Abstract
BACKGROUND
Bevacizumab (Bev) has gained acceptance as an active agent in the treatment of epithelial ovarian cancer (EOC). Data is lacking on survival outcomes in patients who are re-treated with Bev after achieving a complete response to a Bev-containing regimen (BCR). Our primary objective was to compare the progression free survival (PFS) and overall survival (OS) in patients who received Bev after Bev (BAB) versus those who were not re-treated with Bev (NOTBev) after initially experiencing a complete response (CR) to a BCR.
METHODS
This was a retrospective chart review conducted at a single institution. Patients that received Bev in either the front-line or recurrent setting were included. Response was graded according to RECIST criteria or Ca125. PFS was defined as the time from initiation of treatment until progression or date of last contact. OS was defined as the time from initiation of treatment until death or date of last contact.
RESULTS
There were a total of 36 patients who had a CR to a BCR. Of those, 17 received Bev at the time of their subsequent recurrence versus 19 that did not. The rate of primary platinum resistance was similar in both groups (BAB: 35% vs. 21%). More patients in the NOTBev group received Bev as primary therapy (21% vs. 6%, p=0.2), but this was not statistically significant. The median number of regimens prior to the first Bev regimen was 1 (range 0–4) in the NOTBev group versus 2.5 (range 0–6) in the BAB group (p=0.09). Patients in the BAB group had significantly higher mean PFS compared to the NOTBev group (20 vs. 6 months, p=0.0019). On adjusting for covariates, there was a 78% improvement in their PFS (HR 0.22, p=0.0048). No difference in overall survival was noted between the groups (23 vs. 26 months, p=0.7244). The objective response rate (ORR) defined as CR, PR, or SD, was significantly higher in patients that were retreated with bevacizumab, 88% in the BAB group and 50% in the NOTBev group (p = 0.0120).
CONCLUSIONS
Re-treatment with Bev after a prior Bev response is associated with a significantly improved PFS. This is the first of such reports in this patient population. The 14-month improvement in PFS strongly supports the re-use of Bev in patients who demonstrate an initial response to Bev. This strategy should be formally tested in future clinical trials and further investigation should include evaluation of predictors of response to Bev therapy.
INTRODUCTION
Bevacizumab is a humanized VEGF monoclonal antibody that has gained popularity as a targeted biologic agent in the treatment of many cancers including EOC. Recently-published randomized trials have found significant improvement in progression free survival when bevacizumab is used in combination with cytotoxic chemotherapy in the adjuvant treatment of newly diagnosed ovarian cancer. Specifically, the randomized phase III study performed by the Gynecologic Oncology Group (GOG 218) showed that women had a significantly improved PFS (14.1 vs. 10.3 months) when bevacizumab was given with chemotherapy and continued as a single agent in the maintenance phase.1 Similar results were obtained in the Gynaecologic Cancer Intergroup’s trial, ICON7.2 However, neither trial thus far has been able to demonstrate that treatment with bevacizumab translates into a survival advantage. Like many studies, these two have raised many questions about bevacizumab’s role in the treatment of epithelial ovarian cancer.
There is limited data evaluating the use of bevacizumab in the treatment of recurrent epithelial ovarian cancer. A phase II study of single-agent bevacizumab was performed by the GOG (170D) in women with recurrent ovarian, fallopian tube, or primary peritoneal cancer after 1–2 previous cytotoxic chemotherapy regimens. Burger et al., on behalf of the GOG, reported response rates of 21% with median PFS of 4.7 months and median OS of 17 months. Of note, 40% of their patients had PFS that was greater than 6 months. The authors reported that PFS and OS were not associated with prior platinum sensitivity or the number of previous chemotherapy regimens.3 The OCEANS trial reported that the addition of bevacizumab to carboplatinum and gemcitabine followed by maintenance bevacizumab was associated with significantly improved response rate (79 vs. 57%) and PFS (12 vs. 8 months) in patients with recurrent platinum-sensitive ovarian cancer.4
We now have a new population of patients that have been treated with bevacizumab in the front-line setting and an increasing number of patients who have been treated with bevacizumab in general. Thus we are faced with the question of how to treat these patients when they recur. Should patients initially treated with bevacizumab be re-treated at the time of recurrence? The goal of this study was to examine outcomes of women with recurrent EOC who experienced an initial CR to a BCR. Specifically, we aimed to compare PFS and OS between women who were re-treated with bevacizumab at recurrence and those who were not.
