Abstract
We caught solitary foragers of the Australian Jack Jumper ant, Myrmecia croslandi, and released them in three compass directions at distances of 10 and 15 m from the nest at locations they have never been before. We recorded the head orientation and the movements of ants within a radius of 20 cm from the release point and, in some cases, tracked their subsequent paths with a differential GPS. We find that upon surfacing from their transport vials onto a release platform, most ants move into the home direction after looking around briefly. The ants use a systematic scanning procedure, consisting of saccadic head and body rotations that sweep gaze across the scene with an average angular velocity of 90° s−1 and intermittent changes in turning direction. By mapping the ants’ gaze directions onto the local panorama, we find that neither the ants’ gaze nor their decisions to change turning direction are clearly associated with salient or significant features in the scene. Instead, the ants look most frequently in the home direction and start walking fast when doing so. Displaced ants can thus identify home direction with little translation, but exclusively through rotational scanning. We discuss the navigational information content of the ants’ habitat and how the insects’ behaviour informs us about how they may acquire and retrieve that information.
Keywords: visual homing, scanning behaviour, ants, Myrmecia croslandi
1. Introduction
The fact that insects rely on visual memories when returning to places, such as their nests, is well documented (see below). It is less well known that Land & Collett [1] made one of the earliest suggestions about how insects could derive navigational instructions from a comparison of remembered and current views that would guide them back to a place. Provided an insect remembered the apparent size of an array of landmarks as seen from the goal location, it would find back to that location by moving away from landmarks that appeared larger than remembered and by moving towards landmarks that appeared smaller than remembered. Cartwright & Collett [2] later formalized and successfully simulated an extended version of this computational homing mechanism and, since then, many variants have been implemented in simulations and on robotic platforms (reviewed in [3,4]).
It has also become clear since then that panoramic snapshots can provide navigational information without the need for recognizing individual objects, because locations in the natural world are uniquely defined by the visual panorama seen at these locations [5,6]. However, at least two important aspects of insect homing remain little or are just beginning to be understood: the rules that govern the active acquisition of visual spatial memories during the learning flights and the learning walks insects perform when leaving a place for the first time, and the detailed way in which the comparison between learnt views and current scenes provides instructions to guide an insect's return to a goal location (reviewed in [7]; see also [8,9]). The problem with investigating the use of visual memories lies in the need to record in fine spatio-temporal detail an animal's movements, including where it directs its gaze (much like in the pioneering studies by Land and Collett [10,11] on flight control in flies), and to have knowledge of and control over the navigational information available to the animal. Until recently, this has only been possible under controlled laboratory conditions (for review, see [12]), but with the introduction of differential GPS, high-speed digital video, panoramic imagers and three-dimensional modelling tools, it has now become feasible under field conditions to precisely track the movements of navigating insects, in particular ants, and at the same time to quantify the visual information available to them [13–16].
Here, we investigate in the Australian Jack Jumper ant, Myrmecia croslandi, the way in which displaced foragers behave when they first encounter the scene at locations they are unlikely to have been before. How do they recognize where they are, whether they are lost or where they need to go?
Ants of M. croslandi forage individually for insect prey and for sugar secretions from plant-sucking insects, with no evidence of recruitment by trail pheromones or by interaction with other foragers. Foragers from one nest typically move 5–15 m to the closest tree or hunt for insects on the ground within a radius of 2–3 m from the nest [16,17]. When displaced to locations they are unlikely to have been before, 10–15 m away from the nest, most foragers are able to home directly from all compass directions [16]. They do so regardless of the state of their navigational knowledge: ants that were caught at the bottom of their favourite tree possess information on home direction from both path integration and the landmark panorama (full-vector (FV) ants) that is in conflict when they are displaced to locations far away from their normal foraging corridor [16]. This conflict must exist, because M. croslandi foragers do path integrate and walk into the direction of their home vector when released 100 m away from their nest in a landmark-poor environment [16]. In homing directly from all compass directions within 10–15 m from the nest, displaced full-vector ants must ignore the home direction indicated by their path integration system. No such conflict exists for foragers that are caught just before they enter the nest (zero-vector (ZV) ants) when they have run out their path integration vector. Zero-vector ants are also able to home directly from all release stations within 10–15 m from the nest.
We have shown previously that this ability can be explained (as suggested by Graham et al. [18]) by assuming that the ants have memorized nest-oriented panoramic views from a few metres distance at different bearings from the nest [16]. The organization of learning walks in ants suggests that they are designed to systematically acquire such nest-directed views. During the learning walks of Ocymyrmex robustior [19], of the wood ant Formica rufa [20] and of the Jack Jumper ant M. croslandi [21], departing foragers do not move away from the nest or a newly discovered food site in a straight line, but along a spiral path or along increasing spiral segments. As they move away from the goal, they repeatedly turn back to view the scene across the nest or the food site from different directions, and it has been suggested that the ants store panoramic snapshots at these moments [18–20]. Such memorized views contain two pieces of navigational information [5–7,22]: on nest-directed heading direction and on location in space. In its simplest use, the compass orientation of a reference snapshot can be identified by determining the global image difference (mean-squared or root-mean-squared pixel difference) while rotating that snapshot against the current scene. Even at some distance from the reference location, the resulting rotational image difference functions (rotIDFs) have a minimum at the compass direction of the reference image [5,18,22]. Memorized panoramic views also contain information on location, because image differences increase smoothly with distance from a reference location and within a certain range the reference location can be found by moving and comparing views, essentially by a gradient descent in image differences [3,5,23]. We have shown in our previous analysis that in the open grassy woodland habitat of M. croslandi, rotating such reference images against the scene viewed from locations up to 15 m from the nest results in detectable minima of rotIDFs at bearings that point in the home direction [16].
