Abstract
A single central injection of angiotensin II (AngII) potently increases water intake; however, a growing body of research suggests that repeated, acute intracerebroventricular injections of AngII cause a reduction in the dipsogenic response to subsequent AngII. This AngII-induced behavioral desensitization is specific to the effects of angiotensin and mediated by the angiotensin type-1 (AT1) receptor. The neuroanatomical substrate for this phenomenon, however, remains unknown. The anteroventral third ventricle region (AV3V) is an important site for the behavioral and physiological actions of AngII. Therefore, we hypothesized that this region also mediates the effects of repeated central AngII administration. In support of this hypothesis, we found that repeated injections of AngII into the AV3V reduced water intake stimulated by a test injection of AngII given into this region. Moreover, repeated AngII injections in the AV3V reduced water intake after AngII was injected into the lateral ventricle. These studies also demonstrate that activation of the AT1 receptor within the AV3V is required for AngII-induced behavioral desensitization because direct injection of the AT1 receptor antagonist, losartan, into the AV3V blocked the desensitizing effect of repeated AngII injections into the lateral ventricle. These findings provide additional support for a role of the AV3V in the dipsogenic actions of AngII, and suggest that this region is critical for the desensitization that occurs after acute repeated central injections of AngII.
Keywords: AV3V, angiotensin, desensitization, thirst
1. Introduction
Angiotensin II (AngII) acts at a number of peripheral and central sites to engage coordinated physiological and behavioral responses to hypovolemia. In the periphery, AngII causes retention of water and electrolytes through actions at the kidney, and the peptide also acts directly on the cardiovascular system to increase blood pressure [1–3]. This effect on cardiovascular function has made the angiotensin system a useful target for the treatment of hypertension [4, 5]. Within the brain, AngII also engages some of these same cardiovascular responses, but in addition, it causes robust increases in water and salt intake [6–9].
In rats, a single introcerebroventricular (icv) injection of AngII potently increases water intake [8, 10], but repeated injections of the peptide, given within a short time frame, reduce this response [11–16]. This AngII-induced behavioral desensitization has been shown to be specific to the angiotensin system, not the result of a broader behavioral impairment, and dependent on activation of the angiotensin type 1 (AT1) receptor [14, 16]. Although the literature on the effects of AngII-induced desensitization in the behaving animal is growing, to date all of the behavioral studies investigating the effects of repeated administration of AngII have given the peptide icv. This route of administration is useful for studying the broad effects of a compound, but it provides little anatomical specificity. As such, a neuroanatomical locus for this phenomenon remains unknown.
Structures along the lamina terminalis, such as the median preoptic nucleus (MnPO) and the organum vasculosum of the lamina terminalis (OVLT), play an essential role in the neural control of body fluid homeostasis and are responsive to AngII. The MnPO and OVLT express AT1 receptors [17–20], and lesions made in the anteroventral third ventricle region (AV3V), which comprises parts of the MnPO and OVLT, abolish the drinking response to icv AngII [21]. Moreover, c-Fos expression is observed in the AV3V after icv injection of AngII [22, 23], and ventricular obstruction that prevents CSF from reaching the AV3V prevents water intake stimulated by AngII injected into the lateral ventricle [21, 24–26]. Given this important role for the AV3V in mediating the drinking response to icv AngII, it is reasonable to hypothesize that it is similarly involved in the reduced dipsogenic response observed after repeated icv AngII administration. Accordingly, the present studies tested the role of the AV3V in mediating the reduced water intake observed after repeated central administration of AngII. Consistent with our previous studies [14–16], the present experiments used repeated injections of relatively large doses of AngII to explore receptor-mediated responses that may be unobservable under more physiological parameters. The results provide additional support for a role of the AV3V in the drinking response to icv AngII and suggest that this region is both necessary and sufficient for the reduced water intake observed after repeated central injections of AngII.
2. Materials and Methods
2.1. Animals
Adult male Sprague Dawley rats (325–375 g) were obtained from Harlan Laboratories (Indianapolis, IN, USA). Rats were individually housed in stainless steel wire mesh cages in a temperature- and humidity-controlled room on a 12:12 hr light:dark cycle, and all experiments were performed early in the lights-on phase of the cycle. Food and water were available ad libitum, except where otherwise stated. All experimental protocols were approved by the Institutional Animal Care and Use Committee of the State University of New York at Buffalo, and the handling and care of animals was in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Subjects were used only once per experiment, unless otherwise stated.
2.2. Cannula implantation
Rats were anesthetized by an intramuscular injection of ketamine (70 mg/kg) and xylazine (5 mg/kg). Their heads were fixed in a stereotaxic apparatus, a small incision was made to expose the skull, and burr holes were drilled. Rats then were implanted with guide cannulae (26 gauge; Plastics One, Roanoke, VA, USA) aimed at the lateral ventricle, the AV3V, or both the lateral ventricle and the AV3V. Stereotaxic coordinates were 0.9mm posterior to bregma, 1.4 mm lateral to midline, 1.8 mm ventral to the surface of the skull for lateral ventricle injections and 0.11mm anterior to bregma, on midline, 7.8 mm ventral to the surface of the skull for the AV3V. When the experimental design required implantation of two cannulae, one for injection into the lateral ventricle and one for injection into the AV3V, the lateral ventricle cannula used an angled approach (20°) with the following stereotaxic coordinates: 1.4 mm posterior to bregma, 3.1 mm lateral to midline, 3.1 mm ventral to the surface of the skull. Cannulae were affixed to the skull using bone screws and dental cement. Septocaine (topical) and carprofen (5 mg/kg; sc) were administered during surgery for analgesia, and additional carprofen (5 mg/kg; sc) was given as needed for up to three days after surgery to manage pain and inflammation.