METHODS
This was a retrospective chart review conducted at a single institution. Patients with primary or recurrent (any recurrence after completion of adjuvant therapy) epithelial ovarian, fallopian tube, or primary peritoneal cancer whose best response was a complete response (CR) to bevacizumab in combination with cytotoxic chemotherapy from April 2005 through September 2010 were included. Patients who received single-agent bevacizumab as maintenance therapy after combination with cytotoxic chemotherapy were initially excluded because the aim of the study was to evaluate bevacizumab in combination with cytotoxic chemotherapy. Subsequent analysis was performed including patients who received maintenance bevacizumab to evaluate if there was a survival difference. A minimum of two cycles of chemotherapy was required for inclusion.
Response was graded according to RECIST or modified Rustin criteria.5, 6 Disappearance of all target lesions or normalization of previously elevated Ca125, defined as twice the upper limit of normal, was classified as a complete response. Patients that had both measurable disease and an elevated Ca125 were required to have disappearance of all target lesions and normalization of Ca125 to be categorized as a CR. Partial response (PR) was defined as a 30% decrease in the sum of the longest diameter of target lesions or >50% decrease of previously elevated Ca125. The classification of progressive disease (PD) included a 20% increase in the sum of the longest diameter of target lesions or doubling of Ca125. Small changes that did not meet any of the previous criteria were identified as stable disease (SD). Patients were evaluated with a Ca125 before each cycle of chemotherapy. On average, imaging studies for were obtained every 3 cycles for patients enrolled on a study or as otherwise indicated. Progression free survival was defined as the time from initiation of the regimen subsequent to the BCR until progression or death. Patients who had not progressed were censored at their date of last contact. Overall survival was defined as the time from initiation of treatment until death or date of last contact.
This study was approved by and conducted according to standards set forth by the Institutional Review Board at the Ohio State University Wexner Medical Center.
RESULTS
There were 36 patients identified who met inclusion criteria by having a CR to a BCR in either the up-front or recurrent setting. Of those, 17 received bevacizumab at the time of their subsequent recurrence (BAB) and 19 did not (NOTBev). The two groups had similar baseline characteristics (Table 1). Specifically, the median age at diagnosis in both groups was 58. Optimal debulking at primary surgery was achieved in 88% of women in the BAB group and 84% in the NOTBev group (p = 0.7624). In addition, 65% of women in the BAB and 79% of women in the NOTBev group were classified as primary platinum sensitive (p = 0.3403) with a median platinum free interval (PFI) of 11 and 10 months, respectively. With the exception of one patient in the BAB group who was stage II at diagnosis, all of the women in the study were either stage III or IV. The majority of women in both groups had tumors with serous histology (88% in the BAB group and 95% in the NOTBev group, p = 0.4787); the remaining cases were classified as adenocarcinoma not otherwise specified. While not statistically significant, more patients in the NOTBev group were initially treated with bevacizumab as first-line therapy compared to women in the BAB group (21 vs. 6%, p = 0.0637). Furthermore, the BAB was more heavily pre-treated with a median number of prior cytotoxic chemotherapy regimens of 2.5 compared to 1 in the NOTBev group (p = 0.09). There was no difference in the treatment-free interval after initial BCR with the median number of months in the BAB group of 7.5 months (Bev-free interval) and 7.1 months in the NOTBev group (p = 0.8784). Tables 2a and 2b list the chemotherapy regimens that were used in the BAB and NOTBev groups respectively subsequent to their CR to a BCR.
Table 1.
