Abstract
Background
Chylothorax following congenital heart surgery is a common complication with associated morbidities, but consensus treatment guidelines are lacking. Variability exists in the duration of medical treatment and timing for surgical intervention.
Methods
Following institution of a clinical practice guideline for management of post-operative chylothorax at a single center, pediatric cardiothoracic intensive care unit (ICU) in June 2010, we retrospectively analyzed two cohorts of patients: those with chylothorax from 1/2008-5/2010 (early cohort; n = 118) and from 6/2010-8/2011 (late cohort; n = 45). Data collected included demographics, cardiac surgical procedure, treatments for chylothorax, bloodstream infections, hospital mortality, length of hospitalization, duration of mechanical ventilation, and device utilization.
Results
There were no demographic differences between the cohorts. No differences were found in octreotide use or surgical treatments for chylothorax. Significant differences were found in median times to chylothorax diagnosis (9 in early cohort versus 6 days in late cohort, p = 0.004), ICU length of stay (18 vs. 9 days, p = 0.01), hospital length of stay (30 vs. 23 days, p = 0.005), and total durations of mechanical ventilation (11 vs. 5 days, p = 0.02), chest tube use (20 vs. 14 days, p = 0.01), central venous line use (27 vs. 15 days, p = 0.001), and NPO status (9.5 vs. 6 days, p = 0.04).
Conclusions
Institution of a clinical practice guideline for treatment of chylothorax following congenital heart surgery was associated with earlier diagnosis, reduced hospital length of stay, mechanical ventilation, and device utilization for these patients.
Keywords: chyle, pleural effusion, congenital heart disease, practice guidelines
Introduction
Chylothorax, the accumulation of lymphatic fluid within the pleural space, is a common complication following cardiothoracic surgery. Post-operative chylothorax is the most common cause of chylothorax in the pediatric population, exceeding congenital chylothorax and lymphatic malformations. (1) The reported incidence following congenital heart surgery varies from 0.25 to 9.2%. (2, 3) The mechanism may be direct injury of the thoracic duct and tributaries, altering lymphatic drainage. Processes that elevate the central venous pressure (e.g., thrombosis of the subclavian vein, right ventricular diastolic dysfunction, or Fontan physiology) are also associated with the development of chylothorax. Definitive medical or surgical therapy is often required after initial intervention to drain a chylothorax. Prolonged chylothorax places patients at risk for malnutrition, poor wound healing, infections, fluid imbalance, electrolyte abnormalities, prolonged mechanical ventilation and device utilization, and increased length of ICU and hospital stay. (4-6)
Unfortunately, there are few data to guide treatment of post-operative chylothorax, leading to wide practice variation. Most published pediatric studies are limited to small case series and case reports. (1, 7-11) The standard approach for treating chylothorax begins with drainage of the pleural fluid, usually with placement of an indwelling chest tube. (12) Concurrent nutritional interventions are often employed to reduce dietary fat in hopes of decreasing chyle production. These include a low-fat or fat-free diet, or cessation of enteral food and liquid intake (nil per os, NPO) and use of total parenteral nutrition (TPN) including intravenous lipids. Pharmacotherapy with the somatostatin analogue octreotide has been proposed to decrease blood flow to hepatic, portal, and splanchnic circulations, leading to a decrease in lymph flow through the thoracic duct. (13) If medical therapy fails, surgical intervention is considered. Thoracic duct ligation, pleurodesis, and pleuro-peritoneal shunt are reported treatments for persistent chylothorax. (14)
Variability exists around the duration of medical treatment and the choice of timing for surgical intervention. (12) We therefore developed and implemented a clinical practice guideline (CPG) for the diagnosis and treatment of post-operative chylothorax in our pediatric cardiothoracic ICU. The objective of this study was to assess the impact of instituting a CPG on clinical outcomes and resource utilization in patients who developed chylothorax after pediatric cardiac surgery. We hypothesized that patients treated for chylothorax after institution of this CPG would have shorter duration of hospitalization, mechanical ventilation, use of thoracostomy tubes, and fewer days with indwelling central venous lines (CVL).
