Abstract
Short bowel syndrome (SBS) is the most common cause of intestinal failure in infants. In neonates and young infants, necrotizing enterocolitis, gastroschisis, intestinal atresia and intestinal malrotation/volvulus are the leading causes of SBS. Following an acute post-surgical phase, the residual gastrointestinal tract adapts with reorganization of the crypt-villus histoarchitecture and functional changes in nutrient absorption and motility. A cohesive, multidisciplinary approach can allow most neonates with SBS to transition to full enteral feeds and achieve normal growth and development. In this article, we review the clinical features, management, complications, and prognostic factors in SBS.
Keywords: Short gut, NEC, transplantation, intestinal adaptation, intestinal rehabilitation, short bowel syndrome, SBS, necrotizing enterocolitis, malabsorption, small bowel transplant, bowel conservation
Definitions: Intestinal Failure and Short Bowel Syndrome
Intestinal failure is defined as a significant reduction in the functional gut mass below a critical threshold necessary to maintain growth, hydration and/or electrolyte balance (27, 29). Intestinal failure can occur due to surgical resection of bowel, congenital anomalies, or functional/motility disorders. In neonatal intensive care units (NICUs), the most common cause of intestinal failure is the surgical short bowel syndrome (SBS), which is defined as a need for prolonged parenteral nutrition following bowel resection, usually for more than 3 months (78).
Epidemiology of SBS in Neonates and Young Infants
Surgical SBS was recorded in 0.7% (89/12316) of very low birth weight (VLBW) infants born during the period 2002-2005 at the National Institute of Child Health and Development (NICHD) neonatal research network centers (12). The frequency of SBS increased in an inverse relationship with birth weight; the incidence of SBS in infants weighing 401-1000 grams was 1.1% (61/5657), nearly twice that in infants with a birth weight of 1001-1500 grams (28/6659; 0.4%) (12). In Canada, data from a large tertiary NICU show an overall incidence of SBS as 22.1 per 1000 admissions, and 24.5 per 100,000 live births (79). The incidence was higher in infants born at less than 37 weeks gestation (353.7 per 100,000 live births) than in full-term infants (3.5/100,000 live births). The SBS case fatality rate was 37.5% (79). Similarly, in a study from 7 tertiary neonatal units in Italy, intestinal failure was seen in 0.1% (26/30,353) of all live births and 0.5% (26/5088) among those admitted to the NICU (56).
Attempts to estimate the incidence and prevalence of SBS have been constrained by the rarity of the condition, variation in nomenclature, difficulty in providing a clear definition of the study population at tertiary institutions because of complex referral patterns, and paucity of follow-up data (78, 79). Population-based studies are sparse. Although the need for home parenteral nutrition has been used as a surrogate for SBS in some studies, this approach has important limitations; some patients may require parenteral nutrition due to a diagnosis other than SBS, such as malignancy, whereas some infants with SBS who may have been weaned off parenteral nutrition prior to discharge from the hospital may not be included.
Etiology of SBS in Neonates and Young Infants
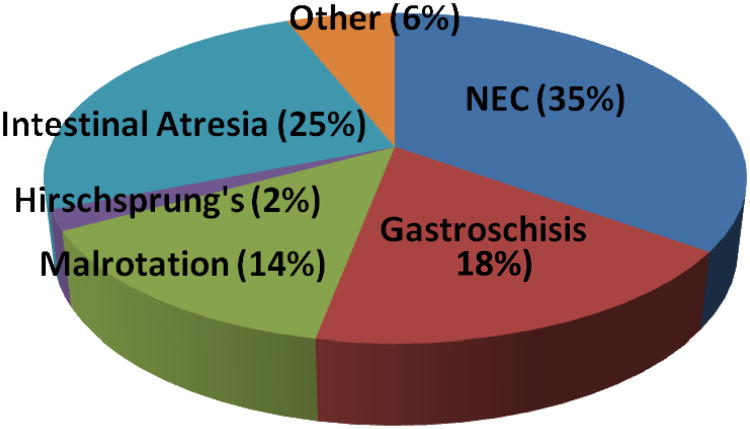
Necrotizing enterocolitis (NEC) is the most common cause of SBS (35%) in neonates, followed by intestinal atresia (25%), gastroschisis (18%), malrotation with volvulus (14%), followed by less common conditions such as Hirschsprung's disease with proximal extension of aganglionosis into the small bowel (2%) (Fig. 1) (29). In another study, infants with SBS who eventually required intestinal transplantation had the following primary diagnoses: gastroschisis (25%), intestinal volvulus (24%), NEC (12%), intestinal pseudo-obstruction (10%), jejunoileal atresia (9%), Hirschsprung's disease (7%), and other conditions (13%) (43). In VLBW infants, NEC remains the predominant cause of SBS. In the NICHD cohort, 96% of cases of SBS were due to NEC. Congenital defects (gastroschisis, intestinal atresia) accounted for 2% and volvulus for the remaining 2% (12).
Figure 1.
Causes of short bowel syndrome in neonates and young infants.
Duro et al.(20) enrolled 473 patients with a diagnosis of NEC. Among the 129 patients who required surgery, 54 (42%) developed SBS, which was significantly more common than in the control group (6/265; 2%; odds ratio (OR) 31.1, 95% confidence interval (CI), 12.9 - 75.1, p < 0.001). Multivariate analysis showed that SBS was associated with variables characteristic of severe NEC: birth weight <750 grams (OR = 9.09, p <0.001), antibiotic use (OR = 16.61, p = 0.022), ventilator use on day of diagnosis (OR = 6.16, p = 0.009); exposure to enteral feeding before the diagnosis of NEC (OR=4.05, p = 0.048); and percentage of small bowel resected (OR = 1.85 per 10 percentage point greater resection, p = 0.031).
Besides NEC, gastroschisis is now emerging as a common cause of pediatric SBS leading to intestinal transplantation (43, 67). The incidence of gastroschisis is increasing and has been estimated in recent studies to be as high as 5 per 10,000 live births (9, 77). In gastroschisis, the fetal bowel eviscerates through a narrow abdominal wall defect and in the absence of a covering membrane, is exposed to the amniotic fluid. Patients with gastroschisis can develop SBS due to associated jejunoileal atresia, malrotation and consequent midgut volvulus, and occasionally, due to refractory intestinal failure (43).
Intestinal atresia, seen in approximately 1 in 5000 newborns, is another important cause of SBS in the NICU (29). Although most patients with jejunoileal atresia have an isolated atretic segment that can be treated by a simple resection and end-to-end anastomosis, some infants with severe disruption develop SBS. When a major blood vessel such as the superior mesenteric artery is occluded in utero, large parts of bowel can become atretic. In about 10% of all cases of intestinal atresia, these disrupted bowel loops lack a dorsal mesentery and can assume a spiral configuration resembling an ‘apple peel’ (72). In a series of 15 infants (23), apple-peel intestinal atresia was associated with increased post-operative morbidity and mortality. Two of these 15 infants had SBS at 24 months. Infants with multiple intestinal atresias (‘sausage-string’ type defect) are also at risk of SBS (57, 58).
SBS can occur in infants with Hirschsprung's disease, particularly in those at the severe end of the spectrum with proximal extension of aganglionosis into the small intestine (41). Some infants with Hirschsprung's disease can develop intestinal failure and SBS after severe enterocolitis and associated tissue necrosis (22).
Clinical Presentation of Infants With SBS
The clinical course of SBS patients can be described in three clinical stages:
Stage I (acute phase)
After recovery from post-operative ileus, most patients go into an acute phase starting at about 1 week after surgery and lasting for up to 3 weeks (24, 62). This phase is characterized by large fluid and electrolyte losses in ostomy effluent/stool, requiring intravenous fluids and parenteral nutrition. Medical management is aimed at maintenance of the fluid and electrolyte balance. Depending on the extent of intestinal resection, the acute phase is generally associated with gastric hypersecretion. Treatment with H2 blockers or proton pump inhibitors may become necessary during this stage.