When displaced ants surface from their transport vials onto a release platform, they look around briefly and within less than 30 s (average 12 s) traverse the 20 cm from the release point to the edge of the platform and exit in approximately the home direction [16]. Here, we ask whether the scanning movements of ants in these first crucial seconds, during which they so successfully determine where they need to go, allow us to identify how ants resolve a conflict between path integration and landmark information, what scene features they attend to and how they compare memorized views with the current scene. We pay particular attention to the relationship between gaze directions and the navigational information content of the scene at six different release stations. Do displaced ants correct a mismatch between remembered and current view by pre-programmed and targeted changes in orientation, as have been described for wood ants [12,24], do they preferentially fixate salient or familiar features in the landmark panorama or do they scan the scene in a way that suggests a more global comparison of views, independent of distinct features in the landmark panorama?
2. Material and methods
(a). Study species
Jack jumper ants, M. croslandi, are large (ca 1 cm body length) and strictly day-active ants that occur along the east coast of Australia [25]. The ants are individually foraging, with no evidence of scout- or trail pheromone-guided recruitment, and are known for their ability to jump and for their potent sting [26]. They have unusually well-developed compound eyes with each eye having nearly 2400 facets [25]. We studied ants from a single nest (35°15′05.59″ S, 149°09′33.18″ E) in an urban park in Canberra, Australia.
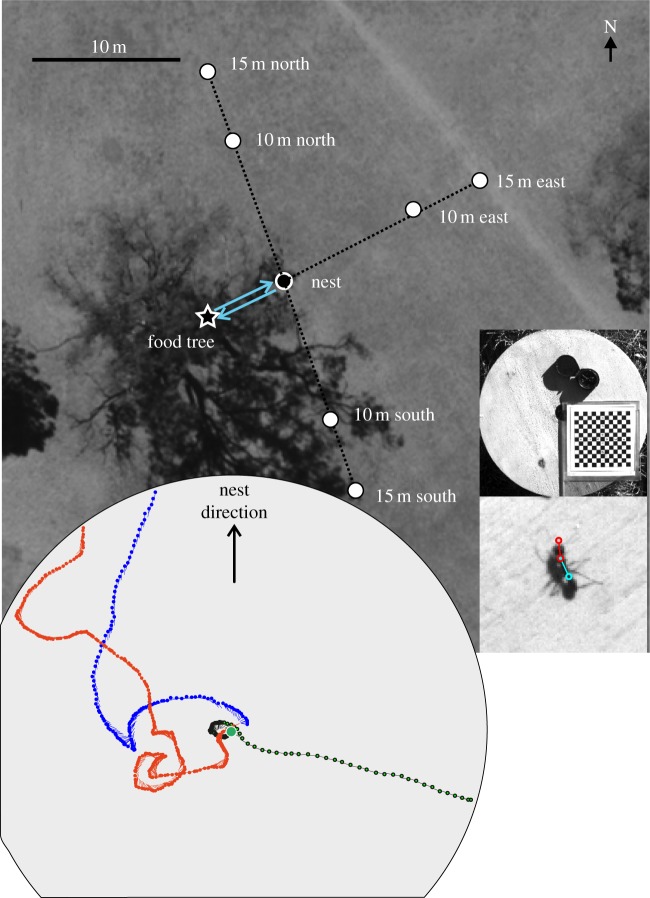
Ants from this nest typically travelled west to a Eucalyptus tree on which they foraged (figure 1). Foraging ants were captured either at the base of the tree (full-vector ants) or as they returned close to the nest (zero-vector ants), in foam-stoppered Perspex tubes. Full-vector ants were first fed with 10% sugar solution (up to 30 min) at the site of capture and were subsequently given live insect prey. Zero-vector ants returning to the nest with prey were released without offering them sugar water. For displacements, the capture tube with an ant was placed in a black nylon sleeve and transferred in the dark to one of six release stations at 10 or 15 m north, south and east of the nest in a random order (figure 1). Overall, we caught and displaced 45 ants on 10 different days between January and April 2013. Full-vector ants were allowed to return to the nest, before we captured them again to release them as zero-vector ants at a different location. Zero-vector ants were in most cases caught after having left the platform at the first release station and transported in the dark to be released a second and a third time at the other release locations. They were finally released close to the nest.
Figure 1.
Experimental area and set-up. Six release stations are shown together with the location of the nest and the nest's foraging tree on an aerial image. The typical foraging corridor for this nest is marked by blue arrows. Square insets on the right show on top the release platform with the central hole to accommodate the transport vials, a compass and a calibration pattern. Bottom inset shows an enlarged image of an ant with red circles indicating the positions on the head and the blue circle the position on the body, the x/y coordinates of which were extracted to determine head and body orientation. Inset on the bottom left shows three example paths of ants on the round release platform with a line indicating head (gaze) orientation pointing into the direction of the dots that mark the position of the front of the head every 40 ms.
