Abstract
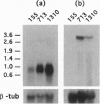
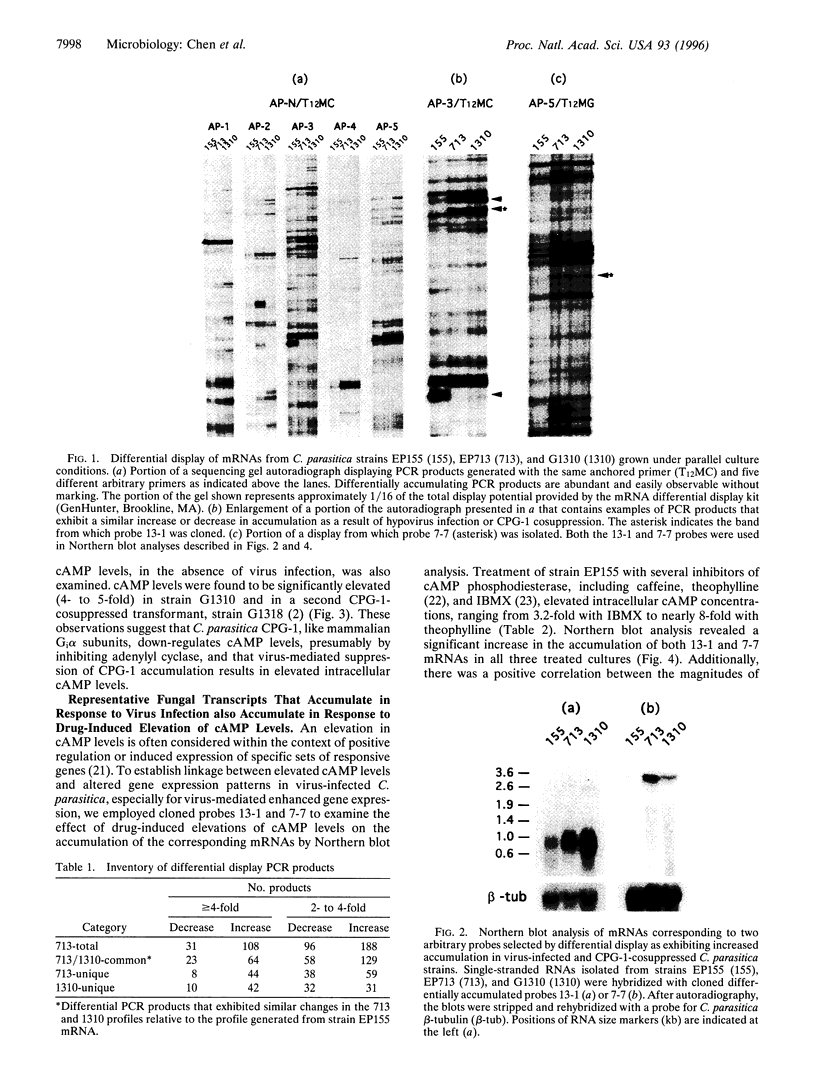
Persistent infection of the chestnut blight fungus Cryphonectria parasitica with the prototypic hypovirus CHVI-713 results in attenuation of fungal virulence (hypo-virulence) and reduced accumulation of the GTP-binding (G) protein a subunit CPG-1. Transgenic cosuppression of CPG-1 accumulation in the absence of virus infection also confers hypovirulence. We now report the use of mRNA differential display to examine the extent to which virus infection alters fungal gene transcript accumulation and to assess the degree to which modification of CPG-1 signal transduction contributes to this alteration. More than 400 PCR products were identified that either increased (296 products) or decreased (127 products) in abundance as a result of virus infection. Significantly, 65% of these products exhibited similar changes as a result of CPG-1 cosuppression in the absence of virus infection. We also report that both virus infection and CPG-1 cosuppression elevate cAMP levels 3- to 5-fold. Additionally, it was possible to mimic the effect of virus infection and CPG-1 cosuppression on transcript accumulation for representative fungal genes by drug-induced elevation of cAMP levels. These results strengthen and extend previous indications that hypovirus infection causes a significant and persistent alteration of fungal gene expression/transcript accumulation. They further show that this alteration is primarily mediated through modification of the CPG-1 signaling pathway and suggest that, similar to mammalian Gi alpha subunits, CPG-1 functions as a negative modulator of adenylyl cyclase. Finally, these results suggest a role for G-protein-regulated cAMP accumulation in hypovirus-mediated alteration of fungal gene expression.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anagnostakis S. L. Biological control of chestnut blight. Science. 1982 Jan 29;215(4532):466–471. doi: 10.1126/science.215.4532.466. [DOI] [PubMed] [Google Scholar]
- Bauer D., Müller H., Reich J., Riedel H., Ahrenkiel V., Warthoe P., Strauss M. Identification of differentially expressed mRNA species by an improved display technique (DDRT-PCR). Nucleic Acids Res. 1993 Sep 11;21(18):4272–4280. doi: 10.1093/nar/21.18.4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B., Choi G. H., Nuss D. L. Attenuation of fungal virulence by synthetic infectious hypovirus transcripts. Science. 1994 Jun 17;264(5166):1762–1764. doi: 10.1126/science.8209256. [DOI] [PubMed] [Google Scholar]