2.3. Drugs and injections
AngII (Biochem Bioscience Inc., King of Prussia, PA, USA) and the AT1 receptor antagonist losartan (losartan potassium; Sigma-Aldrich, St. Luis, MO, USA) were diluted in tris-buffered saline (TBS). Injections were made by hand using a Hamilton syringe (Hamilton Company, Reno, NV, USA) attached to water-filled PE 50 tubing. Tubing was attached to an injector cannula (33 gauge; Plastics One, Roanoke, VA, USA) that was fabricated to extend either 1.5–2.5 mm (lateral ventricle) or 2.5 mm (AV3V) beyond the guide cannula, and the injector was left in place for approximately 30–40 sec after the injection to allow for diffusion of drug. All injections into the AV3V were made using a volume of 0.5 μl, and all icv injections were 1μl.
2.4. Verification of proper cannula placement and AngII responsiveness
Proper lateral ventricle cannula placement was verified by injection of AngII (10 ng). Only data from rats that drank at least 6 ml of water in the 30 min after AngII injection were included. Proper cannula placement in the AV3V was assessed by histological examination and/or injection of AngII (1 ng). Only rats that drank at least 4 ml of water in the 30 min after AngII injection into the parenchyma were considered to have accurate placement in the AV3V.
2.5. Histological verification
After behavioral testing, a subset of rats were anesthetized by inhaled isoflurane and 0.5 μl of dye (Green Food Color, McCormick & Co., Inc., Hunt Valley, MD) was injected into the cannula aimed at the AV3V. Rats then were sacrificed by decapitation, the brains were removed, and 50 μm coronal brain sections were collected on a cryostat for histological examination. Consistent with previous reports [27], the AV3V was defined as an area ventral to the anterior commissure and dorsal to the roof of the third ventricle, extending rostrally along the course of the optic recess to the anterior extent of the OVLT.
2.6. Intake measures
Water intake at the time of behavioral verification of proper cannula placement and for Experiment 1 was assessed using 100 ml graduated bottles with 1 ml gradations. Water intake was calculated as the starting volume minus the volume remaining at the end of the test. In all other experiments, a custom contact lickometer (designed and constructed by the Psychology Electronics Shop, University of Pennsylvania, Philadelphia, PA, USA) was used to determine the number of times the rats licked the water spout. Electric leads connected the lickometer to an electrically isolated water spout. Rats licked through a slot (3.2 mm wide) in the cage to access a recessed water spout in order to minimize non-tongue contact with the spout. Each time the tongue of the rat contacted the spout, a lick was recorded. The lickometer interfaced with a computer that used an integrated digital I/O device (National Instruments, Inc., Austin, TX, USA), and data were processed using a MATLAB (MathWorks, Natick, MA, USA) software environment before being exported for final analysis using Excel (Microsoft Corp., Redmond, WA, USA). Water bottles were weighed before and after each test, and total intake was calculated as the starting bottle weight minus the weight at the end of the test. Total intake was divided by the total number of licks to determine the average volume per lick, and data were broken down into discrete time bins. Binned intake was calculated as the number of licks per bin multiplied by the volume per lick. Lickometer data are represented in 10-min time bins to more accurately capture group differences observed in pilot studies in our laboratory.
2.7. Data analysis
Data were analyzed using STATISTICA software (version 9.0; Statsoft, Tulsa, OK, USA). A Repeated measures ANOVA was used to analyze non-cumulative water intake. Total 30 min intake was analyzed using a one-way ANOVA or a t-test. Statistically significant main or interaction effects were further probed using Student Newman-Keuls post-hoc tests. Comparisons were considered significantly different at p<0.05.
2.8. Experimental Designs
2.8.1. Experiment 1: Dipsogenic response to AngII in the AV3V
To confirm our previous demonstration [28] that a low dose of AngII would not stimulate drinking after injection into the lateral ventricle, rats were given a single icv injection of either vehicle (1 μl TBS) or one of two doses of AngII (1 ng or 10 ng), and water intake was measured for 30 min. To test if the lower of these two doses of AngII stimulated intake after direct injection into the AV3V, a separate group of rats was given a single injection of AngII (1 ng) through a cannula aimed at the AV3V and subsequent 30-min water intake was assessed.
2.8.2. Experiment 2: Effect of repeated injections of AngII into the AV3V
Rats were given an icv treatment regimen comprising 3 injections of either vehicle (TBS) or one of two doses of AngII (300 ng or 100 ng), with each injection separated by 20 min. Twenty minutes after the final treatment regimen injection, all rats received a final icv test injection of AngII (100 ng) and subsequent 30-min water intake was measured. A separate group of rats was given either a vehicle (TBS × 3) or an AngII (100 ng × 3) treatment regimen administered directly into the AV3V, with each injection separated by 20 min. Twenty minutes after the final treatment regimen injection, all rats received an AngII (100 ng) test injection into the AV3V and 30-min water intake was assessed. Consistent with our previous studies [14–16], food and water were removed before the initial treatment regimen injection and were not returned until immediately after the test injection to avoid any influence of intake during the course of the treatment regimen.
2.8.3. Experiment 3: Effect of repeated AngII into the AV3V on water intake stimulated by icv AngII
Rats were given either a vehicle (TBS × 3) or an AngII (100 ng × 3) treatment regimen into the AV3V prior to all rats receiving a test injection of AngII (100 ng) administered through a separate cannula aimed at the lateral ventricle. Again, food and water were unavailable during the entire treatment regimen. After the test injection, food and water were returned, and subsequent 30-min water intake was measured. Subjects were categorized as having either accurate cannula placements in the AV3V or missed placements based on the drinking response to injection of a low dose of AngII and histological assessment. Data generated from these two cohorts were analyzed separately. Verification data for the subjects used in this experiment and an illustration of the approximate location of parenchymal cannula placements are provided in Figure 1B and Figure 1C. The brains of three rats were destroyed before histological assessment was possible, however, so only behavioral data are included for these three subjects.
