Abstract
Stomatopod crustaceans have the most complex and diverse assortment of retinal photoreceptors of any animals, with 16 functional classes. The receptor classes are subdivided into sets responsible for ultraviolet vision, spatial vision, colour vision and polarization vision. Many of these receptor classes are spectrally tuned by filtering pigments located in photoreceptors or overlying optical elements. At visible wavelengths, carotenoproteins or similar substances are packed into vesicles used either as serial, intrarhabdomal filters or lateral filters. A single retina may contain a diversity of these filtering pigments paired with specific photoreceptors, and the pigments used vary between and within species both taxonomically and ecologically. Ultraviolet-filtering pigments in the crystalline cones serve to tune ultraviolet vision in these animals as well, and some ultraviolet receptors themselves act as birefringent filters to enable circular polarization vision. Stomatopods have reached an evolutionary extreme in their use of filter mechanisms to tune photoreception to habitat and behaviour, allowing them to extend the spectral range of their vision both deeper into the ultraviolet and further into the red.
Keywords: Stomatopod, colour vision, ultraviolet vision, polarization vision, spectral filtering, visual ecology
1. Introduction
Stomatopod crustaceans, commonly known as mantis shrimps, have achieved international notoriety for their flamboyant, aggressive behaviour, attractive body coloration, and most of all for their extremely competent eyes (figure 1). Mantis shrimps most often inhabit fairly shallow, marine, tropical waters, where they actively hunt prey from concealed burrows [1]. These animals are built on a standard crustacean plan, with long segmented bodies making them resemble decapod shrimp (or perhaps more accurately, small lobsters), but they are evolutionarily far removed from all other crustacean groups, having detached from the main crustacean line not long after the Cambrian. Over time, they have acquired several unusual characteristics, including their prey-capture apparatus (the raptorial appendages), which resemble the arms of a praying mantis and give them their common name as well as their uniquely designed eyes. The eyes and raptorial appendages are in fact coevolved to foster rapid identification, ranging and capture of passing prey.
Figure 1.

Compound eyes of stomatopod crustaceans assigned to three different superfamilies, all containing six-row midbands. (a) Hemisquilla californiensis (Hemisquilloidea). The eye is elongated, and the midband is fairly prominent. (b) Lysiosquillina sulcata (Lysiosquilloidea). This eye is very tall, and the midband is not very prominent. The dark spots on the eyes are the pseudo-pupils; the animal's right eye is directed at the camera so that all three pseudo-pupils are visible. (c) Odontodactylus scyllarus (Gonodactyloidea). The eye is nearly spherical, with a very prominent midband. Here, the left eye is pointed in the camera's direction and shows three pseudo-pupils. (Online version in colour.)
Stomatopod eye designs vary among species (although they are generally similar within stomatopod superfamilies [2–4]), but they all share a common optical feature that is unique to the group—the eye is subdivided into three parts. These are the extended ommatidial arrays of the dorsal and ventral halves of the eye, each resembling the apposition compound eyes of decapod crustaceans, separated by several parallel rows of ommatidia (a feature called the midband). The number of ommatidial rows in the midband ranges from two to six. It is the six-row midbands that show the greatest set of elaborations associated with filtering, so, in this paper, we will consider only this eye design, concentrating on species in the superfamilies Hemisquilloidea, Lysiosquilloidea and Gonodactyloidea [4] (figure 1).
2. Multiplication of receptor classes in stomatopod retinas
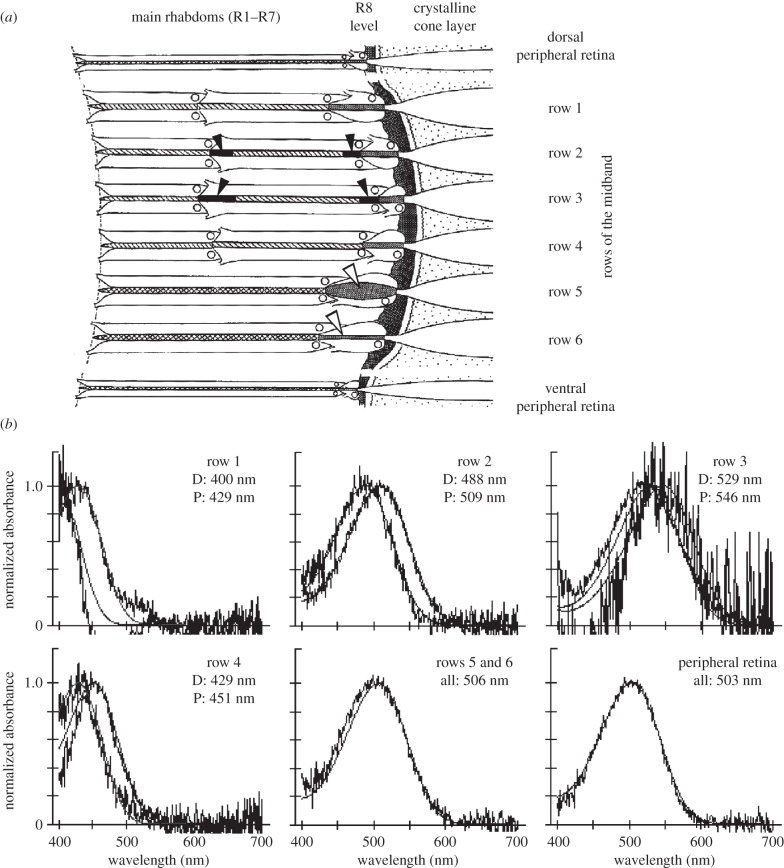
Ommatidial arrangements in a typical stomatopod compound eye with a six-row midband are diagrammed in figure 2a, using the eye of Odontodactylus scyllarus as a case study [5–7]. Ommatidia in the peripheral regions (the dorsal and ventral halves) are built on the standard crustacean plan, with a main rhabdom formed by the rhabdomeres of seven retinular cells (R1–R7) arranged radially, topped by a single rhabdomere formed by an eighth retinular cell (R8). All ommatidia of the midband also have the single R8 cell on top, and those of the two ventral rows (rows 5 and 6) have a single main rhabdom much like those of the periphery [5,7]. The other four rows of the midband, however, are quite unusual and include features not found in any other compound eye. Main rhabdoms in these four dorsal-most ommatidial rows (rows 1–4) are broken up into two tiers of roughly equal length, one formed from contributions of four retinular cells and the other from three [5,7]. In two of the rows (rows 2 and 3), the R8 level is separated from the top of the main rhabdom (i.e. from the distal tier) by a section of strongly coloured material, called an intrarhabdomal filter [5–8]. Depending on the superfamily, there are similar filters separating the proximal tier from the distal one in rows 2 and 3 (lysiosquilloids have the additional filter only in row 2). Evolutionary reconstruction analysis suggests that the common ancestor of all modern stomatopods had already evolved the six-row midband and the full set of four intrarhabdomal filters [4].
Figure 2.