Patient Characteristics.
| Bevacizumab (n=17) | No Bevacizumab (n=19) | P-value | |
|---|---|---|---|
|
| |||
| Age (median) | 58 | 58 | |
| Race | p = .1427 | ||
| White | 17 (100%) | 18 (94.74%) | |
| AA | 1 (5.26%) | ||
|
| |||
| Primary Debulking | p = .7624 | ||
| Surgery | |||
| Optimal | 15 (88.24%) | 16 (84.21%) | |
| Suboptimal | 2 (11.76%) | 3 (15.79%) | |
|
| |||
| Stage | p = .2157 | ||
| I/II | 1 (5.88%) | 0 | |
| III/IV | 16 (94.12%) | 19 (100%) | |
|
| |||
| Histology | p = .4787 | ||
| Serous | 15 (88.24%) | 18 (94.74%) | |
| Other | 2 (11.76%) | 1 (5.26%) | |
|
| |||
| Primary Platinum Sensitive | p = .3403 | ||
| Yes | 11 (64.71%) | 15 (78.95%) | |
| No | 6 (35.29%) | 5 (21.05%) | |
|
| |||
| Bev first line | p = .0637 | ||
| Yes | 1 (5.88%) | 4 (21.05%) | |
| No | 16 (94.12%) | 15 (78.95) | |
|
| |||
| Median # regimens before bev (range) | 2.5 (0–6) | 1 (0–4) | p = .09 |
| Median # months between first bev regimen and subsequent regimen (range) | 7.53 (.4–32.2) | 7.13 (.2–21.6) | p = .8784 |
|
| |||
| Median Ca125 prior to treatment with bev | 94 | 90.5 | |
Table 2a.
Chemotherapy regimens subsequent to BCR.
| Chemotherapy regimens in NOTBev group | N |
|---|---|
| Carboplatinum + Paclitaxel | 1 |
| Gemcitabine + Platinum | 4 |
| Carboplatinum | 1 |
| Doxil | 4 |
| Topotecan | 2 |
| Reolysin + Paclitaxel | 1 |
| ABT-888 + TMZ | 6 |
Table 2b.
| Chemotherapy regimens in BAB group | N |
|---|---|
| Gemcitabine + platinum + bevacizumab | 6 |
| Paclitaxel + platinum + bevacizumab | 2 |
| Weekly Paclitaxel + bevacizumab | 4 |
| Topotecan + bevacizumab | 4 |
| Gemcitabine + bevacizumab | 1 |
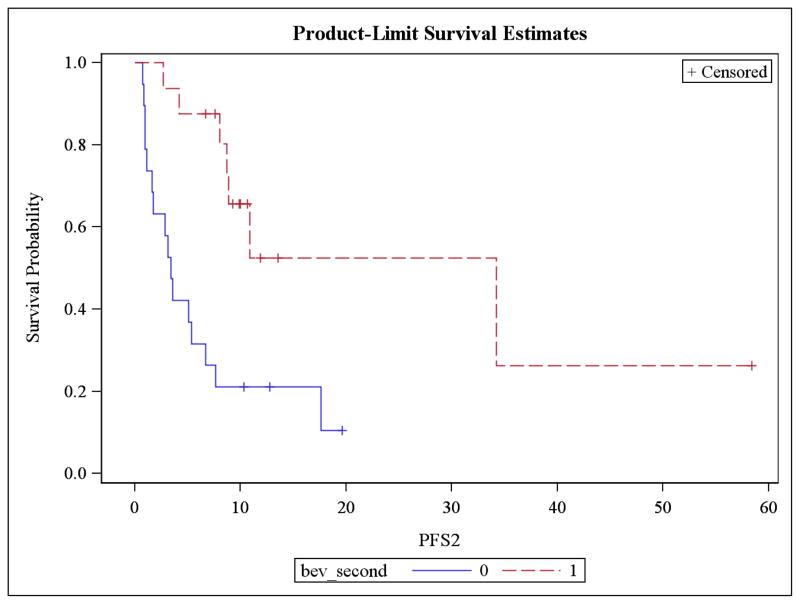
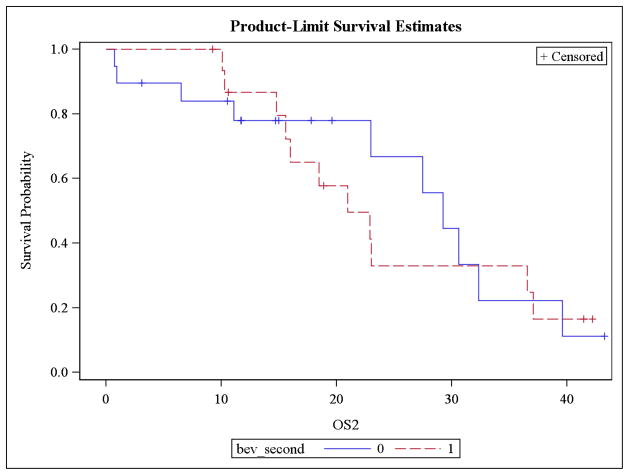
Patients who were re-treated with bevacizumab after experiencing a complete response to a BCR had a significantly longer mean PFS compared to patients in the NOTBev group (20.3 vs. 6.1 months, p = 0.0019; Figure 1). This is equivalent to an 87.3% improvement in PFS (HR 0.127, p = 0.0012) in patients that were re-treated with bevacizumab at the time of recurrence. Median PFS was 10.9 and 3.4, respectively. There was no difference in mean overall survival between the groups; 23.1 vs. 25.8 months in the BAB and NOTBev groups, respectively (p = 0.7244; Figure 2).