Stage II (recovery phase) starts after a few weeks and continues for several months (24, 62). This phase is characterized by gradual improvement in diarrhea and ostomy output. The dependence on parenteral nutrition is related to the degree of initial intestinal loss, condition of the remaining bowel at the time of the surgery, and compensatory histoarchitectural changes in the residual gut mucosa. Clinical management during this stage involves cautious initiation of enteral nutrition and gradual weaning of parenteral nutrition.
Stage III (maintenance phase) indicates successful intestinal adaptation (24, 62). In this stage, enteral nutrition is tolerated and parenteral nutrition can be discontinued. Oral feedings can often be started at this stage. The time required to reach this stage is variable depending upon the infant's clinical course and complications.
The term ‘intestinal adaptation’ is used in the clinical setting to indicate recovery of intestinal function after intestinal resection (70). Intestinal adaptation starts as early as 48 hours after surgery and may continue for up to 18 months. This contrasts with another frequently-used term, ‘intestinal rehabilitation,’ which is used to describe the compensatory changes in the mucosal histoarchitecture and function in the residual bowel, which can increase the absorptive surface area and restore the capacity of the remaining intestine to absorb fluid, electrolytes and nutrients in adequate amounts to meet the growth and maintenance requirements of the body. Typical rehabilitative changes in the mucosa include axial lengthening of the villi, deepening of the crypts, increased enterocyte proliferation, which increases enterocyte counts per villus, and enhanced enterocyte function with increased nutrient uptake (64).
In SBS, the clinical presentation and outcome depend on the length and health of the remaining bowel, age of the patient, gastrointestinal region(s) that were resected and are now missing, presence of ileo-cecal valve, and other co-morbidities (66, 78). The length of the remaining intestine is arguably the most important determinant of clinical outcome in SBS. Age is another important factor, and the potential for intestinal growth is much better in infants than in adults. Intestinal length increases from 142 ± 22 cm at 19-27 weeks of gestation to 217 ± 24 cm at 27-35 weeks, and to 304±44 cm at term (range 176-305 cm) (68, 71). Small bowel growth peaks at 25-35 weeks' gestation and doubles in length during the last 15 weeks of pregnancy. After birth, the intestine continues to grow at a rapid rate until the crown-heel length reaches 60 cm, and then at a slightly slower pace until mature intestinal length of 600-700 cm is reached about when the somatic height approaches 100-140 cm (39).
Studies show that in the absence of surgical bowel lengthening and tapering procedures, an infant with about 35 cm of residual small bowel has a 50% probability of being weaned from parenteral nutrition (1). In other series, the likelihood of developing SBS following bowel resection was greater when the residual intestinal length was less than 25% of the predicted length for a given gestational age (79). In another study, Wilmore et al.(84) reported that an infant with SBS was more likely to survive if the small intestinal length was at least 15 cm in the presence of an intact ileo-cecal valve, or 40 cm in the absence of the ileo-cecal valve. Consistent with these observations, Spencer et al.(66) noted that a bowel length of less than 10% of expected was associated with increased mortality (relative risk 5.74) (66). In this series, 88% (52/59) of all infants retaining ≥10% of their expected normal small intestinal length survived, compared to only 21% (4/19) of those with <10% of expected length. With a given intestinal length, outcomes may also vary depending on the quality of the remaining bowel, region of the gastrointestinal tract, and the gestational age of the patient. There are reports of some infants who were weaned successfully from parenteral nutrition with as little as 10 cm residual intestinal length (1). Generally, premature infants have a greater capacity for intestinal growth and adaptation as compared to full term newborns (1).
In a given patient, the clinical features of SBS can be usually predicted based on the gastrointestinal region(s) that were lost to surgical resection (Fig. 2) (66, 78). Jejunal resection is usually tolerated relatively well because the ileal mucosa can adapt to compensate for the lost absorptive surface area. The loss of jejunum can affect intestinal motility in the post- surgical period, and has also been associated with increased gastric emptying. Decreased production of cholecystokinin, secretin, vasoactive intestinal peptide, and serotonin in these patients can lead to gastrin hyper-secretion, causing increased basal and peak acid output.
Figure 2.
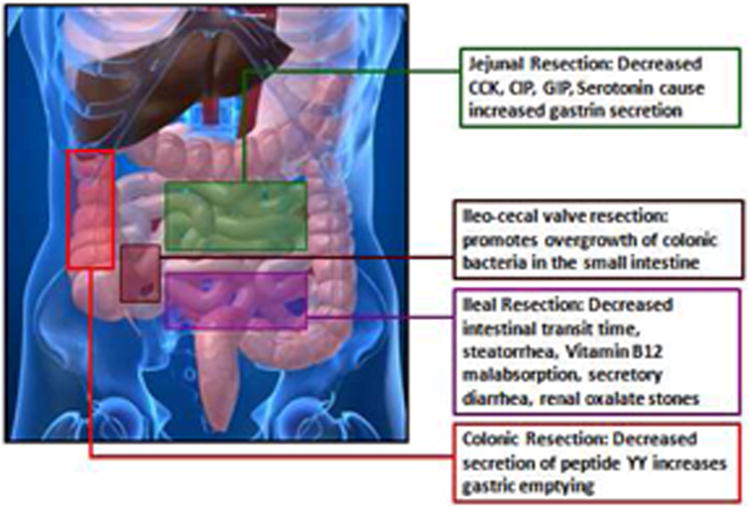
Clinical features of short bowel syndrome can be predicted based on the gastrointestinal region(s) that were lost to surgical resection and resulting physiological changes.
Compared to patients with jejunal resection, ileal resection is more often associated with symptoms as the capacity of jejunum to compensate for the lost ileum is limited (6). Ileal resection is frequently associated with impaired absorption of fluid and electrolytes, bile acids, and vitamin B12. Decreased bile acid re-absorption can result in a reduced bile acid pool, leading to impaired micelle formation, fat malabsorption, and steatorrhea. In these patients, removal of the ileo-cecal valve is another negative predictor of clinical outcome. The ileo-cecal valve is presumed to play an important role in the regulation of gut bacterial flora by preventing retrograde migration and overgrowth of colonic bacteria, which thrive in areas of intestinal dilatation and impaired motility. Colonic bacteria can de-conjugate unabsorbed bile acids, which, in turn, can stimulate the gut epithelium and cause secretory diarrhea.
Colonic resection can increase the risk of fluid and electrolyte depletion and dehydration. Colonic resection can also increase gastric emptying and reduce intestinal transit time due to decreased secretion of peptide YY (PYY), glucagon-like peptide I (GLP-I), and neurotensin, which are important negative regulators of gut motility (51). Overall, preservation of colon is associated with better outcomes in case of SBS patients.
Several studies have shown that plasma levels of citrulline, a non-structural amino acid synthesized in the intestinal mucosa, is a useful marker for estimating the total functional intestinal mass (13, 15). Serum citrulline concentrations correlate with the length of the remaining small intestinal bowel in patients with SBS (13) and also with the ability of the patient to be weaned from parenteral nutrition. Rhoads et al.(53) showed that serum citrulline levels show a linear correlation with percent enteral calories and bowel length. A serum citrulline level ≥19 μmoles/L was associated with enteral tolerance and was predictive of weaning from parenteral nutrition. In another study, Fitzgibbons et al.(25) showed that patients with plasma citrulline levels <12 μmoles/L could not be weaned off parenteral nutrition.
SBS is associated with increased morbidity and mortality. Infants with SBS are at risk of sepsis, prolonged hospitalization, growth delay, and motor developmental delay (8). Infants with SBS have a 3-fold increase in mortality than in controls, who had the same underlying condition but no SBS (37.5% [15/40] vs. 13/3% [18/135]). Infant with SBS have disease-specific mortality rates that are 5 times higher than those without SBS − 20.2 vs. 3.8 per 100 person-years (78, 79). SBS-related mortality tends to be highest in the early postoperative period and then decreases until 200-350 days post-surgery, when a second peak in mortality is associated with the onset of end-stage liver disease (80).