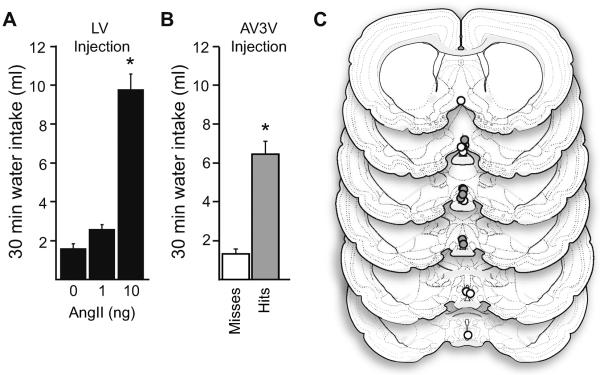
Figure 1.
Injection of a low dose of AngII into the AV3V stimulated water intake. A) When given icv, AngII stimulated a significant increase in 30-min water intake at the 10 ng dose (p<0.05 vs all other groups), but 1 ng AngII did not stimulate intake (p>0.05 vs vehicle). B) When cannulae terminated in the AV3V, rats drank significantly more water to injection of 1 ng AngII than did rats with cannulae that terminated outside of the AV3V (p<0.05). C) Illustration depicting cannula placement in rats from panel B. Closed circles represent accurate cannula placements (hits) and open circles represent placements outside of the AV3V (misses).
2.8.4. Experiment 4: Role of AT1 receptor activation in the AV3V in the desensitizing effect of repeated icv AngII administration
Food and water were removed and rats were given a single pretreatment injection into the AV3V of either vehicle (TBS) or the AT1 receptor antagonist losartan (1 μg). The dose of losartan was chosen because it has previously been found to be ineffective at blocking water intake stimulated by AngII (100 ng) when both drugs were administered icv [14]. Twenty min after the pretreatment injection, rats received either a vehicle (TBS × 3) or an AngII (300 ng × 3) treatment regimen given icv. All rats then received an icv AngII (100 ng) test injection, food and water were returned, and subsequent 30-min water intake was measured.
3. Results
3.1. Experiment 1- Dipsogenic response to AngII in the AV3V
Due to the close proximity of the AV3V to the third ventricle, it was important first to confirm that a low dose of AngII was ineffective at stimulating water intake when administered icv. To this end, rats were given a single icv injection of either vehicle (TBS) or one of two dose of AngII (1 ng or 10 ng) and water intake was measured. A one-way ANOVA on total 30 min intake revealed a significant main effect of Dose (F2,12=79.000, p<0.001; n=5 per group; Figure 1A). Consistent with a previous dose-response analysis from our laboratory [28], post-hoc testing showed that 10 ng AngII was effective at stimulating water intake (p<0.05 vs all other groups), but drinking after 1 ng AngII was no different from controls (p=0.186 vs vehicle).
Using the previous experiment as a guide, we evaluated the drinking response to a low dose of AngII in the AV3V. Rats were administered a single injection of AngII (1 ng) through a cannula aimed at the AV3V and subsequent water intake was measured. Rats drank significantly more water after injection of this low dose of AngII when the cannula terminated in the AV3V than did rats with cannulae that terminated outside of the AV3V (p<0.001; n=8–9 per group; Figure 1B, Figure 1C). Accordingly, subsequent experiments used this as a behavioral verification of proper cannula placement.
3.2. Experiment 2- Effect of repeated injections of AngII into the AV3V
In a similar manner as the experiment above, it was important to find a treatment regimen dose that was ineffective at causing behavioral desensitization when the injections were made icv. Accordingly, rats were given an icv treatment regimen of either vehicle (TBS × 3) or one of two doses of AngII (100 ng × 3 or 300 ng × 3). All rats then received an icv AngII (100 ng) test injection and water intake was measured. A repeated measures ANOVA on non-cumulative intake was performed to test for between subjects effects of Treatment Regimen and within-subjects effects of Time. We did not detect a significant Treatment Regimen × Time interaction (F6,78= 2.145, p=0.057); however, there was a significant main effect of Treatment Regimen (F2,26=4.053, p=0.029; n=9–10 per group; Figure 2A). Post-hoc testing showed that rats given a 300 ng AngII treatment regimen drank less after the test injection than did rats given a vehicle or a 100 ng AngII treatment regimen (p<0.05) before the same AngII test injection. There was no statistical difference in intake between rats that received a vehicle or a 100 ng AngII treatment regimen (p=0.412). There also was a significant main effect of Time [F(3,78)=218.006, p<0.001], which showed that most of the drinking occurred in the first 5–10 min after the test injection.
Figure 2.
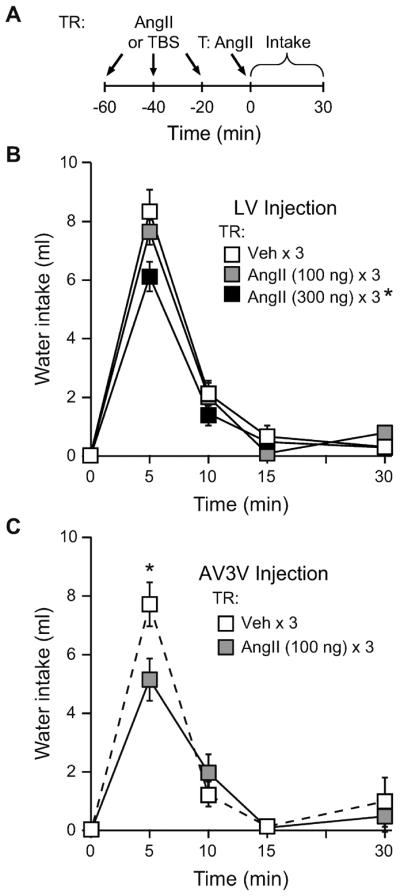
Repeated AngII injections in the AV3V resulted in behavioral desensitization. A) Timeline of the experiment showing treatment regimen (TR) injections and the test injection that immediately preceded the intake measures. B) Rats were given an icv treatment regimen (TR) of either vehicle (TBS) or AngII (300 ng or 100 ng) prior to all rats receiving an icv AngII (100 ng) test injection. Rats that received a 300 ng AngII TR drank less to the test injection than did rats given either a vehicle or a 100 ng AngII TR (p<0.05). A 100 ng AngII TR did not reduce intake stimulated by the test injection (p>0.05 vs vehicle TR). C) When rats were given either an AngII (100 ng) or a vehicle (TBS) TR into the AV3V, rats given an AngII TR drank less water 5 min after an AngII (100 ng) test injection in the AV3V (p<0.05).
