Abstract
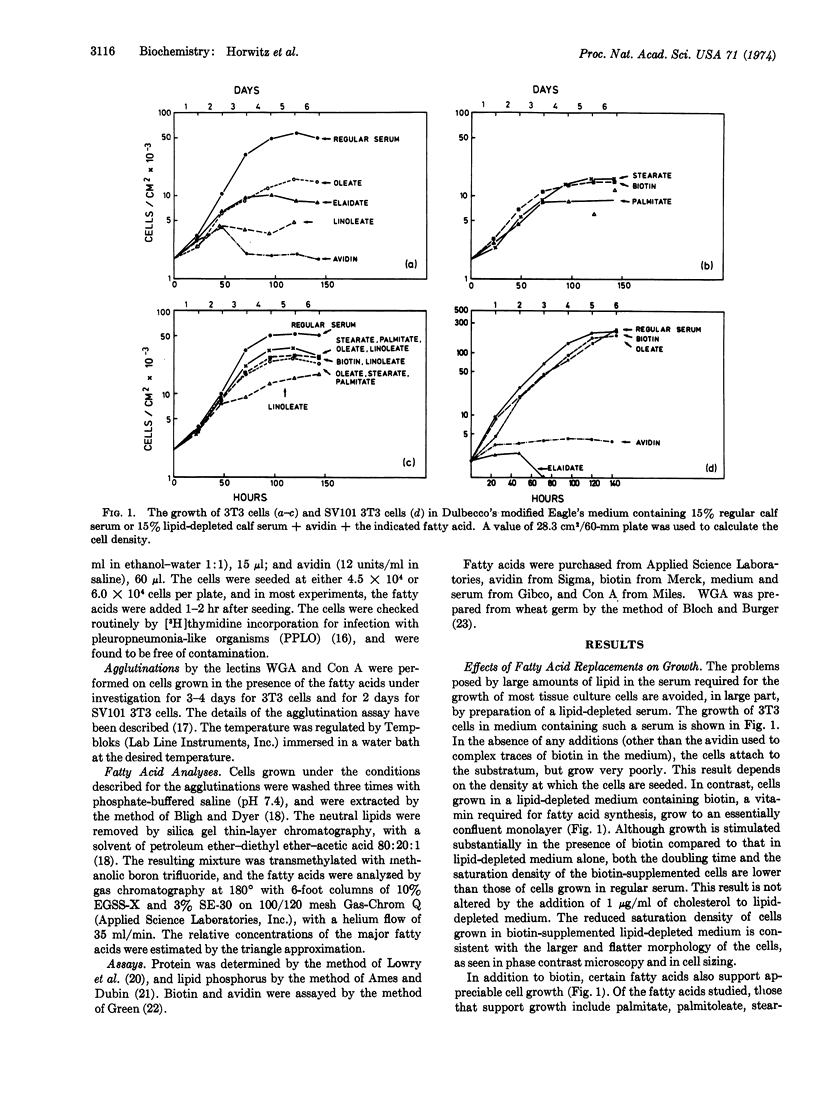
The growth of 3T3 and SV101 3T3 cells in a lipid-depleted medium is enhanced by the addition of biotin or some fatty acids. The extent of enhancement depends on the fatty acid(s) supplied. The presence of linoleate is unique, since it induces a morphological alteration in 3T3 cells resulting in a cell similar to an SV101-transformed 3T3 cell. Analyses of the fatty acids from the membrane phosphatides show that the exogenously supplied fatty acids are incorporated and alter the fatty acid composition. This is most clearly evident with heptadecanoate-grown cells, in which this fatty acid and its derivatives comprise over 45% of the fatty acids in the phospholipids.