(b). Filming technique
Ants were released on a round wooden platform (40 cm diameter) that was raised 15 cm off the ground on aluminium pegs and levelled with a spirit level. The platform had a circular hole in the centre, into which a black nylon sleeve casing including a foam-stoppered Perspex catching tube fitted tightly. Upon release, the foam stopper of the tube was replaced by a flat piece of cardboard with a central hole of 5 mm diameter, through which the ants could reach the platform. We filmed the ants on the platform, including information about true north and nest direction, using a Sony Handycam (HDR-CX550VE, Sony Corp, Japan; at 25 fps, image size 1920 × 1280), a Panasonic Lumix (DMC-FZ200, Panasonic Corp, Osaka, Japan; at 100 fps, image size 1280 × 720) or an Optronis (CR600 × 2, Kehl, Germany; at 200 fps, image size 1024 × 1024) mounted on tripods. We carried out a frame-by-frame analysis at either 40 ms (25 fps) or 5 ms inter-frame interval (200 fps) to determine head and body orientation. For this, we extracted coordinates of head (mandibles), pronotum (first segment of the thorax) and petiole (figure 1 inset) using a custom-written Matlab program (Jan Hemmi & Robert Parker, The Australian National University). Velocity and angular velocity data were smoothed with a five point moving average filter. We estimated the accuracy of determining gaze (head) direction to be within 10°.
(c). Tracking the paths of ants
In a few cases, we tracked the paths of ants after they had left the release platform by placing pins about 20 cm behind each ant, making sure not to disturb their progress. The pin trail was subsequently recorded with differential GPS (for details, see [15,16]).
(d). Capturing panoramic scenes
We recorded panoramic scenes using two methods. In the first, we placed a Sony Bloggie camera (MHS-PM5, Sony Corp., Japan) on the release platform (15 cm off the ground) to record panoramic views at the six release stations and at the nest location. Second, we used a laser scanner/colour camera combination (Z + F IMAGER 5006h, including a colour camera, M-Cam, Zoller + Fröhlich GmbH, Wangen, Germany) to build a three-dimensional model of the ants’ environment and to subsequently generate panoramic views at defined locations within such models (for details, see [27,28]). The GPS coordinates of four landscape features that were easily identifiable in the laser scans were used for aligning the three-dimensional model with the GPS reference system that we used to track ant paths and to locate nest and release sites.
(e). Calculating rotational image difference functions
Concentric panoramic images were un-warped to rectangular panoramas, measuring 2161 × 338 or 1440 × 172 pixels, corresponding to a field of view of 360° × 56°, or 360° × 43°, respectively, with a resolution of six or four pixels per degree, using a custom-written Matlab program. Sun glare and reflection artefacts in the sky were removed by using the colour replacement tool in Corel Photo Paint X5 (Corel Corporation, Ottawa, Canada) to copy adjacent sky patches into the corrupted areas. Eight-bit grey scale images were converted to floating point arrays, low-pass filtered with a 18 × 18 pixel Gaussian filter with σ = 6 pixels (mimicking 1° resolution), or a 36 × 36 pixel Gaussian with σ = 12 pixels (mimicking 3° resolution) before rotIDFs were determined using the Matlab circshift function. For each one pixel shift, the pixel differences were calculated between the reference image and the shifted image, resulting in either 2161 × 338 or 1440 × 172 values that were squared and averaged. For each image shift, we then calculated the root-mean-squared pixel difference.
3. Results
In the following, we document first the unusual mobility of the head in M. croslandi and then go on to ask where animals look when released at unfamiliar locations, whether they behave differently depending on their state as full-vector or zero-vector ants and how their scanning behaviour changes before and after their decision to move. We end by comparing the heading directions of ants on the release platforms immediately upon release and their subsequent paths.
(a). Detailed structure of scanning behaviour
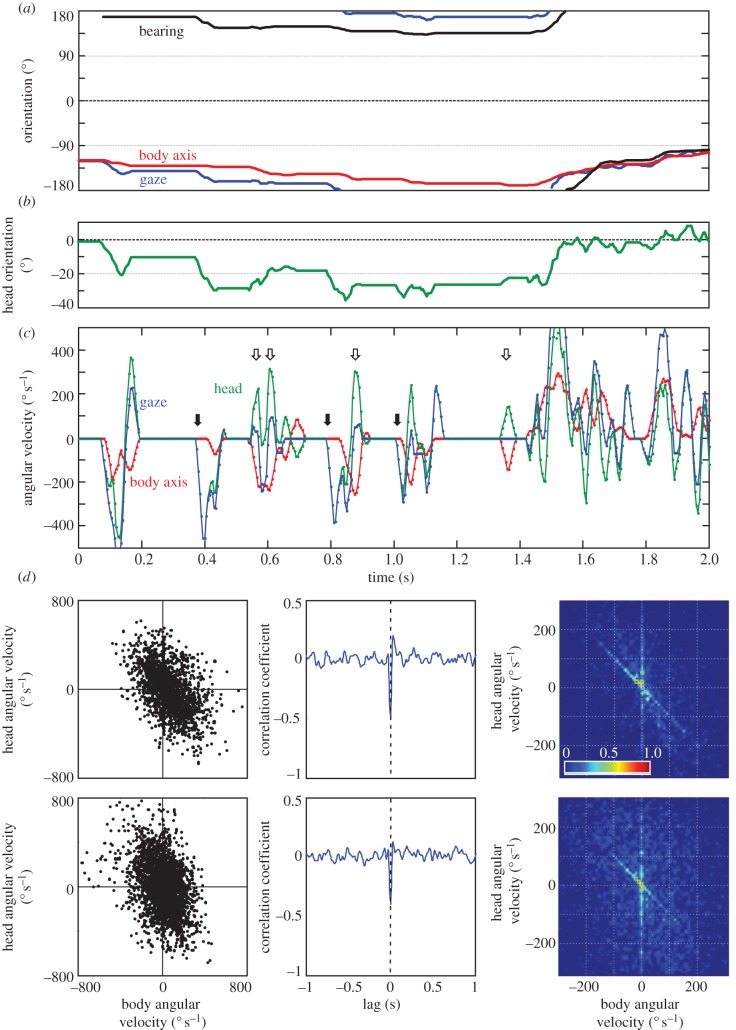
Ants exit their transport vials through a small hole and initially tend to turn on the spot (figure 1 inset). They keep turning in one direction, sometimes through more than 360°, but most often reverse their turning direction earlier. Underlying the fairly constant turning rate with the head turned into the direction of rotation (figure 2a,b) are saccadic head and body movements that interact in complex ways. The time course of head and body angular velocities, illustrated in figure 2c, includes head saccades that occur when the body is at rest, head movements that compensate for the rotation of the body (open arrows), thus keeping gaze direction constant and head saccades at rest that are closely followed by body rotations (black arrows). Head saccades in this example (figure 2c, green line) reach peak angular velocities between 200 and 500° s−1 and body saccades (red line) up to 200° s−1.