(a) A diagrammatic view of the retina of Odontodactylus scyllarus. The crystalline cones are to the right, and the ommatidia of the dorsal half of the eye, the ventral half, and the midband are indicated. R8 rhabdomeres are shaded, and main rhabdoms are hatched. The tiers of the first through the fourth rows are indicated by diagonal hatching; untiered main rhabdoms are crosshatched. The black segments, indicated by closed arrowheads, are the intrarhabdomal filters. Open arrowheads indicate the R8 rhabdomeres in the two most ventral ommatidial rows, which serve as polarization filters. Circles indicate nuclei. (b) Normalized absorbance spectra of visual pigments in main rhabdoms of O. scyllarus, based on between nine and 20 photobleaches per class. Jagged lines show original data, whereas smooth lines are best-fit template spectra. The λmax of each fitted spectrum is given in the panel; D, distal tier; P, proximal tier. Note that in the tiered rows, one through four, the distal visual pigment always has a shorter absorption maximum than the proximal pigment.
When Marshall [5] first reported the complexity of stomatopod photoreceptor arrangements, the design appeared to offer a solution to a long-standing problem related to crustacean colour vision, particularly in the large-bodied malacostracans (stomatopods and decapods, i.e. shrimps, crabs and lobsters). Decapod crustaceans invariably have been found to have a single spectral class of photoreceptor in main rhabdoms, limiting their ability to discriminate colour. An ability to discriminate hue has been demonstrated in some crabs [9,10], where it appears to be based on a simple comparison of receptors in main rhabdoms versus R8 cells. It is also possible that lateral pigments associated with main rhabdoms could give a limited colour sense via comparisons between ommatidia (see the later section on lateral filtering). Stomatopods, using filters, could add new spectral classes to their visual systems, and the tiers could even serve to shift spectral sensitivity in the presence of a single visual pigment. In other words, the visual pigment would remove part of the spectrum of light flowing down the distal tier, altering the proximal tier's spectral response. This hypothesis seemed reasonable, because the only stomatopod characterized by microspectrophotometry (MSP) at the time of Marshall's paper had a single spectral type of main rhabdom [11].
However, the first study of the visual pigments in rhabdoms of a stomatopod species with a six-row midband found that each of the tiered receptor classes apparently contained a different visual pigment, for a total of eight [6]; the untiered rhabdoms in the two ventral midband rows contained another type, and all main rhabdoms of the peripheral retina had yet another for a total of ten photoreceptor classes operating in the visible spectrum, with spectral maxima ranging from approximately 400 to 550 nm (figure 2b, illustrating visual pigments of O. scyllarus). MSP also found a single ultraviolet-sensitive visual pigment, peaking at the unusually short wavelength of approximately 330 nm [12]. Molecular characterization of stomatopod visual pigments was not attempted for another decade, but it quickly revealed that the actual number of expressed opsin proteins that formed these visual pigments was two to three times the number of spectral classes found by MSP [13,14]. Because visual pigments have quite broad absorbance spectra, a limited number of spectral classes—three to four—can supply all information available in the spectral range of stomatopod vision [15]. To use more classes, tuning to narrow the spectral sensitivities and to reduce the spectral overlap of these photoreceptors is necessary. This is primarily the function of the intrarhabdomal filters, although other mechanisms also contribute to the tuning.
3. Filtering in stomatopod retinas: visible-light photoreceptors
Despite their diversity in spectral placement, mantis shrimp visual pigments of the main rhabdoms have maxima only within a range of about 150 nm, from 400 to 550 nm (figure 2b). This results in extreme spectral overlap among receptor classes, and reinforces the requirement for means of both narrowing spectral sensitivities (to reduce spectral overlap) and separating them from others (extending the spectral range that is sampled). This is where the tiering comes into play. Even in the absence of any specialized coloured filters, visual pigments alone can serve a filtering role. In the first and fourth rows of the midband, intrarhabdomal filters are absent, but the visual pigments in the distal tiers are spectrally placed to shorter wavelengths than those of proximal tiers (figure 2b). Thus, visual pigments in the distal tiers operate as long-pass filters, compressing the spectral sensitivities of the proximal tiers into narrower, peakier functions (figure 3b, rows 1 and 4). The presence of ultraviolet-absorbing visual pigments in the R8 rhabdomeres probably filters the distal tier sensitivities to some extent, but this has not been studied in detail.
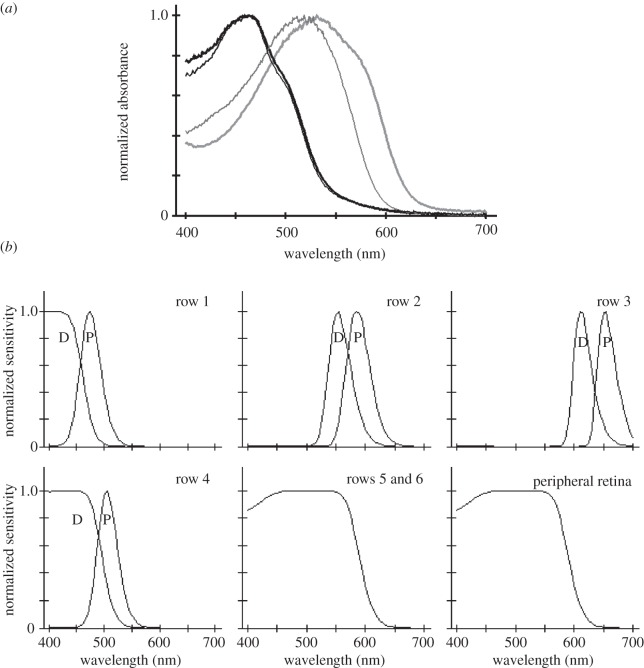
Figure 3.
(a) Normalized absorbance spectra of filter pigments in the third and fourth midband rows of Odontodactylus scyllarus. The distal filters are plotted as thin lines and the proximal filters as thick ones. Row 2 filters (which are identical at the two locations) are plotted in black, and row 3 filters in grey. (b) Computed normalized sensitivity spectra of photoreceptors in main rhabdoms of O. scyllarus, using lengths of each receptor class with a visual pigment density of 0.008 per micrometre together with total filter absorbance for functions in the second and third midband rows. Note that the tiered rows contain pairs of narrow spectral sensitivities, with the distal tier (D) placed to shorter wavelengths than the proximal tier (P). The distal tiers of the first and fourth midband rows are probably narrowed by absorption in the R8 rhabdomeres, but the actual values are not available for these calculations. Sensitivity functions in the untiered rhabdoms are broad due to self-screening by visual pigments in long rhabdoms.
Filtering by visual pigments alone does the job of producing four receptor classes in the violet to green spectral region, but converting a rhabdom loaded with middle-wavelength-absorbing visual pigment into a long-wavelength-sensitive photoreceptor requires further action. Stomatopods universally solve this problem with strongly absorbing photostable pigments placed in segments of the main rhabdom where the tiers meet, forming the intrarhabdomal filters. As noted, only the second and third midband rows ever contain the filters, and the number of types varies among the superfamilies. Hemisquilloids have two, lysiosquilloids usually have three, and gonodactyloids have all four (two in each row) [8,16,17]. Absorbance spectra of filters from O. scyllarus, a gonodactyloid, are given in figure 3a, as are the resulting computed sensitivity functions. Modelled functions such as these are confirmed by single-cell recordings in other species [18]. The computed functions plotted in figure 3b do not include the effects of either the ultraviolet filters or the R8 cell visual pigments, described later.