Complications of SBS
Gastric acid hyper-secretion is seen in approximately 50% of all patients with SBS (31, 83). Proximal intestinal resection may be more likely to increase acid output than distal resection. Increased gastric output in SBS can predispose to acid-peptic injury, exacerbate fluid and electrolyte losses, and impairs intraluminal digestion and cause malabsorption by causing inactivation of pancreatic exocrine enzymes, precipitation of bile salts (and ineffective micelle formation), and by causing physical damage to the small bowel mucosa.
In SBS, increased circulating gastrin levels are widely believed to be the primary reason for acid hyper-secretion. In one study, arterial levels of gastrin were higher than in mesenteric veins draining the distal small bowel, indicating that intestine is a site for gastrin metabolism and that increased gastrin concentrations in patients with SBS may be due to disruption of this process. Another possibility is the loss of an inhibitor that is normally produced in the intestine and blocks gastrin action; secretin, gastrointestinal inhibitory polypeptide, cholecystokinin, and somatostatin have been proposed as candidates (31).
Bacterial Overgrowth is seen in up to 60% of all patients with SBS (29). Expansion of the bacterial flora can result in de-conjugation of bile acids, competition for metabolites, consumption of enteral nutrients and vitamins, accumulation of toxic metabolites such as D-lactate, and bacterial translocation producing blood stream infections (38). Clinical signs of bacterial overgrowth include abdominal pain, anorexia, vomiting, diarrhea, cramps, and metabolic acidosis from accumulation of D-lactic acid.
D-lactic acidosis is a classic complication of bacterial overgrowth (5, 29). Intestinal bacteria produce both L-lactate and D-lactate, of which only L-lactate is metabolized. D-lactate can accumulate to toxic levels, sometimes causing neurological problems ranging from disorientation to coma. D-lactic acidosis is characterized by increased anion gap acidosis with normal L-lactate levels. Strategies to prevent D-lactic acidosis include the administration of antibiotics to control bacterial overgrowth and reducing carbohydrate intake in patients with partial enteral nutrition.
Translocation of enteric bacteria to the blood stream is an important complication of SBS with bacterial overgrowth. In animal models, aerobic bacteria translocate more readily than anaerobic bacteria (38, 60). Treatment of bacterial overgrowth is empiric; broad spectrum antibiotics effective against enteric bacteria are administered for 7-14 days, followed by ‘rest’ for 14-21 days, and then the cycle is repeated. Because many patients with SBS are dependent on parenteral nutrition and have central lines that can provide a nidus for infection, bacterial translocation in these patients increases the risk of central line-associated blood stream infections. The frequency of catheter-related infections in children with SBS has been estimated as 11-26 infections per 1000 catheter days (34, 48). Catheter-associated sepsis may require the removal of the line and with repeated infections, eventually lead to the loss of central venous access sites, accelerated liver failure, and increased mortality. Clinical signs of central line infections include fever, irritability and ileus. Catheter-related infections can be minimized by the use of strict aseptic techniques during insertion, maintenance of sterile occlusive dressings, close surveillance for signs of infection, and the use of antibiotic-impregnated catheters and antibiotic and/or ethanol locks. In a recent study, Jones et al.(34) reported successful use of 70% (v/v) ethanol locks in 23 patients with SBS. Ethanol locks were well-tolerated with no reported adverse effects. Infection rates decreased from 9.9 per 1000 catheter days in historical controls to 2.2 per 1000 catheter days during the period when ethanol locks were used.
Intestinal failure-associated liver disease develops in 40-60% infants who require long-term parenteral nutrition for intestinal failure. The clinical spectrum includes hepatic steatosis, cholestasis, cholelithiasis and hepatic fibrosis (35, 36). Liver disease may progress to biliary cirrhosis and portal hypertension in a minority of patients. The pathogenesis of liver disease in SBS is multi-factorial; in infants, the risk is increased with prematurity, low birth weight, duration of parenteral nutrition, recurrent sepsis, and multiple laparotomies. In patients with no enteral feeding, decreased gut hormone secretion can cause biliary stasis and development of biliary sludge and gallstones, which can further impair hepatic dysfunction. Deficiency of taurine, cysteine, and choline may also contribute to liver toxicity. Finally, conventional parenteral lipid solutions containing sunflower oil may also contribute to liver toxicity in these patients.
In SBS, liver disease usually presents with increasing jaundice, scleral icterus, hepato-splenomegaly, elevated serum transaminases, and eventually, portal hypertension. Monitoring of liver function tests is essential in the management of IFALD. Doppler evaluation of hepatic and portal veins may be useful for assessment of portal hypertension. Liver biopsy is a gold standard to assess the extent of liver damage. Duro et al.(19) recently reported a non-invasive C13 methionine breath test to differentiate cirrhotic vs. non- cirrhotic patients.
Management of SBS
Management of short bowel syndrome requires a multi-disciplinary approach that includes neonatologists, gastroenterologists, surgeons, nutritionists, pharmacists, nurses, and social workers. A cohesive, integrated multidisciplinary approach has been shown to increase survival rates in children with short bowel syndrome (46). The aims of clinical management in SBS are to (a) provide sufficient nutrition to facilitate growth, (b) minimize fluid, nutritional, and electrolyte losses, and (c) maximize intestinal adaptation.
Parenteral Nutrition is used in infants with SBS to provide adequate caloric intake, macronutrients, and micronutrients to optimize growth and development. Parenteral nutrition should provide appropriate caloric intake to promote growth but avoid excessive weight gain without linear growth. Careful attention should be paid to protein intake to prevent catabolism, and some patients may need up to 3.5 g/kg/day (82). Some authors have advocated restricting carbohydrates in the immediate peri-operative period as many patients with SBS are unable to optimally utilize these calories during this period of stress (38). To ensure optimal total caloric intake, fats comprise a critical part of parenteral nutrition. However, there is increasing concern that the cumulative amount of lipid in parenteral nutrition in patients with SBS is an independent risk factor for the development of cholestasis (63).
Current lipid formulations are based on soybean lipids, olive-oil lipids, or fish oil lipids. Patients on long-term parenteral nutrition are at increased risk of cholestasis, fatty liver, and hepatic fibrosis. Limiting soy lipid-based formulations to less than 0.5 g/kg/d has been shown to delay or even prevent cholestasis (63). About 50% of the fatty acids present in soy-based formulations are the omega-6 polyunsaturated fatty acids such as linoleic acid, which is a cause of concern because linoleic acid may have immunosuppressive and pro-inflammatory effects (55). Soy lipid formulations have also been linked to impaired glucose homeostasis, where gluconeogenesis was increased but the normal reduction in glycogenolysis was inhibited (75).
Fish oil-based formulations such as ‘Omegaven’, which are rich in ω-3 fatty acids, have shown promise for reversing parenteral nutrition-associated cholestasis. This formulation is currently available in Europe and is an investigational drug in the United States. The efficacy and safety of fish oil lipid was evaluated in 19 infants who developed cholestasis while on soy lipid-based formula (28). These patients were started fish oil lipid-based formula and the soy lipid-based formula was discontinued. Compared with a historical cohort, infants on fish oil lipids showed reversal of cholestasis at 9.4 weeks, compared to 44.1 weeks in a historical cohort taking soy lipids and also had lower mortality. Administration of fish oil lipids was not associated with fatty acid deficiency, hypertriglyceridemia, infection, growth delay, or coagulopathy (28). Similar findings were reported in other studies (11, 16, 52).
