weight loss maintenance in overweight women. Arch Intern Med. 2008;168(14):1550–1559. doi: 10.1001/archinte.168.14.1550. discussion 1559–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jakicic JM, Marcus BH, Gallagher KI, Napolitano M, Lang W. Effect of exercise duration and intensity on weight loss in overweight, sedentary women: a randomized trial. Jama. 2003;290(10):1323–1330. doi: 10.1001/jama.290.10.1323. [DOI] [PubMed] [Google Scholar]
- 39.Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults--The Evidence Report. National Institutes of Health. Obes Res. 1998;6 (Suppl 2):51S–209S. [PubMed] [Google Scholar]
- 40.Melin I, Karlstrom B, Lappalainen R, Berglund L, Mohsen R, Vessby B. A programme of behaviour modification and nutrition counselling in the treatment of obesity: a randomised 2-y clinical trial. International journal of obesity and related metabolic disorders: journal of the International Association for the Study of Obesity. 2003;27(9):1127–1135. doi: 10.1038/sj.ijo.0802372. [DOI] [PubMed] [Google Scholar]
- 41.Taras HL, Sallis JF, Nader PR, Nelson J. Children’s television-viewing habits and the family environment. American Journal of Diseases of Children. 1990;144(3):357–359. doi: 10.1001/archpedi.1990.02150270107036. [DOI] [PubMed] [Google Scholar]
- 42.Appel LJ, Clark JM, Yeh HC, Wang NY, Coughlin JW, Daumit G, Miller ER, 3rd, Dalcin A, Jerome GJ, Geller S, et al. Comparative effectiveness of weight-loss interventions in clinical practice. N Engl J Med. 2011;365(21):1959–1968. doi: 10.1056/NEJMoa1108660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Working Group Report on Future Research Directions in Childhood Obesity Prevention and Treatment. [ http://www.nhlbi.nih.gov/meetings/workshops/child-obesity/index.htm]
- 44.Wen X, Gillman MW, Rifas-Shiman SL, Sherry B, Kleinman K, Taveras EM. Decreasing prevalence of obesity among young children in Massachusetts from 2004 to 2008. Pediatrics. 2012;129(5):823–831. doi: 10.1542/peds.2011-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Salsa Sabor y Salud. Healthy Lifestyles for Latino Families. [ http://www.nlci.org/salsa/indexSSS.htm]
- 46.National Association for Sport and Physcial Education. Active Start: A Statement of Physical Activity Guidelines for Children from Birth to Age 5. 2. 2009. [Google Scholar]
- 47.Institute of Medicine Committee on Health Literacy. Health Literacy: A Prescription to End Confusion. Washington DC: The National Academies Press; 2004. [PubMed] [Google Scholar]
- 48.Richard WO, Thompson JR, Rothman RL, McDougald Scott AM, Heerman WJ, Sommer EC, Barkin SL. A Health Literate Approach to the Prevention of Childhood Overweight & Obesity. Patient Education and Couseling. 2013 doi: 10.1016/j.pec.2013.08.010. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Doak C, Doak Leonard, Root Jane. Teaching Patients with Low Literacy Skills. Philadelphia: Lippincott Williams & Wilkins; 1996. [Google Scholar]
- 50.Collins LM, Murphy SA, Bierman KL. A conceptual framework for adaptive preventive interventions. Prev Sci. 2004;5(3):185–196. doi: 10.1023/b:prev.0000037641.26017.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Neuman SB, Celano Donna. Every Child Ready to Read. 2. Association for Library Service to Children (ALSC) and the Public Library Association (PLA); 2011. [Google Scholar]
- 52.Chrispeels JH, WJ, Rivero E. Evaluation summary of the Impact of the Parent Institue for Quality Education on Parent’s Engagement with their Children’s Schooling. Santa Barbara: University of California; 2000. [Google Scholar]
- 53.Tunis SR, Stryer DB, Clancy CM. Practical clinical trials: increasing the value of clinical research for decision making in clinical and health policy. Jama. 2003;290(12):1624–1632. doi: 10.1001/jama.290.12.1624. [DOI] [PubMed] [Google Scholar]