The unusual feature of this type of filtering in stomatopod ommatidia is that it continues over a series of steps, with each stage successively narrowing the spectrum of light passing down the photoreceptor. Consider the third midband row of O. scyllarus, assuming that full-spectrum light enters at the top of the main rhabdom (and ignoring the effects of ultraviolet filtering). Passage through the distal, orange–red filter removes nearly all light below 575–600 nm. This significantly sharpens sensitivity of the distal tier, because its visual pigment absorbs only on its long-wavelength tail (the visual pigment itself absorbs maximally at 529 nm). The visual pigment's absorption continues to remove middle-wavelength light, so that the spectrum entering the proximal filter is further long-wavelength shifted, and trimmed again by the red proximal filter at the junction of the rhabdomal tiers. The remnant of the bright white light that started down the rhabdom interacts with the green-absorbing visual pigment of the proximal tier (λmax, 546 nm), producing a far-red-sensitive photoreceptor. The same series of events occurs in the second midband row, with its shorter-wavelength visual pigments and filter classes, shaping a receptor pair most sensitive to green and yellow light. Ocular filters are used widely among animals, both vertebrate and invertebrate [19], but stomatopods are unique in their use of a series of many filtering stages in a single receptor unit. This of course creates an assemblage of exquisitely well-tuned receptor sensitivities and paradoxically simplifies the required processing further into the optic lobes, because the receptors are sorted into a large number of spectral classes right in the retina.
Intrarhabdomal filters in stomatopod retinas are strong absorbers of light, with densities commonly in excess of 0.2 density units per micrometre at the peak, often in the range of 0.5 OD per micrometre [16]. Because filter lengths range from 5 to nearly 50 µm in some cases [7,8,20], the total densities at the peak can be enormous—frequently exceeding 10 OD. While being nearly transparent at long wavelengths, they nevertheless exact a huge cost in sensitivity, especially when mated with medium-wavelength-absorbing visual pigments. This problem will be explored in §4, on the visual ecology of stomatopod filtering. For now, we want to consider the filtering materials that underlie this unusually efficient light capture—orders of magnitude greater than that of visual pigments.
Vertebrate oil droplets most often use carotenoid pigments for filtering [21,22]. Some of the transmitted colours of stomatopod filters, e.g. yellow, orange and red, are spectrally similar to those of vertebrate cone oil droplets (figure 4a–d). However, stomatopod filters are constructed from tightly packed tiny vesicles (figure 4e), unlike typical oil droplets in vertebrate cones, and they often have spectral features not seen in carotenoids. Crustaceans often incorporate carotenoids into carotenoproteins, most commonly the ketocarotenoid astaxanthin [23–25]. For example, the blue and red colours of decapod crustaceans, and probably of stomatopods too, are due to such pigmentation. The same compounds could be filter pigments. Intrarhabdomal filters are blue, purple or red in solution, just like crustacean astaxanthin-based carotenoproteins (e.g. crustacyanin). Crustacyanin, the blue pigment in lobster carapace, is a candidate for a filter pigment because its absorption properties vary with its polymeric state, so different forms could be used to make different filter classes. In addition, some of the red filters have absorption spectra such as that of pure astaxanthin, so it is possible that free carotenoids are present in some cases. Until carefully isolated preparations of filters are subjected to high performance liquid chromatographic analysis, the identities of the filter pigments will unfortunately remain a matter for speculation. It has been estimated that the concentrations of the pigments in rhabdomeric membranes are similar to opsin concentrations [16], so they could be packed into the vesicle membranes as opsins are into microvilli. It is interesting that the pigments used for the filters, as betrayed by their absorption spectra measured by MSP, are consistent within taxonomic groups of stomatopods, suggesting that whatever their nature, the biochemical pathways used to synthesize and deposit them in vesicles are broadly conserved within stomatopod families and superfamilies, but vary between them [4,16].
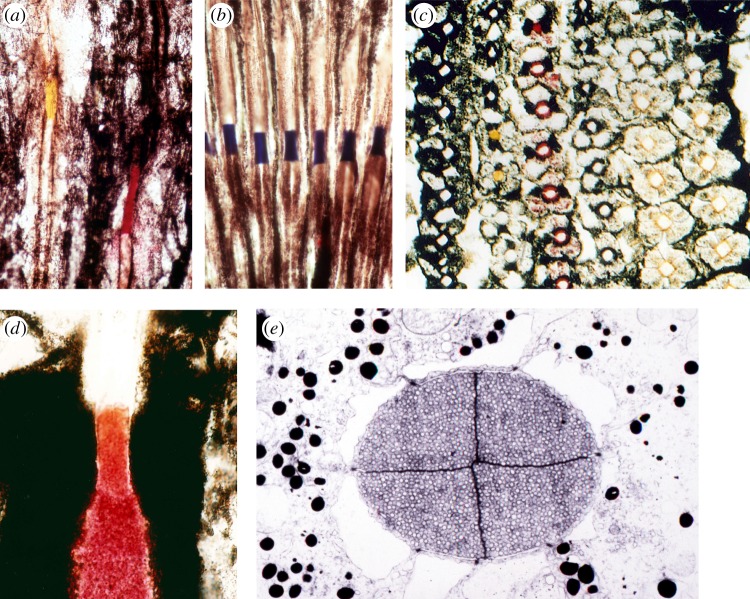
Figure 4.
(a–d) Photographs of intrarhabdomal filters and lateral filtering pigments in cryosections of fresh retinas of various stomatopod crustaceans. (a) Longitudinal section showing proximal filters in second row (yellow) and third row (red) ommatidia of Odontodactylus scyllarus. Note the dense coloration of the filters and their relatively extended lengths. The clear sections above and below the filters are the rhabdoms. (b) Blue-coloured filters in third row ommatidia of a species of Gonodactylus. (c) A cross section through the midband of the lysiosquilloid stomatopod C. scolopendra, showing the six rows with the dorsal-most row to the left. Yellow intrarhabdomal filters are visible in the second row, and a variety of coloured lateral filtering pigments can be seen, especially the red lateral filters in the third row and golden ones in the fifth and sixth rows. (d) Longitudinal section of the distal filter and associated lateral screening pigment at the junction of the distal tier and R8 rhabdomere in the third midband row. Note that the lateral screen extends down along the distal tier, acting together with the intrarhabdomal filter to tune the associated receptor. The colour of the screening pigment resembles that of the pigment in the filter. (e) An electron micrograph of the distal filter in a second-row ommatidium of Neogonodactylus oerstedii, showing the four-part structure (from the four retinular cells contributing to the receptor at that level) and the collection of vesicles, presumably containing the coloured pigment, in the filter.
4. Visual ecology of intrarhabdomal filters
Because the filter pigments are strong absorbers, they do an excellent job of shaping finely tuned receptors (figure 3). In doing so, they create a new set of problems. As in other crustaceans, visual pigments in stomatopod main rhabdoms achieve maxima at wavelengths of 550 nm at most. The evolution of the filters opened the possibility for these animals to form very-long-wave-sensitive photoreceptor classes. By pairing filter pigments with visual pigments having λmax ranging from 500 to 550 nm, they can produce receptor sets maximally sensitive well beyond 600 nm (in extreme cases, nearly 700 nm at the peak). This comes at a huge cost in sensitivity, because the filters block almost the entire absorption range of visual pigments, leaving only the very tip of the long-wavelength tail of the absorption bandpass to capture light; the overall effect is to reduce potential photon capture by well over 90%. This penalty is perhaps acceptable in the bright, broad-spectrum illumination of intertidal or barely subtidal habitats on coral reefs. It becomes truly problematical as long-wavelength light is filtered away at even moderate depths, compounding very low sensitivity with similarly low photon availability. Many mantis shrimps inhabit water far below the intertidal, with some species common at depths exceeding 50 m. They also exist at moderate depths in coastal waters, where water transparency is distinctly lower than that of coral reefs. Maintaining visual function in these habitats is a challenge, one that many stomatopod species have solved.
