Abstract
Objective: Brain–computer interfaces (BCIs) could potentially be used to interact with pathological brain signals to intervene and ameliorate their effects in disease states. Here, we provide proof-of-principle of this approach by using a BCI to interpret pathological brain activity in patients with advanced Parkinson disease (PD) and to use this feedback to control when therapeutic deep brain stimulation (DBS) is delivered. Our goal was to demonstrate that by personalizing and optimizing stimulation in real time, we could improve on both the efficacy and efficiency of conventional continuous DBS.
Methods: We tested BCI-controlled adaptive DBS (aDBS) of the subthalamic nucleus in 8 PD patients. Feedback was provided by processing of the local field potentials recorded directly from the stimulation electrodes. The results were compared to no stimulation, conventional continuous stimulation (cDBS), and random intermittent stimulation. Both unblinded and blinded clinical assessments of motor effect were performed using the Unified Parkinson's Disease Rating Scale.
Results: Motor scores improved by 66% (unblinded) and 50% (blinded) during aDBS, which were 29% (p = 0.03) and 27% (p = 0.005) better than cDBS, respectively. These improvements were achieved with a 56% reduction in stimulation time compared to cDBS, and a corresponding reduction in energy requirements (p < 0.001). aDBS was also more effective than no stimulation and random intermittent stimulation.
Interpretation BCI-controlled DBS is tractable and can be more efficient and efficacious than conventional continuous neuromodulation for PD. Ann Neurol 2013;74:449–457
Deep brain stimulation (DBS) is an established treatment for severe Parkinson disease (PD), dystonia, and tremor, and has an emerging role in a range of other neurological and neuropsychiatric conditions.1 However, its widespread adoption is at present limited by cost, side effects, and partial efficacy.2 In many brain disorders, for example PD, symptoms fluctuate on a moment-by-moment basis depending on factors such as cognitive and motor load and concurrent drug therapy. If it were feasible to track these fluctuations with a suitable feedback signal and stimulate only when necessary, it might be possible to improve therapeutic efficacy while preserving battery life and limiting side effects.3,4 A recent study in nonhuman primates suggested that adaptively controlled DBS triggered by feedback from the spikes of a single motor cortical neurone was even more effective than standard continuous high-frequency stimulation in a model of PD.6
In developing adaptive DBS (aDBS) for clinical use, two challenges must be overcome. First, the feedback signal must be robust over time. Second, neurosurgical intervention in the brain should be minimized so as to limit surgical risks, preferably only using a single surgical site. One possible solution to these issues is to record the local field potential (LFP) directly from the stimulating electrode and to use this as the feedback signal to control when stimulation is delivered. Increasing evidence suggests that beta frequency band (13–30Hz) oscillations in the LFP can be consistently picked up in the subthalamic nucleus (STN) of patients with PD and that their level correlates with motor impairment, with and without treatment.2 The LFP recorded in this way is robust over time, and developments in amplifier systems have enabled the recordings of beta oscillations in the LFP while simultaneously delivering high-frequency stimulation, despite the voltages used for the latter being around 1 million times greater than the LFP oscillations.7,8
Here we test whether a brain–computer interface (BCI) system that uses the beta activity in the LFP recorded directly from the stimulating electrode in the STN to control when stimulation is delivered can be more energy efficient than and clinically superior to current standard continuous DBS.
Patients and Methods
We recorded 8 patients (Table1) with advanced idiopathic PD with motor fluctuations and/or dyskinesias who gave their informed consent to take part in the study, which was approved by the National Research Ethics Service Committee South Central–Oxford A. Patients underwent DBS surgery on the STN as previously described.10 In Cases 2, 7, and 8 (see Table1), the locations of the electrodes were confirmed with immediate postoperative fast spin-echo T2-weighted magnetic resonance imaging (MRI) with a Leksell frame still in situ. In the remaining cases, locations were confirmed with immediate postoperative computed tomography (CT) with a Leksell or CRW frame (Integra Radionics, Burlington, MA) still in situ. CT scans were then fused with preoperative T2-weighted MRI.11 Electrode extension cables were externalized through the scalp to enable recordings prior to connection to a subcutaneous DBS pacemaker, implanted in a second operative procedure up to 7 days later. The permanent quadripolar macroelectrode used was model 3389 (Medtronic Neurologic Division, Minneapolis, MN), featuring 4 platinum–iridium cylindrical surfaces. Its contacts are numbered 0, 1, 2, and 3, with 0 being the most caudal and contact 3 being the most cranial.
Table 1.