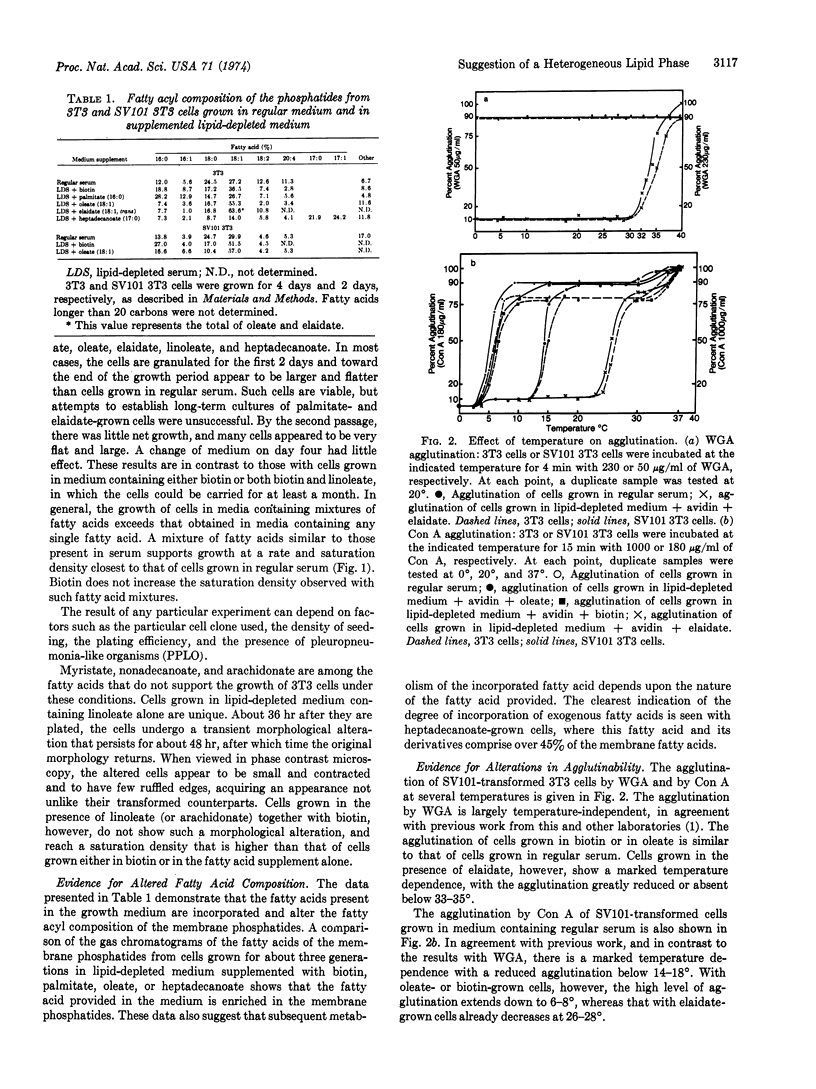
The fatty acid replacements have a striking effect on the temperature dependence of agglutination by wheat germ agglutinin and concanavalin A, implying that fluidity is involved in agglutination. These temperature dependencies and the effect of fatty acid replacements on them were different for the two lectins, but similar for both transformed and untransformed cells. These observations are interpreted as suggesting that the lipid phase is heterogeneous, and that transformed and untransformed cell membranes have regions of similar fluidity.
Keywords: agglutination, essential fatty acids
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bailey J. M., Dunbar L. M. Essential fatty acid requirements of cells in tissue culture: a review. Exp Mol Pathol. 1973 Apr;18(2):142–161. doi: 10.1016/0014-4800(73)90013-0. [DOI] [PubMed] [Google Scholar]
- Bretscher M. S. Asymmetrical lipid bilayer structure for biological membranes. Nat New Biol. 1972 Mar 1;236(61):11–12. doi: 10.1038/newbio236011a0. [DOI] [PubMed] [Google Scholar]
- Cronan J. E., Vagelos P. R. Metabolism and function of the membrane phospholipids of Escherichia coli. Biochim Biophys Acta. 1972 Feb 14;265(1):25–60. doi: 10.1016/0304-4157(72)90018-4. [DOI] [PubMed] [Google Scholar]
- Hammarström S., Samuelsson B., Bjursell G. Prostaglandin levels in normal and transformed baby-hamster-kidney fibroblasts. Nat New Biol. 1973 May 9;243(123):50–51. [PubMed] [Google Scholar]
- Jacobs R. A., Majerus P. W. The regulation of fatty acid synthesis in human skin fibroblasts. Inhibition of fatty acid synthesis by free fatty acids. J Biol Chem. 1973 Dec 25;248(24):8392–8401. [PubMed] [Google Scholar]
- Kohler S. J., Horwitz A. F., Klein M. P. Magnetic resonance studies on membranes and model membrane systems. IV. A comparison of yeast and egg lecithin dispersions. Biochem Biophys Res Commun. 1972 Dec 18;49(6):1414–1421. doi: 10.1016/0006-291x(72)90496-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levine E. M., Burleigh I. G., Boone C. W., Eagle H. An altered pattern of RNA synthesis in serially propagated human diploid cells. Proc Natl Acad Sci U S A. 1967 Feb;57(2):431–438. doi: 10.1073/pnas.57.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden C. D., Wright K. L., McConnell H. M., Fox C. F. Lateral phase separations in membrane lipids and the mechanism of sugar transport in Escherichia coli. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2271–2275. doi: 10.1073/pnas.70.8.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavis R. D., Vagelos P. R. The effect of phospholipid fatty acid composition in membranous enzymes in Escherichia coli. J Biol Chem. 1972 Feb 10;247(3):652–659. [PubMed] [Google Scholar]