Patients and Methods
Study Population and Setting
The analytic cohort included all patients diagnosed with chylothorax after cardiothoracic surgery in the University of Michigan Pediatric Cardiothoracic ICU from January 2008 to August 2011. The chylothorax CPG was implemented on June 1, 2010. We sought to compare outcomes between those treated prior to CPG initiation (early cohort, EC) to those treated after (late cohort, LC). The study was approved by the Health Sciences and Behavioral Sciences Institutional Review Boards. There were 1944 surgical admissions to the ICU during the study period, and patients with the diagnosis of chylothorax were identified using a keyword search of the electronic medical record. Patients with more than one post-operative admission and more than one instance of chylothorax within the study time frame had only their earliest admission included. We excluded patients with surgical repair of coarctation of the aorta or vascular ring via lateral thoracotomy (as these patients were not included by the CPG). Following a thorough chart review, 163 patients were included in the study. During the study period, there were no systematic or institutional changes to surgical practices or post-operative care strategies such as ICU extubation or feeding protocols. There were also no significant changes to the institution's congenital heart surgeons or attending cardiologists, who influence surgical practices and post-operative strategies during this study period.
CPG Development
A multidisciplinary team (including physicians, nurse practitioners, and dieticians) designed and created a CPG for the management of post-operative chylothorax. The flow diagram (Figure 1) demonstrates the recommended progressive therapies, which are based on volume of chest tube drainage at time of chylothorax diagnosis. Low volume drainage was considered less than or equal to 20 ml/kg/day of chest tube output, while high volume drainage was greater than 20 ml/kg/day. Patients with low volume drainage are placed on a high medium chain triglyceride diet. Patients with high volume drainage are made NPO and given TPN and octreotide. If the NPO and octreotide therapies fail to decrease the drainage to less than 10 ml/kg/day within 7 to 10 days, the patient undergoes a surgical thoracic duct ligation followed by 3 days of NPO and TPN. These additional 3 days of NPO and TPN represent a consensus-based decision at our institution. If the patient continues to have greater than 10 ml/kg/day of chest tube output, a second round of NPO with TPN and octreotide is instituted for 7 days. If this also fails to decrease the chest tube output below 10 ml/kg/day, the patient undergoes a mechanical pleurodesis followed by 3 days of NPO and TPN. Supplemental recommendations include obtaining an echocardiogram to evaluate for correctable abnormalities, monitoring serum albumin levels, coagulation studies, serum immunoglobulin levels, and pleural fluid analysis if the diagnosis of chylothorax is in question.
Figure 1.
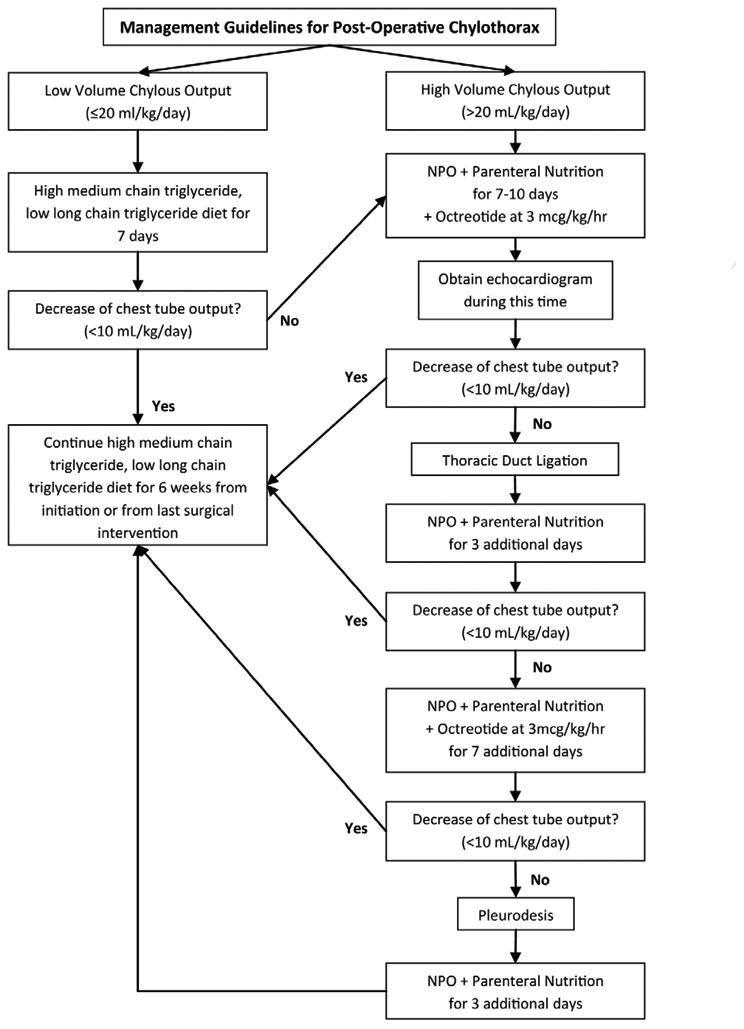
Flow diagram demonstrating the recommended progressive therapies.