We next tested whether repeated AngII injections given to the AV3V was sufficient to produce desensitization. Rats were given either a vehicle (TBS × 3) or an AngII (100 ng × 3) treatment regimen into the AV3V prior to all rats receiving a test injection of AngII (100 ng) administered into this same site, and water intake was measured. A repeated measures ANOVA on non-cumulative intake found a significant Treatment Regimen × Time interaction (F3,33=2.964, p=0.046; n=6–7 per group; Figure 2B). Post-hoc testing revealed that these differences occurred 5 min after the test injection, at which time rats given an AngII treatment regimen drank significantly less water than did rats given a vehicle treatment regimen (p=0.004).
3.3. Experiment 3- Effect of repeated AngII into the AV3V on water intake stimulated by icv AngII
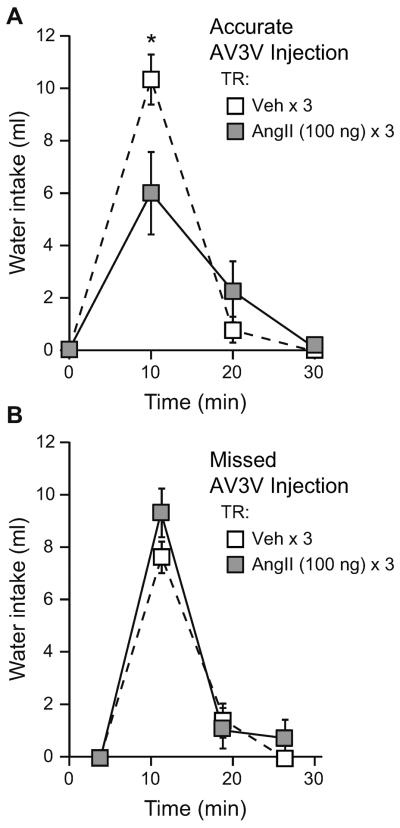
To test whether desensitization in the AV3V was sufficient to reduce water intake stimulated by injection of AngII into the lateral ventricle, rats (n=10 per group) were given either a vehicle (TBS × 3) or an AngII (100 ng × 3) treatment regimen into the AV3V prior to all rats receiving a lateral ventricle test injection of AngII (100 ng), and subsequent intake was assessed. Histological examination and the drinking response to 1 ng AngII in a separate procedure were used to categorize rats as hits or misses based on proper placement of the AV3V injector. From the initial 20 rats that were included in the study, it was determined that 11 had proper cannula placement in the AV3V while 9 had missed placements that terminated in sites outside the AV3V. When intake by rats with proper AV3V cannula placement (hits) was analyzed, a repeated measures ANOVA on non-cumulative water intake after the test injection revealed a significant Treatment Regimen × Time interaction (F2,18=25.127, p=0.038; n=5–6 per group; Figure 3A). Post-hoc testing on the interaction showed that this effect was due to differences in intake 10 min after the test injection, at which time rats given an AngII treatment regimen had reduced water intake (p=0.004). Analysis of data from rats with AV3V injections that missed the intended target found no difference in intake after an icv AngII challenge in rats given either a vehicle (TBS × 3) or an AngII (100 ng × 3) treatment regimen (main effect of Treatment Regimen, F1,7=1.126, p=0.328; Treatment Regimen × Time interaction, F2,14=1.640, p=0.229; n=4–5 per group; Figure 3B).
Figure 3.
Repeated AngII administration in the AV3V caused reduced water intake stimulated by an icv AngII challenge. A) When cannulae terminated in the AV3V, rats given an AngII (100 ng) treatment regimen (TR) drank less water 10 min after an icv AngII (100 ng) test injection than did rats given a vehicle (TBS) TR prior to the same test injection (p<0.05). B) When cannulae terminated outside the AV3V, there was no difference in intake between rats that received either an AngII (100 ng) or a vehicle (TBS) TR in the parenchyma prior to an icv AngII (100 ng) test injection (p>0.05).
3.4. Experiment 4- Role of AT1 receptor activation in the AV3V in the desensitizing effect of repeated icv AngII administration
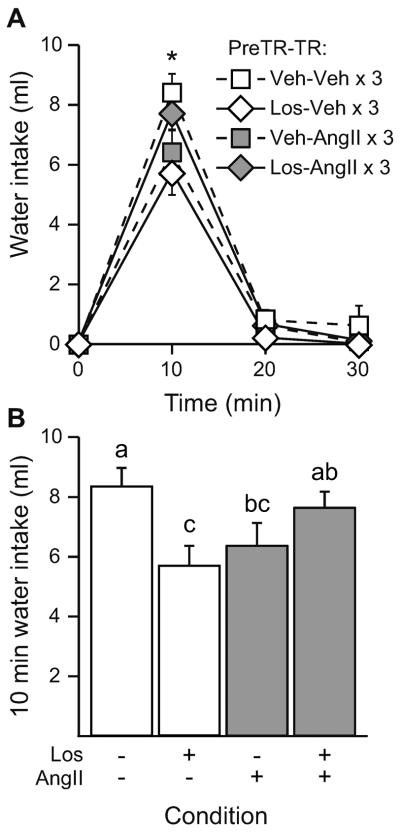
To test if AT1 receptor activation in the AV3V is required for AngII-induced behavioral desensitization, rats were given a single pretreatment injection of either vehicle (TBS) or losartan (1 μg) into the AV3V prior to receiving either a vehicle (TBS × 3) or an AngII (300 ng × 3) treatment regimen given icv. All rats then received an icv AngII test injection and water intake was measured. A repeated measures ANOVA performed on non-cumulative water intake after the test injection showed a significant Pretreatment × Treatment Regimen interaction (F1,39=9.381, p=0.004) which indicated that pretreatment with losartan reduced water intake in rats that received a vehicle treatment regimen (p=0.015 vs vehicle pretreatment/vehicle treatment regimen). There also was a significant Pretreatment × Treatment Regimen × Time interaction (F2,78=4.124, p=0.020; n=10–11 per group; Figure 4A). Post-hoc testing on the 3-way interaction showed that there were significant differences between groups 10 min after the test injection. Analysis of the 10 min time point showed that, in rats pretreated with vehicle, the AngII treatment regimen reduced water intake (p=0.011 vs vehicle treatment regimen; Figure 4B). Losartan pretreatment reduced water intake when given before a vehicle treatment regimen (p=0.001 vs vehicle pretreatment/vehicle treatment regimen), but losartan completely blocked the reduction in water intake otherwise observed after an AngII treatment regimen (p=0.300 vs vehicle pretreatment/vehicle treatment regimen). Furthermore, intake after the final test injection was different between the two groups that received losartan pretreatment. Specifically, after pretreatment with losartan, rats given an AngII treatment regimen drank more water than did rats given a vehicle treatment regimen (p=0.012).