- Chen B., Choi G. H., Nuss D. L. Mitotic stability and nuclear inheritance of integrated viral cDNA in engineered hypovirulent strains of the chestnut blight fungus. EMBO J. 1993 Aug;12(8):2991–2998. doi: 10.1002/j.1460-2075.1993.tb05967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Iyengar R. Inhibition of cloned adenylyl cyclases by mutant-activated Gi-alpha and specific suppression of type 2 adenylyl cyclase inhibition by phorbol ester treatment. J Biol Chem. 1993 Jun 15;268(17):12253–12256. [PubMed] [Google Scholar]
- Choi G. H., Chen B., Nuss D. L. Virus-mediated or transgenic suppression of a G-protein alpha subunit and attenuation of fungal virulence. Proc Natl Acad Sci U S A. 1995 Jan 3;92(1):305–309. doi: 10.1073/pnas.92.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi G. H., Larson T. G., Nuss D. L. Molecular analysis of the laccase gene from the chestnut blight fungus and selective suppression of its expression in an isogenic hypovirulent strain. Mol Plant Microbe Interact. 1992 Mar-Apr;5(2):119–128. doi: 10.1094/mpmi-5-119. [DOI] [PubMed] [Google Scholar]
- Choi G. H., Nuss D. L. Hypovirulence of chestnut blight fungus conferred by an infectious viral cDNA. Science. 1992 Aug 7;257(5071):800–803. doi: 10.1126/science.1496400. [DOI] [PubMed] [Google Scholar]
- Feldman J. F., Thayer J. P. Cyclic AMP-induced tyrosinase synthesis in Neurospora crassa. Biochem Biophys Res Commun. 1974 Dec 11;61(3):977–982. doi: 10.1016/0006-291x(74)90251-4. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Johnson R. R., Cranston H. J., Chaverra M. E., Dyer W. E. Characterization of cDNA clones for differentially expressed genes in embryos of dormant and nondormant Avena fatua L. caryopses. Plant Mol Biol. 1995 Apr;28(1):113–122. doi: 10.1007/BF00042043. [DOI] [PubMed] [Google Scholar]
- Kaziro Y., Itoh H., Kozasa T., Nakafuku M., Satoh T. Structure and function of signal-transducing GTP-binding proteins. Annu Rev Biochem. 1991;60:349–400. doi: 10.1146/annurev.bi.60.070191.002025. [DOI] [PubMed] [Google Scholar]
- Kazmierczak P., Pfeiffer P., Zhang L., Van Alfen N. K. Transcriptional repression of specific host genes by the mycovirus Cryphonectria hypovirus 1. J Virol. 1996 Feb;70(2):1137–1142. doi: 10.1128/jvi.70.2.1137-1142.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson T. G., Choi G. H., Nuss D. L. Regulatory pathways governing modulation of fungal gene expression by a virulence-attenuating mycovirus. EMBO J. 1992 Dec;11(12):4539–4548. doi: 10.1002/j.1460-2075.1992.tb05555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson T. G., Nuss D. L. Altered transcriptional response to nutrient availability in hypovirus-infected chestnut blight fungus. EMBO J. 1994 Dec 1;13(23):5616–5623. doi: 10.1002/j.1460-2075.1994.tb06899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang P., Averboukh L., Pardee A. B. Distribution and cloning of eukaryotic mRNAs by means of differential display: refinements and optimization. Nucleic Acids Res. 1993 Jul 11;21(14):3269–3275. doi: 10.1093/nar/21.14.