For patients with chylothorax following lateral thoracotomy surgery, injury to the thoracic duct is assumed to be the cause and patients go directly for thoracic duct ligation, bypassing the CPG. Due to expected high output drainage in the first week after Fontan palliation, the CPG is initiated no earlier than one week following surgery. Patients with heterotaxy syndrome frequently have bilateral thoracic ducts, so bilateral thoracic ductal ligation was recommended in that population.
Measurements
We collected demographics, cardiac diagnosis, primary surgical procedure, chest tube output at time of chylothorax diagnosis, medical and surgical treatments for chylothorax, hospital mortality, ICU length of stay, total hospital length of stay, duration of mechanical ventilation and numbers of intubation, duration and number of chest tube placements, duration and number of CVL placements, duration of time NPO and TPN use, and bloodstream infections (BSI) (as identified by Centers for Disease Control and Prevention criteria for central line associated BSI). Surgical repairs were categorized as two ventricle with arch repair, two ventricle without arch repair, single ventricle with arch repair, and single ventricle without arch repair to simplify description of patient complexity and case mix. We also recorded Risk Adjustment for Congenital Heart Surgery (RACHS-1) category. (15) Compliance with the CPG was audited by reviewing appropriate placement of patients into high versus low volume chylothorax pathways, duration of medical treatments, and time to surgical treatments.
Statistical analysis
Data are presented as frequency (with percentage) for categorical variables and median (with interquartile range) for continuous variables. Group comparisons performed on demographic and clinical characteristics, medical and surgical treatment for chylothorax, and clinical outcomes were made using Chi-square tests or Fisher's exact tests, as appropriate, for nominal variables, Mantel-Haenszel Chi-square test for ordinal variables, and Wilcoxon Rank Sum test for continuous variables. All analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC, USA), with statistical significance set at p-values less than 0.05 using two-sided tests.
Results
Among 163 patients included in the analysis, there were 118 EC patients and 45 LC patients. There were no significant differences between the groups in distribution of gender, age, weight, cardiac diagnosis, surgical complexity, or chest tube output at time of diagnosis. The overall incidence of postoperative chylothorax was 8.9% during the study period, with no difference in incidence between the two study groups (9.2% EC vs. 8.2% LC, p = 0.45). 90 patients had bilateral chylothorax and none had chylopericardium. 87% of the LC patients were in compliance with the CPG. Of the 6 LC patients who did not follow the CPG; 3 patients were incorrectly categorized as low volume chylous output, 2 patients had delay in timing of thoracic duct ligation, and 1 patient was continued on breast milk despite low volume chylous output. The LC showed a shorter time to recognition and diagnosis of chylothorax (6 days versus 9 days following cardiac surgery, p = 0.004) (Table 1).
Table 1. Demographic and clinical characteristics in patients diagnosed and treated for chylothorax before and after initiation of clinical practice guideline (N=163).