There are three ways that long-wavelength photoreception might be improved when light becomes dim and blue-shifted without major optical changes in ommatidial design. The first is to reduce overall filter absorption, most easily by limiting filter length. This has not been studied across species systematically, but it is known that in several species of mantis shrimps that occupy a large depth range, individuals found at greater depths have shorter filters, at least in the third midband row (which has the longest-wavelength sensitivity range) [20]. More often, species use the second solution, changing the filter sets used. Thus, deeper-living types having blue-shifted filter sets, particularly in the third row [17,18,26]. No species has ever been found to completely lose any type of filter in deep water. In fact, in most species that have the full set of four filter classes, the entire long-wavelength photoreceptor set degenerates when light becomes limited [27,28]. Apparently, if too few photons are captured by a particular photoreceptor, then it simply withers away. Species in stomatopod superfamilies with fewer than four filter classes could in a way be pre-adapted for life in more challenging photic environments. Indeed, lysiosquilloids (with two or three types of filters) and hemisquilloids (with two types) frequently inhabit murky coastal waters and are often found at depths beyond 10 m, where red light is greatly attenuated.
The other way to adapt to a narrowing spectral environment is to exchange filters for others that have shorter-wavelength transmission properties (in a way, this is like the interspecific patterns mentioned a couple of paragraphs ago, but it would occur in the same species). This is an active mechanism for tuning an individual's visual system, and it is common in stomatopod species that inhabit a range of depths [20,29,30]. Despite their enormous supply of different genes for their visual pigments [13,14], mantis shrimps seem to have a very limited ability to vary the visual pigment complement for tuning to depth [28]. In these animals, while species-level adaptations among visual pigments to environmental light are fairly common [17,26,27], the only known tuning mechanism within species involves changes in filter absorption spectra. Most surprisingly, it can happen in individual animals when they change to a new photic environment, even under laboratory conditions [20,30,31]. The tuning can occur either during development or at any later time in life, and filter absorption spectra can even vary within a single retina (figure 5a,b). It is not known whether this local variation is an individual adaptation within each ommatidium or whether it represents a snapshot of filters changing as an animal shifts light environments (for instance, from a dimly lit environment in the field to the much brighter and broad-spectrum photic world of the laboratory). The flexibility is clearly associated with the ecology of each species, because members of the same genus vary in their ability to adjust; species with restricted depth ranges are the least flexible [20].
Figure 5.
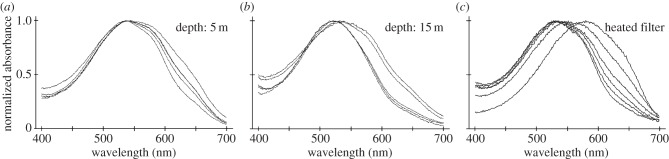
Normalized absorbance spectra taken in cryosections of third-row, proximal intrarhabdomal filters. (a,b) Scans from filters in retinas of two individuals of Gonodactylopsis spongicola, one collected at a depth of 5 m and the other at 15 m, showing the differences between the two sample sets and the variability within a single filter class in a single retina. (c) Successive scans of a single filter (from Neogonodactylus oerstedii) taken after various times of warming the sample on a hotplate. The spectrum of the original, blue-coloured filter (e.g. figure 4b) is the rightmost scan; the final scan of a red-appearing filter is to the left. Note that these spectra are quite similar to those from the untreated cryosections from G. spongicola.
Gonodactylopsis spongicola, some of whose filter spectra are illustrated in figure 5, is a mantis shrimp species inhabiting an unusually large depth range, from near the surface to depths exceeding 50 m. As might be expected, this species has highly flexible tuning of its filter pigments, whereas its visual pigments show no evidence of variation with depth [28,29]. The effects of filter changes on spectral sensitivity are among the most extreme that we have seen, particularly in the proximal tier of the third midband row, the photoreceptor class with the longest-wavelength sensitivity of all (figure 6). By varying its filter pigments in the third row as habitat depth increases, the estimated spectral sensitivity peak of the distal tier is shifted downwards by about 30–40 nm, whereas the proximal tier shifts by about 75 nm. The blue shift moves the sensitivity range into the range of available light, and perhaps more significantly, it exposes more of the long-wavelength tails of visual pigments in this row. The effect can increase photon capture in photoreceptors of the proximal tier by an estimated factor of more than 30 times [29], clearly an enormous advantage in dim waters deficient in long-wavelength photons.
Figure 6.
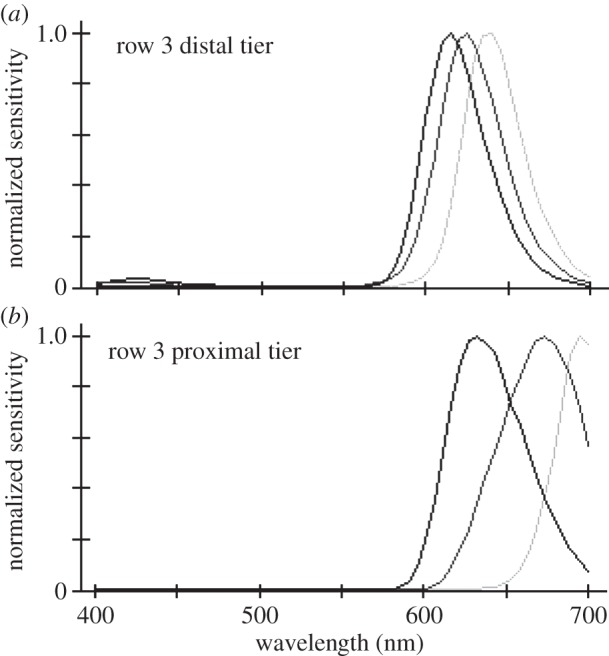
(a,b) Normalized sensitivity spectra of distal and proximal tier rhabdoms from the third ommatidial row of midbands of individual Gonodactylopsis spongicola collected at various depths from near the surface to approximately 32 m depth. The light grey curve is from the shallow-living individual, the mid-grey grey curve for an animal living at moderate depth, and the black curve for the deep-living individual. Note how changes in filter pigments greatly affect tuning of underlying photoreceptors. The spectra were computed as for figure 3b.
At present, the mechanisms used in adaptive filter tuning are unknown, but they are presumably related to the level of stimulation of receptor classes and are not a response to environmental spectra as such. Limiting the spectral environment to blue wavelengths, or just reducing lighting levels overall without spectral changes, produces similar filter shifts under laboratory conditions [30]. Therefore, it appears that receptors respond somehow via their adaptation state, perhaps through a signal related to phototransduction or physiological adaptation. Some, and possibly all, tuning apparently involves changes either in carotenoprotein aggregations or in the way the chromophore is associated with the protein. The astaxanthin-binding pigment crustacyanin, for example, changes from a bluish tone to a red–purple colour as it changes from a polymeric state (the α-form) to a dimeric association (the β-form) [25,32], and if the protein is denatured then the red colour of the chromophore appears. Filters that are gently heated, or even stored on microscope slides in the dark, produce colour changes similar to those seen in vivo (figures 5c and 7). During these changes, the intermediate spectra that appear can be matched by spectra generated from binary mixes of the two end-products [16], and they look remarkably similar to the various filter spectra measured from variable filters in retinal sections (figure 5a,b). Note that the fixed filters on the slides (figure 7) passed from blue through purple (presumably β-crustaxanthin) to red, which is the colour of astaxanthin. As mentioned already, if the light environment cannot provide sufficient photons to maintain the photoreceptor, then the entire photoreceptive region of the ommatidium degenerates.