Olive oil-based parenteral nutrition formulas have also been shown to be safe and well-tolerated in infants (26). Compared to soy-lipid formulations, olive oil-based lipids contain lower levels of linoleic acid, thereby avoiding some of the adverse effects associated with n-6 PUFAs (55). Furthermore, olive oil formulations do not appear to have the same effects on glucose metabolism that soy lipid formulations do (75). Olive oil-based formulations can also decrease total cholesterol and low-density lipoproteins, with no significant differences in liver functions or adverse events (26).
Patients with SBS need careful monitoring for electrolyte balance. Infants with ileostomy or poor colonic function can lose considerable amounts of sodium in stool. In the absence of adequate sodium replacement, compensatory hyper-aldosteronism may ensue (61) and result in increased urinary potassium losses. Generally, parenteral sodium replacement is considered adequate if urinary sodium levels are more than 30 mEq/L (38).
Trace element depletion is not unusual in patients with SBS on parenteral nutrition. Zinc is of particular importance in infants with extensive intestinal resection, as substantial amounts of zinc could be lost in ileostomy fluid (as high as 17 mg/L ileostomy fluid). Infants with SBS who are dependent on parenteral nutrition often require 300-500 μg/kg/day of zinc (82).
Enteral Nutrition
There is little agreement in the literature regarding the timing of initiation or the rate of advancement of enteral feedings in SBS. Most clinicians prefer to start enteral feedings as soon as possible, hoping to promote intestinal adaptation, achieve full enteral feeds sooner, and prevent parenteral nutrition-associated adverse events (29, 38). In SBS, continuous administration of enteral nutrition is the preferred method because of lower risk of osmotic diarrhea and overall better tolerance than bolus feeds (76).
The optimal formula of enteral feeds has not yet been established. Most clinicians prefer human milk or a semi-elemental or amino acid-based formula containing medium-chained and long-chained triglycerides (38, 54). In experimental models, formulations containing complex proteins, disaccharides, and long-chain triglycerides increased intestinal adaptation, but concerns remain about increased gut permeability to complex proteins and the risk of sensitization. Human breast milk is associated with a shorter duration of parenteral nutrition when compared to cow's milk or protein hydrolysates (1). The difference between hydrolyzed protein and non-hydrolyzed protein has been evaluated in infants receiving some enteral and parenteral nutrition, and no difference was noted in energy intake, intestinal permeability, or nitrogen balance (40).
The rate of advancement of enteral feedings should be individualized with careful monitoring of stool/stoma output, vomiting, and abdominal distention. Generally, stool/stoma output should be limited to 40-50 mL/kg/d, although higher outputs may be permissible if hydration, acid-base status, and electrolyte balance can be maintained (82). We typically increase enteral feedings to volumes where the stool output is at maximally-tolerated levels, and then as the bowel adapts, increase the volume and/or concentration of feeds (54).
Pharmacological Interventions in SBS
Acid suppressing agents
Patients with SBS frequently develop hyper-gastrinemia, which leads to increased gastric acid secretion. H2-receptor blockers and proton pump inhibitors are often used to suppress gastric acid secretion, especially for first six months after surgery.
Prokinetic agents
Intestinal dysmotility is an important cause of morbidity in patients with SBS (17). Symptoms of intestinal dysmotility include abdominal distention, vomiting, feeding intolerance, and inability to progress enteral feeds. Bacterial overgrowth, lactic acidosis, bacterial translocation, and systemic infection are complications of intestinal dysmotility and distension. Various pharmacologic agents have been used to treat intestinal dysmotility:
Erythromycin increases gut motility by activating motilin receptors. In physiological studies, prokinetic effects of erythromycin are observed at doses as low as 10 to 20 mg/kg/day. However, in a systematic review examining 10 randomized controlled studies, both high and low dose erythromycin failed to show improvement in feeding intolerance in preterm infants (49). In a recent review (50), erythromycin use was associated with shorter time to full feeds, decreased duration of parenteral nutrition, and decreased incidence of cholestasis.
Metoclopramide, a dopamine receptor antagonist with moderate serotonergic activity, can improve lower esophageal sphincter tone, improve gastric emptying, and facilitate antro-pyloro-duodenal contractions. Adverse effects of metoclopramide include extra-pyramidal reactions in up to 30% patients. In 2009, FDA added labeling changes including limiting metoclopramide use to less than 12 weeks. Data on use of metoclopramide in SBS patients is limited (17). When used, metoclopramide is given enterally at a dose of 0.1 mg/kg 3-4 times a day for 2 to 3 weeks.
Anti-diarrheal agents are used to reduce gut motility in selected patients with high ostomies:
Opoid agents decrease peristalsis, increase absorption of fluid and electrolytes, increase tone in the large intestine and increase anal sphincter tone. In pediatric SBS, loperamide, diphenoxylate/atropine and opium alkaloid codeine have been tried, although no data are available to compare these agents in children. Loperamide may be superior to codeine in reducing electrolyte losses and is the preferred agent to treat diarrhea in these patients (17).
Absorbent agents, such as pectin and guar gum agents act by absorbing fluids and compounds and binding potential intestinal toxins from the gut. These agents can reduce the intestinal transit time and reduce diarrhea (18). Caution should be used in patients with significant dysmotility as it may lead to bacterial overgrowth and lactic acidosis (17).
Ursodeoxycholic acid (UDCA)
UDCA is a hydrophilic derivative of chenodeoxycholic acid, which acts by displacement of endogenous, more hydrophobic, toxic bile acids. UDCA can arrest rising alkaline phosphatase and also lower serum bilirubin levels in patients with SBS and cholestasis (14). UDCA is usually initiated orally at a dose of 20 mg/kg/day divided in 2 doses and is given for a period of 4 to 6 weeks.
Cholestyramine
In patients with SBS, particularly those with loss of ileum, there is disruption of enterohepatic circulation of bile acids (65). Unabsorbed bile acids reach colon and cause irritation leading to secretory diarrhea. Cholestyramine is beneficial in treatment of bile acid-induced secretory diarrhea (17).
Pharmacological approaches to enhance intestinal adaptation
Various pharmacologic agents have been studied to accelerate intestinal adaptation in animal models and humans. Epidermal growth factor (EGF), growth hormone, glucagon like peptide-2 ( GLP2), keratinocyte growth factor, and insulin like growth factor 1 (IGF-1) have been studied in animal models. Growth hormone and GLP2 have been studied in humans (38). GH in combination with glucagon has shown some improvement in intestinal function in adults but has not been evaluated in pediatric SBS. A GLP-2 analog (teduglutide) has been shown to reduce the need for parenteral nutrition by more than 20% in double blind randomized human trials (33).
Surgical Management of SBS
Surgical interventions in SBS include bowel conservation at the time of initial presentation, bowel lengthening operations and intestinal transplantation.
Bowel conservation
The goal of the initial surgical procedure is to limit bowel loss and excise only the obviously-compromised bowel. Any bowel of marginal viability is kept in situ. A “second-look” procedure in 24 hours can be used to assess the viability of the remaining bowel (29). During this period, a temporary “silo” can be used to prevent abdominal compartment syndrome, and closure can be performed once intestinal edema has resolved.
Longitudinal intestinal lengthening and tailoring (LILT) procedure
The LILT procedure was first described by Bianchi in 1980 (3). This procedure involves splitting the intestine into two longitudinal halves, and then anastomosing the halves in series with the rest of the intestine. This procedure doubles the length of the bowel and at the same time, improves transit time and peristalsis by decreasing the diameter of the dilated bowel. All mucosa is preserved. However, there is no increase in the absorptive surface area. A modification of LILT was described in 1998 (10), where the intestine was divided in the middle of the dilated segment, staples were placed obliquely, then longitudinally, and then obliquely again to the opposite edge of the intestine. This procedure resulted in two intestinal segments that remained connected to the proximal and distal ends of the intestine. The free ends were then anastomosed. Only 1 anastomosis was needed, as opposed to 3 in the original LILT procedure (10).