Figure 1.
Progression free survival significantly higher in BAB vs NOTBev group.
Figure 2.
No difference in OS between BAB and NOTBev groups.
Univariate analysis was performed to assess for possible confounding factors in estimation of PFS. Variables that proved to be significant confounders were bevacizumab used in the first line setting and the number of prior cytotoxic chemotherapy regimens. These factors maintained their significance in multivariate analysis as well. After adjusting for these covariates, patients who were re-treated with bevacizumab at recurrence experienced a 78% improvement in PFS (HR 0.22, p = 0.0048). As previously stated, the average time between completion of the first BCR and initiation of the second, the “bev-free interval,” was 7.4 months. There was no correlation between the “bev free interval” and response (CR, PR or SD) to the subsequent BCR.
There were four patients who received maintenance bevacizumab who were not included in the above analysis since the objective of the study was to compare outcomes after receiving bevacizumab containing cytotoxic regimens. We performed a subsequent analysis to include these four patients to assure there was would not be a significant change to the findings. Two of the 4 patients were retreated with bevacizumab at the time of recurrence and two were not. Survival analysis (PFS and OS) was similar to previous findings when these four patients were included in the analysis.
We evaluated ORR (CR and PR) and clinical benefit (CR, PR and SD) in our patients. Figure 3 shows the clinical response distribution between the two groups. The ORR was significantly better in the BAB versus Not Bev patients (47% versus 27%, p=0.0**). Higher number of patients achieved clinical benefit (CR, PR and SD) that were retreated with bevacizumab, 88% as compared to 50% in the NOTBev group (p = 0.0120).
Figure 3.
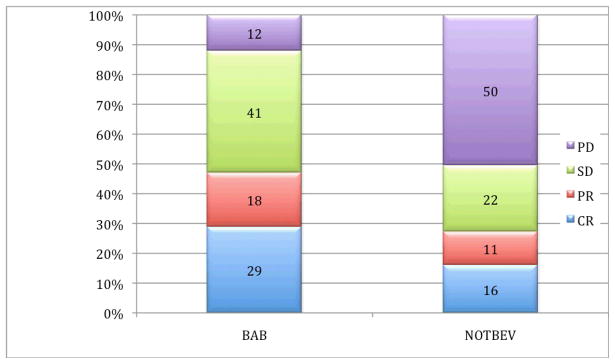
Clinical response distribution in BAB and NOTBev groups.