Figure 4.
AT1 receptor activation in the AV3V was required for the reduced water intake observed after repeated icv AngII administration. Rats were given pretreatment (PreTR) of either vehicle (TBS) or losartan (1 μg) into the AV3V prior to receiving an icv treatment regimen (TR) of either AngII (300 ng) or vehicle (TBS). All rats then received an icv test injection of AngII (100 ng). A) There was a significant Time × PreTR × TR interaction 10 min after the test injection (p<0.05). B) At 10 min after the test injection, rats pretreated with vehicle had reduced water intake after an AngII TR (p<0.05). PreTR with losartan reduced water intake after a vehicle TR (p<0.05 vs veh/veh), but losartan PreTR blocked the desensitizing effect of the AngII TR (p>0.05 vs veh/veh). Bars with different letters are significantly different from each other (p<0.05).
4. Discussion
Repeated icv administration of AngII causes a reduction in water intake stimulated by an icv injection of AngII given later [11–16]. This AngII-induced behavioral desensitization is specific to the angiotensin system and not the result of a broader behavioral impairment [16]. The present studies identified the AV3V as a key neural substrate in this response by showing that 1) repeated AngII injections into the AV3V reduced drinking after a subsequent AngII injection into the AV3V, 2) repeated injections into the AV3V reduced drinking after a subsequent AngII injection into the lateral ventricle, and 3) AV3V injection of losartan prevented the behavioral desensitization that otherwise occurred after repeated injections of AngII into the lateral ventricle. These findings add to an existing literature implicating the AV3V in the drinking response to icv AngII and suggest that this region is both necessary and sufficient for the reduced water intake observed after repeated central AngII administration.
The multiple approaches used here provide a better perspective on the role of the AV3V in behavioral desensitization to AngII. Although the results of Experiment 2 show that AngII in the AV3V was sufficient to produce behavioral desensitization of the response to AngII in the AV3V, Experiment 3 was critical to show the importance of the AV3V in the response to repeated injections of AngII into the lateral ventricle. Indeed, these experiments suggest that other sites, at least those affected by AngII administered to the lateral ventricle, were unable to compensate for the desensitization of the AV3V. This finding is consistent with the hypothesis that the AV3V is the first order site in the network that produces drinking behavior after icv injection of AngII.
Although other studies that have tested the behavioral effects of AngII-induced desensitization have found a greater reduction in intake using lower doses of AngII and fewer injections (12,13), the interpretation of those studies is complicated by the fact that rats were permitted water access after each injection of the drug. Therefore, it is difficult to determine whether the reduced intake results from decreased efficacy of AngII, or from drinking-related inhibitory feedback [29]. Previous work from our lab, however, shows that multiple injections of a large dose of AngII are required to cause behavioral desensitization when water is absent [14]. In spite of the clearly pharmacological approach, the behavioral effect observed may serve as a faithful proxy of AngII-induced receptor desensitization and has provided useful information to guide studies of underlying mechanisms of desensitization.
The importance of AT1 receptors in the desensitizing response to AngII was suggested previously [14], and the present studies highlight the AV3V as a specifically relevant population of these receptors. It is worth noting that we used a dose of losartan that did not block the desensitizing effects of an AngII treatment regimen when the losartan was given to the lateral ventricle [14]. As such, it is unlikely that any effect of losartan in the present studies was due to seepage into the ventricular system. The results of these studies were not as straightforward, however, as the other present experiments. Specifically, we found that pretreatment with losartan reduced drinking by rats in the vehicle treatment regimen condition. This suggests that losartan has a particularly potent effect on AngII-induced intake when given directly in the AV3V. The critical comparison in this experiment, however, is the effect of losartan pretreatment on the AngII treatment regimen. Therefore, it is noteworthy that losartan prevented any differences in intake between rats in the control condition and rats receiving an AngII treatment regimen. Accordingly, any effect of losartan in the vehicle treatment regimen group should be of little concern because the effect of losartan in AngII-treated rats is in the opposite direction from what would be predicted if losartan were simply inhibiting intake after the test injection. These data provide further support that the effect of repeated AngII injections requires the AT1 receptor and that the critical population of AT1 receptors involved in behavioral desensitization resides in the AV3V.
