Abstract
The purpose of this simple study was to characterize a panel of clinical isolates of Mycobacterium tuberculosis obtained from the Western Cape region of South Africa where new clinical vaccine trials are beginning, in the low dose aerosol guinea pig infection model. Most of the strains tested grew well in the lungs and other organs of these animals, and in most cases gave rise to moderate to very severe lung damage. We further observed that the current BCG vaccine was highly protective against two randomly selected strains, giving rise to significantly prolonged survival.
1. Introduction
The global epidemic of disease caused by Mycobacterium tuberculosis shows no signs of abating, and is particularly serious in Sub-Saharan Africa, much of it driven by the HIV co-epidemic [1]. The seriousness of the situation, in part at least, reflects the large range of the efficacy of the current BCG vaccine [2, 3] and the increasing drug resistance of many strains of Mycobacterium tuberculosis [4–6].
The past decade has seen strenuous efforts to develop new more effective vaccines against tuberculosis, and the establishment of a pipeline of new candidates. Leading this list are recombinant BCG, a prime boost [BCG/adenovirus] strategy, a recombinant vaccinia virus, and a fusion protein candidate delivered in a potent new adjuvant [7–9].
Clinical trials of new tuberculosis vaccines have started to be conducted at various sites, including in the Western Cape of South Africa. As yet however none of the current lead candidates have been tested against the newly emerging high virulence clinical isolates that represent the heart of the problem in this area. In this regard in fact, a recent expert panel suggested [2] that new candidates entering trials should first be tested against representative local isolates, but this has not been done yet. This is far from trivial, given our recent observations that many newly emerging strains [particularly W-Beijing strains] are of extremely high virulence in small animal models [10, 11]. These models also reveal that these clinical strains, unlike the laboratory strains used to screen vaccines, seem to be able to elicit a much broader T cell response including potentially high levels of Foxp3+ regulatory T cells and IL-17 secreting cells [12, 13], which has the potential to interfere with vaccine efficacy [12].
In this study we show that a panel of representative clinical isolates obtained from patients attending clinics in the Western Cape region of South Africa mostly grow very well in guinea pigs exposed to low dose aerosol infection, and rapidly caused extensive to severe lung damage. Encouragingly however, prior vaccination with BCG was highly effective against two strains tested [picked randomly], reducing the lung burden and significantly extending animal survival. However if this represents a general trend for strains from this region it suggests that achieving an improvement by a new vaccine over the existing BCG vaccine may be difficult. This hypothesis is discussed further below in the context of the disappointing results very recently reported regarding the Phase II trial of the MVA85A vaccine candidate [14].
2. Materials and methods
2.1.1. Animals
Female outbred Hartley guinea pigs (~450–500 g in weight) were purchased from the Charles River Laboratories (North Wilmington, MA) and held under barrier conditions in a biosafety level III animal laboratory. The specific-pathogen-free nature of the guinea pig colonies was demonstrated by testing sentinel animals. All experimental protocols were approved by the Animal Care and Usage Committee of Colorado State University and comply with NIH guidelines.
2.1.2. Experimental Design
Ten clinical strains from the Western Cape in South Africa were selected from an existing strain collection which is maintained in the Division of Molecular Biology and Human Genetics at Stellenbosch University [Table 1]. Six of these strains, designated R3180, R2139, R3239, R5727, R5688, and R2135 were rifampicin mono-resistant by culture and have a prominent mutation in the rpoB gene. Four strains designated SAWC954, SAWC1063, SAWC3382 and TT372 were completely drug sensitive with a wild type rpoB gene. These strains represent the most prominent drug sensitive and drug resistant genotypes in the region and are described in detail elsewhere [15–20]. All strains were grown in 7H9 broth containing 0.05% Tween 80 and OADC. When a strain had an OD600 reading of 0.60–1.00 it was bottled, frozen, and then titered. Thawed aliquots of frozen cultures were diluted in sterile saline to the desired inoculum concentration of 1×106 cfu/ml. The infection inoculum was determined for all the bacterial strains tested by plating serial dilutions of inoculum on nutrient 7H11 agar containing 10 μg/ml cycloheximide and 50 μg/ml of carbenicillin to prevent contamination. Colonies were counted after 3 weeks incubation at 37°C. No significant differences in terms of the infection dose were seen among any of the strains tested. A Madison chamber aerosol generation device was used to expose the animals to M. tuberculosis. This device was calibrated to deliver approximately 10–20 bacilli into the lungs [21].