LILT procedure can be technically challenging; complications include fistula formation, anastomotic stenosis, and anastomotic leakage (30, 69). A high incidence of sepsis, progressive intestinal failure, and surgical complications has also been reported (7). In a review of 20 patients (4), 6-year survival rates were 45%. Survivors had residual bowel length greater than 40 cm and no liver disease, whereas the non-survivors had shorter bowel lengths and significant cholestasis. The Bianchi procedure is not recommended is neonates, those with liver disease, or intestine length less than 50 cm (7).
Serial Transverse Enteroplasty (STEP)
The STEP procedure was first described in 2003 (37). This procedure involves application of surgical staples in a trans-mesenteric transverse fashion, which creates a longer and narrower intestinal lumen (45). The STEP procedure has several advantages over the Bianchi procedure in being less technically challenging, resulting in a uniform lumen, and in the ability to be repeated if re-dilatation of the bowel occurs (21, 47).
Outcomes from the STEP procedure have been encouraging. In a series of 38 patients (45), the average increase in intestinal length was 69% and enteral caloric intake was noted to increase from 31% to 67% in median 1 year period. Complications included staple line leak and intra-operative aspiration, bowel obstruction, hypertension, hematoma, abscess, and pleural effusions. Three patients died and another 3 received transplantation beyond 30 days post-operatively (45). Similar results have been reported elsewhere (32, 81). In a group of 14 children who underwent the STEP procedure in Toronto, Canada, the total increase in intestinal length was 49% (81). Parenteral nutrition calories decreased from 71% to 36% within 1 month and to 12% after 1 year. Compared to patients who underwent LILT, the STEP procedure allowed faster weaning off parenteral nutrition and lower need for later transplants. No difference was found in early complications, growth rates, or survival rates (69).
Intestinal Transplantation
Transplantation is a treatment of last resort, indicated mainly in patients with irreversible hepatic and intestinal failure (44, 73, 74). With improved parenteral nutrition and consequent prevention of liver disease, the need for transplants has decreased (29). Types of transplantation include isolated intestine, isolated liver, combined liver and intestine, and multi-visceral. Current indications for intestinal transplantation, combined intestine/liver transplantation or multi-visceral transplantation for patients with irreversible intestinal failure are: (a) impending liver failure as manifested by jaundice, elevated liver injury tests, clinical findings (splenomegaly, varices, coagulopathy), history of stomal bleeding, or hepatic cirrhosis on biopsy; (b) loss of major venous access defined as more than two thromboses in the great vessels (subclavian, jugular and femoral veins); (c) frequent central line-related sepsis consisting of more than two episodes of systemic sepsis per year, or one episode of line-related fungemia; (d) recurrent episodes of severe dehydration despite intravenous fluid management (42). Complications of transplantation include acute rejection, infection, graft-versus-host disease, and post-transplant lympho-proliferative disease. Factors contributing to poor outcomes include multiple pre-transplant surgeries, end-stage liver disease, inferior vena cava thrombosis, and hospitalization versus home care (59). One-year patient and intestine graft survival is 89% and 79% for intestine only recipients and 72% and 69% for liver-intestine recipients, respectively. By 10 years, patient and intestine graft survival falls to 46% and 29% for intestine only recipients, and 42% and 39% for liver-intestine, respectively (44). More recently, living donor intestinal transplantation and CLDILT combined living donor intestine/liver transplant have been done successfully in pediatric patients with intestinal and liver failure, and have a major advantage of virtual elimination of waiting time (2, 73).
Conclusion
SBS is the most common cause of intestinal failure in infants. Advancements in parenteral nutrition, strategies to prevent infections, surgical technique, and intestinal transplantation have greatly increased survival in patients with short bowel syndrome. With a cohesive and integrated management plan and a multidisciplinary approach, most neonates with short bowel syndrome can be transitioned to enteral feeding and to achieve normal growth and development.
Brief summary of important points.
In neonatal intensive care units (NICUs), the most common cause of intestinal failure is surgical short bowel syndrome (SBS), which is defined as a need for prolonged parenteral nutrition following bowel resection, usually for more than 3 months.
The clinical course of SBS patients can be described in three clinical stages: acute, recovery, and maintenance.
A cohesive, multidisciplinary approach can allow most infants with SBS to transition to full enteral feeds and achieve normal growth and development.
Acknowledgments
Funding: National Institutes of Health award R01HD059142 (to A.M.)
Footnotes
Conflicts of interest: The authors disclose no conflicts.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Sachin C. Amin, Email: sachina@uic.edu.
Cleo Pappas, Email: cleop76@gmail.com.
Hari Iyengar, Email: hiyengar@gmail.com.
Akhil Maheshwari, Email: akhil1@uic.edu.
References
- 1.Andorsky DJ, Lund DP, Lillehei CW, Jaksic T, Dicanzio J, Richardson DS, Collier SB, Lo C, Duggan C. Nutritional and other postoperative management of neonates with short bowel syndrome correlates with clinical outcomes. The Journal of pediatrics. 2001;139:27–33. doi: 10.1067/mpd.2001.114481. [DOI] [PubMed] [Google Scholar]
- 2.Berg CL, Steffick DE, Edwards EB, Heimbach JK, Magee JC, Washburn WK, Mazariegos GV. Liver and intestine transplantation in the United States 1998-2007. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2009;9:907–931. doi: 10.1111/j.1600-6143.2009.02567.x. [DOI] [PubMed] [Google Scholar]
- 3.Bianchi A. Intestinal loop lengthening--a technique for increasing small intestinal length. Journal of pediatric surgery. 1980;15:145–151. doi: 10.1016/s0022-3468(80)80005-4. [DOI] [PubMed] [Google Scholar]
- 4.Bianchi A. Longitudinal intestinal lengthening and tailoring: results in 20 children. Journal of the Royal Society of Medicine. 1997;90:429–432. doi: 10.1177/014107689709000804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bongaerts G, Tolboom J, Naber T, Bakkeren J, Severijnen R, Willems H. D-lactic acidemia and aciduria in pediatric and adult patients with short bowel syndrome. Clinical chemistry. 1995;41:107–110. [PubMed] [Google Scholar]
- 6.Buchman AL, Scolapio J, Fryer J. AGA technical review on short bowel syndrome and intestinal transplantation. Gastroenterology. 2003;124:1111–1134. doi: 10.1016/s0016-5085(03)70064-x. [DOI] [PubMed] [Google Scholar]
- 7.Bueno J, Guiterrez J, Mazariegos GV, Abu-Elmagd K, Madariaga J, Ohwada S, Kocoshis S, Reyes J. Analysis of patients with longitudinal intestinal lengthening procedure referred for intestinal transplantation. Journal of pediatric surgery. 2001;36:178–183. doi: 10.1053/jpsu.2001.20047. [DOI] [PubMed] [Google Scholar]