As stated above, in addition to the relevance to AngII-induced behavioral desensitization, the present data provide additional evidence that the AV3V contains the primary neurons in the circuit that responds to central AngII injections. This hypothesis is supported by several independent lines of research. Although the SFO was initially implicated in the response to centrally administered AngII [30], this was challenged by evidence that the reduction in AngII responsiveness after SFO ablation was a function of the resulting debris that collected in the ventricular foramen after SFO lesion, thereby blocking the flow of CSF from the lateral ventricle to the third ventricle [24, 25]. This interpretation was supported by a series of studies using insertion of a grease plug into various locations in the cerebral ventricles, thereby obstructing the flow of CSF to different AngII-sensitive brain sites [21, 24–26], and by studies using periventricular ablation of the tissue surrounding the anteroventral third ventricle [21]. These studies strongly suggest that the first order neurons involved in mediating the drinking response to central AngII reside in the AV3V. A more direct test of this is found in the report of Fitts & Mason [31] that demonstrated water intake after injections of AngII into sites near the AV3V, but the use of relatively large doses of AngII and rather large volumes of their injections make it difficult to determine the specificity of this effect for the AV3V. The present data add to this literature by demonstrating a clear drinking response to injection of AngII in the AV3V and, to the best of our knowledge, show for the first time that AT1 receptors in this brain area play an important role in this behavioral response.
The lamina terminalis comprises multiple nuclei along the anterior wall of the third ventricle, including the MnPO, OVLT, and SFO. This is a region of vast interconnectedness and is an essential component in the neural circuitry subserving fluid balance [1, 3, 32–34]. The lamina terminalis is rich in angiotensin receptors, has been shown to possess angiotensin precursors, and likely releases angiotensin as a neurocrine signal [35]. The present data suggest that AngII-induced desensitization can occur in the AV3V and that this area is likely responsible for mediating the behavioral effects observed after repeated icv AngII administration. Although the AV3V may be viewed as a less specific anatomical term than the OVLT and the MnPO, which are structures comprised by the AV3V [21, 24, 25, 36], the AV3V nomenclature may provide more appropriate functional relevance. In this respect, it is possible that the cytoarchitectural criteria that may separate these structures are less important than the functional similarities that unite them. For instance, the OVLT and the MnPO both express angiotensin receptors [19, 37] and both are involved in the central effects of AngII [18]. Further complicating the issue, early studies investigating the role of the AV3V primarily relied upon electrolytic lesion techniques. The small size of the brain areas, relative to the larger lesions, prevented any ability to distinguish between effects solely attributed to the OVLT or to the MnPO. Nevertheless, it is likely that these earlier findings were the result of specific damage to the AV3V because we found that injection of a low dose of AngII into the AV3V stimulated a significant increase in water intake compared to intake by rats given the same injection through cannulae that were slightly outside of the AV3V. Notably, we observed this effect using a dose of AngII that was ineffective at stimulating intake when given icv. This control experiment was critical due to the close proximity of the AV3V to the 3rd ventricle, which could potentially permit the infused drug to seep into the ventricular system and contact distant sites [10]. Accordingly, we were able to determine that our effects were restricted to the AV3V, and, even when injections were made into the ventral 3rd ventricle, it was insufficient to account for any observed differences.
Future studies will be needed to further elucidate a mechanism for desensitization in the behaving animal and to place this mechanism in the context of the neural circuitry underlying this phenomenon. Previous in vitro research has shown that AngII receptor binding causes phosphorylation of the receptor which can lead to uncoupling of the G protein [38, 39] and receptor internalization [40]. Initial attempts by Torsoni et al. [12] to clarify a set of cellular events that give rise to AngII-induced desensitization suggest the requirement of SOCS-3 expression, and this may involve inhibitory feedback on JAK/STAT signaling. Work from our laboratory added to this proposed pathway by demonstrating the requirement of p44/42 MAPK [15], and others have shown the importance of MAPK in JAK/STAT signaling after AngII exposure [41, 42]. A clear link between these pathways and how this relates to changes in AngII-responsiveness in vivo remains an open area of research. The present findings provide a potentially useful anatomical reference for these future studies, but additional research is needed to test if the desensitizing effects of AngII reside wholly within the AV3V, or if these effects occur throughout a distributed neural circuit.
4.1 Conclusions
The present studies provide the first evidence that the AV3V is critical for the behavioral desensitization that occurs after repeated central injections of AngII. It is unknown, however, whether AngII-induced desensitization occurs in other brain nuclei, and what, if any, effect this would have on behavior and physiology. Previous findings using cell culture models in cardiomyocytes and vascular smooth muscle [43] suggest that this phenomenon occurs in other tissues, making it likely that desensitization is a general property of the AT1 receptor and that this phenomenon likely occurs in other AngII-responsive brain nuclei (e.g., the SFO and the paraventricular nucleus of the hypothalamus [17–20]). Further research is required to systematically test these hypotheses and determine how any such sensitization affects fluid intake. Nevertheless, given the known therapeutic utility of drugs that target the angiotensin system [4, 5], any additional information regarding the processes that govern angiotensin signaling may prove to be especially informative in the future treatment of hypertension and other human health concerns. The phenomenon of AngII-induced desensitization provides a useful model for studying the actions of this peptide, and research on desensitization of the angiotensin system has provided important insights into the behavioral and physiological effects of AngII. The present data add to an existing body of research implicating the AV3V as a critical component in the central regulation of body fluid homeostasis and the response to AngII. Furthermore, these data point to an important role of the AV3V in mediating the effects of repeated central AngII administration.
Highlights
-
□
Injection of a low dose of AngII into the AV3V stimulated water intake
-
□
Repeated AV3V AngII injections reduced drinking after AngII injection into the AV3V
-
□
Effect was similar when drinking was stimulated by AngII in the lateral ventricle
-
□
Losartan in the AV3V prevented the effect of repeated central injections of AngII
Acknowledgements
Anikó Marshall provided helpful technical assistance. Peter J. Vento is currently affiliated with the Department of Neurosciences, Medical University of South Carolina, Charleston, SC.