Figure 2.
The dynamics of ant scanning movements. (a) Gaze direction (blue), body axis orientation (red) and bearing (black) relative to the release point with the true nest direction at 0° for a 2 s segment about 3 s after the ant had entered the platform. (b) Head orientation relative to the body longitudinal axis for the same sequence. (c) Time course of the angular velocity of the head relative to the body (green), the longitudinal body axis (red) and the gaze direction (blue) during the same sequence as shown in (a,b). Open arrows mark instances when head movements compensate for body rotations and black arrows mark instances when the head rotation is followed by body rotation. Sampling rate was 200 fps. (d) The relationship between the angular velocity of the body and the angular velocity of the head relative to the body for two example sequences (top row and bottom row). Left panels: scatter diagrams of head angular velocity over body angular velocity showing overall negative correlation. Centre panels: cross-correlation between head and body angular velocities, showing zero lag for the smallest negative correlation coefficient. Right panels: two-dimensional histograms of the centre section of the scatter plots on the left, showing high frequencies of head movements compensating for body rotations (maximal values along the diagonal) and high frequencies of head movements while the body is still (maximal values along vertical at zero body angular velocity).
Overall, head movements tend to compensate for body rotations. Example scatter diagrams of the angular velocity of the head relative to the body over the angular velocity of the body (figure 2d) show a negative correlation, with its smallest correlation coefficient at zero lag (figure 2d, centre panels). Most frequently, head movements either compensate for body rotations (diagonal maximum in scatter plot histograms; right panels figure 2d) or are made while body orientation is constant (vertical maximum at zero body angular velocity in scatter plot histograms; right panels figure 2d).
Both full-vector and zero-vector ants spend about 30–40% of their time on the platform fixating the panorama (at angular velocities smaller than 50° s−1): at 10 m release stations, zero-vector ants for on average 4.3 ± 4.5 s (mean ± s.d.; n = 53) and full-vector ants for on average 3.2 ± 1.9 s (n = 10). Equivalent times for 15 m release locations are for zero-vector ants 3.2 ± 2.7 s (n = 36) and for full-vector ants 2.5 ± 3.3 s (n = 8). There is no significant difference between full-vector and zero-vector ants in this respect (10 m: FV versus ZV: t-test, p = 0.3792, t = 0.8858, d.f. = 61; 15 m: FV versus ZV: p = 0.5336, t = 0.6277, d.f. = 42).
It is possible that saccadic changes in gaze direction are directed to either familiar or to unfamiliar features in the scene, which would, for instance, allow ants to detect the extent of mismatch between what they remember and what they currently see. We would then expect to see a correlation between structure in the scene and the gaze directions of ants (see below). Alternatively, ants may systematically scan across the scene to detect more global information on heading direction, provided by a global image comparison [5,22]. Indeed, the observation that the ants keep turning with relatively constant velocity in one direction before reversing the direction of the scan (see examples in figure 3a,b) appears to indicate that saccades may not be driven by panorama features, but instead reflect the operation of a systematic scanning procedure.
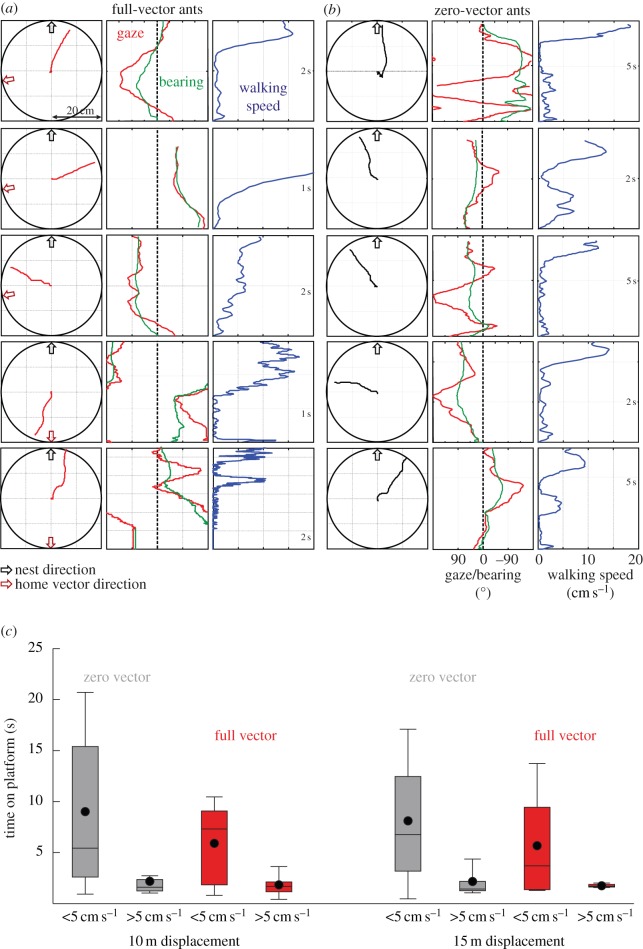
Figure 3.
