Abstract
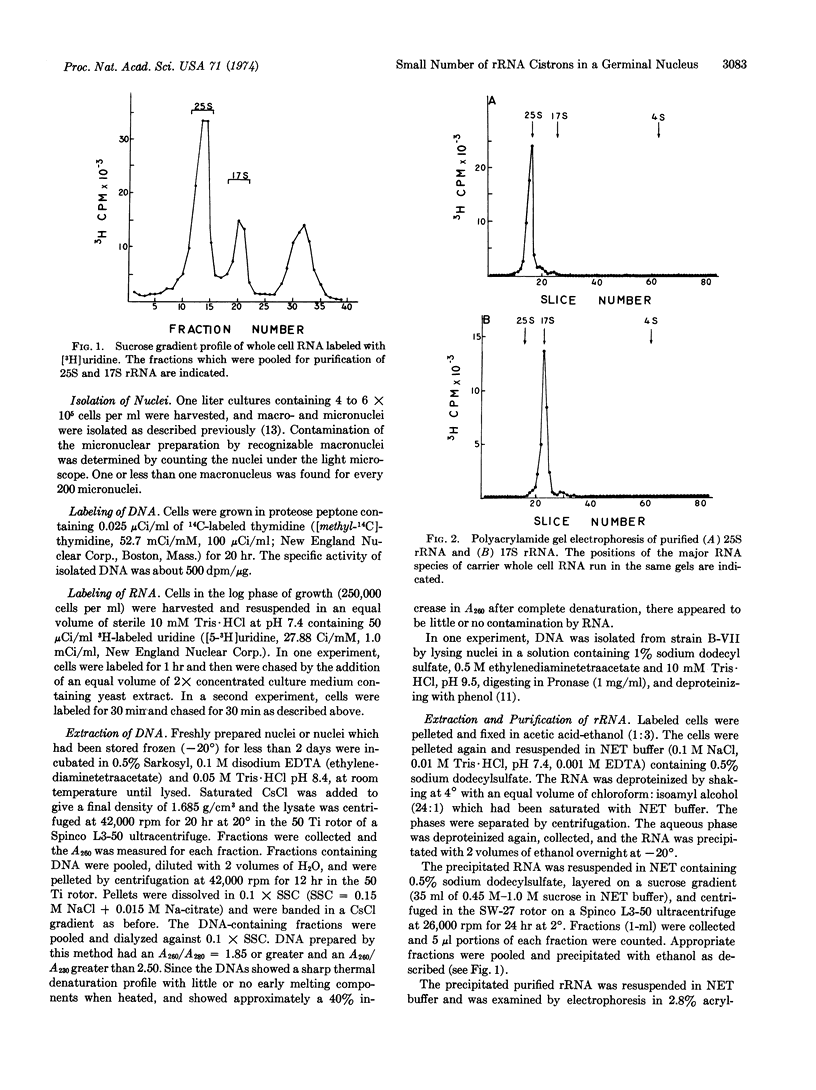
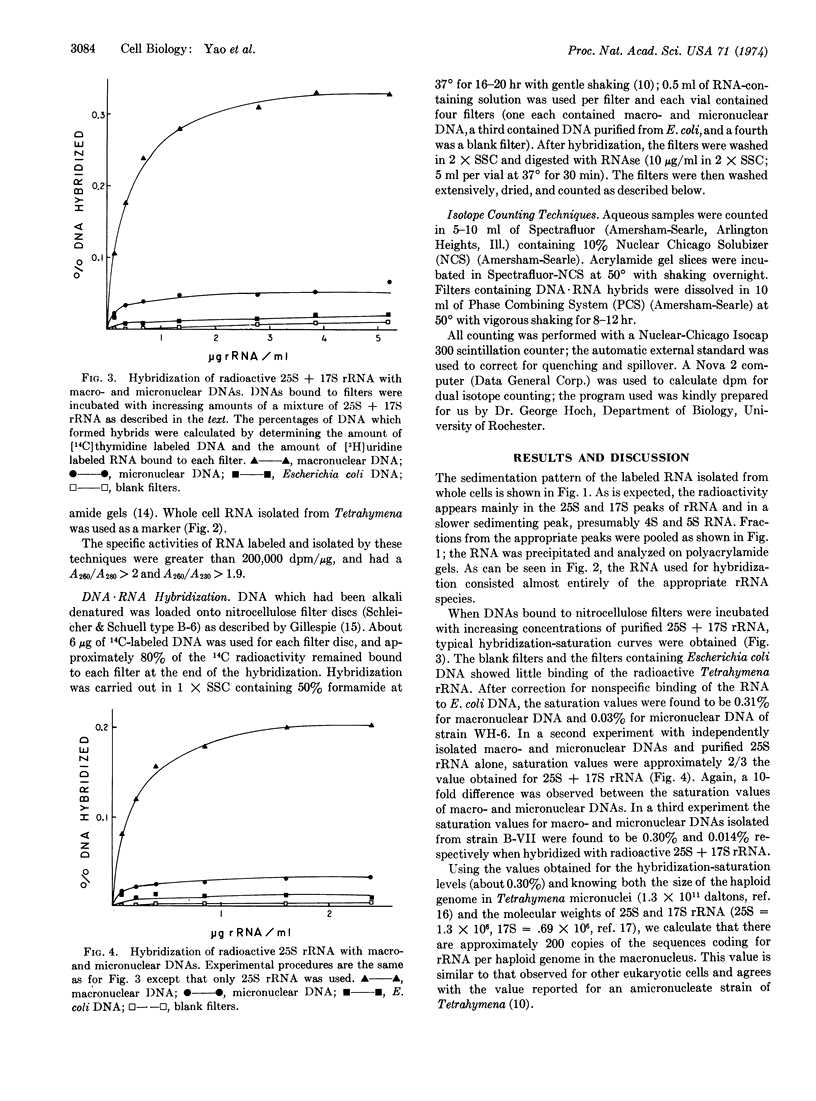
The percentage of DNA complementary to 25S and 17S rRNA has been determined for both the macro- and micronucleus of the ciliated protozoan, Tetrahymena pyriformis. Saturation levels obtained by DNA·RNA hybridization indicate that approximately 200 copies of the gene for rRNA per haploid genome were present in macronuclei. The saturation level obtained with DNA extracted from isolated micronuclei was only 5-10% of the level obtained with DNA from macronuclei. After correction for contamination of micronuclear DNA by DNA from macronuclei, only a few copies (possibly only 1) of the gene for rRNA are estimated to be present in micronuclei. Micronuclei are germinal nuclei. Macronuclei serve as somatic nuclei during vegetative growth but are destroyed every sexual generation and are reformed from products of meiosis, fertilization, and division of the micronuclei. Thus, the hybridization data suggest that the gene for rRNA must be amplified during macronuclear formation with each sexual generation. These observations also demonstrate that the multiple copies of a repeated gene in a somatic nucleus of a eukaryote can be generated from a small number of copies of that gene in a germinal nucleus.
Keywords: evolution of repeated genes, amplification, DNA·RNA hybridization, macro- and micronuclei
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnstiel M. L., Chipchase M., Speirs J. The ribosomal RNA cistrons. Prog Nucleic Acid Res Mol Biol. 1971;11:351–389. doi: 10.1016/s0079-6603(08)60332-3. [DOI] [PubMed] [Google Scholar]
- Brown D. D., Blackler A. W. Gene amplification proceeds by a chromosome copy mechanism. J Mol Biol. 1972 Jan 14;63(1):75–83. doi: 10.1016/0022-2836(72)90522-0. [DOI] [PubMed] [Google Scholar]
- Brown D. D., Dawid I. B. Specific gene amplification in oocytes. Oocyte nuclei contain extrachromosomal replicas of the genes for ribosomal RNA. Science. 1968 Apr 19;160(3825):272–280. doi: 10.1126/science.160.3825.272. [DOI] [PubMed] [Google Scholar]
- Brown D. D., Sugimoto K. 5 S DNAs of Xenopus laevis and Xenopus mulleri: evolution of a gene family. J Mol Biol. 1973 Aug 15;78(3):397–415. doi: 10.1016/0022-2836(73)90464-6. [DOI] [PubMed] [Google Scholar]