Clinical Details
| Case | Age, yr | Disease Duration, yr | UPDRS Off | UPDRS On | Site | First Symptom | DBS Indication | Drugs (total daily dose) |
|---|---|---|---|---|---|---|---|---|
| 1 | 59 | 12 | 42 | 20 | Oxford | Right arm bradykinesia | On/off fluctuations, tremor bradykinesia | L-dopa 900mg, rasagiline 1mg |
| 2 | 62 | 10 | 20 | 8 | UCLH | Left arm bradykinesia/tremor | On/off fluctuations, tremor | L-dopa 1,000mg, trihexyphenidyl 3mg |
| 3 | 67 | 7 | 43 | 14 | Oxford | Right side rigidity/pain | On/off fluctuations, dyskinesias | L-dopa 1,000mg, ropinirole 10mg, amantadine 200mg |
| 4 | 49 | 10 | 42 | 6 | Oxford | Right arm tremor | Tremor | L-dopa 300mg, trihexyphenidyl 2mg |
| 5 | 49 | 10 | 58 | 23 | Kings | Right arm rigidity/pain | On/off fluctuations, tremor | L-dopa 1,100mg |
| 6 | 63 | 3 | 18 | 8 | Oxford | Tremor | Tremor/bradykinesia | L-dopa 800mg |
| 7 | 67 | 14 | 63 | 24 | UCLH | Shoulder pain/stiffness | On/off fluctuations | L-dopa 650mg, pergolide 9mg |
| 8 | 57 | 8 | 43 | 17 | UCLH | Stiffness/tremor | Severe off periods, on/off fluctuations | L-dopa 1,500mg, rotigotine 16mg, rasagiline 1mg, entacapone 1,000mg |
DBS = deep brain stimulation; UCLH = University College London Hospitals; UPDRS = United Parkinson's Disease Rating Scale.
We recorded bipolar LFP activity from contacts 0–2 and 1–3 of the electrodes in the STN after overnight withdrawal of antiparkinsonian medication between operations for electrode placement and pacemaker implantation. LFPs were band-pass filtered between 3 and 37Hz and amplified (×9,100) using a 3-stage common mode rejection amplifier. The system and its validation have previously been described in detail.12 Recordings from all the STNs exhibited beta activity in the LFP.
We determined which contact pair of 0–2 and 1–3 exhibited the greater beta (13–35Hz) amplitude on the side contralateral to the most affected upper limb from recordings made with the patient at rest, unstimulated and off medication. We then determined the frequency of the peak beta value in the frequency spectrum of the LFP from the selected bipole and filtered the signal around this as specified for each subject in Table2. Next, the voltage for test stimulation was determined using continuous DBS (cDBS) at 130Hz. The contact selected for stimulation was that which lay in between the bipolar contact selected above for recording (eg, contact 1 or contact 2).12 Stimulation was begun at 0.5V and increased by 0.5V increments every 3 to 4 minutes until clinical benefit was seen, without side effects such as paresthesia. This voltage was then fixed across the subsequent test conditions. Finally, prior to presentation of test blocks, the stimulation trigger threshold that achieved a reduction of stimulation time of approximately 50% while maintaining clinical effect was heuristically determined.
Table 2.
Stimulation Details
| Online Filter, Range (Hz) | Stim, V | Stim Site | Stim Contact | aDBS, % Time on Stim | Random, % Time on Stim | aDBS, Time between Stim Bursts, s | Random, Time between Stim Bursts, s | |
|---|---|---|---|---|---|---|---|---|
| Case 1 | 16–22 | 2.7 | L | 1 | 44.2 | 44.5 | 1.09 | 1.19 |
| Case 2 | 19–25 | 1.8 | R | 1 | 35.5 | 34.1 | 0.64 | 0.75 |
| Case 3 | 23–29 | 1.8 | R | 2 | 43.4 | 42.6 | 0.47 | 0.69 |
| Case 4 | 17–24 | 1.6 | L | 2 | 46.4 | 46.5 | 0.45 | 0.50 |
| Case 5 | 16–18 | 2.1 | L | 1 | 42.1 | 45.2 | 0.94 | 0.86 |
| Case 6 | 28–34 | 2.6 | R | 1 | 57.7 | 45.8 | 0.73 | 0.64 |
| Case 7 | 17–22 | 2.4 | R | 2 | 37.1 | 40.8 | 0.64 | 0.65 |
| Case 8 | 16–20 | 2.7 | R | 1 | 47.6 | 46.7 | 1.75 | 1.53 |
| Mean | 22 | 2.1 | 44.3 | 43.3 | 0.84 | 0.85 | ||
| SEM | 1.8 | 0.2 | 2.4 | 1.5 | 0.2 | 0.1 | ||
| p | 0.58 | 0.81 |
Two-tailed, paired t tests showed no difference between time on stimulation in aDBS and random stimulation modes or length of time between stimulation bursts. Stimulation voltage was the same for all stimulation conditions.
aDBS = adaptive deep brain stimulation; L = left; R = right; SEM = standard error of the mean; Stim = stimulation.