TABLE ONE.
Clinical strains used in this study.
| Isolate nr. | Strain Family | Isoniazid Resistance | Rifampicin Resistance | Resistance pattern | rpoB |
|---|---|---|---|---|---|
| R5727 | X-Family (DRF150) | S | R | Rifampicin Mono resistant | +S/526TAC |
| R3239 | Atypical Beijing | S | R | Rifampicin Mono resistant | +S/526TAC |
| TT372 | Atypical Beijing | S | S | Sensitive | |
| R5688 | LAM3 | S | R | Rifampicin Mono resistant | +S/526CTC |
| R2139 | S family | S | R | Rifampicin Mono resistant | +S/526TAC |
| SAWC1063 | S family | S | S | Sensitive | |
| R3180 | Typical Beijing | S | R | Rifampicin Mono resistant | +S/531TTG |
| SAWC3382 | Typical Beijing | S | S | Sensitive | |
| R2135 | Typical Beijing (cluster R220) | S | R | Rifampicin Mono resistant | +S/531TTG |
| SAWC954 | Typical Beijing (cluster R220) | S | S | Sensitive |
2.1.3. Bacterial Load
On days 30 and 60 after aerosol exposure, the bacterial load in the right cranial lung lobe, spleen, and medistinal lymph nodes were determined by plating serial dilutions of tissue homogenates on nutrient 7H11 agar containing 10 μg/ml cycloheximide and 50 μg/ml of carbenicillin. Colonies were counted after 3 weeks of incubation at 37°C and data expressed as log10 CFU.
2.1.4. Histological analysis