- 54.Zwarenstein M, Treweek S, Gagnier JJ, Altman DG, Tunis S, Haynes B, Oxman AD, Moher D. Improving the reporting of pragmatic trials: an extension of the CONSORT statement. Bmj. 2008;337:a2390. doi: 10.1136/bmj.a2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Daniels SR, Khoury PR, Morrison JA. The utility of body mass index as a measure of body fatness in children and adolescents: Differences by race and gender. Pediatrics. 1997;99(6):804–807. doi: 10.1542/peds.99.6.804. [DOI] [PubMed] [Google Scholar]
- 56.Pietrobelli A, Faith MS, Allison DB, Gallagher D, Chiumello G, Heymsfield SB. Body mass index as a measure of adiposity among children and adolescents: a validation study. J Pediatr. 1998;132(2):204–210. doi: 10.1016/s0022-3476(98)70433-0. [DOI] [PubMed] [Google Scholar]
- 57.Dezenberg CV, Nagy TR, Gower BA, Johnson R, Goran MI. Predicting body composition from anthropometry in pre-adolescent children. Int J Obes (Lond) 1999;23(3):253–259. doi: 10.1038/sj.ijo.0800802. [DOI] [PubMed] [Google Scholar]
- 58.Bray GA, DeLany JP, Harsha DW, Volaufova J, Champagne CC. Evaluation of body fat in fatter and leaner 10-y-old African American and white children: the Baton Rouge Children’s Study. American Journal of Clinical Nutrition. 2001;73(4):687–702. doi: 10.1093/ajcn/73.4.687. [DOI] [PubMed] [Google Scholar]
- 59.Scott CR, Smith JM, Cradock MM, Pihoker C. Characteristics of youth-onset noninsulin-dependent diabetes mellitus and insulin-dependent diabetes mellitus at diagnosis. Pediatrics. 1997;100(1):84–91. doi: 10.1542/peds.100.1.84. [DOI] [PubMed] [Google Scholar]
- 60.Pinhas-Hamiel O, Dolan LM, Daniels SR, Standiford D, Khoury PR, Zeitler P. Increased incidence of non-insulin-dependent diabetes mellitus among adolescents. J Pediatr. 1996;128(5 Pt 1):608–615. doi: 10.1016/s0022-3476(96)80124-7. [DOI] [PubMed] [Google Scholar]
- 61.Freedman DS, Dietz WH, Srinivasan SR, Berenson GS. The relation of overweight to cardiovascular risk factors among children and adolescents: The Bogalusa Heart Study. Pediatrics. 1999;103:1175–1182. doi: 10.1542/peds.103.6.1175. [DOI] [PubMed] [Google Scholar]
- 62.Dwyer JT, Stone EJ, Yang M, Feldman H, Webber LS, Must A, Perry CL, Nader PR, Parcel GS. Predictors of overweight and overfatness in a multiethnic pediatric population. Child and Adolescent Trial for Cardiovascular Health Collaborative Research Group. Am J Clin Nutr. 1998;67(4):602–610. doi: 10.1093/ajcn/67.4.602. [DOI] [PubMed] [Google Scholar]
- 63.Teixeira PJ, Sardinha LB, Going SB, Lohman TG. Total and regional fat and serum cardiovascular disease risk factors in lean and obese children and adolescents. Obes Res. 2001;9(8):432–442. doi: 10.1038/oby.2001.57. [DOI] [PubMed] [Google Scholar]
- 64.Mcgill HC, Mcmahan CA, Malcom GT, Oalmann MC, Strong JP, Wissler RW, Robertson AL, Cornhill JF, Gay S, Gay RE, et al. Relation of Glycohemoglobin and Adiposity to Atherosclerosis in Youth. Arterioscl Throm Vas. 1995;15(4):431–440. doi: 10.1161/01.atv.15.4.431. [DOI] [PubMed] [Google Scholar]
- 65.Berenson GS, Srinivasan SR, Bao WH, Newman WP, Tracy RE, Wattigney WA, Study BH. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. New Engl J Med. 1998;338(23):1650–1656. doi: 10.1056/NEJM199806043382302. [DOI] [PubMed] [Google Scholar]
- 66.Gesell SB, Bess KD, Barkin SL. Understanding the Social Networks That Form within the Context of an Obesity Prevention Intervention. Journal of Obesity. 2012;2012:749832. doi: 10.1155/2012/749832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Valente TW. Network interventions. Science. 2012;337(6090):49–53. doi: 10.1126/science.1217330. [DOI] [PubMed] [Google Scholar]