Filtered LFPs were rectified and smoothed using a moving average filter of 400-millisecond duration to produce an online value of beta amplitude. The latter was used to control triggering of stimulation via a user-defined threshold through a portable computer (Fig 1). The trigger output was passed via an optically isolated input to the stimulator. The delay between crossing of threshold to stimulation onset was 30 to 40 milliseconds. Stimulation was delivered by a custom-built battery-powered (±9V) stimulator specially constructed for this series of experiments. It used an embedded microprocessor and a digital-to-analog converter for stimulation control that delivered a biphasic charge balanced symmetrical pulse waveform. The stimulator was extensively tested in vitro, and its design was subject to external review (Dr S. Wang, Suzhou Institute of Biomedical Engineering and Technology, Chinese Academy of Sciences). Stimulation once triggered was sustained until beta amplitude fell below threshold again (Fig 2). Stimulation was delivered monopolarly, at 130Hz, with a pulse duration of 100 microseconds and ramped up and down over 250 milliseconds at onset and offset. The 250-millisecond ramping was necessary to avoid paresthesias induced during the switching on and off of stimulation (see Table2). The stimulator provided a continuous readout of the stimulation voltage, which could then be tracked throughout the experiment. For safety, charge densities were limited to <30μQ/cm2, and DC currents were blocked with a DC-blocking capacitor. The input–output voltage function was linear, and there was no pulse shape distortion when this was tested in vitro (0.5kΩ impedance). All connections to the patient were optically isolated, and the stimulator met the EN60601-1 medical safety standard. Estimates of time on stimulation in the aDBS mode included an additional 250 milliseconds per stimulation block to allow for the linear ramping up and down.
Figure 1.
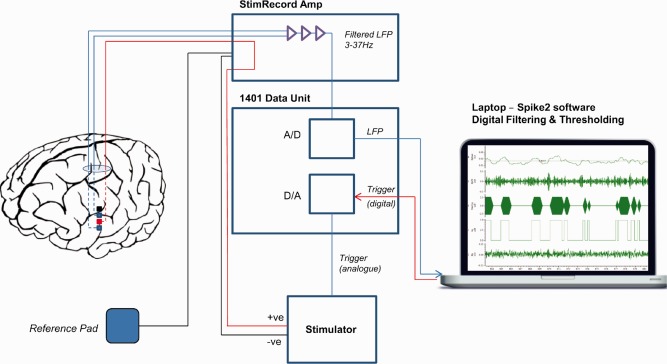
Experimental setup for adaptive deep brain stimulation in externalized subjects. Bipolar local field potential (LFP) is passed through a custom built StimRecord amplifier that filters (3–37Hz) and amplifies (×9,100). The analogue (A) output is passed to a 1401 data acquisition unit (Cambridge Electronic Design, Cambridge, UK), which converts it to a digital (D) signal that is displayed on a portable computer using Spike2 software (Cambridge Electronic Design, Cambridge, UK). The signal is digitally filtered around the beta peak in real time and converted to beta amplitude by rectifying and smoothing. A threshold is set that triggers stimulation in a monopolar montage between the 2 bipolar recording electrodes when beta power crosses the threshold. Stimulation terminates when beta power drops again below threshold. [Color figure can be viewed in the online issue, which is available at www.annalsofneurology.org.]
Figure 2.
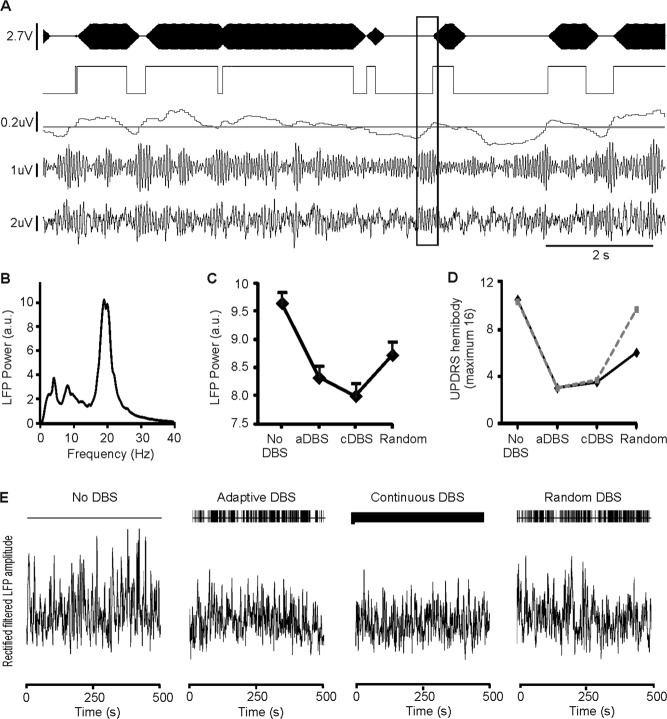
Results in Case 1. (A) Sample section of adaptive deep brain stimulation (aDBS) recordings. Channels are from bottom to top: analogue filtered local field potential (LFP; bipolar contacts 0 and 2), LFP digitally filtered about the beta peak, running average of rectified, beta filtered, amplitude with triggering threshold superimposed, stimulation trigger signal and stimulation (130Hz, 100μS, monopolar contact 1). Boxed area shows a beta burst. Time for this to cross threshold depends on LFP amplitude at onset of beta burst, but thereafter there was only a 30- to 40-millisecond delay to stimulation onset. Note stimulation is ramped, and can be triggered by bursts of beta that are of variable duration, but often last <1 second (see also Table2). (B) LFP power spectrum without DBS. (C) LFP power changes in different stimulation modes. (D) Motor impairment in different stimulation modes. Solid black and interrupted gray lines are unblinded clinical and blinded video scores, respectively. (E) Sections (500 seconds) of rectified beta-filtered LFP amplitude in each stimulation mode. a.u. = arbitrary units; cDBS = continuous DBS; UPDRS = United Parkinson's Disease Rating Scale.