Right caudal lung lobes from each guinea pig were fixed with 4% paraformaldehyde in phosphate-buffered saline. Paraffin-embedded sections from these tissues were cut to 5 μm, mounted on glass slides, de-paraffinized, and stained using hematoxylin and eosin.
2.1.5. BCG vaccination
Guinea pigs were vaccinated intradermally with 1×104 Mycobacterium bovis BCG, strain Pasteur, or mock vaccinated with saline. M. bovis BCG was grown in Proskauer-Beck broth. Animals were challenged by aerosol exposure as above 6 weeks later.
2.1.6. Kaplan Meier survival assay
Survival of animals was monitored by weighing and observation based on a modified Karnofsky scale. A guinea pig was euthanized if the animal showed extensive labored breathing, was lethargic, had a matted or scruffy coat, and if the weight loss was greater than 20% of weight at the time of challenge.
3. Results
3.1.1. Course of infection in target organs
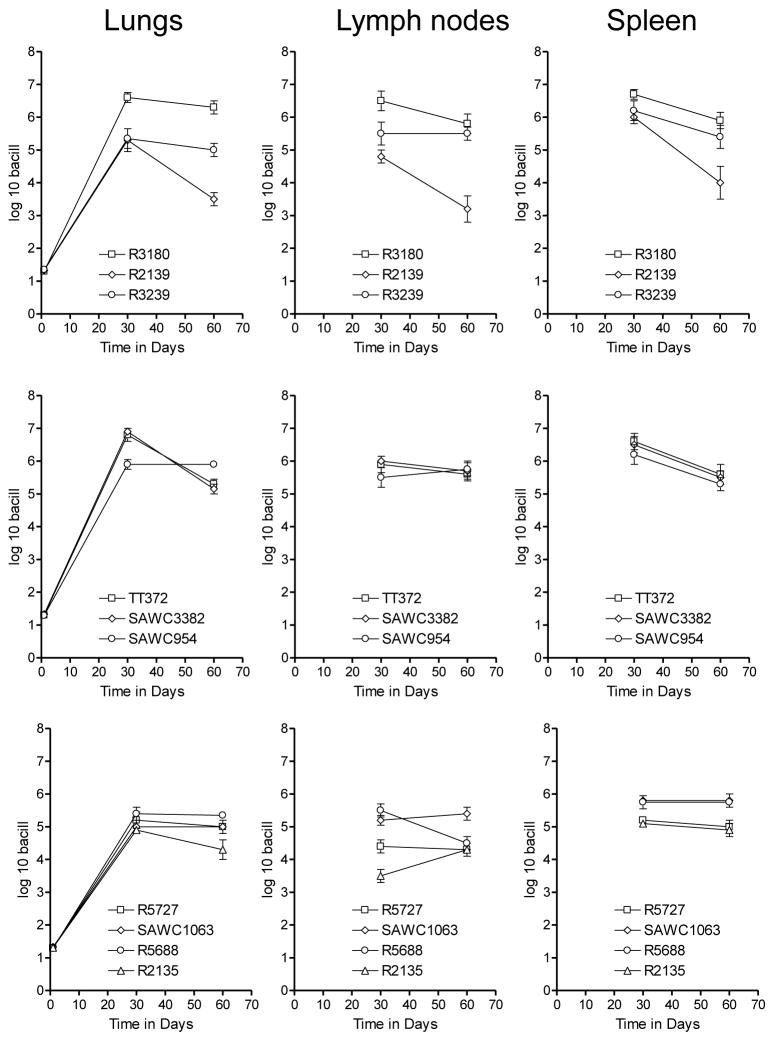
The capacity of each isolate to grow in guinea pigs after deposition of approximately 20 bacilli into the lungs is shown in Fig 1. All ten strains grew progressively in the lungs, reaching between 5 and 7-log in terms of the bacterial burden. Thereafter the growth of the infection was contained, although only one strain, the rifampicin mono-resistant strain R2139, showed evidence of any significant clearance. All ten strains showed