There were three bowel perforations in the entire cohort (8.3%). Two of the perforations were in the NOTBev group during their initial treatment with bevacizumab. The third perforation occurred in a patient in the BAB group. Thus, the rate of bowel perforation was 10.53% in the NOTBev group vs. 5.77% in the BAB group (p = 0.6109). Progression was the major reason for discontinuation of both the BAB and NOTBev regimens (47.1% and 76.8.%, respectively).
CONCLUSIONS
The data presented here suggests that women who initially have a CR to bevacizumab plus chemotherapy have a statistically significant longer PFS and improved ORR with their subsequent regimen if it includes bevacizumab. The exact explanation for these findings remains elusive. One possibility is simply that adding another agent results in added benefit; we know that combination chemotherapy has superior response rate and PFS when compared to single-agent therapy.13–15 However, this explanation isn’t necessarily congruent with the findings of GOG218 where there was no difference in the control arm and the second arm that had the addition of bevacizumab during the treatment phase but not the maintenance phase.1 Another possible explanation is tumor biology, i.e. that there is an inherent difference between tumors that respond to BCR and those that don’t. This too is not consistent with our findings, since the baseline characteristics between the BAB and NOTBev groups were similar. Data from preclinical studies provides some basis for a hypothesis that the addition of bevacizumab sensitizes cells to cytotoxic chemotherapy. Preclinical studies have shown that treatment with bevacizumab alters microvessel architecture such that drug delivery to tumor is enhanced.7 Thus, it may be that patients retreated with bevacizumab have improved PFS as a result of improved delivery of cytotoxic chemotherapy. This is highlighted by the fact that the BAB group was more heavily pretreated; bevacizumab, potentially via improved drug delivery, sensitizes tumor cells to cytotoxic chemotherapy that otherwise may not have provided clinical benefit. The colorectal literature supports this theory as well with reports of clinical responses to BCR in patients that had otherwise chemotherapy-resistant disease8–10 While we know many of the mechanisms behind resistance to cytotoxic chemotherapy, resistance to targeted agents such as Bevacizumab has not even been defined.
This is the first study to address re-treatment with bevacizumab in EOC. This topic has been explored in other disease sites including colorectal cancer, specifically in the BRiTE study of metastatic colorectal cancer. In the original prospective observational study all patients were treated with bevacizumab in the front-line setting and had a longer than expected OS. A follow-up study evaluated the role of re-treatment with bevacizumab in patients who initially had disease progression while on study. The authors found a significant improvement in OS in patients who received bevacizumab at the time of progression compared to those who did not (31.8 vs. 19.9 months) and receiving bevacizumab beyond progression was significantly associated with improved OS.11 A similar question is being asked in patients with lung cancer. An international randomized phase III trial is currently recruiting participants with non-squamous non-small-cell lung cancer who have progressed on bevacizumab and randomizing them to cytotoxic chemotherapy with or without bevacizumab.12
The PFS for the BAB group is comparable to that which was reported from the OCEANS trial (median PFS 10.9 and 12.4 months, respectively).4 However, our patient cohort was more heavily pre-treated with a median number of prior chemotherapy regimens of 2.5 in the BAB group as compared to 1 prior non-bev-containing regimen required in the OCEANs study. In GOG 170D two-thirds of patients had received 2 other chemotherapy regimens prior to treatment with single-agent bevacizumab. Interestingly, the median PFS in that study was 4.7 months.3 Our data therefore suggests an added benefit of cytotoxic chemotherapy to bevacizumab in the treatment of patients with heavily pre-treated recurrent EOC. Interestingly, the OCEANs data also demonstrated an improved response rate in patients that were treated with cytotoxic and bevacizumab (79 vs. 57%) over those patients treated with cytotoxic alone.