The movements and gaze directions of ants on release platforms. Five examples each of full-vector (a) and zero-vector (b) ant behaviour upon release on 10 m distant platforms. Left columns: ant paths (red lines, FV; black lines, ZV) with nest direction indicated by black arrow, the direction of the path integration vector indicated by red arrow. Centre columns: gaze directions (red lines) and bearings (green lines) relative to the true home direction at 0°. Right columns: walking speed of ants. Note that time runs from bottom to top on the vertical axis. (c) The average time ants spend on the platform at low and high walking speeds. Box plot conventions: lower whisker: 10% quartile; box bottom: first quartile; horizontal line: median; black dot: mean; box top: third quartile; top whisker: 90% quartile. Zero-vector ants (grey boxes) do not spend significantly more time walking slowly compared with the full-vector ants (red boxes). See text for the results of a statistical analysis.
(b). The decision to move
Both full-vector ants (figure 3a, left column, red paths) and zero-vector ants (figure 3b, left column, black paths) scan the panorama on the release platform with an angular speed of about 90° s−1 and at some stage they decide to move. In many cases, such as the ones shown in figure 3, we record a sudden and marked increase in walking speed (right column panels, figure 3a,b) that brings the ants to the edge of the platform. There is no significant difference in the time full-vector and zero-vector ants spend scanning the panorama at walking speeds less than 5 cm s−1 before deciding to move (figure 3c; 10 m: t-test, p = 0.264, t = 1.127, d.f. = 61; ZV: 9.0 ± 8.4 s (mean ± s.d., n = 53), FV: 5.9 ± 3.8 s (n = 10); 15 m: t-test, p = 0.334, t = 0.9768, d.f. = 42; ZV = 8.1 ± 6.5 s (mean ± s.d., n = 36); FV: 5.7 ± 4.5 s (n = 8)).
The platform paths relative to the true home direction of zero-vector (black) and full-vector ants (red) at the different release stations are shown in figure 4a. We asked to what degree the increase in walking speed depended on where the ants looked. The histograms in figure 4b show the gaze directions of ants relative to the true home direction at 0° depending on their walking speed, black for walking speeds slower than 5 cm s−1 and blue for walking speeds faster than 5 cm s−1. The histograms in the first column of figure 4b show the averages of individually normalized gaze direction histograms for all zero-vector ants released at the three release stations at 10 m distance from the nest (top) and for those at the 15 m distance from the nest (bottom). Gaze directions are narrowly distributed around the true nest direction when ants move fast (blue histograms), whereas their gaze directions are much more widely distributed across the panorama when they move slowly or turn on the spot (black curves). Within 20 cm of their release point, the ants thus identify the home direction through rotational movements only. They do not need to translate significant distances in order to access this navigational information.
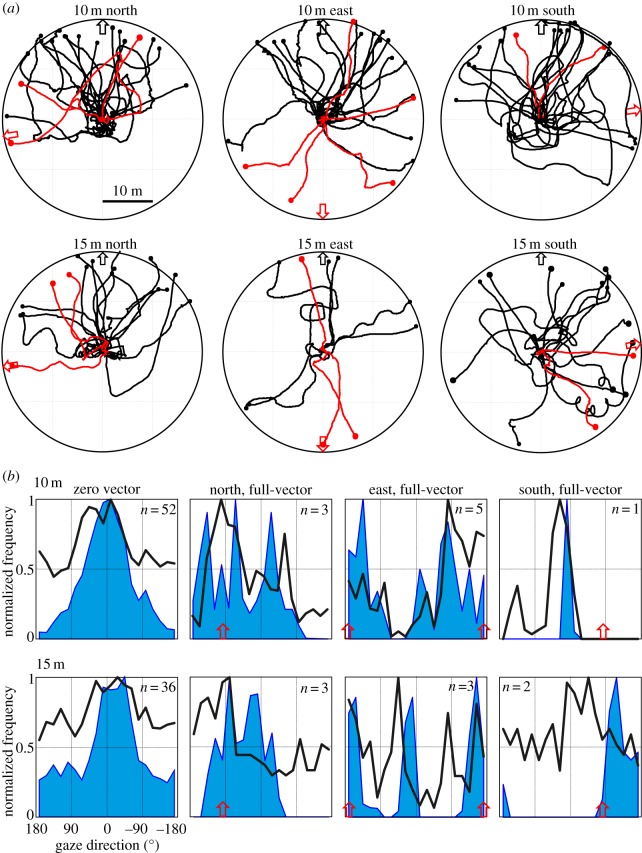
Figure 4.
(a) Paths of ants on release platforms 10 m from the nest (top row) and 15 m from the nest (bottom row). Black lines, zero-vector ants; red lines, full-vector ants. Nest direction is indicated by black arrow, the direction of the path integration vector (for red paths) is indicated by red arrows. (b) Gaze directions of ants on release platforms. Column 1: normalized frequency histograms of gaze directions relative to the true home direction at 0° of zero-vector ants released at 10 m (top) and at 15 m (bottom) from the nest, when walking slower than 5 cm s−1 (black lines) and faster than 5 cm s−1 (blue filled areas). Columns 2–4: same for full-vector ants, with the direction supplied by the path integrator indicated by red arrows. n indicates the number of releases at the different release stations.