evidence of extra-pulmonary dissemination to the spleen and draining lymph nodes. In the latter organs the bacterial load diminished, probably reflecting the increasingly severe lymphadenopathy and tissue destruction, as we have previously described [22].
Figure 1.
Growth of selected Western Cape strains in guinea pigs after exposure to approximately 20 bacilli. Data shown as log mean numbers in target organs ± SEM [n=5].
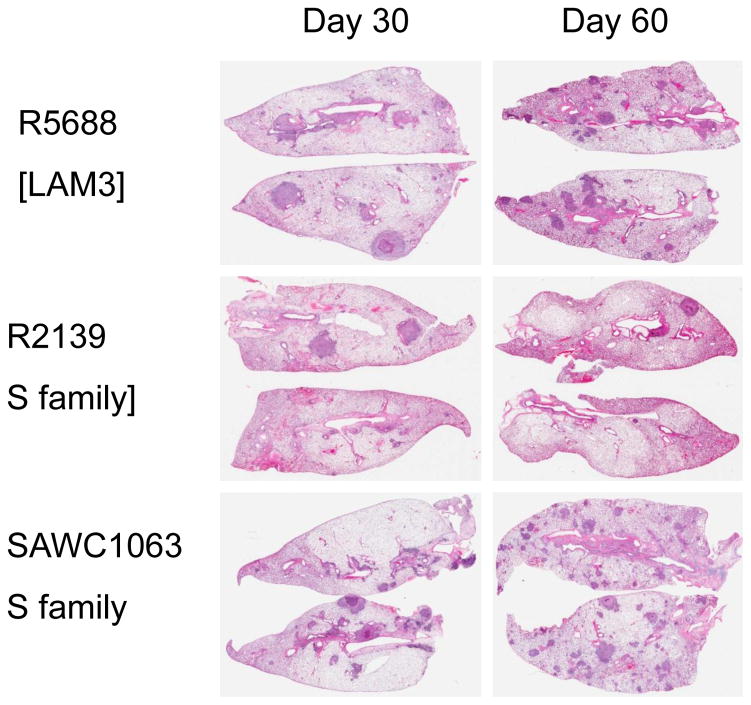
3.1.2. Lung pathology
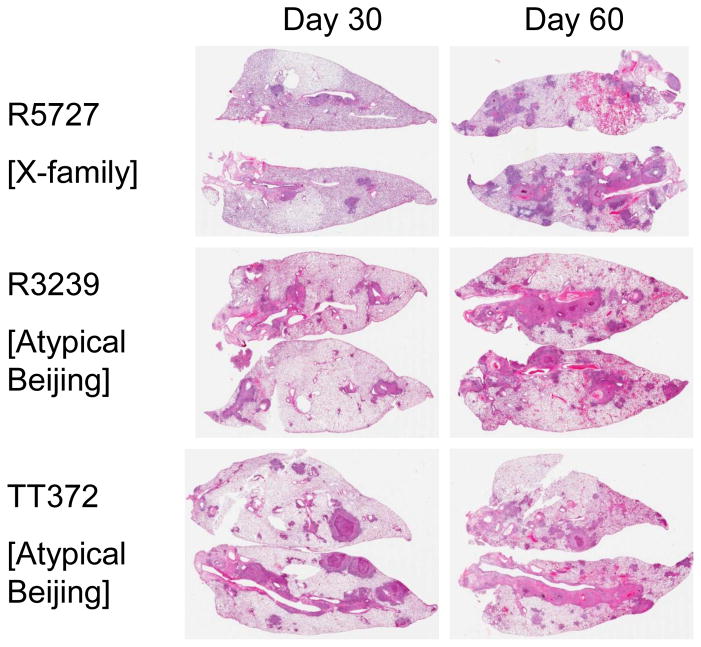
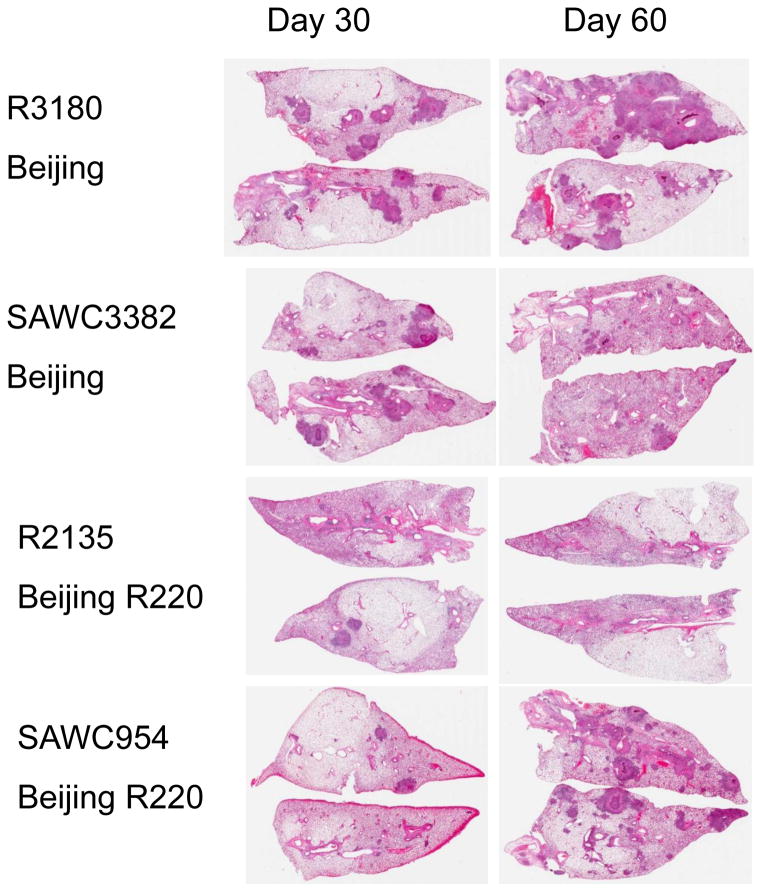
In all cases, infection with the panel strains caused progressive lung damage due to granulomatous inflammation [Figs 2–4]. By day-60 lung consolidation was extensive, particularly in the cases of R3180 and R5737, with moderate to severe consolidation and damage also seen in animals infected with TT372, SAWC3382, R3239, R5688, and SAWC954. More moderate lung damage was seen in guinea pigs infected with SAWC1063, R2135, and R2139.
Figure 2.
Lung pathology in guinea pigs infected with an X-family strain and two Atypical Beijing strains. Hematoxylin and eosin staining. Low power whole organ scan.
Figure 4.
Lung pathology in guinea pigs infected with two Beijing strains and two Beijing R220 strains. Hematoxylin and eosin staining. Low power whole organ scan.