The weaknesses of this study are the inherent limitation of retrospective reports. The fact that it is a single-institutional review also results in selection bias, however it allows us to compare patients with similar treatment paradigms. In addition, approximately 50% of patients in this retrospective study were enrolled in a clinical trial that was either through the Gynecologic Oncology Group (GOG) or other institutionally-based study. This has the potential to bias the current study in a few ways. First, the requirement of a good performance status (PS) for enrollment selects for patients that are likely going to be more tolerant of chemotherapy. It is also possible that the frequent imaging required on clinical trials may detect recurrences earlier than if no imaging were performed as in the case of many patients treated off of protocol. However, at this institution Ca125 is checked with every cycle of chemotherapy and every 3 months after completion of chemotherapy (similar to Ca125 surveillance on the majority of clinical trials). In addition, we know that in the majority of cases Ca125 precedes clinical progression and that the false positive rate of CT scan for recurrence can be has high as 14% as opposed to 1.6% for Ca125.16 Furthermore, the conclusions of this study are not intended to be treatment-changing, but rather hypothesis-generating. These results suggest that there may be a role for a randomized controlled trial asking the question of whether patients treated with bevacizumab should be re-treated at the time of recurrence. Another important question that this study raises is whether or not bevacizumab resistance is an entity and if so, how should it be defined. In our dataset, we did not find any relationship between the “bev-free interval” and response to subsequent BCR, but larger numbers are needed to truly answer this question. Furthermore, knowing that treatment with bevacizumab comes with a high cost and not necessarily an OS advantage17, it behooves the community to try and identify which patients will exhibit a response to bevacizumab.
Re-treatment with Bev after a prior Bev response is associated with a significantly improved PFS and ORR. This is the first of such reports in this patient population. The 14-month improvement in PFS strongly supports the re-use of Bev in subsequent regimens in patients who demonstrate an initial response to Bev. This strategy should be formally tested in future clinical trials and further investigation should include evaluation of predictors of response to Bev therapy.
References
- 1.Burger RA, Brady MF, Bookman MA, Fleming GF, Monk BJ, Huang H, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365(26):2473–83. doi: 10.1056/NEJMoa1104390. [DOI] [PubMed] [Google Scholar]
- 2.Perren TJ, Swart AM, Pfisterer J, Ledermann JA, Pujade-Lauraine E, Kristensen G, et al. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med. 2011;365(26):2484–96. doi: 10.1056/NEJMoa1103799. [DOI] [PubMed] [Google Scholar]
- 3.Burger RA, Sill MW, Monk BJ, Greer BE, Sorosky JI. Phase II trial of bevacizumab in persistent or recurrent epithelial ovarian cancer or primary peritoneal cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2007;25(33):5165–71. doi: 10.1200/JCO.2007.11.5345. [DOI] [PubMed] [Google Scholar]
- 4.Aghajanian CFN, Rutherford T, et al. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol. 2011;29(18 s, part II of II):781s. doi: 10.1200/JCO.2012.42.0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guppy AE, Rustin GJ. CA125 response: can it replace the traditional response criteria in ovarian cancer? Oncologist. 2002;7(5):437–43. doi: 10.1634/theoncologist.7-5-437. [DOI] [PubMed] [Google Scholar]