- Michaelson D. M., Horwitz A. F., Klein M. P. Head group modulation of membrane fluidity in sonicated phospholipid dispersions. Biochemistry. 1974 Jun 4;13(12):2605–2612. doi: 10.1021/bi00709a021. [DOI] [PubMed] [Google Scholar]
- Noonan K. D., Burger M. M. Binding of ( 3 H)concanavalin A to normal and transformed cells. J Biol Chem. 1973 Jun 25;248(12):4286–4292. [PubMed] [Google Scholar]
- Noonan K. D., Burger M. M. Induction of 3T3 cell division at the monolayer stage. Early changes in macromolecular processes. Exp Cell Res. 1973 Aug;80(2):405–414. doi: 10.1016/0014-4827(73)90313-3. [DOI] [PubMed] [Google Scholar]
- Noonan K. D., Burger M. M. The relationship of concanavalin A binding to lectin-initiated cell agglutination. J Cell Biol. 1973 Oct;59(1):134–142. doi: 10.1083/jcb.59.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overath P., Träuble H. Phase transitions in cells, membranes, and lipids of Escherichia coli. Detection by fluorescent probes, light scattering, and dilatometry. Biochemistry. 1973 Jul 3;12(14):2625–2634. doi: 10.1021/bi00738a012. [DOI] [PubMed] [Google Scholar]
- Phillips M. C., Hauser H., Paltauf F. The inter- and intra-molecular mixing of hydrocarbon chains in lecithin-water systems. Chem Phys Lipids. 1972 Mar;8(2):127–133. doi: 10.1016/0009-3084(72)90024-2. [DOI] [PubMed] [Google Scholar]
- Rosenblith J. Z., Ukena T. E., Yin H. H., Berlin R. D., Karnovsky M. J. A comparative evaluation of the distribution of concanavalin A-binding sites on the surfaces of normal, virally-transformed, and protease-treated fibroblasts. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1625–1629. doi: 10.1073/pnas.70.6.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanu A. M., Edelstein C. Solubility in aqueous solutions of ethanol of the small molecular weight peptides of the serum very low density and high density lipoproteins: relevance to the recovery problem during delipidation of serum lipoproteins. Anal Biochem. 1971 Dec;44(2):576–588. doi: 10.1016/0003-2697(71)90247-8. [DOI] [PubMed] [Google Scholar]
- Shimshick E. J., McConnell H. M. Lateral phase separation in phospholipid membranes. Biochemistry. 1973 Jun 5;12(12):2351–2360. doi: 10.1021/bi00736a026. [DOI] [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Wisnieski B. J., Williams R. E., Fox C. F. Manipulation of fatty acid composition in animal cells grown in culture. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3669–3673. doi: 10.1073/pnas.70.12.3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood R., Falch J. Lipids of cultured hepatoma cells. II. Effect of media lipids on cellular phospholipids. Lipids. 1973 Dec;8(12):702–710. doi: 10.1007/BF02531836. [DOI] [PubMed] [Google Scholar]