3.1.3. Efficacy of BCG vaccination
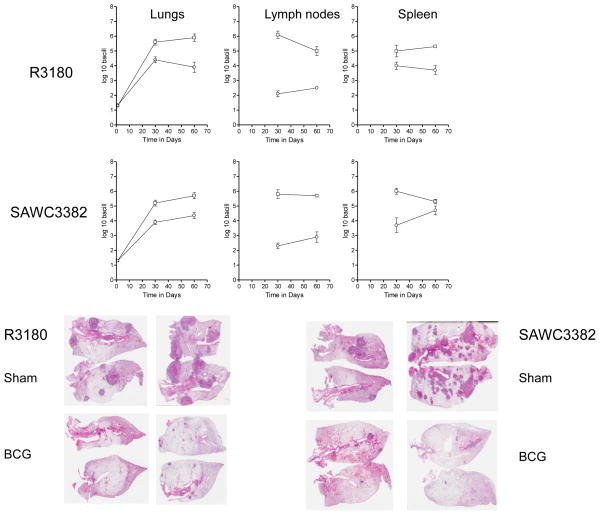
As shown in Fig. 5, prior BCG Pasteur vaccination was highly effective against two randomly selected strains [R3180 and SAWC3382]. The bacterial load in the lungs was reduced by at least ten-fold, and dissemination, particularly in the spleen, severely limited. Pathologic analysis of the lungs [Fig. 5] of these animals showed very extensive lesion consolidation and lesion necrosis developing in the control animals by day-60. In contrast very few lesions were seen in animals vaccinated with BCG.
Figure 5.
Efficacy of BCG vaccination against two representative Beijing strains. Guinea pigs were inoculated with saline [squares] or BCG Pasteur [circles] and the bacterial load in target organs measured 30 and 60 days later. Data shown as log mean numbers in target organs ± SEM [n=5]. Also shown is representative lung pathology in guinea pigs inoculated with saline [sham] or BCG and challenged with two Beijing strains. Hematoxylin and eosin staining. Low power whole organ scan.
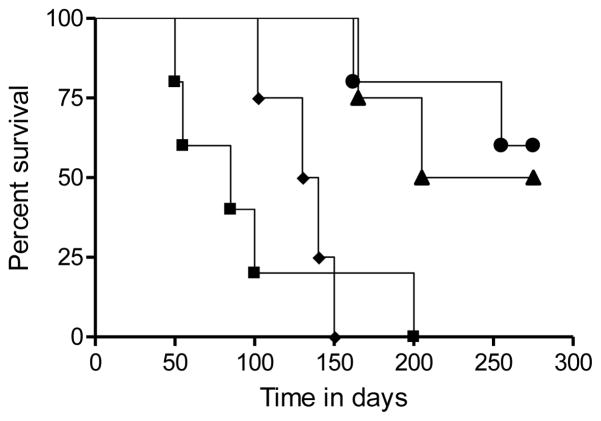
As a consequence, the survival of the animals given BCG was significantly prolonged [P<0.008] with half the challenged guinea pigs still alive and healthy at day-320 of the infection [Fig. 6].
Figure 6.
Kaplan Meier survival analysis comparing survival of control animals [squares R3180; diamonds SWAC3382] and BCG vaccinated animals [circles R3180; triangles SAWC3382].
4. Discussion
The results of this simple study show that a representative panel of ten strains obtained from the region of South Africa where new clinical studies are ongoing grew well in the lungs of guinea pigs after low dose aerosol exposure, and gave rise to moderate to very severe lung damage over the two months of the experimental infection. In addition, encouragingly, BCG vaccination was highly protective when tested against two randomly selected isolates.
The study observed a range of progressive pathological damage to the lungs, with seven of the isolates causing extensive to severe granulomatous inflammation and necrosis, while only three showed more modest lung damage that is more usually seen in response to laboratory strains. This reinforces our growing feeling, based on our recent experience with several panels of clinical strains, that while major differences are not always seen in terms of bacterial growth in the lungs after low dose exposure, the rapidity and severity of lung damage is a better indicator of virulence [23].