- 6.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 7.Dickson PV, Hamner JB, Sims TL, Fraga CH, Ng CY, Rajasekeran S, et al. Bevacizumab-induced transient remodeling of the vasculature in neuroblastoma xenografts results in improved delivery and efficacy of systemically administered chemotherapy. Clin Cancer Res. 2007;13(13):3942–50. doi: 10.1158/1078-0432.CCR-07-0278. [DOI] [PubMed] [Google Scholar]
- 8.Shitara K, Munakata M, Muto O, Sakata Y. Metastatic rectal cancer responding to third-line therapy employing bevacizumab after failure of oxaliplatin and irinotecan: case report. Jpn J Clin Oncol. 2008;38(7):493–6. doi: 10.1093/jjco/hyn046. [DOI] [PubMed] [Google Scholar]
- 9.Kang BW, Kim TW, Lee JL, Ryu MH, Chang HM, Yu CS, et al. Bevacizumab plus FOLFIRI or FOLFOX as third-line or later treatment in patients with metastatic colorectal cancer after failure of 5-fluorouracil, irinotecan, and oxaliplatin: a retrospective analysis. Med Oncol. 2009;26(1):32–7. doi: 10.1007/s12032-008-9077-8. [DOI] [PubMed] [Google Scholar]
- 10.Chen HX, Mooney M, Boron M, Vena D, Mosby K, Grochow L, et al. Phase II multicenter trial of bevacizumab plus fluorouracil and leucovorin in patients with advanced refractory colorectal cancer: an NCI Treatment Referral Center Trial TRC-0301. J Clin Oncol. 2006;24(21):3354–60. doi: 10.1200/JCO.2005.05.1573. [DOI] [PubMed] [Google Scholar]
- 11.Grothey A, Sugrue MM, Purdie DM, Dong W, Sargent D, Hedrick E, et al. Bevacizumab beyond first progression is associated with prolonged overall survival in metastatic colorectal cancer: results from a large observational cohort study (BRiTE) J Clin Oncol. 2008;26(33):5326–34. doi: 10.1200/JCO.2008.16.3212. [DOI] [PubMed] [Google Scholar]
- 12.Gridelli C, Bennouna J, de Castro J, Dingemans AM, Griesinger F, Grossi F, et al. Randomized Phase IIIb Trial Evaluating the Continuation of Bevacizumab Beyond Disease Progression in Patients with Advanced Non-Squamous Non-Small-Cell Lung Cancer after First-Line Treatment with Bevacizumab Plus Platinum-Based Chemotherapy: Treatment Rationale and Protocol Dynamics of the AvaALL (MO22097) Trial. Clin Lung Cancer. 2011;12(6):407–11. doi: 10.1016/j.cllc.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 13.Parmar MK, Ledermann JA, Colombo N, du Bois A, Delaloye JF, Kristensen GB, et al. Paclitaxel plus platinum-based chemotherapy versus conventional platinum-based chemotherapy in women with relapsed ovarian cancer: the ICON4/AGO-OVAR-2.2 trial. Lancet. 2003;361(9375):2099–106. doi: 10.1016/s0140-6736(03)13718-x. [DOI] [PubMed] [Google Scholar]
- 14.Pfisterer J, Plante M, Vergote I, du Bois A, Hirte H, Lacave AJ, et al. Gemcitabine plus carboplatin compared with carboplatin in patients with platinum-sensitive recurrent ovarian cancer: an intergroup trial of the AGO-OVAR, the NCIC CTG, and the EORTC GCG. J Clin Oncol. 2006;24(29):4699–707. doi: 10.1200/JCO.2006.06.0913. [DOI] [PubMed] [Google Scholar]
- 15.Alberts DS, Liu PY, Wilczynski SP, Clouser MC, Lopez AM, Michelin DP, et al. Randomized trial of pegylated liposomal doxorubicin (PLD) plus carboplatin versus carboplatin in platinum-sensitive (PS) patients with recurrent epithelial ovarian or peritoneal carcinoma after failure of initial platinum-based chemotherapy (Southwest Oncology Group Protocol S0200) Gynecol Oncol. 2008;108(1):90–4. doi: 10.1016/j.ygyno.2007.08.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van der Burg ME, Lammes FB, Verweij J. The role of CA 125 and conventional examinations in diagnosing progressive carcinoma of the ovary. Surg Gynecol Obstet. 1993;176(4):310–4. [PubMed] [Google Scholar]
- 17.Cohn DE, Kim KH, Resnick KE, O’Malley DM, Straughn JM., Jr At what cost does a potential survival advantage of bevacizumab make sense for the primary treatment of ovarian cancer? A cost-effectiveness analysis. J Clin Oncol. 2011;29(10):1247–51. doi: 10.1200/JCO.2010.32.1075. [DOI] [PubMed] [Google Scholar]