- 8.Casaccia G, Trucchi A, Spirydakis I, Giorlandino C, Aite L, Capolupo I, Catalano OA, Bagolan P. Congenital intestinal anomalies, neonatal short bowel syndrome, and prenatal/neonatal counseling. Journal of pediatric surgery. 2006;41:804–807. doi: 10.1016/j.jpedsurg.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 9.Chabra S, Gleason CA, Seidel K, Williams MA. Rising prevalence of gastroschisis in Washington State. Journal of toxicology and environmental health Part A. 2011;74:336–345. doi: 10.1080/15287394.2011.534424. [DOI] [PubMed] [Google Scholar]
- 10.Chahine AA, Ricketts RR. A modification of the Bianchi intestinal lengthening procedure with a single anastomosis. Journal of pediatric surgery. 1998;33:1292–1293. doi: 10.1016/s0022-3468(98)90171-3. [DOI] [PubMed] [Google Scholar]
- 11.Chung PH, Wong KK, Wong RM, Tsoi NS, Chan KL, Tam PK. Clinical experience in managing pediatric patients with ultra-short bowel syndrome using omega-3 fatty acid. European journal of pediatric surgery : official journal of Austrian Association of Pediatric Surgery [et al] = Zeitschrift fur Kinderchirurgie. 2010;20:139–142. doi: 10.1055/s-0029-1238283. [DOI] [PubMed] [Google Scholar]
- 12.Cole CR, Hansen NI, Higgins RD, Ziegler TR, Stoll BJ. Very low birth weight preterm infants with surgical short bowel syndrome: incidence, morbidity and mortality, and growth outcomes at 18 to 22 months. Pediatrics. 2008;122:e573–582. doi: 10.1542/peds.2007-3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crenn P, Coudray-Lucas C, Cynober L, Messing B. Post-absorptive plasma citrulline concentration: a marker of intestinal failure in humans. Transplantation proceedings. 1998;30:2528. doi: 10.1016/s0041-1345(98)00711-8. [DOI] [PubMed] [Google Scholar]
- 14.De Marco G, Sordino D, Bruzzese E, Di Caro S, Mambretti D, Tramontano A, Colombo C, Simoni P, Guarino A. Early treatment with ursodeoxycholic acid for cholestasis in children on parenteral nutrition because of primary intestinal failure. Alimentary pharmacology & therapeutics. 2006;24:387–394. doi: 10.1111/j.1365-2036.2006.02972.x. [DOI] [PubMed] [Google Scholar]
- 15.Diamanti A, Panetta F, Gandullia P, Morini F, Noto C, Torre G, Lezo A, Goffredo B, Daniele A, Gambarara M. Plasma citrulline as marker of bowel adaptation in children with short bowel syndrome. Langenbeck's archives of surgery / Deutsche Gesellschaft fur Chirurgie. 2011;396:1041–1046. doi: 10.1007/s00423-011-0813-8. [DOI] [PubMed] [Google Scholar]
- 16.Diamond IR, Sterescu A, Pencharz PB, Kim JH, Wales PW. Changing the paradigm: omegaven for the treatment of liver failure in pediatric short bowel syndrome. Journal of pediatric gastroenterology and nutrition. 2009;48:209–215. doi: 10.1097/MPG.0b013e318182c8f6. [DOI] [PubMed] [Google Scholar]
- 17.Dicken BJ, Sergi C, Rescorla FJ, Breckler F, Sigalet D. Medical management of motility disorders in patients with intestinal failure: a focus on necrotizing enterocolitis, gastroschisis, and intestinal atresia. Journal of pediatric surgery. 2011;46:1618–1630. doi: 10.1016/j.jpedsurg.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 18.Drenckpohl D, Hocker J, Shareef M, Vegunta R, Colgan C. Adding dietary green beans resolves the diarrhea associated with bowel surgery in neonates: a case study. Nutrition in clinical practice : official publication of the American Society for Parenteral and Enteral Nutrition. 2005;20:674–677. doi: 10.1177/0115426505020006674. [DOI] [PubMed] [Google Scholar]
- 19.Duro D, Fitzgibbons S, Valim C, Yang CF, Zurakowski D, Dolan M, Bechard L, Yu YM, Duggan C, Jaksic T. [13C]Methionine breath test to assess intestinal failure-associated liver disease. Pediatric research. 2010;68:349–354. doi: 10.1203/PDR.0b013e3181ed15e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duro D, Kalish LA, Johnston P, Jaksic T, McCarthy M, Martin C, Dunn JC, Brandt M, Nobuhara KK, Sylvester KG, Moss RL, Duggan C. Risk factors for intestinal failure in infants with necrotizing enterocolitis: a Glaser Pediatric Research Network study. The Journal of pediatrics. 2010;157:203–208 e201. doi: 10.1016/j.jpeds.2010.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ehrlich PF, Mychaliska GB, Teitelbaum DH. The 2 STEP: an approach to repeating a serial transverse enteroplasty. Journal of pediatric surgery. 2007;42:819–822. doi: 10.1016/j.jpedsurg.2006.12.056. [DOI] [PubMed] [Google Scholar]
- 22.Elhalaby EA, Teitelbaum DH, Coran AG, Heidelberger KP. Enterocolitis associated with Hirschsprung's disease: a clinical histopathological correlative study. Journal of pediatric surgery. 1995;30:1023–1026. doi: 10.1016/0022-3468(95)90334-8. discussion 1026-1027. [DOI] [PubMed] [Google Scholar]
- 23.Festen S, Brevoord JC, Goldhoorn GA, Festen C, Hazebroek FW, van Heurn LW, de Langen ZJ, van Der Zee DC, Aronson DC. Excellent long-term outcome for survivors of apple peel atresia. Journal of pediatric surgery. 2002;37:61–65. doi: 10.1053/jpsu.2002.29428. [DOI] [PubMed] [Google Scholar]
- 24.Finkel Y, Goulet O. Short Bowel Syndrome. In: Kleinman RE, Sanderson IR, Goulet O, Sherman PM, Mieli-Vergani G, Shneider BL, editors. Walker's Pediatric Gastrointestinal Disease. Hamilton, ON: BC Decker, Inc.; 2008. pp. 601–612. [Google Scholar]
- 25.Fitzgibbons S, Ching YA, Valim C, Zhou J, Iglesias J, Duggan C, Jaksic T. Relationship between serum citrulline levels and progression to parenteral nutrition independence in children with short bowel syndrome. Journal of pediatric surgery. 2009;44:928–932. doi: 10.1016/j.jpedsurg.2009.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goulet O, de Potter S, Antebi H, Driss F, Colomb V, Bereziat G, Alcindor LG, Corriol O, Le Brun A, Dutot G, Forget D, Perennec V, Ricour C. Long-term efficacy and safety of a new olive oil-based intravenous fat emulsion in pediatric patients: a double-blind randomized study. The American journal of clinical nutrition. 1999;70:338–345. doi: 10.1093/ajcn/70.3.338. [DOI] [PubMed] [Google Scholar]
- 27.Goulet O, Ruemmele F. Causes and management of intestinal failure in children. Gastroenterology. 2006;130:S16–28. doi: 10.1053/j.gastro.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 28.Gura KM, Lee S, Valim C, Zhou J, Kim S, Modi BP, Arsenault DA, Strijbosch RA, Lopes S, Duggan C, Puder M. Safety and efficacy of a fish-oil-based fat emulsion in the treatment of parenteral nutrition-associated liver disease. Pediatrics. 2008;121:e678–686. doi: 10.1542/peds.2007-2248. [DOI] [PubMed] [Google Scholar]
- 29.Gutierrez IM, Kang KH, Jaksic T. Neonatal short bowel syndrome. Seminars in fetal & neonatal medicine. 2011;16:157–163. doi: 10.1016/j.siny.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 30.Huskisson LJ, Brereton RJ, Kiely EM, Spitz L. Problems with intestinal lengthening. Journal of pediatric surgery. 1993;28:720–722. doi: 10.1016/0022-3468(93)90041-i. [DOI] [PubMed] [Google Scholar]
- 31.Hyman PE, Everett SL, Harada T. Gastric acid hypersecretion in short bowel syndrome in infants: association with extent of resection and enteral feeding. Journal of pediatric gastroenterology and nutrition. 1986;5:191–197. [PubMed] [Google Scholar]