The next three columns of panels in figure 4b show walking-speed sorted gaze direction histograms for full-vector ants at the release stations to the north (second column), the east (third column) and to the south of the nest (fourth column). The histograms are again centred on the true home direction at zero, and the different directions of the path integration vector are marked by red arrows. The numbers of full-vector animals are admittedly small, but their gaze directions are interesting: at both the 10 m and 15 m release stations to the north of the nest, the ants tend to look into the direction indicated by their path integrator (approx. +90°), but the final gaze direction of ants when they start moving indicates that some follow the instructions of the path integrator and some ignore them and move towards the true nest direction (see also the paths in figure 4a). At the release stations to the east of the nest (third column, figure 4b), the situation is slightly different, because information from the landmark panorama and from the path integrator is in conflict by 180°. Ants in this situation look and move into three different directions: into the home vector direction at 180° relative to the true nest direction, into the nest direction at 0° and into a direction half-way between (±90°). To the south of the nest (fourth column, figure 4b), the full-vector ant released at 10 m ignores the information from the path integrator and looks and moves into the nest direction. The two full-vector ants released at 15 m do look into the nest direction, but eventually decide to follow the instruction of their path integrator (see red paths in figure 4a).
(c). Scanning and the panorama
Both full-vector and zero-vector ants are thus able to establish the bearing of their nest within 20 cm of their release at distances up to 15 m from the nest in three different compass directions. They must extract this information from the landmark panorama, and we ask next: what is the navigational information content of their particular habitat that allows these ants to determine homing direction from these distances? We investigate here two possibilities: one is that the ants look at unfamiliar salient features or recognize familiar landmarks such as their normal foraging tree in the panorama and move away from or towards such features; the second possibility is that the ants determine heading direction by comparing remembered nest-directed panoramic snapshots and the scene they experience when being released at our recording locations.
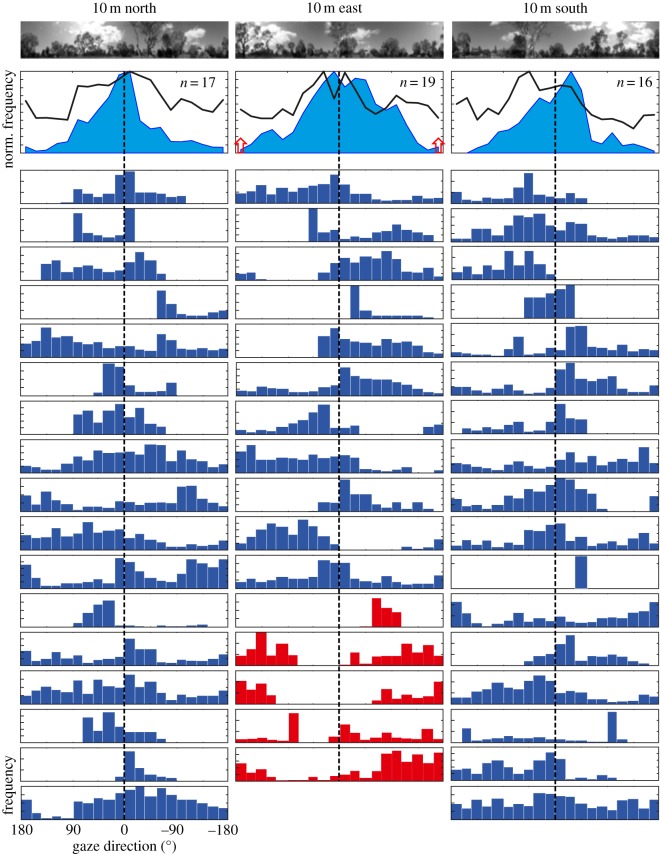
We explore these two possibilities by asking whether there is a detectable relationship between gaze directions and salient or significant features of the landmark panorama, or between such features and the gaze directions at which the ants decide to reverse their scanning direction. Figure 5 shows in the top row the landmark panorama at the three 10 m release sites and in the second row the histograms of gaze directions of all zero-vector ants released there, depending on whether they moved faster than 5 cm s−1 (blue filled area histograms) or slower than 5 cm s−1 (black line histograms). Each of the following rows shows in blue the frequency distribution of gaze directions of individual ants released at all three sites during their time on the platform, with nest direction being indicated by a vertical dashed line. Red histograms show the gaze directions of five full-vector ants released 10 m east of the nest. Clearly, individual ants do not agree where to direct their frontal visual field in detail, but they all tend to concentrate on the nest-directed part of the panorama. This is also evident from the compound histograms of all the zero-vector ants that we released at the three sites just below the panoramic images. At this level of analysis, we thus do not find clear evidence that gaze is directed at landscape features.
Figure 5.
Scanning gaze direction and the local panorama at 10 m release stations. Top row shows the nest-oriented panoramic scenes at the north (left), east (centre) and south (right) release stations oriented south, west and north, respectively. Second row shows compound gaze histograms of all zero-vector ants released at the three sites, sorted depending on whether the ants walked slower than 5 cm s−1 (black lines) or faster than 5 cm s−1 (blue filled areas). Histograms show means of individual ant histograms that were normalized to one. Path integration directions are indicated by red arrows. Blue-barred histograms are gaze directions for the whole time each of 17 individual ants spent on the platform, each released at three stations (rows 1–11) or at two stations (rows 12–17). Dashed lines at 0° indicate true nest direction. Red-barred histograms for the 10 m east release station are the gaze directions of five full-vector ants.