- Brown D. D., Wensink P. C., Jordan E. A comparison of the ribosomal DNA's of Xenopus laevis and Xenopus mulleri: the evolution of tandem genes. J Mol Biol. 1972 Jan 14;63(1):57–73. doi: 10.1016/0022-2836(72)90521-9. [DOI] [PubMed] [Google Scholar]
- Charret R. L'ADN nucléolaire chez Tetrahymena pyriformis: chronoloe de sa réplication. Exp Cell Res. 1969 Mar;54(3):353–361. doi: 10.1016/0014-4827(69)90214-6. [DOI] [PubMed] [Google Scholar]
- Engberg J., Nilsson J. R., Pearlman R. E., Leick V. Induction of nucleolar and mitochondrial DNA replication in Tetrahymena pyriformis. Proc Natl Acad Sci U S A. 1974 Mar;71(3):894–898. doi: 10.1073/pnas.71.3.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engberg J., Pearlman R. E. The amount of ribosomal RNA genes in Tetrahymena pyriformis in different physiological states. Eur J Biochem. 1972 Apr 11;26(3):393–400. doi: 10.1111/j.1432-1033.1972.tb01779.x. [DOI] [PubMed] [Google Scholar]
- Flamm W. G., McCallum M., Walker P. M. The isolation of complementary strands from a mouse DNA fraction. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1729–1734. doi: 10.1073/pnas.57.6.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall J. G. Differential synthesis of the genes for ribosomal RNA during amphibian oögenesis. Proc Natl Acad Sci U S A. 1968 Jun;60(2):553–560. doi: 10.1073/pnas.60.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall J. G. Free ribosomal RNA genes in the macronucleus of Tetrahymena. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3078–3081. doi: 10.1073/pnas.71.8.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorovsky M. A. Macro- and micronuclei of Tetrahymena pyriformis: a model system for studying the structure and function of eukaryotic nuclei. J Protozool. 1973 Feb;20(1):19–25. doi: 10.1111/j.1550-7408.1973.tb05995.x. [DOI] [PubMed] [Google Scholar]
- Gorovsky M. A. Studies on nuclear structure and function in Tetrahymena pyriformis. II. Isolation of macro- and micronuclei. J Cell Biol. 1970 Dec;47(3):619–630. doi: 10.1083/jcb.47.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hourcade D., Dressler D., Wolfson J. The amplification of ribosomal RNA genes involves a rolling circle intermediate. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2926–2930. doi: 10.1073/pnas.70.10.2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell S. H. The differential synthesis and degradation of ribosomal DNA during the vegetative cell cycle in Chlamydomonas reinhardi. Nat New Biol. 1972 Dec 27;240(104):264–267. doi: 10.1038/newbio240264a0. [DOI] [PubMed] [Google Scholar]
- Loening U. E. Molecular weights of ribosomal RNA in relation to evolution. J Mol Biol. 1968 Dec;38(3):355–365. doi: 10.1016/0022-2836(68)90391-4. [DOI] [PubMed] [Google Scholar]
- Loening U. E. The fractionation of high-molecular-weight ribonucleic acid by polyacrylamide-gel electrophoresis. Biochem J. 1967 Jan;102(1):251–257. doi: 10.1042/bj1020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newlon C. S., Sonenshein G. E., Holt C. E. Time of synthesis for ribosomal ribonucleic acid in Physarum. Biochemistry. 1973 Jun 5;12(12):2338–2345. doi: 10.1021/bi00736a024. [DOI] [PubMed] [Google Scholar]
- Prescott D. M., Murti K. G., Bostock C. J. Genetic apparatus of Stylonychia sp. Nature. 1973 Apr 27;242(5400):576, 597-600. doi: 10.1038/242576a0. [DOI] [PubMed] [Google Scholar]
- Ritossa F. M., Atwood K. C., Lindsley D. L., Spiegelman S. On the chromosomal distribution of DNA complementary to ribosomal and soluble RNA. Natl Cancer Inst Monogr. 1966 Dec;23:449–472. [PubMed] [Google Scholar]
- Sinclair J. H., Brown D. D. Retention of common nucleotide sequences in the ribosomal deoxyribonucleic acid of eukaryotes and some of their physical characteristics. Biochemistry. 1971 Jul 6;10(14):2761–2769. doi: 10.1021/bi00790a017. [DOI] [PubMed] [Google Scholar]
- Smith G. P. Unequal crossover and the evolution of multigene families. Cold Spring Harb Symp Quant Biol. 1974;38:507–513. doi: 10.1101/sqb.1974.038.01.055. [DOI] [PubMed] [Google Scholar]
- Wensink P. C., Brown D. D. Denaturation map of the ribosomal DNA of Xenopus laevis. J Mol Biol. 1971 Sep 14;60(2):235–247. doi: 10.1016/0022-2836(71)90290-7. [DOI] [PubMed] [Google Scholar]
- Woodard J., Kaneshiro E., Gorovsky M. A. Cytochemical studies on the problem of macronuclear subnuclei in tetrahymena. Genetics. 1972 Feb;70(2):251–260. doi: 10.1093/genetics/70.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]