Funding Sources Support for this work was provided by NIH research grant HL091911 to DD and by a grant from the University at Buffalo Mark Diamond Research Fund awarded to PJV.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Fitzsimons JT. Angiotensin, thirst, and sodium appetite. Physiol Rev. 1998;78:583–686. doi: 10.1152/physrev.1998.78.3.583. [DOI] [PubMed] [Google Scholar]
- [2].Johnson AK, Thunhorst RL. The neuroendocrinology of thirst and salt appetite: visceral sensory signals and mechanisms of central integration. Front Neuroendocrinol. 1997;18:292–353. doi: 10.1006/frne.1997.0153. [DOI] [PubMed] [Google Scholar]
- [3].McKinley MJ, Johnson AK. The physiological regulation of thirst and fluid intake. News Physiol Sci. 2004;19:1–6. doi: 10.1152/nips.01470.2003. [DOI] [PubMed] [Google Scholar]
- [4].Goodfriend TL, Elliott ME, Catt KJ. Angiotensin receptors and their antagonists. N Engl J Med. 1996;334:1649–54. doi: 10.1056/NEJM199606203342507. [DOI] [PubMed] [Google Scholar]
- [5].de Gasparo M, Levens N. Does blockade of angiotensin II receptors offer clinical benefits over inhibition of angiotensin-converting enzyme? Pharmacol Toxicol. 1998;82:257–71. doi: 10.1111/j.1600-0773.1998.tb01572.x. [DOI] [PubMed] [Google Scholar]
- [6].Booth DA. Effects of intrahypothalamic glucose injection on eating and drinking elicited by insulin. J Comp Physiol Psychol. 1968;65:13–6. doi: 10.1037/h0025396. [DOI] [PubMed] [Google Scholar]
- [7].Epstein AN, Fitzsimons JT, Rolls BJ. Drinking induced by injection of angiotensin into the brain of the rat. J Physiol. 1970;210:457–74. doi: 10.1113/jphysiol.1970.sp009220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Severs WB, Summy-Long J, Taylor JS, Connor JD. A central effect of angiotensin: release of pituitary pressor material. J Pharmacol Exp Ther. 1970;174:27–34. [PubMed] [Google Scholar]
- [9].Buggy J, Fisher AE. Evidence for a dual central role for angiotensin in water and sodium intake. Nature. 1974;250:733–5. doi: 10.1038/250733a0. [DOI] [PubMed] [Google Scholar]
- [10].Johnson AK, Epstein AN. The cerebral ventricles as the avenue for the dipsogenic action of intracranial angiotensin. Brain Res. 1975;86:399–418. doi: 10.1016/0006-8993(75)90891-4. [DOI] [PubMed] [Google Scholar]
- [11].Quirk WS, Wright JW, Harding JW. Tachyphylaxis of dipsogenic activity to intracerebroventricular administration of angiotensins. Brain Res. 1988;452:73–8. doi: 10.1016/0006-8993(88)90010-8. [DOI] [PubMed] [Google Scholar]
- [12].Torsoni MA, Carvalheira JB, Calegari VC, Bezerra RM, Saad MJ, Gontijo JA, et al. Angiotensin II (AngII) induces the expression of suppressor of cytokine signaling (SOCS)-3 in rat hypothalamus - a mechanism for desensitization of AngII signaling. J Endocrinol. 2004;181:117–28. doi: 10.1677/joe.0.1810117. [DOI] [PubMed] [Google Scholar]
- [13].Zapparoli A, Figueiredo JF, Boer PA, Rocha Gontijo JA. Impaired dipsogenic and renal response to repetitive intracerebroventricular angiotensin II (AngII) injections in rats. J Renin Angiotensin Aldosterone Syst. 2011;12:161–8. doi: 10.1177/1470320310392617. [DOI] [PubMed] [Google Scholar]
- [14].Vento PJ, Daniels D. Repeated administration of angiotensin II reduces its dipsogenic effect without affecting saline intake. Exp Physiol. 2010;95:736–45. doi: 10.1113/expphysiol.2010.052191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Vento PJ, Daniels D. Mitogen-activated protein kinase is required for the behavioural desensitization that occurs after repeated injections of angiotensin II. Exp Physiol. 2012;97:1305–14. doi: 10.1113/expphysiol.2012.065771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Vento PJ, Myers KP, Daniels D. Investigation into the specificity of angiotensin II-induced behavioral desensitization. Physiol Behav. 2012;105:1076–81. doi: 10.1016/j.physbeh.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].McKinley MJ, Allen A, Clevers J, Denton DA, Mendelsohn FA. Autoradiographic localization of angiotensin receptors in the sheep brain. Brain Res. 1986;375:373–6. doi: 10.1016/0006-8993(86)90761-4. [DOI] [PubMed] [Google Scholar]
- [18].McKinley MJ, McAllen RM, Pennington GL, Smardencas A, Weisinger RS, Oldfield BJ. Physiological actions of angiotensin II mediated by AT1 and AT2 receptors in the brain. Clin Exp Pharmacol Physiol Suppl. 1996;3:S99–104. [PubMed] [Google Scholar]
- [19].Mendelsohn FA, Quirion R, Saavedra JM, Aguilera G, Catt KJ. Autoradiographic localization of angiotensin II receptors in rat brain. Proc Natl Acad Sci U S A. 1984;81:1575–9. doi: 10.1073/pnas.81.5.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Song K, Allen AM, Paxinos G, Mendelsohn FA. Mapping of angiotensin II receptor subtype heterogeneity in rat brain. J Comp Neurol. 1992;316:467–84. doi: 10.1002/cne.903160407. [DOI] [PubMed] [Google Scholar]
- [21].Buggy J, Johnson AK. Angiotensin-induced thirst: effects of third ventricle obstruction and periventricular ablation. Brain Res. 1978;149:117–28. doi: 10.1016/0006-8993(78)90592-9. [DOI] [PubMed] [Google Scholar]