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang P., Pardee A. B. Differential display of eukaryotic messenger RNA by means of the polymerase chain reaction. Science. 1992 Aug 14;257(5072):967–971. doi: 10.1126/science.1354393. [DOI] [PubMed] [Google Scholar]
- Mou L., Miller H., Li J., Wang E., Chalifour L. Improvements to the differential display method for gene analysis. Biochem Biophys Res Commun. 1994 Mar 15;199(2):564–569. doi: 10.1006/bbrc.1994.1265. [DOI] [PubMed] [Google Scholar]
- Powell W. A., Jr, Van Alfen N. K. Two nonhomologus viruses of Cryphonectria (Endothia) parasitica reduce accumulation of specific virulence-associated polypeptides. J Bacteriol. 1987 Nov;169(11):5324–5326. doi: 10.1128/jb.169.11.5324-5326.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell W. A., Van Alfen N. K. Differential accumulation of poly(A)+ RNA between virulent and double-stranded RNA-induced hypovirulent strains of Cryphonectria (Endothia) parasitica. Mol Cell Biol. 1987 Oct;7(10):3688–3693. doi: 10.1128/mcb.7.10.3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigling D., Van Alfen N. K. Regulation of laccase biosynthesis in the plant-pathogenic fungus Cryphonectria parasitica by double-stranded RNA. J Bacteriol. 1991 Dec;173(24):8000–8003. doi: 10.1128/jb.173.24.8000-8003.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taussig R., Iñiguez-Lluhi J. A., Gilman A. G. Inhibition of adenylyl cyclase by Gi alpha. Science. 1993 Jul 9;261(5118):218–221. doi: 10.1126/science.8327893. [DOI] [PubMed] [Google Scholar]
- Wang P., Nuss D. L. Induction of a Cryphonectria parasitica cellobiohydrolase I gene is suppressed by hypovirus infection and regulated by a GTP-binding-protein-linked signaling pathway involved in fungal pathogenesis. Proc Natl Acad Sci U S A. 1995 Dec 5;92(25):11529–11533. doi: 10.1073/pnas.92.25.11529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcikiewicz R. J., Dobson P. R., Brown B. L. Muscarinic acetylcholine receptor activation causes inhibition of cyclic AMP accumulation, prolactin and growth hormone secretion in GH3 rat anterior pituitary tumour cells. Biochim Biophys Acta. 1984 Sep 14;805(1):25–29. doi: 10.1016/0167-4889(84)90032-6. [DOI] [PubMed] [Google Scholar]
- Zhang L., Churchill A. C., Kazmierczak P., Kim D. H., Van Alfen N. K. Hypovirulence-associated traits induced by a mycovirus of Cryphonectria parasitica are mimicked by targeted inactivation of a host gene. Mol Cell Biol. 1993 Dec;13(12):7782–7792. doi: 10.1128/mcb.13.12.7782. [DOI] [PMC free article] [PubMed] [Google Scholar]