| Characteristic | All | Early Cohort (n=118) |
Late Cohort (n=45) |
P-value§ |
|---|---|---|---|---|
| Weight at admission, kg | 4.4 (3.2-8.9) | 4.1 (3.1-9.2) | 5.2 (3.5-7.9) | 0.24 |
|
| ||||
| Male sex | 99 (60.7) | 71 (60.2) | 28 (62.2) | 0.81 |
|
| ||||
| Age at surgery, days | 90 (7-278) | 72 (7-354) | 123 (9-229) | 0.55 |
|
| ||||
| Number of patients with age at surgery ≤ 30days | 67 (41.1) | 52 (44.1) | 15 (33.3) | 0.21 |
|
| ||||
| Primary diagnosis/procedure | ||||
| Single ventricle without arch repair | 52 (31.9) | 38 (32.2) | 14 (31.1) | 0.92 |
|
| ||||
| Single ventricle with arch repair | 26 (16.0) | 20 (17.0) | 6 (13.3) | |
|
| ||||
| Two ventricles without arch repair | 70 (42.9) | 49 (41.5) | 21 (46.7) | |
|
| ||||
| Two ventricles with arch repair | 15 (9.2) | 11 (9.3) | 4 (8.9) | |
|
| ||||
| RACHS-1 classification | ||||
| 1-3 | 99 (60.7) | 69 (58.5) | 30 (66.7) | 0.43† |
|
| ||||
| 4-6 | 61 (37.4) | 46 (39.0) | 15 (33.3) | |
|
| ||||
| Zero | 3 (1.8) | 3 (2.5) | 0 (0.0) | |
|
| ||||
| Age at chylothorax diagnosed, days | 95 (18-288) | 79 (18-387) | 127 (24-237) | 0.84 |
|
| ||||
| Time from surgery to chylothorax diagnosis, days | 8 (5-11) | 9 (5-12) | 6 (4-8) | 0.004 |
|
| ||||
| Chest tube output, mL/kg/day | 16.2 (8.8-28.1) | 17 (8.5-28.5) | 15.1 (9-27) | 0.94 |
|
| ||||
| Patients with bilateral chylothorax | 90 (55.2%) | 68 (57.6%) | 22 (48.9%) | 0.32 |
|
| ||||
| Low/High output | ||||
| Low | 97 (59.5) | 70 (59.3) | 27 (60.0) | 0.98 |
|
| ||||
| High | 65 (39.9) | 47 (39.8) | 18 (40.0) | |
|
| ||||
| Missing data | 1 (0.6) | 1 (0.8) | . | |
|
| ||||
| Patients with measured triglyceride levels | 56 (34.4) | 41 (34.7) | 15 (33.3) | 0.87 |
|
| ||||
| Pleural triglyceride levels, mg/dL | 309.5 (228-462.5) | 311 (235-426) | 286 (227-600) | 0.83 |
|
| ||||
| Clinical Practice Guidelines followed | N/A | N/A | 39 (86.7) | N/A |
|
| ||||
Data presented as N (%) for categorical variables and median (interquartile range) for continuous variables.
P-value from Chi-square test for categorical variables and Wilcoxon Rank Sum test for continuous variables on comparison of each characteristic between patients in both cohorts.
RACHS-1 = Risk Adjustment for Congenital Heart Surgery.
In comparing the two cohorts, there were no differences in the frequency or timing of surgical treatment of chylothorax and frequency of octreotide treatment (Table 2). There were no differences in hospital mortality or duration of TPN use (Table 3). Compared with the EC, the LC demonstrated a shorter median ICU length of stay (9 days vs. 18 days, p = 0.01) and total hospital length of stay (23 days vs. 30 days, p = 0.005). Several other clinical outcomes were found to be significantly improved, including duration of mechanical ventilation (5 days vs. 11 days, p = 0.02) and frequency of repeat intubations (single intubation in 71% vs. 48%, p = 0.01). The frequency of chest tube insertion (need for only a single chest tube in 16% vs. 3%, p = 0.01) and duration (14 days vs. 20 days, p = 0.01) was also improved in the LC. We also saw an improvement in the LC in duration of CVL (15 days vs. 27 days, p = 0.001) and days spent NPO (6 days vs. 9.5 days, p = 0.04). The LC had a shorter interval from resumption of enteral nutrition and hospital discharge (11 days vs. 16 days, p=0.02). Finally, the LC had shorter intervals from cardiac surgery to initiation of octreotide (9 days vs. 20.5 days, p=0.007) and NPO (9 day vs. 15 days, p=0.006) as treatments for chylothorax. None of the patients in the study had a central line associated BSI.
Table 2. Comparison of medical and surgical treatments for chylothorax in patients diagnosed and treated for chylothorax before and after initiation of clinical practice guideline (N=163).