- [22].Rowland NE, Li BH, Rozelle AK, Smith GC. Comparison of fos-like immunoreactivity induced in rat brain by central injection of angiotensin II and carbachol. Am J Physiol. 1994;267:R792–8. doi: 10.1152/ajpregu.1994.267.3.R792. [DOI] [PubMed] [Google Scholar]
- [23].McKinley MJ, Badoer E, Vivas L, Oldfield BJ. Comparison of c-fos expression in the lamina terminalis of conscious rats after intravenous or intracerebroventricular angiotensin. Brain Res Bull. 1995;37:131–7. doi: 10.1016/0361-9230(94)00266-4. [DOI] [PubMed] [Google Scholar]
- [24].Buggy J, Fisher AE, Hoffman WE, Johnson AL, Phillips MI. Ventricular obstruction: effect on drinking induced by intracranial injection of angiotensin. Science. 1975;190:72–4. doi: 10.1126/science.1166302. [DOI] [PubMed] [Google Scholar]
- [25].Buggy J, Fisher AE. Anteroventral third ventricle site of action for angiotensin induced thirst. Pharmacol Biochem Behav. 1976;4:651–60. doi: 10.1016/0091-3057(76)90216-1. [DOI] [PubMed] [Google Scholar]
- [26].Hoffman WE, Phillips MI. Regional study of cerebral ventricle sensitive sites to angiotensin II. Brain Res. 1976;110:313–30. doi: 10.1016/0006-8993(76)90405-4. [DOI] [PubMed] [Google Scholar]
- [27].Johnson AK, Buggy J. Periventricular preoptic-hypothalamus is vital for thirst and normal water economy. Am J Physiol. 1978;234:R122–9. doi: 10.1152/ajpregu.1978.234.3.R122. [DOI] [PubMed] [Google Scholar]
- [28].Daniels D, Mietlicki EG, Nowak EL, Fluharty SJ. Angiotensin II stimulates water and NaCl intake through separate cell signalling pathways in rats. Exp Physiol. 2009;94:130–7. doi: 10.1113/expphysiol.2008.044446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Miller NE, Sampliner RI, Woodrow P. Thirst-reducing effects of water by stomach fistula vs. water by mouth measured by both a consummatory and an instrumental response. J Comp Physiol Psychol. 1957;50:1–5. doi: 10.1037/h0046009. [DOI] [PubMed] [Google Scholar]
- [30].Simpson JB, Routtenberg A. Subfornical organ: site of drinking elicitation by angiotensin II. Science. 1973;181:1172–5. doi: 10.1126/science.181.4105.1172. [DOI] [PubMed] [Google Scholar]
- [31].Fitts DA, Masson DB. Preoptic angiotensin and salt appetite. Behav Neurosci. 1990;104:643–50. doi: 10.1037//0735-7044.104.4.643. [DOI] [PubMed] [Google Scholar]
- [32].McKinley MJ, Pennington GL, Oldfield BJ. Anteroventral wall of the third ventricle and dorsal lamina terminalis: headquarters for control of body fluid homeostasis? Clin Exp Pharmacol Physiol. 1996;23:271–81. doi: 10.1111/j.1440-1681.1996.tb02823.x. [DOI] [PubMed] [Google Scholar]
- [33].McKinley MJ, Allen AM, May CN, McAllen RM, Oldfield BJ, Sly D, et al. Neural pathways from the lamina terminalis influencing cardiovascular and body fluid homeostasis. Clin Exp Pharmacol Physiol. 2001;28:990–2. doi: 10.1046/j.1440-1681.2001.03592.x. [DOI] [PubMed] [Google Scholar]
- [34].Lind RW, Johnson AK. Subfornical organ-median preoptic connections and drinking and pressor responses to angiotensin II. J Neurosci. 1982;2:1043–51. doi: 10.1523/JNEUROSCI.02-08-01043.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].McKinley MJ, Albiston AL, Allen AM, Mathai ML, May CN, McAllen RM, et al. The brain renin-angiotensin system: location and physiological roles. Int J Biochem Cell Biol. 2003;35:901–18. doi: 10.1016/s1357-2725(02)00306-0. [DOI] [PubMed] [Google Scholar]
- [36].Bealer SL, Phillips MI, Johnson AK, Schmid PG. Anteroventral third ventricle lesions reduce antidiuretic responses to angiotensin II. Am J Physiol. 1979;236:E610–5. doi: 10.1152/ajpendo.1979.236.6.E610. [DOI] [PubMed] [Google Scholar]
- [37].Song K, Allen AM, Paxinos G, Mendelsohn FA. Angiotensin II receptor subtypes in rat brain. Clin Exp Pharmacol Physiol. 1991;18:93–6. doi: 10.1111/j.1440-1681.1991.tb01414.x. [DOI] [PubMed] [Google Scholar]
- [38].Boulay G, Chretien L, Richard DE, Guillemette G. Short-term desensitization of the angiotensin II receptor of bovine adrenal glomerulosa cells corresponds to a shift from a high to a low affinity state. Endocrinology. 1994;135:2130–6. doi: 10.1210/endo.135.5.7956936. [DOI] [PubMed] [Google Scholar]
- [39].Tang H, Shirai H, Inagami T. Inhibition of protein kinase C prevents rapid desensitization of type 1B angiotensin II receptor. Circ Res. 1995;77:239–48. doi: 10.1161/01.res.77.2.239. [DOI] [PubMed] [Google Scholar]
- [40].Sasamura H, Dzau VJ, Pratt RE. Desensitization of angiotensin receptor function. Kidney Int. 1994;46:1499–501. doi: 10.1038/ki.1994.429. [DOI] [PubMed] [Google Scholar]
- [41].Kodama H, Fukuda K, Pan J, Makino S, Sano M, Takahashi T, et al. Biphasic activation of the JAK/STAT pathway by angiotensin II in rat cardiomyocytes. Circ Res. 1998;82:244–50. doi: 10.1161/01.res.82.2.244. [DOI] [PubMed] [Google Scholar]
- [42].Bhat GJ, Baker KM. Angiotensin II stimulates rapid serine phosphorylation of transcription factor Stat3. Mol Cell Biochem. 1997;170:171–6. doi: 10.1023/a:1006865721939. [DOI] [PubMed] [Google Scholar]
- [43].Thomas WG. Regulation of angiotensin II type 1 (AT1) receptor function. Regul Pept. 1999;79:9–23. doi: 10.1016/s0167-0115(98)00140-2. [DOI] [PubMed] [Google Scholar]