The basis for this damage, we now hypothesize, is the severity and magnitude of the immune response within the lungs. There is clearly a strong TH1 effector response to the W-Beijing strains, as we previously showed, but this appears to wane after the first month [24, 25]. This is then replaced by other CD4 T cell populations, including Foxp3+ regulatory T cells and TH17 cells. This can be demonstrated in the mouse model directly by flow cytometry [12] and indirectly in the guinea pig by RT-PCR [13].
The implications for vaccine efficacy are far from clear. In a mouse model we previously showed that the protective effect of BCG was quickly lost in animals infected with two W-Beijing isolates, and that this was associated with a substantial influx of Foxp3+ cells into the lungs at that time [12]. Similarly, guinea pigs infected with a separate panel of high virulence strains showed a increase in Foxp3 mRNA message in the lungs [13]. In our studies here however BCG was highly protective, and this resistance had not diminished at the day-60 assay point. Unfortunately we did not have the resources in this study to monitor the host response, so we do not know if the two strains we picked [randomly] here have a lower capacity to generate immunoregulatory T cell subsets, allowing memory immunity generated by the BCG vaccine to operate in an optimal manner, and this certainly deserves further investigation. One should also note however that BCG prolonged the survival of the vaccinated guinea in our study, and some still died; this is in contrast to conventional studies where H37Rv is usually the challenge strain and survival after vaccination can often be 100% over the duration of the study. In other words, if laboratory adapted strains are used for these types of assays, the vaccine efficacy can be over-estimated.
The variability of the efficacy of BCG has been long recognized, and various theories have been advanced to explain this, the most recent being the suggestion from our laboratory that the vaccine is inefficient in generating sufficient numbers of central memory CD4 T cells [26] [unfortunately, due to lack of reagents, we cannot as yet do this type of analysis in the guinea pig model]. Having said that, the BCG vaccine has given reasonable protection in some cases, especially in children [3]. This raises the issue that if a new vaccine candidate is tested in a trial in parallel to the existing BCG vaccine, and the trial population is infected by local isolates that are inhibited by BCG [such as here] then to show an increased efficacy or benefit of the new vaccine creates an obvious statistical power limitation.
These studies were conducted prior to the recent publication of the results of the MVA85A Phase II efficacy trial conducted in the Western Cape region [14]. As noted above, our results with BCG were very surprising given our previous experience with virulent Beijing strains, in which BCG was poorly effective – here, instead, we observed about the maximum protection that can be achieved in this animal model in the lungs, as well as a spectacular reduction in lung pathology. One can only speculate given the fact that the vaccine trial did not contain a “no-BCG” control arm [for obvious reasons] but it appears that BCG vaccination of infants by itself was reasonably protective [noting the caveat that BCG Danish was used in the trial, as opposed to Pasteur used here]. If this is the case, it is unlikely that protective immunity provided by any “BCG-boosting” vaccine would be detectable to the point where a statistical improvement could be demonstrated.
We suggest, based on this, that dropping MVA85A in priority could be a mistake. The animal data and early clinical data for this vaccine has consistently been highly encouraging [27]. If MVA85A were tested in a population in which the efficacy of BCG was in fact poor, we suspect a powerful boosting effect of MVA85A could potentially be observed. This could easily be tested in animal models, using Beijing strains against which the protective effect of BCG is minimal by itself, of which we now have multiple examples.
Figure 3.
Lung pathology in guinea pigs infected with a LAM-family strain and two S-family strains. Hematoxylin and eosin staining. Low power whole organ scan.
Highlights.
With one exception, all the Cape strains tested were virulent
BCG gave very good protection, something we have not seen using Beijing strains isolated in the USA.
If this pattern of protection is widespread, it explains why McShane could not boost BCG in her trial. This is VERY important in our view.
Acknowledgments
5. Funding: This study was supported by NIH grants AI070456 and AI092002, the South African National Research Foundation, and the Wellcome Trust.
Footnotes
Conflict of Interest statements: All authors have no competing interests.
Ethical approval: These studies were approved by the Biosafety Officer and the Institutional Animal Care and Usage committee at Colorado State University, and at Stellenbosch University.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Crystal A. Shanley, Email: crystal.shanley@colostate.edu.