- 68.Gesell SB, Barkin SL, Valente TW. Social Network Diagnostics: A Tool for Monitoring Group Interventions. Implementation Science. 2013 doi: 10.1186/1748-5908-8-116. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ng DP, Koh D, Choo S, Chia KS. Saliva as a viable alternative source of human genomic DNA in genetic epidemiology. Clin Chim Acta. 2006;367(1–2):81–85. doi: 10.1016/j.cca.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 70.Environmental Supports for Physical Activity Questionnaire. [ http://prevention.sph.sc.edu/tools/docs/Env_Supports_for_PA.pdf]
- 71.Kirtland KA, Porter DE, Addy CL, Neet MJ, Williams JE, Sharpe PA, Neff LJ, Kimsey CD, Jr, Ainsworth BE. Environmental measures of physical activity supports: perception versus reality. Am J Prev Med. 2003;24(4):323–331. doi: 10.1016/s0749-3797(03)00021-7. [DOI] [PubMed] [Google Scholar]
- 72.Reinert KR, Po’e EK, Barkin SL. The relationship between executive function and obesity in children and adolescents: a systematic literature review. Journal of Obesity. 2013;2013:820956. doi: 10.1155/2013/820956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sonneville KR, Rifas-Shiman SL, Kleinman KP, Gortmaker SL, Gillman MW, Taveras EM. Associations of obesogenic behaviors in mothers and obese children participating in a randomized trial. Obesity (Silver Spring) 2012;20(7):1449–1454. doi: 10.1038/oby.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bellg AJ, Borrelli B, Resnick B, Hecht J, Minicucci DS, Ory M, Ogedegbe G, Orwig D, Ernst D, Czajkowski S. Enhancing treatment fidelity in health behavior change studies: best practices and recommendations from the NIH Behavior Change Consortium. Health Psychol. 2004;23(5):443–451. doi: 10.1037/0278-6133.23.5.443. [DOI] [PubMed] [Google Scholar]
- 75.Agras WS, Hammer LD, McNicholas F, Kraemer HC. Risk factors for childhood overweight: a prospective study from birth to 9. 5 years. J Pediatr. 2004;145(1):20–25. doi: 10.1016/j.jpeds.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 76.Pryor LE, Tremblay RE, Boivin M, Touchette E, Dubois L, Genolini C, Liu X, Falissard B, Cote SM. Developmental trajectories of body mass index in early childhood and their risk factors: an 8-year longitudinal study. Arch Pediatr Adolesc Med. 2011;165(10):906–912. doi: 10.1001/archpediatrics.2011.153. [DOI] [PubMed] [Google Scholar]
- 77.Spybrook J, Raudenbush SW, Congdon R, Martinez A. Software. 2011. Optimal Design for Longitudinal and Multilevel Research: Documentation for the “Optimal Design”. [Google Scholar]
- 78.Raudenbush SW, Xiao-Feng L. Effects of study duration, frequency of observation, and sample size on power in studies of group differences in polynomial change. Psychol Methods. 2001;6(4):387–401. [PubMed] [Google Scholar]
- 79.Raudenbush S, Liu X. Statistical power and optimal design for multisite randomized trials. Psychol Methods. 2000;5(2):199–213. doi: 10.1037/1082-989x.5.2.199. [DOI] [PubMed] [Google Scholar]
- 80.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Baker DW. The meaning and the measure of health literacy. J Gen Intern Med. 2006;21(8):878–883. doi: 10.1111/j.1525-1497.2006.00540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Leunissen RW, Kerkhof GF, Stijnen T, Hokken-Koelega A. Timing and tempo of first-year rapid growth in relation to cardiovascular and metabolic risk profile in early adulthood. Jama. 2009;301(21):2234–2242. doi: 10.1001/jama.2009.761. [DOI] [PubMed] [Google Scholar]
- 83.Nader PR, O’Brien M, Houts R, Bradley R, Belsky J, Crosnoe R, Friedman S, Mei Z, Susman EJ. Identifying risk for obesity in early childhood. Pediatrics. 2006;118(3):e594–601. doi: 10.1542/peds.2005-2801. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.