Figure 7.
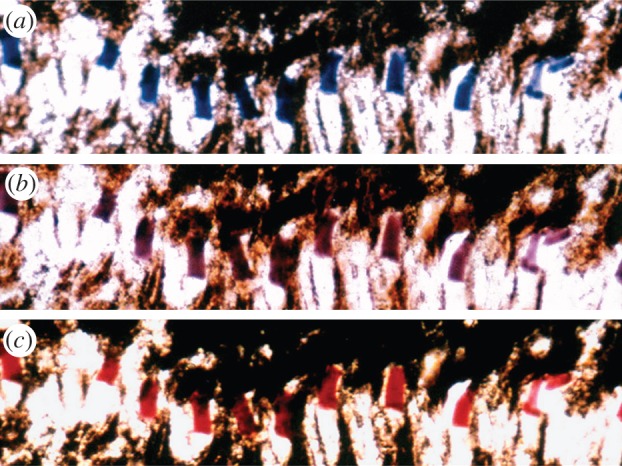
Photographs taken at various times of a cryosection from a retina of Neogonodactylus oerstedii in mounting medium on a microscope slide. The section shows a series of proximal filters from the third ommatidial row. (a) A photograph of the freshly mounted section. (b) The same section a few days later, and (c) again after several weeks. As the filter slowly denatured on the slide, its transmitted colour changed from blue through purple to red, following the same series of changes as the heated filter in figure 5c.
5. Filtering of ultraviolet photoreceptors
In addition to the filtering properties evident in the main rhabdom photoreceptors of mantis shrimp, there is also compelling evidence for the presence of ultraviolet filters that affect the spectral sensitivity of the R8 receptors. In two species of gonodactyloids, Neogonodactylus oerstedii and O. scyllarus, electrophysiological recordings of the R8s have revealed at least five spectrally distinct sensitivities, peaking between 310 and 380 nm [33,34]. Their sensitivities could be modelled by pairwise combinations of various UV visual pigments having different λmax values with different long-pass filters assumed to be located in the cornea, crystalline cones or the actual rhabdomeres [33]. However, only a single ultraviolet visual pigment has previously been identified in mantis shrimp, with a λmax of 330 nm [12], and transcriptome sequencing suggests the presence of a maximum of two short-wavelength-sensitive opsin transcripts expressed in mantis shrimp retinas [14]. Coupled with the extremely narrow sensitivity spectra evident in certain populations of R8s, particularly the four receptor types that peak between 310 and 340 nm, these observations suggest that mantis shrimp use specialized ultraviolet long- and also short-pass optical filters located laterally or distally to the R8s, perhaps in the cornea or crystalline cones.
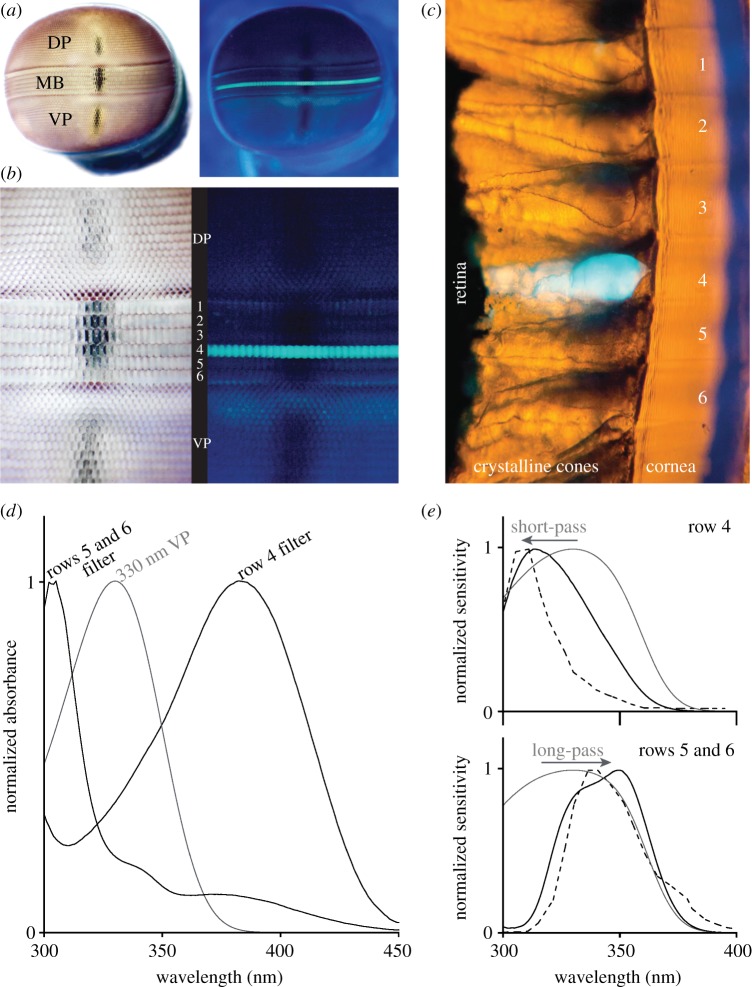
The presence of specialized ultraviolet filters is also demonstrated by fluorescence photography and microscopy of mantis shrimp eyes. If eyes of members of the genus Odontodactylus are illuminated with ultraviolet light, the fourth row of the midband emits brilliant blue-green fluorescence (figure 8a,b). Significantly, the fluorescence overlies the pseudo-pupil, indicating that an ultraviolet-absorbing pigment is situated in the optical path. Cross sectioning of the retina localizes the pigment to the crystalline cones (figure 8c). MSP examination of thick tangential sections (200 μm) of the crystalline cone layer of O. scyllarus reveals the presence of at least two different ultraviolet filters (figure 8d). The fourth midband row contains one of these pigments, with an absorption λmax of 382 nm and a maximum OD of 1.3. In addition, the crystalline cones of the fifth and sixth midband rows share another pigment with a λmax of 305 nm and a maximum OD of 1.7. Neither of these filter pigments absorbs light beyond 450 nm. Crystalline cones in the first, second and third midband rows in O. scyllarus may contain additional ultraviolet filters, but the pigments appear to denature rapidly following sectioning, making it difficult to characterize them.
Figure 8.
Ultraviolet filters in Odontodactylus scyllarus. (a,b) The eye (a) and a magnified view of the midband region (b) of O. scyllarus under white (left panel) and ultraviolet (right panel) illumination. The fourth midband row fluoresces blue-green under ultraviolet illumination and overlays the dark pseudo-pupil, indicating that the fluorophore lies in the optical path. (c) A cross section of the midband of O. scyllarus with yellow transmitted illumination and ultraviolet epi-illumination, indicating that the blue-green fluorescence in midband row four is localized to the crystalline cones. (d) Normalized absorbance spectra of the crystalline cones in midband rows four, five and six (black lines), and the absorbance spectra of a hypothetical 330 nm visual pigment (VP) [12] expressed in the underlying R8s (grey line). The absorbance spectra were measured using microspectrophotometry with 200-μm-thick sections of the crystalline cone layer oriented along their optical path. (e) Tuning effects of the crystalline cone ultraviolet filters found in midband rows four (top panel) and five and six (bottom panel) on a hypothetical shared visual pigment with a λmax of 330 nm (grey line, plotting absorptance of the R8, computed using rhabdom lengths measured in [7]). The plot shows the modelled normalized sensitivity of the R8 (black line, computed from the absorptance of the R8 and the transmittance of the filter) versus the spectral sensitivities measured electrophysiologically (dashed line, adapted from [34]).