Elizma M. Streicher, Email: lizma@sun.ac.za.
Robin M. Warren, Email: rw@sun.ac.za.
Thomas C. Victor, Email: tv@sun.ac.za.
Ian M. Orme, Email: ian.orme@colostate.edu.
References
- 1.Dye C, Glaziou P, Floyd K, Raviglione M. Prospects for tuberculosis elimination. Annual Review of Public Health. 2013;34:271–86. doi: 10.1146/annurev-publhealth-031912-114431. [DOI] [PubMed] [Google Scholar]
- 2.McShane H, Jacobs WR, Fine PE, Reed SG, McMurray DN, Behr M, et al. BCG: Myths, realities, and the need for alternative vaccine strategies. Tuberculosis (Edinburgh, Scotland) 2012;92:283–8. doi: 10.1016/j.tube.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith KC, Orme IM, Starke J. The BCG Vaccine. In: Plotkin S, Orenstein W, Offit P, editors. Vaccines. 6. WB Saunders; 2012. [Google Scholar]
- 4.Raviglione MC, Gupta R, Dye CM, Espinal MA. The burden of drug-resistant tuberculosis and mechanisms for its control. Annals of the New York Academy of Sciences. 2001 Dec;953:88–97. doi: 10.1111/j.1749-6632.2001.tb11364.x. [DOI] [PubMed] [Google Scholar]
- 5.Raviglione MC, Smith IM. XDR tuberculosis--implications for global public health. New England Journal of Med. 2007;356:656–9. doi: 10.1056/NEJMp068273. [DOI] [PubMed] [Google Scholar]
- 6.Dye C, Williams BG. Slow elimination of multidrug-resistant tuberculosis. Science Tanslational Medicine. 2009;1:3ra8. doi: 10.1126/scitranslmed.3000346. [DOI] [PubMed] [Google Scholar]
- 7.Kaufmann SH, Gengenbacher M. Recombinant live vaccine candidates against tuberculosis. Current Opinion in Biotechnology. 2012;23:900–7. doi: 10.1016/j.copbio.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 8.McShane H. Tuberculosis vaccines: beyond bacille Calmette-Guerin. Philosophical transactions of the Royal Society of London. 2011;366:2782–9. doi: 10.1098/rstb.2011.0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ottenhoff TH, Kaufmann SH. Vaccines against tuberculosis: where are we and where do we need to go? PLoS pathogens. 2012;8:e1002607. doi: 10.1371/journal.ppat.1002607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palanisamy GS, DuTeau N, Eisenach KD, Cave DM, Theus SA, Kreiswirth BN, et al. Clinical strains of Mycobacterium tuberculosis display a wide range of virulence in guinea pigs. Tuberculosis (Edinburgh, Scotland) 2009;89:203–9. doi: 10.1016/j.tube.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Palanisamy GS, Smith EE, Shanley CA, Ordway DJ, Orme IM, Basaraba RJ. Disseminated disease severity as a measure of virulence of Mycobacterium tuberculosis in the guinea pig model. Tuberculosis (Edinburgh, Scotland) 2008;88:295–306. doi: 10.1016/j.tube.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ordway DJ, Shang S, Henao-Tamayo M, Obregon-Henao A, Nold L, Caraway M, et al. Mycobacterium bovis BCG-mediated protection against W-Beijing strains of Mycobacterium tuberculosis is diminished concomitant with the emergence of regulatory T cells. Clinical and Vaccine Immunology. 2011;18:1527–35. doi: 10.1128/CVI.05127-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shang S, Harton M, Tamayo MH, Shanley C, Palanisamy GS, Caraway M, et al. Increased Foxp3 expression in guinea pigs infected with W-Beijing strains of M. tuberculosis. Tuberculosis (Edinburgh, Scotland) 2011;91:378–85. doi: 10.1016/j.tube.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tameris MD, Hatherill M, Landry BS, Scriba TJ, Snowden MA, Lockhart S, et al. Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomised, placebo-controlled phase 2b trial. Lancet. 2013;381:1021–8. doi: 10.1016/S0140-6736(13)60177-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dramowski A, Morsheimer MM, Jordaan AM, Victor TC, Donald PR, Schaaf HS. Rifampicin-monoresistant Mycobacterium tuberculosis disease among children in Cape Town, South Africa. International Journal of Tuberculosis and Lung Disease. 2012;16:76–81. doi: 10.5588/ijtld.11.0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson R, Warren RM, van der Spuy GD, Gey van Pittius NC, Theron D, Streicher EM, et al. Drug-resistant tuberculosis epidemic in the Western Cape driven by a virulent Beijing genotype strain. International Journal of Tuberculosis and Lung Disease. 2010;14:119–21. [PubMed] [Google Scholar]