| Treatment | All | Early Cohort (n=118) |
Late Cohort (n=45) |
P-value§ |
|---|---|---|---|---|
| Surgical intervention | ||||
|
| ||||
| Mechanical pleurodesis | 7 (4.3) | 6 (5.1) | 1 (2.2) | 0.67 |
| Time from surgery to mechanical pleurodesis, days | 32 (20-54) | 31 (20-33) | 54 | 0.36 |
|
| ||||
| Time from chylothorax diagnosis to mechanical pleurodesis, days | 24 (10-46) | 22.5 (10-25) | 46 | 0.36 |
|
| ||||
| Thoracic duct ligation | 21 (12.9) | 16 (13.6) | 5 (11.1) | 0.68 |
| Time from surgery to thoracic duct ligation, days | 20 (19-27) | 21.5 (19.5-31.5) | 20 (15-27) | 0.57 |
|
| ||||
| Time from chylothorax diagnosis to thoracic duct ligation, days | 13 (10-20) | 13 (10-22) | 14 (9-19) | 0.90 |
|
| ||||
| Octreotide | ||||
|
| ||||
| Treated with octreotide | 29 (17.8) | 18 (15.3) | 11 (24.4) | 0.17 |
|
| ||||
| Time from surgery to octreotide treatment, days | 14 (10-23) | 20.5 (14-29) | 9 (6-10) | 0.007 |
|
| ||||
| Duration on octreotide, days | 10 (6-16) | 11 (7-20) | 7 (6-14) | 0.28 |
|
| ||||
Data presented as N (%) for categorical variables and median (interquartile range) for continuous variables.
P-value from Chi-square test or Fisher's exact test for categorical variables and Wilcoxon Rank Sum test for continuous variables on comparison of each characteristic between patients in both cohorts.
Table 3. Comparisons of clinical outcomes in patients diagnosed and treated for chylothorax before and after initiation of clinical practice guideline (N=163).
| Outcome | All | Early Cohort (n=118) |
Late Cohort (n=45) |
P-value§ |
|---|---|---|---|---|
| ICU stay | ||||
|
| ||||
| Total number(s) of ICU admission | ||||
| One | 128 (78.5) | 92 (78.0) | 36 (80.0) | 0.78 |
|
| ||||
| ≥ Two | 35 (21.5) | 26 (22.0) | 9 (20.0) | |
|
| ||||
| ICU length post-extubation, days | 3 (1-5) | 3 (1-6) | 3 (2-4) | 0.71 |
|
| ||||
| Total ICU length of stay, days | 16 (6-31) | 18 (7-39) | 9 (5-18) | 0.01 |
|
| ||||
| Hospital stay | ||||
|
| ||||
| Total hospital length of stay, days | 28 (18-53) | 30 (22-54) | 23 (14-34) | 0.005 |
|
| ||||
| Weight at discharge, kg | 5.2 (3.7-9.5) | 5.0 (3.6-9.6) | 5.5 (4.1-8.1) | 0.63 |
|
| ||||
| Mortality | ||||
|
| ||||
| Death during initial ICU stay | 10 (6.1) | 9 (7.6) | 1 (2.2) | 0.29 |
|
| ||||
| Hospital death | 13 (8.0) | 10 (8.5) | 3 (6.7) | 1.00 |
|
| ||||
| Mechanical ventilation | ||||
|
| ||||
| Total number of intubations | ||||
|
|
||||
| One | 89 (54.6) | 57 (48.3) | 32 (71.1) | 0.01 |
|
| ||||
| ≥ Two | 74 (45.4) | 61 (51.7) | 13 (28.9) | |
|
| ||||
| Total duration of mechanical ventilation, days | 9 (3-20) | 11 (3-24) | 5 (3-12) | 0.02 |
|
| ||||
| Chest tubes | ||||
|
| ||||
| Total number of chest tubes placed | ||||
|
|
||||
| One | 11 (6.8) | 4 (3.4) | 7 (15.6) | 0.01 |
|
| ||||
| ≥ Two | 152 (93.2) | 114 (96.6) | 38 (84.4) | |
|
| ||||
| Total duration of chest tubes, days | 18 (11-27) | 20 (12-30) | 14 (10-23) | 0.01 |
|
| ||||
| Chest tube removal to discharge, days | 6 (2-17) | 8 (3-20) | 4 (1-13) | 0.08 |
|
| ||||
| Central venous lines | ||||
|
| ||||
| Total number(s) of central venous lines | ||||
| None | 1 (0.6) | 1 (0.9) | 0 (0.0) | 0.94† |
|
| ||||
| One | 65 (39.9) | 47 (39.8) | 18 (40.0) | |
|
| ||||
| ≥ Two | 97 (59.5) | 70 (59.3) | 27 (60.0) | |
|
| ||||
| Total duration of central venous lines, days | 23.5 (12-50) | 27 (16-54) | 15 (8-26) | 0.001 |
|
| ||||
| NPO | ||||
|
| ||||
| Total number(s) of time(s) NPO | ||||
| One | 65 (39.9) | 44 (37.3) | 21 (46.7) | 0.08 |
|
| ||||
| Two | 42 (25.8) | 28 (23.7) | 14 (31.1) | |
|
| ||||
| ≥ Three | 56 (34.4) | 46 (39.0) | 10 (22.2) | |
|
| ||||
| Treated with NPO for chylothorax | 76 (46.6) | 58 (49.2) | 18 (40) | 0.30 |
|
| ||||
| Total duration NPO, days | 9 (3-18) | 9.5 (4-19) | 6 (2-14) | 0.04 |
|
| ||||
| Interval from surgery to NPO treatment, days | 13 (10-20.5) | 15 (11-22) | 9 (7-13) | 0.006 |
|
| ||||
| Resumption of enteral nutrition to discharge, days | 14 (8-27) | 16 (10-30) | 11 (6-19) | 0.02 |
|
| ||||
| Total parenteral nutrition | ||||
|
| ||||
| Total duration on total parenteral nutrition, days | 12 (5-22) | 13 (5-24) | 10 (4-21) | 0.29 |
|
| ||||
Data presented as N (%) for categorical variables and median (interquartile range) for continuous variables.