The ultraviolet-absorbing pigments in rows four through six could serve, respectively, as long- and short-pass optical filters for a single hypothetical 330 nm visual pigment shared across the underlying R8s (figure 8e). In the fourth midband row, the filter acts as a short pass filter, attenuating the longer ultraviolet wavelengths and shifting the maximum sensitivity of the R8 receptor down from 330 to 315 nm. To the best of our knowledge, this is the first example of the use of a short-pass biological optical filter in nature. Conversely, the filter in midband rows five and six acts as a long-pass filter on the same 330 nm visual pigment, attenuating short ultraviolet light and shifting the maximum sensitivity of the R8 rhabdomere from 330 to 340 nm. Thus, in these midband rows, mantis shrimp create two spectrally distinct ultraviolet receptors incorporating the same visual pigment, but with either short- or long-pass filters in the overlying crystalline cones. Additionally, the pigment in the cone of the fourth row probably serves double duty, acting as a short-pass filter for the underlying R8 as described above, as well as a long-pass filter for the violet receptor in the distal tier of the main rhabdom of that row, which expresses a visual pigment with a λmax of 429 nm (figure 2).
6. Other types of filters used in stomatopod retinas
(a). Lateral filters
So far, we have reviewed filter types used by mantis shrimps that are placed in series with photoreceptors, so that light must transit the filter before reaching underlying photoreceptors. In addition to these, mantis shrimp retinas are abundantly endowed with many other strongly coloured filtering materials that act to tune photoreceptors in less direct ways [8]. A particularly diverse set of pigments is used as lateral filters, which lie along the edges of photoreceptors (figure 4). Filters such as these occur in other crustacean retinas [35–37], and they are used effectively by butterflies to sharpen spectral sensitivities in their tiered colour receptors [38,39]. In stomatopods, the lateral filters ensheath rhabdoms, extending into the palisade bridges immediately adjacent to the photosensitive microvilli. Unlike the pupillary pigments in stomatopods, which exist near the distal tip of the rhabdom and migrate for rapid light and dark adaptation [40,41], or the pigments that line crab rhabdoms [35,36], the screening pigments are thought to be relatively fixed in position. Being optically coupled to the rhabdom, they act both to sharpen and to tune spectral sensitivities of enshrouded photoreceptors by capturing light travelling in the evanescent wave along the lengths of photoreceptors [8,39,42].
The lateral pigments are potentially powerful spectral filters when their absorbance spectra vary sharply with wavelength (making them appear strongly coloured), and pigments such as these are particularly common in lysiosquilloid retinas (figure 4c), although they are found in those of other stomatopods. In Coronis scolopendra, bright red pigment granules line the distal rhabdoms in the third midband row (figure 4c). Their absorbance spectrum resembles that of the red distal intrarhabdomal filter in that row, suggesting that they probably contain the same substance, and modelling indicates that they could effectively modify the spectral sensitivities of their associated photoreceptors [8]. A similar situation exists in distal tiers of third row receptors in the hemisquilloid Hemisquilla californiensis (figure 4d). The use of these pigments in regions adjacent to rhabdoms, in the same locations as other pigments in various decapod crustaceans, suggests to us that they could have been an intermediate state in the evolution of the more effective intrarhabdomal filters. Significantly, both the lysiosquilloids and hemisquilloids lack proximal intrarhabdomal filters in the rows where these pigments are found, whereas gonodactyloids (which have the proximal filter class) have not been found to employ strongly coloured lateral filters in their retinas [8]. One advantage of lateral filtering is that it does not reduce overall sensitivity as much as dense intrarhabdomal filters, an advantage for vision in the deeper-living lysiosquilloids and hemisquilloids (which also tend to inhabit more turbid waters than do gonodactyloids).
(b). Larval retinal pigments
The retinas of larvae of some mantis shrimp species contain colourful pigments in the retinular cells. These are well characterized only in larvae of two species of the lysiosquilloid Pullosquilla [43]. The pigment forms clumps of bright yellow material that absorbs light very similarly to some of the filter pigments of adult retinas of various stomatopods, suggesting that it could similarly be a carotenoid or (more likely) a carotenoprotein. The fact that the pigment is found mostly in the distal regions of retinular cells, near the top of the retina, and that it surrounds the retina, indicates that it could not serve to filter light entering the rhabdom but rather acts as a lateral screen. However, its absorption spectrum completely overlaps the larval rhodopsin's spectrum, ruling out the possibility that it is a lateral filter. Rather, it appears really to be a screening pigment, taking over (at least in part) from the dark ommochromes the role of blocking stray light from entering the rhabdom off-axis. Based on the yellow pigment's spectral absorption and its location in retinular cells, Jutte et al. [43] offer it as another candidate ancestral form of what eventually became the intrarhabdomal filters of adult stomatopods.
The role of this pigment in the larvae is likely to be associated with the strong selective pressure on pelagic animals to be as invisible as possible, and the typical solution for plankton is to become transparent [44]. Because retinal photoreceptors (and screening pigments) must absorb light to function, obviating any possibility of complete transparency, they can at least reduce light absorption to just that required for the visual pigment. The spectrally matched yellow larval pigments might act in this way, providing transparency outside the visual pigment's absorption band. In conjunction with reflecting pigments at the retina's outer margins, larval stomatopod eyes could be nearly invisible against the bright background light, giving them some protection against visual predators.
(c). Birefringent filters: a role in polarization vision
The final example of filtering in mantis shrimp retinas takes us from the realm of spectral filtering to polarizational filtering. Stomatopods have an unexcelled diversity of polarized-light receptor classes [7], rivalling (or even exceeding) their spectral photoreceptor diversity. Some species can analyse and behaviourally respond to circularly polarized light, an ability found thus far in no other animal [45]. The circular polarization photoreceptors are created by filtering light before it enters conventional linear polarization receptors, which are built like those of all other crustaceans (and many other animals) using orthogonal arrays of parallel microvilli [7]. The filtering, amazingly, is itself done by a parallel microvillar array, the photoreceptive microvilli of the R8 cells of the two ventral rows of the midband (figure 2a). Their inherent birefringence (produced by organized lipids in membranes) and form birefringence (produced by the microvillar arrays themselves) together, given the length of the R8 rhabdomere, produce a nearly achromatic quarter-wave delay filter [45,46]. The filter converts circularly polarized light to linearly polarized light, which can then be analysed by the underlying typical crustacean polarization ommatidia. This is the only known example of a photoreceptor for one visual modality (ultraviolet sensitivity) acting as a filter for a different one (circular polarization sensitivity).