- 17.Mukinda FK, Theron D, van der Spuy GD, Jacobson KR, Roscher M, Streicher EM, et al. Rise in rifampicin-monoresistant tuberculosis in Western Cape, South Africa. International Journal of Tuberculosis and Lung Disease. 2012;16:196–202. doi: 10.5588/ijtld.11.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seddon JA, Hesseling AC, Marais BJ, Jordaan A, Victor T, Schaaf HS. The evolving epidemic of drug-resistant tuberculosis among children in Cape Town, South Africa. International Journal of Tuberculosis and Lung Disease. 2012;16:928–33. doi: 10.5588/ijtld.11.0679. [DOI] [PubMed] [Google Scholar]
- 19.Strauss OJ, Warren RM, Jordaan A, Streicher EM, Hanekom M, Falmer AA, et al. Spread of a low-fitness drug-resistant Mycobacterium tuberculosis strain in a setting of high human immunodeficiency virus prevalence. Journal of Clinical Microbiology. 2008;46:1514–6. doi: 10.1128/JCM.01938-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Victor TC, Streicher EM, Kewley C, Jordaan AM, van der Spuy GD, Bosman M, et al. Spread of an emerging Mycobacterium tuberculosis drug-resistant strain in the western Cape of South Africa. International Journal of Tuberculosis and Lung Disease. 2007;11:195–201. [PubMed] [Google Scholar]
- 21.Kraft SL, Dailey D, Kovach M, Stasiak KL, Bennett J, McFarland CT, et al. Magnetic resonance imaging of pulmonary lesions in guinea pigs infected with Mycobacterium tuberculosis. Infection and Immunity. 2004;72:5963–71. doi: 10.1128/IAI.72.10.5963-5971.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Basaraba RJ, Dailey DD, McFarland CT, Shanley CA, Smith EE, McMurray DN, et al. Lymphadenitis as a major element of disease in the guinea pig model of tuberculosis. Tuberculosis (Edinb) 2006;86:386–94. doi: 10.1016/j.tube.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 23.Kato-Maeda M, Shanley CA, Ackart D, Jarlsberg LG, Shang S, Obregon-Henao A, et al. Beijing sublineages of Mycobacterium tuberculosis differ in pathogenicity in the guinea pig. Clinical and Vaccine Immunololgy. 2012;19:1227–37. doi: 10.1128/CVI.00250-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ordway D, Henao-Tamayo M, Harton M, Palanisamy G, Troudt J, Shanley C, et al. The hypervirulent Mycobacterium tuberculosis strain HN878 induces a potent TH1 response followed by rapid down-regulation. Journal of Immunology. 2007;179:522–31. doi: 10.4049/jimmunol.179.1.522. [DOI] [PubMed] [Google Scholar]
- 25.Ordway D, Palanisamy G, Henao-Tamayo M, Smith EE, Shanley C, Orme IM, et al. The cellular immune response to Mycobacterium tuberculosis infection in the guinea pig. Journal of Immunology. 2007;179:2532–41. doi: 10.4049/jimmunol.179.4.2532. [DOI] [PubMed] [Google Scholar]
- 26.Orme IM. The Achilles heel of BCG. Tuberculosis (Edinburgh, Scotland) 2010;90:329–32. doi: 10.1016/j.tube.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 27.McShane H, Pathan AA, Sander CR, Goonetilleke NP, Fletcher HA, Hill AV. Boosting BCG with MVA85A: the first candidate subunit vaccine for tuberculosis in clinical trials. Tuberculosis (Edinb) 2005;85:47–52. doi: 10.1016/j.tube.2004.09.015. [DOI] [PubMed] [Google Scholar]