P-value from Chi-square test or Fisher's exact test for nominal variables, Mantel-Haenszel Chi-square test for ordinal variables, and Wilcoxon Rank Sum test for continuous variables on comparison of each outcome between patients in both cohorts.
Comparison was made as None/One (combined ‘None’ into ‘One’ category) vs. Two and p-value was from Chi-square test.
ICU = intensive care unit; NPO = nil per os
Comment
This CPG provided a uniform approach to the treatment of post-operative chylothorax with the goals of decreasing unnecessary practice variation, improving patient outcomes while minimizing harm, and promoting cost-effective practices. This retrospective analysis demonstrates improvement in several measured clinical and resource utilization outcomes following implementation of the CPG.
Treating chylothorax requires patience, as conservative medical therapy is variably effective and may take several days to weeks before benefits are seen. While surgical thoracic duct ligation and pleurodesis have shown to be successful in some refractory patients (11, 12), it is unclear when this therapeutic option should be exercised given the associated risks. Several authors advocate for early thoracic duct ligation or pleurodesis in patients failing conservative medical treatment. (16-18) It is not yet possible to identify the subset of patients who will drain for long times and consequently be at risk for protein malnutrition, electrolyte, and immunologic abnormalities. We believe that spontaneous resolution of chylothorax is unlikely when initial drainage volume is excessive. This was the rationale for separating the patients into high and low volume groups to guide initial therapy in the CPG. Although octreotide has shown to be useful in treating children with chylothorax with few side effects (e.g. flu-like symptoms, nausea, abdominal distension, diarrhea, cutaneous flushing, transient hypoglycemia), the optimal timing and duration of octreotide treatment in children is unknown with data limited to case reports and small retrospective studies. (12, 13, 19) If aggressive medical therapy does not result in decreasing chest tube drainage, surgical treatment with thoracic duct ligation is instituted within 2 weeks of starting therapy.
Our analysis showed improvement in multiple outcomes after institution of the chylothorax CPG. Most notably, there was a decrease in total hospital and ICU length of stay, and device utilization was also significantly improved (e.g., mechanical ventilation, chest tubes, and CVL). These improvements were noted despite finding no difference in frequency of time to surgical treatment for chylothorax. Thus, the observed results do not appear to be due to a greater proportion of LC patients having surgical treatment for persistent chylothorax. The lack of a measurable difference in frequency of surgical intervention may be due to the low number of patients in each cohort who required surgical treatment for persistent chylothorax.