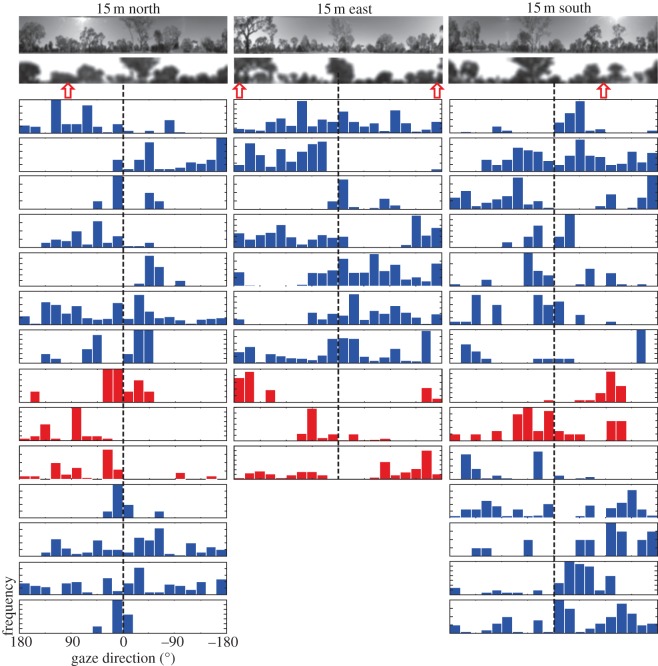
We find a similar pattern of results when considering the directions in which ants fixate the scene at the end points of saccadic gaze changes. In figure 6, we plot for the 15 m release stations the frequency distributions of gaze directions for those instances where the angular velocity of gaze was less than 50° s−1. The histograms are aligned with the camera-based and the reconstructed panorama that emphasizes the skyline at the release locations. Blue-barred histograms are for zero-vector ants and red-barred histograms for full-vector ants. Again, there is little evidence that the ants’ gaze comes to rest at distinct features of the panorama. Some, but not all full-vector ants, however, do align their gaze with the home-vector direction (see red-barred histograms in figure 6).
Figure 6.
The panorama and gaze directions during fixation. Images on top show the scene at release locations 15 m north, east and south of the nest both as camera-based images (top row) and as reconstructed model views (second row, scene filtered with 3° of resolution). The nest direction is indicated by a dashed line and home vector directions by red arrow heads. Histograms show gaze directions of individual ants on the release platform for those instances when the angular velocity of gaze changed by less than 50° s−1. Blue, zero-vector ants; red, full-vector ants.
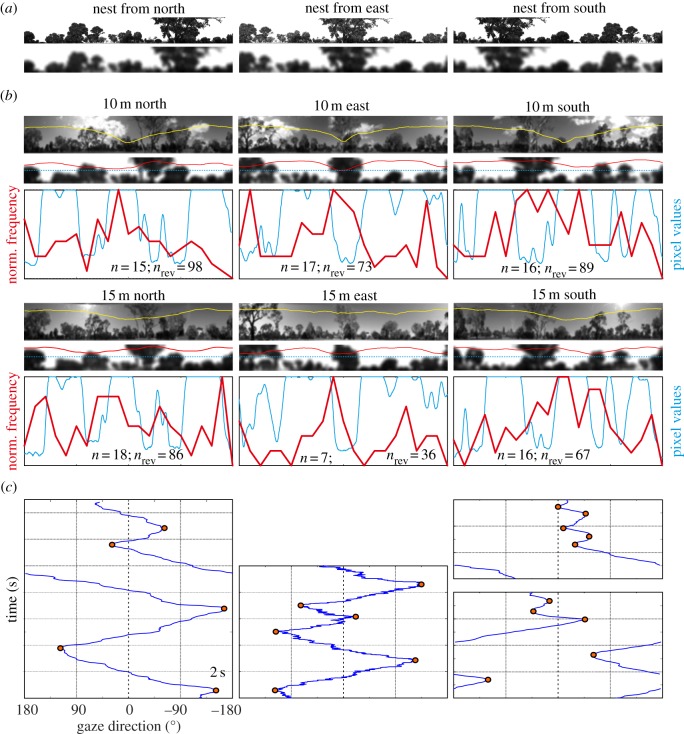
As the examples of time courses of gaze directions in figure 3 demonstrate, the ants change the direction of scanning from time to time. It may, therefore, be reasonable to ask whether it is these reversals of scanning direction that are related to particular features in the panoramic scene. We attempt to answer this question by mapping the gaze directions of ants at the moment they reverse scanning direction onto the six different panoramas they experienced at our six release sites 10 and 15 m from the nest (figure 7) and to the rotIDFs at these sites. We determined rotIDFs by comparing the nest-directed panoramas at the different release sites with the panorama at the nest centred on south for the northern release sites, on west for the eastern release sites and on north for the southern release sites (figure 7a). We used both camera-based panoramic images and panoramic views reconstructed in our three-dimensional model of the area to account for the influence of the non-uniform brightness distribution in the sky and of varying cloud cover. Reconstructed views also highlight the dominant features in the landmark panorama that has been shown to be used by ants to determine heading direction [29,30]. Figure 7b shows rotIDFs (yellow and red lines) overlaid on the recorded and reconstructed panoramic scenes together with the histograms of gaze directions at the moment ants reverse scanning direction (labelled by red dots in figure 7c). The rotIDFs show rather shallow minima, partly because we used as reference the scene directly above the nest and not off-set at some distance from the nest as happens during learning walks [16,19,20].