- 32.Javid PJ, Kim HB, Duggan CP, Jaksic T. Serial transverse enteroplasty is associated with successful short-term outcomes in infants with short bowel syndrome. Journal of pediatric surgery. 2005;40:1019–1023. doi: 10.1016/j.jpedsurg.2005.03.020. discussion 1023-1014. [DOI] [PubMed] [Google Scholar]
- 33.Jeppesen PB, Gilroy R, Pertkiewicz M, Allard JP, Messing B, O'Keefe SJ. Randomised placebo-controlled trial of teduglutide in reducing parenteral nutrition and/or intravenous fluid requirements in patients with short bowel syndrome. Gut. 2011;60:902–914. doi: 10.1136/gut.2010.218271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones BA, Hull MA, Richardson DS, Zurakowski D, Gura K, Fitzgibbons SC, Duro D, Lo CW, Duggan C, Jaksic T. Efficacy of ethanol locks in reducing central venous catheter infections in pediatric patients with intestinal failure. Journal of pediatric surgery. 2010;45:1287–1293. doi: 10.1016/j.jpedsurg.2010.02.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kelly DA. Intestinal failure-associated liver disease: what do we know today? Gastroenterology. 2006;130:S70–77. doi: 10.1053/j.gastro.2005.10.066. [DOI] [PubMed] [Google Scholar]
- 36.Kelly DA. Preventing parenteral nutrition liver disease. Early human development. 2010;86:683–687. doi: 10.1016/j.earlhumdev.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 37.Kim HB, Fauza D, Garza J, Oh JT, Nurko S, Jaksic T. Serial transverse enteroplasty (STEP): a novel bowel lengthening procedure. Journal of pediatric surgery. 2003;38:425–429. doi: 10.1053/jpsu.2003.50073. [DOI] [PubMed] [Google Scholar]
- 38.Kocoshis SA. Medical management of pediatric intestinal failure. Seminars in pediatric surgery. 2010;19:20–26. doi: 10.1053/j.sempedsurg.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 39.Koffeman GI, van Gemert WG, George EK, Veenendaal RA. Classification, epidemiology and aetiology. Best practice & research Clinical gastroenterology. 2003;17:879–893. doi: 10.1016/s1521-6918(03)00099-4. [DOI] [PubMed] [Google Scholar]
- 40.Ksiazyk J, Piena M, Kierkus J, Lyszkowska M. Hydrolyzed versus nonhydrolyzed protein diet in short bowel syndrome in children. Journal of pediatric gastroenterology and nutrition. 2002;35:615–618. doi: 10.1097/00005176-200211000-00005. [DOI] [PubMed] [Google Scholar]
- 41.Laughlin DM, Friedmacher F, Puri P. Total colonic aganglionosis: a systematic review and meta-analysis of long-term clinical outcome. Pediatric surgery international. 2012;28:773–779. doi: 10.1007/s00383-012-3117-3. [DOI] [PubMed] [Google Scholar]
- 42.Mazariegos GV. Intestinal transplantation: current outcomes and opportunities. Current opinion in organ transplantation. 2009;14:515–521. doi: 10.1097/MOT.0b013e328330680d. [DOI] [PubMed] [Google Scholar]
- 43.Mazariegos GV, Squires RH, Sindhi RK. Current perspectives on pediatric intestinal transplantation. Current gastroenterology reports. 2009;11:226–233. doi: 10.1007/s11894-009-0035-1. [DOI] [PubMed] [Google Scholar]
- 44.Mazariegos GV, Steffick DE, Horslen S, Farmer D, Fryer J, Grant D, Langnas A, Magee JC. Intestine transplantation in the United States, 1999-2008. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2010;10:1020–1034. doi: 10.1111/j.1600-6143.2010.03044.x. [DOI] [PubMed] [Google Scholar]
- 45.Modi BP, Javid PJ, Jaksic T, Piper H, Langer M, Duggan C, Kamin D, Kim HB. First report of the international serial transverse enteroplasty data registry: indications, efficacy, and complications. Journal of the American College of Surgeons. 2007;204:365–371. doi: 10.1016/j.jamcollsurg.2006.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Modi BP, Langer M, Ching YA, Valim C, Waterford SD, Iglesias J, Duro D, Lo C, Jaksic T, Duggan C. Improved survival in a multidisciplinary short bowel syndrome program. Journal of pediatric surgery. 2008;43:20–24. doi: 10.1016/j.jpedsurg.2007.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morikawa N, Kuroda T, Kitano Y, Tanaka H, Takayasu H, Fujino A, Shibata Y, Tanemura H, Muto M, Honna T. Repeat STEP procedure to establish enteral nutrition in an infant with short bowel syndrome. Pediatric surgery international. 2009;25:1007–1011. doi: 10.1007/s00383-009-2456-1. [DOI] [PubMed] [Google Scholar]
- 48.Mouw E, Chessman K, Lesher A, Tagge E. Use of an ethanol lock to prevent catheter-related infections in children with short bowel syndrome. Journal of pediatric surgery. 2008;43:1025–1029. doi: 10.1016/j.jpedsurg.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 49.Ng E, Shah VS. Erythromycin for the prevention and treatment of feeding intolerance in preterm infants. Cochrane Database Syst Rev. 2008:CD001815. doi: 10.1002/14651858.CD001815.pub2. [DOI] [PubMed] [Google Scholar]
- 50.Ng PC. Use of oral erythromycin for the treatment of gastrointestinal dysmotility in preterm infants. Neonatology. 2009;95:97–104. doi: 10.1159/000153093. [DOI] [PubMed] [Google Scholar]
- 51.Nightingale JM, Kamm MA, van der Sijp JR, Ghatei MA, Bloom SR, Lennard-Jones JE. Gastrointestinal hormones in short bowel syndrome. Peptide YY may be the ‘colonic brake’ to gastric emptying. Gut. 1996;39:267–272. doi: 10.1136/gut.39.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Puder M, Valim C, Meisel JA, Le HD, de Meijer VE, Robinson EM, Zhou J, Duggan C, Gura KM. Parenteral fish oil improves outcomes in patients with parenteral nutrition-associated liver injury. Annals of surgery. 2009;250:395–402. doi: 10.1097/SLA.0b013e3181b36657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rhoads JM, Plunkett E, Galanko J, Lichtman S, Taylor L, Maynor A, Weiner T, Freeman K, Guarisco JL, Wu GY. Serum citrulline levels correlate with enteral tolerance and bowel length in infants with short bowel syndrome. The Journal of pediatrics. 2005;146:542–547. doi: 10.1016/j.jpeds.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 54.Rudolph JA, Squires R. Current concepts in the medical management of pediatric intestinal failure. Current opinion in organ transplantation. 2010;15:324–329. doi: 10.1097/MOT.0b013e32833948be. [DOI] [PubMed] [Google Scholar]
- 55.Sala-Vila A, Barbosa VM, Calder PC. Olive oil in parenteral nutrition. Current opinion in clinical nutrition and metabolic care. 2007;10:165–174. doi: 10.1097/MCO.0b013e32802bf787. [DOI] [PubMed] [Google Scholar]
- 56.Salvia G, Guarino A, Terrin G, Cascioli C, Paludetto R, Indrio F, Lega L, Fanaro S, Stronati M, Corvaglia L, Tagliabue P, De Curtis M. Neonatal onset intestinal failure: an Italian Multicenter Study. The Journal of pediatrics. 2008;153:674–676. 676 e671–672. doi: 10.1016/j.jpeds.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 57.Sapin E, Carricaburu E, De Boissieu D, Goutail-Flaud MF, Benammar S, Helardot PG. Conservative intestinal surgery to avoid short-bowel syndrome in multiple intestinal atresias and necrotizing enterocolitis: 6 cases treated by multiple anastomoses and Santulli-type enterostomy. European journal of pediatric surgery : official journal of Austrian Association of Pediatric Surgery [et al] = Zeitschrift fur Kinderchirurgie. 1999;9:24–28. doi: 10.1055/s-2008-1072207. [DOI] [PubMed] [Google Scholar]