7. Summary and conclusion
Stomatopod crustaceans or mantis shrimps have geometrically complex arrangements of ommatidia in their apposition compound eyes. They similarly have perhaps the most complex assemblage of retinal photoreceptor types of all animals. Species with six ommatidial rows in the midbands of their compound eyes create 16 functional classes of photoreceptors using unusual receptor arrangements, including two or three tiers of rhabdom segments in each ommatidium—an R8 cell on top of every rhabdom and a main rhabdom of seven retinular cells formed into one or two tiers. The arrangement creates abundant opportunities for spectral filtering and tuning of photoreceptors, which is amplified by the use of photostable filter pigments paired with specific sets of photoreceptors. The pigments that filter the ultraviolet receptor classes exist in the crystalline cones of appropriate ommatidia, whereas those acting on the visual pigments in the main rhabdoms are inserted between the receptor tiers in ommatidia of the second and third rows of the midband. Thus, filters operate at several levels in many ommatidia, and particularly in those of the midband. The tiering alone, even in the absence of the filters, permits visual pigments in the R8 photoreceptors and the distal tiers to filter light reaching deeper levels of an ommatidium.
Photostable pigments are also used as lateral filters lining the rhabdoms of some species, and even occur in some larval types. The pigments of the intrarhabdomal filters (i.e. those within actual rhabdoms) and of the lateral filters are variable among species and the intrarhabdomal filters can even change within an individual mantis shrimp to adapt to a specific photic environment. As a result, there is adaptation between species and often within species to tune photoreceptors to the spectral properties of the environments inhabited by these animals.
While photoreceptor tuning is widespread among animals, both vertebrate and invertebrate, the mantis shrimps seem to have taken it to an extreme, even incorporating polarization filters in eyes of some species to form visual receptors sensitive to circularly polarized light. At present, however, we know rather little about the specific pigments used in the filters and even less concerning how the filters develop, how they become fitted into the rhabdoms, and how they are altered when the receptor's stimulating light environment changes. The evolution, physiology and biochemistry of these unusual optical devices are fertile research topics.
Acknowledgement
Much of the research reported here was made possible by the Lizard Island Research Station in Queensland, Australia.
Funding statement
This work is based on research supported by the US National Science Foundation, The Australian Research Council, The Asian Office of Aerospace Research and Development and also by the Air Force Office of Scientific Research, most recently under grant nos. AOARD-12-4063 and FA9550-12-1-0321.
References
- 1.Caldwell RL, Dingle H. 1975. Ecology and evolution of agonistic behavior in stomatopods. Naturwissenschaften 65, 214–222. ( 10.1007/BF00603166) [DOI] [Google Scholar]
- 2.Manning RB, Schiff H, Abbott BC. 1984. Eye structure and the classification of stomatopod Crustacea. Zool. Scr. 13, 41–44. ( 10.1111/j.1463-6409.1984.tb00021.x) [DOI] [Google Scholar]
- 3.Harling C. 2000. Re-examination of eye design in the classification of stomatopod crustaceans. J. Crust. Biol. 20, 172–185. [Google Scholar]
- 4.Porter ML, Zhang Y, Desai S, Caldwell RL, Cronin TW. 2010. Evolution of anatomical and physiological specialization in the compound eyes of stomatopod crustacans. J. Exp. Biol. 213, 3473–3486. ( 10.1242/jeb.046508) [DOI] [PubMed] [Google Scholar]
- 5.Marshall NJ. 1988. A unique colour and polarization vision system in mantis shrimps. Nature 333, 557–560. ( 10.1038/333557a0) [DOI] [PubMed] [Google Scholar]
- 6.Cronin TW, Marshall NJ. 1989. A retina with at least ten spectral types of photoreceptors in a stomatopod crustacean. Nature 339, 137–140. ( 10.1038/339137a0) [DOI] [Google Scholar]
- 7.Marshall NJ, Land MF, King CA, Cronin TW. 1991. The compound eyes of mantis shrimps (Crustacea, Hoplocarida, Stomatopoda). I. Compound eye structure: the detection of polarized light. Phil. Trans. R. Soc. Lond. B 334, 33–56. ( 10.1098/rstb.1991.0096) [DOI] [Google Scholar]
- 8.Marshall NJ, Land MF, King CA, Cronin TW. 1991. The compound eyes of mantis shrimps (Crustacea, Hoplocarida, Stomatopoda). II. Coloured pigments in the eyes of stomatopod crustaceans: polychromatic vision by serial and lateral filtering. Phil. Trans. R. Soc. Lond. B 334, 57–84. ( 10.1098/rstb.1991.0097) [DOI] [Google Scholar]
- 9.Hyatt GW. 1975. Physiological and behavioral evidence for color discrimination by fiddler crabs (Brachyura, Ocypodidae, genus Uca). In Physiological ecology of estuarine organisms (ed. Vernberg FJ.), pp. 333–365. Columbia, SC: University of South Carolina Press. [Google Scholar]
- 10.Baldwin J, Johnsen S. 2012. The male blue crab, Callinectes sapidus, uses both chromatic and achromatic cues during mate choice . J. Exp. Biol. 215, 1184–1191. ( 10.1242/jeb.067512) [DOI] [PubMed] [Google Scholar]
- 11.Cronin TW. 1985. The visual pigment of a stomatopod crustacean, Squilla empusa . J. Comp. Physiol. 156, 679–687. ( 10.1007/BF00619117) [DOI] [Google Scholar]
- 12.Cronin TW, Marshall NJ, Quinn CA, King CA. 1994. Ultraviolet photoreception in mantis shrimp. Vision Res. 34, 1443–1452. ( 10.1016/0042-6989(94)90145-7) [DOI] [PubMed] [Google Scholar]
- 13.Porter ML, Bok M, Robinson PR, Cronin TW. 2009. Molecular diversity of visual pigments in Stomatopoda (Crustacea). Vis. Neurosci. 26, 255–266. ( 10.1017/S0952523809090129) [DOI] [PubMed] [Google Scholar]
- 14.Porter ML, Speiser DI, Zaharoff S, Caldwell RL, Cronin TW, Oakley TH. 2013. The evolution of complexity in the visual systems of stomatopods: insights from transcriptomics. Integr. Comp. Biol. 53, 39–49. ( 10.1093/icb/ict060) [DOI] [PubMed] [Google Scholar]
- 15.Barlow HB. 1982. What causes trichromacy? A theoretical analysis using comb-filtered spectra. Vision Res. 22, 635–643. ( 10.1016/0042-6989(82)90099-2) [DOI] [PubMed] [Google Scholar]
- 16.Cronin TW, Marshall NJ, Caldwell RL. 1994. The intrarhabdomal filters in the retinas of mantis shrimps. Vision Res. 34, 279–291. ( 10.1016/0042-6989(94)90087-6) [DOI] [PubMed] [Google Scholar]