Patients were clinically tested off stimulation and with conventional cDBS, aDBS, and random stimulation (random bursts of stimulation not triggered by beta amplitude rise). Experimental conditions were randomized in order across patients, and each condition was performed once. About 5 minutes rest without stimulation was given before each experimental condition. The mean amplitude threshold for triggering stimulation in this mode was 3.9 ± 3.8% above the mean beta amplitude of the LFP and corresponded to a peak-to-peak amplitude of the beta filtered signal of 2.6 ± 0.6μV. Mean ± standard error of the mean of duration of rest, aDBS, cDBS, and random blocks was 629 ± 102 seconds, 640 ± 143 seconds, 507 ± 37 seconds, and 515 ± 32 seconds, respectively. Subjects were clinically assessed at least 300 seconds into each condition. Temperli et al have shown that the change in tremor, rigidity, and bradykinesia following the onset or offset of STN DBS follows an approximately exponential course, with, judging from their published figure, about 50% of the total change achieved after 5 minutes (300 seconds),13 and others have made similar observations.14–15 For clinical assessment, we used a subsection of the hemibody motor United Parkinson's Disease Rating Scale (UPDRS; items 20, 22, and 23) for the upper limb contralateral to the side of stimulation. These assessments were also recorded with a digital video recorder and blindly rated by 3 experienced movement disorder specialists who were not part of the clinical or research team, with an inter-rater reliability of 0.52.16 Although patients were implanted bilaterally, we only evaluated stimulation contralateral to the worse affected hemibody, so as to limit patient fatigue and due to time constraints in the perioperative period. Total electrical energy delivered per unit of time was calculated assuming an impedance of 0.5kΩ.17–18 Physiological data were analyzed in MATLAB (version 7.10; MathWorks, Natick, MA) using custom-written scripts and wavelet convolution. Statistical analyses were performed in SPSS Statistics 19 (SPSS, Chicago, IL). Clinical data were normally distributed (single-sample Kolmogorov–Smirnov tests p > 0.05), and therefore means, standard error of the means, and parametric statistical analyses are presented. Where Mauchly's test of sphericity was significant (p < 0.05) in repeated measures analyses of variance (ANOVAs), Greenhouse–Geisser corrections were applied (and degrees of freedom adjusted accordingly).
Results
Clinical Effect
Mean baseline (without stimulation or medication) UPDRS hemibody subscores (bradykinesia, rigidity, tremor) were 5.8 ± 0.8 and 6.3 ± 0.7 in unblinded and blinded postoperative clinical assessments. Repeated measures ANOVA demonstrated a significant main effect of stimulation condition within subjects in both unblinded and blinded assessments (Fdf 1.7,11.9 = 13.7, p = 0.001 and Fdf 3,21 = 10.5, p < 0.001, respectively).
Both aDBS and cDBS improved motor scores (Fig 3). The mean reduction in unblinded and blinded UPDRS scores relative to the unstimulated state was 66.2% (unblinded assessment) and 49.7% (blinded assessment) for aDBS. However, the improvement with cDBS was significantly less at 54.3% (paired t test, 2-way, t7 = 2.78, p = 0.028) and 30.5% (t7 = 3.7, p = 0.007) in unblinded and blinded assessments, respectively. The average improvement in motor scores in the aDBS condition compared to the cDBS condition was 28.7 ± 10.6% (p = 0.03) and 27.0 ± 7.8% (p = 0.005) in unblinded and blinded assessments, respectively. This effect was maintained if rigidity scores were excluded from the blinded assessments, with a 22 ± 10.6% (p = 0.04) improvement in motor scores with aDBS compared to cDBS.
Figure 3.
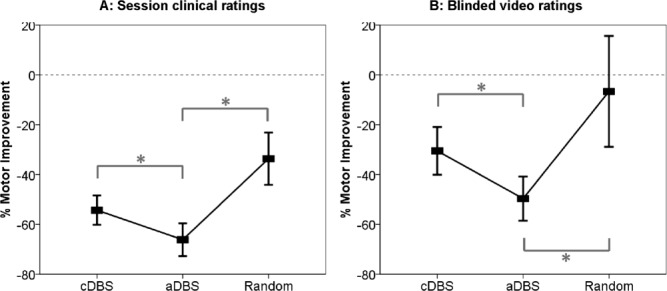
Clinical improvements. Mean ± standard error of the mean percentage change in hemibody United Parkinson's Disease Rating Scale scores (items 20, 22, and 23) with different stimulation conditions as assessed unblinded during the experimental sessions (A) or from video recordings by blinded experts (B). Asterisks denote significant differences following correction for multiple comparisons by the false discovery rate procedure. All changes were significant from the unstimulated state, with the exception of the blinded score for random stimulation. aDBS = adaptive deep brain stimulation; cDBS = continuous deep brain stimulation.