- 58.Sapin E, Joyeux L. Multiple intestinal anastomoses to avoid short bowel syndrome and stimulate bowel maturity in type IV multiple intestinal atresia and necrotizing enterocolitis. Journal of pediatric surgery. 2012;47:628. doi: 10.1016/j.jpedsurg.2011.12.027. [DOI] [PubMed] [Google Scholar]
- 59.Sauvat F, Dupic L, Caldari D, Lesage F, Cezard JP, Lacaille F, Ruemmele F, Hugot JP, Colomb V, Jan D, Hubert P, Revillon Y, Goulet O. Factors influencing outcome after intestinal transplantation in children. Transplantation proceedings. 2006;38:1689–1691. doi: 10.1016/j.transproceed.2006.05.033. [DOI] [PubMed] [Google Scholar]
- 60.Schimpl G, Feierl G, Linni K, Uitz C, Ozbey H, Hollwarth ME. Bacterial translocation in short-bowel syndrome in rats. European journal of pediatric surgery : official journal of Austrian Association of Pediatric Surgery [et al] = Zeitschrift fur Kinderchirurgie. 1999;9:224–227. doi: 10.1055/s-2008-1072249. [DOI] [PubMed] [Google Scholar]
- 61.Schwarz KB, Ternberg JL, Bell MJ, Keating JP. Sodium needs of infants and children with ileostomy. The Journal of pediatrics. 1983;102:509–513. doi: 10.1016/s0022-3476(83)80175-9. [DOI] [PubMed] [Google Scholar]
- 62.Serrano MS, Schmidt-Sommerfeld E. Nutrition support of infants with short bowel syndrome. Nutrition. 2002;18:966–970. doi: 10.1016/s0899-9007(02)00986-3. [DOI] [PubMed] [Google Scholar]
- 63.Shin JI, Namgung R, Park MS, Lee C. Could lipid infusion be a risk for parenteral nutrition-associated cholestasis in low birth weight neonates? European journal of pediatrics. 2008;167:197–202. doi: 10.1007/s00431-007-0454-7. [DOI] [PubMed] [Google Scholar]
- 64.Sidhu GS, Narasimharao KL, Rani VU, Sarkar AK, Chakravarti RN, Mitra SK. Morphological and functional changes in the gut after massive small bowel resection and colon interposition in rhesus monkeys. Digestion. 1984;29:47–54. doi: 10.1159/000199008. [DOI] [PubMed] [Google Scholar]
- 65.Sinha L, Liston R, Testa HJ, Moriarty KJ. Idiopathic bile acid malabsorption: qualitative and quantitative clinical features and response to cholestyramine. Alimentary pharmacology & therapeutics. 1998;12:839–844. doi: 10.1046/j.1365-2036.1998.00388.x. [DOI] [PubMed] [Google Scholar]
- 66.Spencer AU, Neaga A, West B, Safran J, Brown P, Btaiche I, Kuzma-O'Reilly B, Teitelbaum DH. Pediatric short bowel syndrome: redefining predictors of success. Annals of surgery. 2005;242:403–409. doi: 10.1097/01.sla.0000179647.24046.03. discussion 409-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Squires RH, Duggan C, Teitelbaum DH, Wales PW, Balint J, Venick R, Rhee S, Sudan D, Mercer D, Martinez JA, Carter BA, Soden J, Horslen S, Rudolph JA, Kocoshis S, Superina R, Lawlor S, Haller T, Kurs-Lasky M, Belle SH. Natural History of Pediatric Intestinal Failure: Initial Report from the Pediatric Intestinal Failure Consortium. The Journal of pediatrics. 2012 doi: 10.1016/j.jpeds.2012.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Struijs MC, Diamond IR, de Silva N, Wales PW. Establishing norms for intestinal length in children. Journal of pediatric surgery. 2009;44:933–938. doi: 10.1016/j.jpedsurg.2009.01.031. [DOI] [PubMed] [Google Scholar]
- 69.Sudan D, Thompson J, Botha J, Grant W, Antonson D, Raynor S, Langnas A. Comparison of intestinal lengthening procedures for patients with short bowel syndrome. Annals of surgery. 2007;246:593–601. doi: 10.1097/SLA.0b013e318155aa0c. discussion 601-594. [DOI] [PubMed] [Google Scholar]
- 70.Sukhotnik I, Siplovich L, Shiloni E, Mor-Vaknin N, Harmon CM, Coran AG. Intestinal adaptation in short-bowel syndrome in infants and children: a collective review. Pediatric surgery international. 2002;18:258–263. doi: 10.1007/s003830100695. [DOI] [PubMed] [Google Scholar]
- 71.Touloukian RJ, Smith GJ. Normal intestinal length in preterm infants. Journal of pediatric surgery. 1983;18:720–723. doi: 10.1016/s0022-3468(83)80011-6. [DOI] [PubMed] [Google Scholar]
- 72.Trobs RB, Tannapfel A. Apple peel small bowel. Imaging case book. Journal of perinatology : official journal of the California Perinatal Association. 2009;29:832–833. doi: 10.1038/jp.2009.91. [DOI] [PubMed] [Google Scholar]
- 73.Tzvetanov IG, Oberholzer J, Benedetti E. Current status of living donor small bowel transplantation. Current opinion in organ transplantation. 2010;15:346–348. doi: 10.1097/MOT.0b013e3283398fa4. [DOI] [PubMed] [Google Scholar]
- 74.Ueno T, Wada M, Hoshino K, Yonekawa Y, Fukuzawa M. Current status of intestinal transplantation in Japan. Transplantation proceedings. 2011;43:2405–2407. doi: 10.1016/j.transproceed.2011.05.043. [DOI] [PubMed] [Google Scholar]
- 75.van Kempen AA, van der Crabben SN, Ackermans MT, Endert E, Kok JH, Sauerwein HP. Stimulation of gluconeogenesis by intravenous lipids in preterm infants: response depends on fatty acid profile. American journal of physiology Endocrinology and metabolism. 2006;290:E723–730. doi: 10.1152/ajpendo.00303.2005. [DOI] [PubMed] [Google Scholar]
- 76.Vanderhoof JA, Matya SM. Enteral and parenteral nutrition in patients with short-bowel syndrome. European journal of pediatric surgery : official journal of Austrian Association of Pediatric Surgery [et al] = Zeitschrift fur Kinderchirurgie. 1999;9:214–219. doi: 10.1055/s-2008-1072247. [DOI] [PubMed] [Google Scholar]
- 77.Vu LT, Nobuhara KK, Laurent C, Shaw GM. Increasing prevalence of gastroschisis: population-based study in California. The Journal of pediatrics. 2008;152:807–811. doi: 10.1016/j.jpeds.2007.11.037. [DOI] [PubMed] [Google Scholar]
- 78.Wales PW, Christison-Lagay ER. Short bowel syndrome: epidemiology and etiology. Seminars in pediatric surgery. 2010;19:3–9. doi: 10.1053/j.sempedsurg.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 79.Wales PW, de Silva N, Kim J, Lecce L, To T, Moore A. Neonatal short bowel syndrome: population-based estimates of incidence and mortality rates. Journal of pediatric surgery. 2004;39:690–695. doi: 10.1016/j.jpedsurg.2004.01.036. [DOI] [PubMed] [Google Scholar]
- 80.Wales PW, de Silva N, Kim JH, Lecce L, Sandhu A, Moore AM. Neonatal short bowel syndrome: a cohort study. Journal of pediatric surgery. 2005;40:755–762. doi: 10.1016/j.jpedsurg.2005.01.037. [DOI] [PubMed] [Google Scholar]
- 81.Wales PW, de Silva N, Langer JC, Fecteau A. Intermediate outcomes after serial transverse enteroplasty in children with short bowel syndrome. Journal of pediatric surgery. 2007;42:1804–1810. doi: 10.1016/j.jpedsurg.2007.07.029. [DOI] [PubMed] [Google Scholar]
- 82.Wessel JJ, Kocoshis SA. Nutritional management of infants with short bowel syndrome. Seminars in perinatology. 2007;31:104–111. doi: 10.1053/j.semperi.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 83.Williams NS, Evans P, King RF. Gastric acid secretion and gastrin production in the short bowel syndrome. Gut. 1985;26:914–919. doi: 10.1136/gut.26.9.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wilmore DW. Factors correlating with a successful outcome following extensive intestinal resection in newborn infants. The Journal of pediatrics. 1972;80:88–95. doi: 10.1016/s0022-3476(72)80459-1. [DOI] [PubMed] [Google Scholar]