There are likely multiple factors that led to the findings of decreased ICU and hospital length of stay, including earlier recognition and shorter time to diagnosis with earlier intervention. The earlier time to diagnosis in the LC does not by itself account for the observed decrease in ICU and hospital length of stay. Another potential explanation is decreased duration of mechanical ventilation and earlier extubation in the LC, which decreases the need for continuous sedation infusions and CVL, and results in shorter ICU and hospital stays. There was no difference between EC and LC in duration of ICU stay following extubation. Additionally, the LC had a significantly shorter interval from cardiac surgery to NPO treatment of chylothorax; this earlier initiation of NPO treatment may lead to the decrease in overall hospital length of stay. The total duration of NPO restriction was also decreased, which may have resulted in better overall nutritional status, facilitating extubation, and therefore decreasing length of hospitalization. Placement of additional chest tubes was significantly less in the LC, suggesting fewer recurrent effusions following CPG implementation. We feel that institution of the chylothorax CPG created a regimented change in ICU culture and placed the diagnosis and treatment in the forefront of daily medical plans. Though not statistically significant, there was a trend in the LC for shorter duration from time of final chest tube removal to hospital discharge. There was a difference with shorter interval in the LC for resumption of enteral nutrition to hospital discharge, supporting the notion that the CPG focuses attention to treatment of chylothorax in daily medical plans, resulting in early recognition, guided treatment strategies, and progressively routine nature of managing post-operative chylothorax. Limitations:
The retrospective design of the study limits our ability to make causal inferences. Though we attempted to identify all important data variables to include in the analysis, it is possible that unmeasured confounders explain some of the differences in outcomes between the two cohorts. This study was performed at a single center; hence, the results may not be applicable to all patients who develop chylothorax after congenital heart surgery.
Confirmatory pleural fluid analysis (e.g. elevated triglycerides, lymphocyte predominance, or presence of chylomicrons) is the accepted method for diagnosis of chylothorax, especially when the etiology of the pleural effusion is unclear. In this study, diagnosis of chylothorax was made by either clinical observation of chylous appearing chest tube drainage with or without pleural fluid analysis for triglycerides, or only by pleural fluid drainage with elevated triglyceride levels (>110 mg/dL). These methods of diagnosis did not change between EC and LC periods. Approximately one third of patients had pleural fluid triglyceride levels examined at time of diagnosis, and the CPG had no effect on clinician practice in ordering this test. The rationale behind this practice is that confirmatory testing was thought to be unnecessary as it would not alter treatment in our specific patient population.
Because routine pleural fluid analysis was not routinely obtained, some false positives may be present in our study patients. This may also be a reason for the apparent high incidence rate of chylothorax, which was 8.9%. Identifying false negative cases would not have been practical or possible within this study design.
Lastly, the study did not include data for patients readmitted for chylothorax, but this represented a very small number of patients.
Conclusions
The initiation of a CPG for the treatment of chylothorax in children recovering from cardiac surgery was associated with earlier time to diagnosis of chylothorax, decrease in ICU and total hospital length of stay, shorter duration of mechanical ventilation, decrease in repeat intubations, decreased total days of chest tube and CVL use, and decrease in days NPO. Further prospective research is needed to validate the benefits of our CPG in other clinical settings. This analysis broadly demonstrates the potential value of using CPGs in pediatric cardiac intensive care units.
Acknowledgments
The authors would like to acknowledge Dr. David Hanauer for assistance with the Electronic Medical Record Search Engine tool provided by the University of Michigan Cancer Center's Biomedical Informatics Core with partial support from the National Institutes of Health through the University of Michigan Cancer Center's Support Grant (CA46592).
Abbreviations and Acronyms
- BSI
bloodstream infections
- CPG
clinical practice guideline
- CVL
central venous line
- EC
early cohort
- ICU
intensive care unit
- LC
late cohort
- NPO