Random intermittent stimulation lead to a motor score reduction of 33.7% and 6.7% in unblinded and blinded assessments, which was significantly less than the improvement found with aDBS (paired t test, 2-way, unblinded t7 = 4.2, p = 0.004; blinded t7 = 2.17, p = 0.03).
Power Savings and Adaptive Effect
The mean total electrical energy delivered with aDBS (132 ± 21μW) was significantly less than that with cDBS (270 ± 37μW; t7 = 7.4, p < 0.0001), so that the better improvements in clinical score were achieved with less than half the total electrical energy delivered. Similarly, when averaged over the whole block of stimulation, aDBS was only on 44.2 ± 2.4% of the time. This was well matched by random DBS, which was on 43.3% ± 1.5% of the time (paired t test, 2-way t7 = 0.57, p = 0.59; see Table2). Moreover, time on stimulation tended to progressively drop during stimulation in the aDBS mode. This was despite the use of a fixed beta threshold, suggesting that beta bursts became less frequent over the course of aDBS. The mean correlation coefficient between percentage stimulation time over each 10 seconds and total duration of DBS up to that point was −0.23 across subjects (2-tailed, 1-sample t test, t7 = 3.2, p = 0.01). This correlation was also individually significant in 3 of the 8 cases (Pearson test, p < 0.01; Fig 4).
Figure 4.
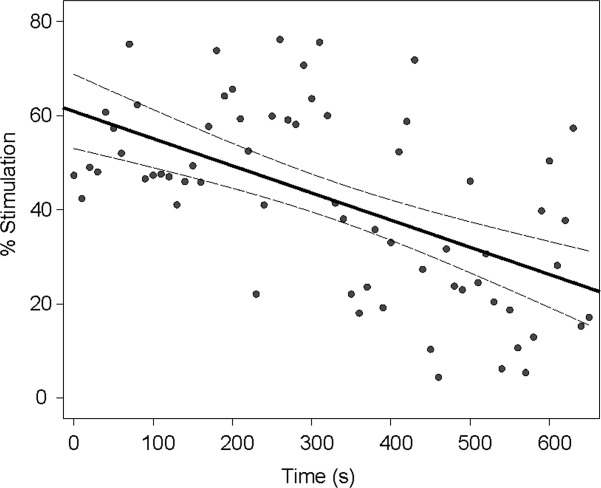
Decline in triggered stimulation duration over time. Dependency of proportion of time stimulated (% per 10-second block) on duration of adaptive deep brain stimulation is shown for Subject 5. Solid and interrupted lines are result of linear regression and 95% confidence intervals, respectively. r = −0.567, p < 0.001.
Beta Suppression
The suppression of beta peak amplitude averaged across the duration of a given block type and then averaged across subjects correlated with the mean clinical improvement in those blocks (Pearson test, R2 = 0.99, p < 0.001, and R2 = 0.85, p = 0.08 for blinded and unblinded clinical assessments, respectively).
Discussion
In this proof-of-principle study, we demonstrate successful BCI-controlled deep brain stimulation in patients with PD. We show that this approach can be about 30% more effective than conventional high-frequency stimulation, despite delivering <50% of the stimulation of current DBS. The latter is of critical importance, as it impacts on both side effects and the lifetime of implanted battery systems.
The scale of our treatment effects is particularly encouraging, as we only tested unilateral stimulation. However, it should be noted that we evaluated only selected motor UPDRS items over stimulation blocks of about 10 minutes. These methodological features stemmed from time constraints related to fatigue in postoperative patients and the nonambulatory nature of our BCI-controlled control system. Nevertheless, aDBS had beneficial effects on all 3 of the cardinal signs of Parkinsonism: rest tremor, bradykinesia, and rigidity. Moreover, the scale of (contralateral) improvement with cDBS was similar to that previously reported, despite the above limitations related to postoperative assessment. Most studies of the efficacy of chronic continuous bilateral DBS use unblinded clinical assessments off medication and rate improvement as between 28 and 71%, which compares favorably with the 54% improvement seen here with continuous stimulation.19 Relatively few trials have assessed the acute benefit of continuous DBS in the chronically implanted system through the blinded assessment of videos. These report lower treatment effects ranging from between 25.3 and 30%,12–13 similar to the 30.5% reduction shown with cDBS determined through blinded assessment in the current study, although less than the 49.7% reduction found with aDBS.20–21
The random stimulation mode, in which random intermittent DBS was delivered for similar periods of time as aDBS, served to exclude the possibility that intermittency itself is the key determinate of the efficacy observed with aDBS. This might arise, for example, if the effect of continuous high-frequency stimulation was subject to accommodation. However, the random mode was less than half as efficient as aDBS, implying that intermittent stimulation has to be triggered by beta bursts to have its optimal effect.