- 17.Cronin TW, Marshall NJ, Caldwell RL, Shashar N. 1994. Specialization of retinal function in the compound eyes of mantis shrimps. Vision Res. 34, 2639–2656. ( 10.1016/0042-6989(94)90221-6) [DOI] [PubMed] [Google Scholar]
- 18.Cronin TW, Marshall J. 2004. The unique visual world of mantis shrimps. In Complex worlds from simpler nervous systems (ed. Prete FR.), pp. 239–268. Cambridge, MA: MIT Press. [Google Scholar]
- 19.Douglas RH, Marshall NJ. 1999. A review of vertebrate and invertebrate ocular filters. In Adaptive mechanisms in the ecology of vision (eds Archer SA, Djamgoz MBA, Loew ER, Partridge JC, Vallerga S.), pp. 95–162. London, UK: Kluwer Academic Publishers. [Google Scholar]
- 20.Cheroske AG, Barber PH, Cronin TW. 2006. Evolutionary variation in the expression of phenotypically plastic color vision in Caribbean mantis shrimps, genus Neogonodactylus . Mar. Biol. 150, 213–220. ( 10.1007/s00227-006-0313-5) [DOI] [Google Scholar]
- 21.Lipetz L. 1984. Pigment types, densities and concentrations in cone oil droplets of Emydoidea blandingii . Vision Res. 24, 605–612. ( 10.1016/0042-6989(84)90115-9) [DOI] [PubMed] [Google Scholar]
- 22.Goldsmith TH, Collins JS, Licht S. 1984. The cone oil droplets of avian retinas. Vision Res. 24, 1661–1671. ( 10.1016/0042-6989(84)90324-9) [DOI] [PubMed] [Google Scholar]
- 23.Cheeseman DF, Lee WL, Zagalsky PF. 1990. Carotenoproteins in invertebrates. Biol. Rev. 42, 132–160. [Google Scholar]
- 24.Zagalsky PF, Ceccaldi JJY, Daumas R. 1970. Comparative studies on some decapod crustacean carotenoproteins. Comp. Biochem. Physiol. 34, 579–607. ( 10.1016/0010-406X(70)90287-2) [DOI] [PubMed] [Google Scholar]
- 25.Zagalsky PG, Eliopoulos EE, Findlay JBC. 1990. The architecture of invertebrate carotenoproteins. Comp. Biochem. Physiol. B 97, 1–81. ( 10.1016/0305-0491(90)90171-O) [DOI] [PubMed] [Google Scholar]
- 26.Cronin TW, Marshall NJ, Caldwell RL. 2000. Spectral tuning and the visual ecology of mantis shrimps. Phil. Trans. R. Soc. Lond. B 355, 1263–1267. ( 10.1098/rstb.2000.0680) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cronin TW, Marshall NJ, Caldwell RL. 1996. Visual pigment diversity in two genera of mantis shrimps implies rapid evolution (Crustacea; Stomatopoda). J. Comp. Physiol. A 179, 371–384. ( 10.1007/BF00194991) [DOI] [Google Scholar]
- 28.Cronin TW, Caldwell RL, Erdmann M. 2002. Tuning of photoreceptor function in mantis shrimp species that inhabit a range of depths. I. Visual pigments. J. Comp. Physiol. A 188, 179–186. ( 10.1007/s00359-002-0291-0) [DOI] [PubMed] [Google Scholar]
- 29.Cronin TW, Caldwell RL. 2002. Tuning of photoreceptor function in three mantis shrimp species that inhabit a range of depths. II. Filter pigments. J. Comp. Physiol. A 188, 187–197. ( 10.1007/s00359-002-0292-z) [DOI] [PubMed] [Google Scholar]
- 30.Cheroske AG, Cronin TW, Caldwell RL. 2003. Adaptive color vision in Pullosquilla litoralis (Stomatopoda, Lysiosquilloidea) associated with spectral and intensity changes in light environment. J. Exp. Biol. 206, 373–379. ( 10.1242/jeb.00084) [DOI] [PubMed] [Google Scholar]
- 31.Cronin TW, Caldwell RL, Marshall NJ. 2001. Tunable color vision in a mantis shrimp. Nature 411, 547–548. ( 10.1038/35079184) [DOI] [PubMed] [Google Scholar]
- 32.Ciani M, Rizkallah PJ, Olczak A, Raftery J, Chayen NE, Zagalsky PF, Helliwell JR. 2002. The molecular basis of the coloration mechanism in lobster shell: β-Crustacyanin at 3.2-Å resolution. Proc. Natl Acad. Sci. USA 99, 9795–9800. ( 10.1073/pnas.152088999) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marshall J, Oberwinkler J. 1999. The colourful world of the mantis shrimp. Nature 401, 873–874. ( 10.1038/44751) [DOI] [PubMed] [Google Scholar]
- 34.Kleinlogel S, Marshall NJ. 2009. Ultraviolet polarisation sensitivity in the stomatopod crustacean Odontodactylus scyllarus. J. Comp. Physiol. A 195, 1153–1162. ( 10.1007/s00359-009-0491-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leggett LMW. 1979. A retinal substrate for colour discrimination in crabs . J. Comp. Physiol. 133, 159–166. ( 10.1007/BF00657531) [DOI] [Google Scholar]
- 36.Stowe S. 1980. Spectral sensitivity and retinal pigment movement in the crab Leptograpsus variegatus (Fabricus). J. Exp. Biol. 87, 73–98. [DOI] [PubMed] [Google Scholar]
- 37.Jordão JM, Cronin TW, Oliveira RF. 2007. Spectral sensitivity of four species of fiddler crabs (Uca pugnas, Uca pugilator, Uca vomeris, and Uca tangeri) measured by in situ microspectrophotometry. J. Exp. Biol. 210, 447–453. ( 10.1242/jeb.02658) [DOI] [PubMed] [Google Scholar]
- 38.Arikawa K, Stavenga DG. 1997. Random array of colour filters in the eyes of butterflies. J. Exp. Biol. 200, 2501–2506. [DOI] [PubMed] [Google Scholar]
- 39.Arikawa K, Scholten DGW, Kinoshita K, Stavenga DG. 1999. Tuning of photoreceptor spectral sensitivities by red and yellow pigments in the butterfly Palilio xuthus . Zool. Sci. 16, 17–24. ( 10.2108/zsj.16.17) [DOI] [Google Scholar]
- 40.Cronin TW. 1989. Application of intracellular optical techniques to the study of stomatopod crustacean vision. J. Comp. Physiol. A 164, 737–749. ( 10.1007/BF00616746) [DOI] [Google Scholar]
- 41.King CA, Cronin TW. 1993. Cytoskeleton of retinular cells from the stomatopod, Gonodactylus oerstedii: possible roles in pigment granule migration. Cell Tissue Res. 274, 315–328. ( 10.1007/BF00318750) [DOI] [Google Scholar]
- 42.Snyder AW, Menzel R, Laughlin SB. 1973. Structure and function of the fused rhabdom. J. Comp. Physiol. 87, 99–135. ( 10.1007/BF01352157) [DOI] [Google Scholar]
- 43.Jutte PA, Cronin TW, Caldwell RL. 1998. Photoreception in the planktonic larvae of two species of Pullosquilla, a lysiosquilloid stomatopod crustacean. J. Exp. Biol. 201, 2481–2487. [DOI] [PubMed] [Google Scholar]
- 44.Johnsen S. 2001. Hidden in plain sight: the ecology and physiology of organismal transparency. Biol. Bull. 201, 301–318. ( 10.2307/1543609) [DOI] [PubMed] [Google Scholar]
- 45.Chiou T-H, Kleinlogel S, Cronin T, Caldwell R, Loeffler B, Siddiqi A, Goldizen A, Marshall J. 2008. Circular polarization vision in a stomatopod crustacean. Curr. Biol. 18, 429–434. ( 10.1016/j.cub.2008.02.066) [DOI] [PubMed] [Google Scholar]
- 46.Roberts NW, Chiou T-H, Marshall NJ, Cronin TW. 2009. A biological quarter-wave retarder with excellent achromaticity in the visible wavelength region. Nat. Photon. 3, 641–644. ( 10.1038/nphoton.2009.189) [DOI] [Google Scholar]