nil per os
- RACHS-1
Risk Adjustment for Congenital Heart Surgery
- TPN
total parenteral nutrition
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Buttiker V, Fanconi S, Burger R. Chylothorax in children: Guidelines for diagnosis and management. Chest. 1999;116(3):682–687. doi: 10.1378/chest.116.3.682. [DOI] [PubMed] [Google Scholar]
- 2.Cannizzaro V, Frey B, Bernet-Buettiker V. The role of somatostatin in the treatment of persistent chylothorax in children. Eur J Cardiothorac Surg. 2006;30(1):49–53. doi: 10.1016/j.ejcts.2006.03.039. [DOI] [PubMed] [Google Scholar]
- 3.Biewer ES, Zurn C, Arnold R, et al. Chylothorax after surgery on congenital heart disease in newborns and infants -risk factors and efficacy of mct-diet. J Cardiothorac Surg. 2010;5:127. doi: 10.1186/1749-8090-5-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Panthongviriyakul C, Bines JE. Post-operative chylothorax in children: An evidence-based management algorithm. J Paediatr Child Health. 2008;44(12):716–721. doi: 10.1111/j.1440-1754.2008.01412.x. [DOI] [PubMed] [Google Scholar]
- 5.Allen EM, van Heeckeren DW, Spector ML, Blumer JL. Management of nutritional and infectious complications of postoperative chylothorax in children. J Pediatr Surg. 1991;26(10):1169–1174. doi: 10.1016/0022-3468(91)90325-n. [DOI] [PubMed] [Google Scholar]
- 6.Brown KL, Ridout DA, Goldman AP, Hoskote A, Penny DJ. Risk factors for long intensive care unit stay after cardiopulmonary bypass in children. Crit Care Med. 2003;31(1):28–33. doi: 10.1097/00003246-200301000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Beghetti M, La Scala G, Belli D, Bugmann P, Kalangos A, Le Coultre C. Etiology and management of pediatric chylothorax. J Pediatr. 2000;136(5):653–658. doi: 10.1067/mpd.2000.104287. [DOI] [PubMed] [Google Scholar]
- 8.Chan SY, Lau W, Wong WH, Cheng LC, Chau AK, Cheung YF. Chylothorax in children after congenital heart surgery. Ann Thorac Surg. 2006;82(5):1650–1656. doi: 10.1016/j.athoracsur.2006.05.116. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen DM, Shum-Tim D, Dobell AR, Tchervenkov CI. The management of chylothorax/chylopericardium following pediatric cardiac surgery: A 10-year experience. J Card Surg. 1995;10(4 Pt 1):302–308. doi: 10.1111/j.1540-8191.1995.tb00616.x. [DOI] [PubMed] [Google Scholar]
- 10.Chan EH, Russell JL, Williams WG, Van Arsdell GS, Coles JG, McCrindle BW. Postoperative chylothorax after cardiothoracic surgery in children. Ann Thorac Surg. 2005;80(5):1864–1870. doi: 10.1016/j.athoracsur.2005.04.048. [DOI] [PubMed] [Google Scholar]
- 11.Milonakis M, Chatzis AC, Giannopoulos NM, et al. Etiology and management of chylothorax following pediatric heart surgery. J Card Surg. 2009;24(4):369–373. doi: 10.1111/j.1540-8191.2008.00781.x. [DOI] [PubMed] [Google Scholar]
- 12.Soto-Martinez M, Massie J. Chylothorax: Diagnosis and management in children. Paediatr Respir Rev. 2009;10(4):199–207. doi: 10.1016/j.prrv.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 13.Caverly L, Rausch CM, da Cruz E, Kaufman J. Octreotide treatment of chylothorax in pediatric patients following cardiothoracic surgery. Congenit Heart Dis. 2010;5(6):573–578. doi: 10.1111/j.1747-0803.2010.00464.x. [DOI] [PubMed] [Google Scholar]
- 14.Engum SA, Rescorla FJ, West KW, Scherer LR, 3rd, Grosfeld JL. The use of pleuroperitoneal shunts in the management of persistent chylothorax in infants. J Pediatr Surg. 1999;34(2):286–290. doi: 10.1016/s0022-3468(99)90192-6. [DOI] [PubMed] [Google Scholar]
- 15.Jenkins KJ, Gauvreau K. Center-specific differences in mortality: Preliminary analyses using the risk adjustment in congenital heart surgery (rachs-1) method. The Journal of thoracic and cardiovascular surgery. 2002;124(1):97–104. doi: 10.1067/mtc.2002.122311. [DOI] [PubMed] [Google Scholar]
- 16.Le Nue R, Molinaro F, Gomes-Ferreira C, et al. Surgical management of congenital chylothorax in children. Eur J Pediatr Surg. 2010;20(5):307–311. doi: 10.1055/s-0030-1254164. [DOI] [PubMed] [Google Scholar]
- 17.Stager V, Le L, Wood RE. Postoperative chylothorax successfully treated using conservative strategies. Proc (Bayl Univ Med Cent) 2010;23(2):134–138. doi: 10.1080/08998280.2010.11928601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nath DS, Savla J, Khemani RG, Nussbaum DP, Greene CL, Wells WJ. Thoracic duct ligation for persistent chylothorax after pediatric cardiothoracic surgery. Ann Thorac Surg. 2009;88(1):246–251. doi: 10.1016/j.athoracsur.2009.03.083. discussion 251-242. [DOI] [PubMed] [Google Scholar]
- 19.Roehr CC, Jung A, Proquitte H, et al. Somatostatin or octreotide as treatment options for chylothorax in young children: A systematic review. Intensive Care Med. 2006;32(5):650–657. doi: 10.1007/s00134-006-0114-9. [DOI] [PubMed] [Google Scholar]