Improvements in motor deficits were mirrored by proportionate changes in beta power across the different stimulation conditions. The efficacy of triggering DBS off beta bursts (over and above that of random intermittent stimulation), together with the correlation between treatment effects and beta power reduction, provides further evidence that the oscillatory synchronization indexed by beta activity in the STN LFP is at the very least a faithful biomarker of Parkinsonian impairment, if not causally important.22 An additional interesting observation that needs to be substantiated in further trials is that the proportion of time involving stimulation in the aDBS mode tended to progressively fall as this mode was maintained. Given the constant trigger threshold, this result suggests that aDBS may lead to positive adaptive effects whereby pathological Parkinsonian networks become less prone to produce phasic increases in beta activity. Such an antikindling effect has also been noted in computational studies of desynchronizing brain stimulation, including DBS.23 The presence of adaptive effects further raises the possibility that clinical improvement with aDBS may be even greater when this intervention is sustained for periods longer than those utilized in our study.
Improvements in clinical efficacy were achieved despite significant reductions in power usage, with an overall reduction in stimulation time in the aDBS condition of 56%. This equated to a mean decrease in total electrical energy delivered of 132μW, and energy saving would have been even more marked had we employed higher-stimulation voltages, as energy is proportional to voltage squared. Against this should, however, be offset the additional power requirements of the low-energy circuits necessary to deliver feedback-controlled stimulation and ramping in a clinical system. For a single-channel power classifier, this should be no more than about 10μW, leaving the aDBS mode overall power savings sufficient to double the battery life of the implantable pulse generator in addition to the improvements in clinical efficacy shown.24 Conventional cDBS is associated with an average battery life of <4 years, with replacement usually necessitating general anesthesia and incurring substantial hardware costs.25 Therefore, a halving of power consumption would reduce the number of replacement battery operations required, limiting surgical risk and significantly reducing costs of overall treatment. Alternatively, the interval between recharging would double if rechargeable implantable pulse generators are used instead, although these are not suitable for all patients with PD.26
The delivery of substantially less stimulation energy with aDBS has an additional implication, not evaluated in the current study. DBS-related side effects critically depend on stimulation parameters, including the energy delivered. Thus, aDBS may be associated with fewer side effects than conventional stimulation, although this remains to be confirmed. In particular, periods of nearly normal functioning will not be compromised by DBS when the delivery of this is controlled by a BCI.27 Such periods of normal functioning include those induced by antiparkinsonian medication, which when effective is associated with suppression of beta activity in the STN.28–33
We have demonstrated that it is possible to track an LFP biomarker from the site of stimulation and use this to successfully control stimulation in patients with a continuously fluctuating neurological condition such as PD. Power savings were substantial, and efficacy was found to be superior to standard stimulation. Although not directly tested in this study, it is hoped that the reduced time on stimulation will result in side effects being proportionately reduced once chronic aDBS is possible. Our approach does not complicate the surgical procedure, which still involves implantation of a single brain target. This and other observations suggest chronic aDBS is feasible; beta activity in the LFP is robust over time,7–8 and reported in a mean of 95% of STN at rest in the off-medication state by 5 groups,2 and of 60% of STN by 1 group.8–35 Implantable DBS systems with the capability to deliver feedback-controlled stimulation are already in technical development.24 Thus, our acute study should in time be replicable in a chronic setting.
Several aDBS parameters, including the level of smoothing of beta activity to be applied and the target level of on-stimulation time, remain to be optimized, so that the gains in terms of efficacy and efficiency seen here may be further improved in time. Our threshold crossing of beta amplitude as the stimulation trigger affords a good starting point for algorithmic control, although this may be further improved upon in the future with more sophisticated classifiers based on the use of multiple LFP features to track clinical state. Equally, our intervention, discontinuous but regular stimulation at high frequency, may be bettered in time by stimulation regimes that specifically target pathological rhythms through phase cancellation or disruption.36–37 Nevertheless, our simple and tractable system was able to outperform standard continuous stimulation in efficacy and power consumption, potentially offering a major advance in electrical neuromodulation therapy for PD. The same approach may also prove beneficial in other fluctuating movement and neuropsychiatric disorders.
Acknowledgments
This study was funded by a clinical research training grant (SL - 093929/Z/10/Z) from the Wellcome Trust, in addition to support from the Medical Research Council, National Institute for Health Research Oxford Biomedical Research Centre, and Rosetrees Trust.
We thank A. Batla, T. Saifee, and P. Kassavetis for their assistance with the blinded video ratings.
Potential Conflicts of Interest
L.Z., M.H.: consultancy, travel support, speaking fees, Medtronic, St Jude. T.F.: consultancy, AbbVie Pharmaceuticals, St Jude, and Medtronic. P.L.: consultancy, speaking fees, Medtronic, St Jude. K.A.: grants/grants pending, Medtronic, St Jude; speaking fees, Medtronic. A.L.G.: patents, method and apparatus for treating respiratory disease. T.Z.A.: consultancy, grants/grants pending, speaking fees, Medtronic. P.B.: consultancy, Medtronic, Sapiens; travel expenses, Movement Disorders congress.
References
- Hariz M. Twenty-five years of deep brain stimulation: celebrations and apprehensions. Mov Disord. 2012;27:930–933. doi: 10.1002/mds.25007. [DOI] [PubMed] [Google Scholar]
- Little S, Brown P. What brain signals are suitable for feedback control of deep brain stimulation in Parkinson's disease? Ann N Y Acad Sci. 2012;1265:9–24. doi: 10.1111/j.1749-6632.2012.06650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modolo J, Beuter A, Thomas AW, Legros A. Using “smart stimulators” to treat Parkinson's disease: re-engineering neurostimulation devices. Front Comput Neurosci. 2012;6:69. doi: 10.3389/fncom.2012.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovych OV, Tass PA. Desynchronizing electrical and sensory coordinated reset neuromodulation. Front Hum Neurosci. 2012;6:58. doi: 10.3389/fnhum.2012.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priori A, Foffani G, Rossi L, Marceglia S. Adaptive deep brain stimulation (aDBS) controlled by local field potential oscillations. Exp Neurol. 2013;245:77–86. doi: 10.1016/j.expneurol.2012.09.013. [DOI] [PubMed] [Google Scholar]
- Rosin B, Slovik M, Mitelman R, et al. Closed-loop deep brain stimulation is superior in ameliorating parkinsonism. Neuron. 2011;72:370–384. doi: 10.1016/j.neuron.2011.08.023. [DOI] [PubMed] [Google Scholar]
- Abosch A, Lanctin D, Onaran I, et al. Long-term recordings of local field potentials from implanted deep brain stimulation electrodes. Neurosurgery. 2012;71:804–814. doi: 10.1227/NEU.0b013e3182676b91. [DOI] [PubMed] [Google Scholar]
- Giannicola G, Rosa M, Servello D, et al. Subthalamic local field potentials after seven-year deep brain stimulation in Parkinson's disease. Exp Neurol. 2012;237:312–317. doi: 10.1016/j.expneurol.2012.06.012. [DOI] [PubMed] [Google Scholar]
- Eusebio A, Cagnan H, Brown P. Does suppression of oscillatory synchronisation mediate some of the therapeutic effects of DBS in patients with Parkinson's disease? Front Integr Neurosci. 2012;6:47. doi: 10.3389/fnint.2012.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foltynie T, Hariz MI. Surgical management of Parkinson's disease. Expert Rev Neurother. 2010;10:903–914. doi: 10.1586/ern.10.68. [DOI] [PubMed] [Google Scholar]
- O'Gorman RL, Jarosz JM, Samuel M, et al. CT/MR image fusion in the postoperative assessment of electrodes implanted for deep brain stimulation. Stereotact Funct Neurosurg. 2009;87:205–210. doi: 10.1159/000225973. [DOI] [PubMed] [Google Scholar]
- Eusebio A, Thevathasan W, Doyle Gaynor L, et al. Deep brain stimulation can suppress pathological synchronization in parkinsonian patients. J Neurol Neurosurg Psychiatry. 2011;82:569–573. doi: 10.1136/jnnp.2010.217489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temperli P, Ghika J, Villemure JG, et al. How do parkinsonian signs return after discontinuation of subthalamic DBS? Neurology. 2003;60:78–81. doi: 10.1212/wnl.60.1.78. [DOI] [PubMed] [Google Scholar]
- Lopiano L, Torre E, Benedetti F, et al. Temporal changes in movement time during the switch of the stimulators in Parkinson's disease patients treated by subthalamic nucleus stimulation. Eur Neurol. 2003;50:94–99. doi: 10.1159/000072506. [DOI] [PubMed] [Google Scholar]
- Blahak C, Bäzner H, Capelle HH, et al. Rapid response of parkinsonian tremor to STN-DBS changes: direct modulation of oscillatory basal ganglia activity? Mov Disord. 2009;24:1221–1225. doi: 10.1002/mds.22536. [DOI] [PubMed] [Google Scholar]
- Ebel RL. Estimation of the reliability of ratings. Psychometrika. 1951;16:407–424. [Google Scholar]
- Blahak C, Capelle HH, Baezner H, et al. Battery lifetime in pallidal deep brain stimulation for dystonia. Eur J Neurol. 2011;18:872–875. doi: 10.1111/j.1468-1331.2010.03290.x. [DOI] [PubMed] [Google Scholar]
- Volkmann J, Herzog J, Kopper F, Deuschl G. Introduction to the programming of deep brain stimulators. Mov Disord. 2002;17:S181–S187. doi: 10.1002/mds.10162. [DOI] [PubMed] [Google Scholar]
- Benabid AL, Chabardes S, Mitrofanis J, Pollak P. Deep brain stimulation of the subthalamic nucleus for the treatment of Parkinson's disease. Lancet Neurol. 2009;8:67–81. doi: 10.1016/S1474-4422(08)70291-6. [DOI] [PubMed] [Google Scholar]
- Ford B, Winfield L, Pullman S, et al. Subthalamic nucleus stimulation in advanced Parkinson's disease: blinded assessments at one year follow up. J Neurol Neurosurg Psychiatry. 2004;75:1255–1259. doi: 10.1136/jnnp.2003.027557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrioto A, Lozano AM, Poon Y, et al. Ten-year outcome of subthalamic stimulation in Parkinson disease: a blinded evaluation. Arch Neurol. 2011;68:1550–1556. doi: 10.1001/archneurol.2011.182. [DOI] [PubMed] [Google Scholar]
- Jenkinson N, Brown P. New insights into the relationship between dopamine, beta oscillations and motor function. Trends Neurosci. 2011;34:611–618. doi: 10.1016/j.tins.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Hauptmann C, Tass PA. Therapeutic rewiring by means of desynchronizing brain stimulation. Biosystems. 2007;89:173–181. doi: 10.1016/j.biosystems.2006.04.015. [DOI] [PubMed] [Google Scholar]
- Afshar P, Khambhati A, Stanslaski S, et al. A translational platform for prototyping closed-loop neuromodulation systems. Front Neural Circuits. 2013;6:117. doi: 10.3389/fncir.2012.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bin-Mahfoodh M, Hamani C, Sime E, Lozano AM. Longevity of batteries in internal pulse generators used for deep brain stimulation. Stereotact Funct Neurosurg. 2003;80:56–60. doi: 10.1159/000075161. [DOI] [PubMed] [Google Scholar]
- Timmermann L, Schüpbach M, Hertel F, et al. A new rechargeable device for deep brain stimulation: a prospective patient satisfaction survey. Eur Neurol. 2013;69:193–199. doi: 10.1159/000342236. [DOI] [PubMed] [Google Scholar]
- Chen CC, Brücke C, Kempf F, et al. Deep brain stimulation of the subthalamic nucleus: a two-edged sword. Curr Biol. 2006;16:952–953. doi: 10.1016/j.cub.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Brown P, Oliviero A, Mazzone P, et al. Dopamine dependency of oscillations between subthalamic nucleus and pallidum in Parkinson's disease. J Neurosci. 2001;21:1033–1038. doi: 10.1523/JNEUROSCI.21-03-01033.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priori A, Foffani G, Pesenti A, et al. Rhythm-specific pharmacological modulation of subthalamic activity in Parkinson's disease. Exp Neurol. 2004;189:369–379. doi: 10.1016/j.expneurol.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Alonso-Frech F, Zamarbide I, Alegre M, et al. Slow oscillatory activity and levodopa-induced dyskinesias in Parkinson's disease. Brain. 2006;129:1748–1757. doi: 10.1093/brain/awl103. [DOI] [PubMed] [Google Scholar]
- Kühn AA, Kupsch A, Schneider G, Brown P. Reduction in subthalamic 8-35 Hz oscillatory activity correlates with clinical improvement in Parkinson's disease. Eur J Neurosci. 2006;23:1956–1960. doi: 10.1111/j.1460-9568.2006.04717.x. [DOI] [PubMed] [Google Scholar]
- Kühn AA, Tsui A, Aziz T, et al. Pathological synchronisation in the subthalamic nucleus of patients with Parkinson's disease relates to both bradykinesia and rigidity. Exp Neurol. 2009;215:380–387. doi: 10.1016/j.expneurol.2008.11.008. [DOI] [PubMed] [Google Scholar]
- Ray NJ, Jenkinson N, Wang S, et al. Local field potential beta activity in the subthalamic nucleus of patients with Parkinson's disease is associated with improvements in bradykinesia after dopamine and deep brain stimulation. Exp Neurol. 2008;213:108–113. doi: 10.1016/j.expneurol.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Giannicola G, Marceglia S, Rossi L, et al. The effects of levodopa and ongoing deep brain stimulation on subthalamic beta oscillations in Parkinson's disease. Exp Neurol. 2010;226:120–127. doi: 10.1016/j.expneurol.2010.08.011. [DOI] [PubMed] [Google Scholar]
- Rosa M, Giannicola G, Servello D, et al. Subthalamic local field beta oscillations during ongoing deep brain stimulation in Parkinson's disease in hyperacute and chronic phases. Neurosignals. 2011;19:151–162. doi: 10.1159/000328508. [DOI] [PubMed] [Google Scholar]
- Brittain JS, Probert-Smith P, Aziz TZ, Brown P. Tremor suppression by rhythmic transcranial current stimulation. Curr Biol. 2013;23:436–440. doi: 10.1016/j.cub.2013.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tass PA, Qin L, Hauptmann C, et al. Coordinated reset has sustained aftereffects in Parkinsonian monkeys. Ann Neurol. 2012;72:816–820. doi: 10.1002/ana.23663. [DOI] [PubMed] [Google Scholar]