Figure 7.
The panorama and gaze directions at moments when ants reverse scanning direction. (a) The panoramic scene at the nest facing south (left), west (centre) and north (right) as reconstructed within the three-dimensional model of the area. Bottom row shows the scene filtered with 3° of resolution. (b) Local panoramic views acquired from the panoramic imager (top row images) and as reconstructed in the three-dimensional model (second row images). Yellow and red lines are the rotational image difference functions at these locations relative to the nest views shown in (a). Below images: frequency histograms of gaze directions (red line histograms) at the moment of reversal of scanning direction (see red dots in (c)) at the different release sites. Blue line shows pixel values (from 0 to 255) along horizontal transects through the reconstructed views (indicated by blue dotted lines in second row of panoramas). Reversals were determined over the whole scanning period on platforms. Number of ants given as n, number of reversals as nrev. (c) Examples of gaze directions over time (time running from bottom to top along the vertical axis) at the three release sites. Reversal of scanning directions are marked by red dots.
Again, we do not find a clear pattern of correspondence between gaze directions in these instances and the salient features of the panorama. To facilitate comparison, we show horizontal transects (blue lines, figure 7b) through the reconstructed views (indicated by a blue dotted line across the images above) together with the histograms of gaze direction (red lines, figure 7b) at the moment scanning direction changes. Note in particular that the histograms of gaze directions at the 10 m and the 15 m sites, north, east and south, do not have peaks corresponding to similar panorama features and that there is no obvious correspondence between the shape of histograms and objects or gaps in the skyline. Histogram maxima in most cases, however, coincide with the home direction and with minima in the rotIDFs.
What then determines the change in rotation direction? Inspecting figure 7b,c, there are some indications that reversals of scanning direction tend to happen whenever gaze encounters increasing image differences. Speaking against this interpretation is the finding that scanning direction reversals happen most frequently when ants look into the home direction that coincides roughly with the minimum of rotIDFs in most cases. The maxima of gaze histograms may, however, be due to small and frequent changes in scanning direction close to the rotIDF minimum (e.g. top panel on the right in figure 7c) that may reflect the animals’ search for the most accurate bearing.
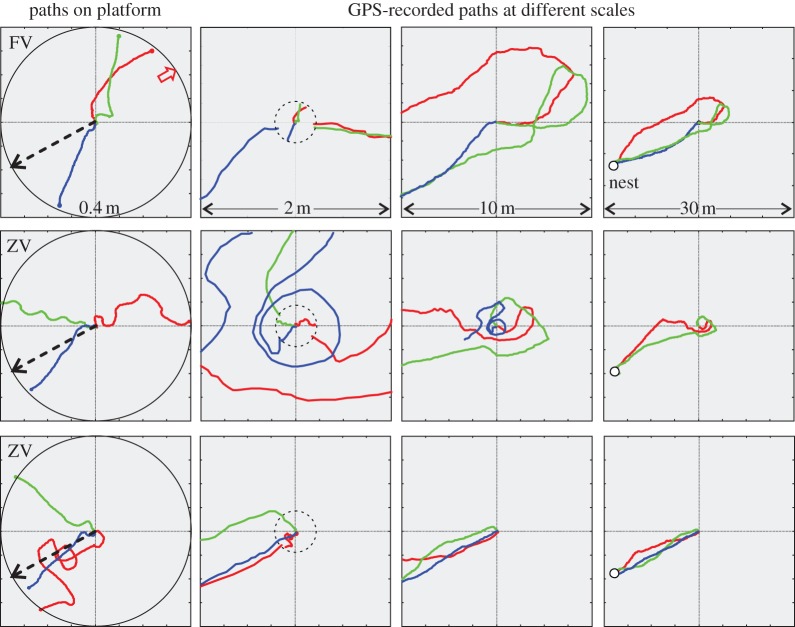
(d). The relationship between paths on the release platform and the subsequent path
The scatter of ant bearings as they leave the release platforms is quite large, as can be seen in figure 4a. However, most of the ants, including full-vector ants that initially exit in a direction indicated by their path integrator, eventually do correct their heading direction and make their way home. To document this observation, we filmed a number of ants on the release platform 15 m east of the nest and subsequently recorded their path with differential GPS (figure 8). We find that full-vector ants that initially followed their home vector direction (green and red paths, left-most panel, row 1, figure 8), turn around after a few metres and, without search, are able to home directly from a distance that is even further away from the nest than the initial release site. The paths of six zero-vector ants shown in rows 2 and 3 (figure 8) also demonstrate that platform exit directions can be misleading in relation to the homing success of ants and that initially well-directed ants can be completely lost after exiting the platform (blue path in row 2). The initial directions chosen by displaced ants thus do not always correspond with the direction of the ants’ subsequent path or even their homing success, indicating that the ants constantly monitor the available navigational information and correct their heading direction accordingly.
Figure 8.
Example paths of ants on the release platform (left column) and after leaving the platform at increasing scales from second to fourth column. Nest direction is indicated by dashed arrows. Red, green and blue colours label ant identity from left to right. Note that the platform paths in the second column are not always contiguous with the GPS paths, because ants often spend some time underneath the platform after moving over its edge. Top row shows three examples each of full-vector (FV) ants, whereas second and bottom row shows three examples each of zero-vector (ZV) ants. Note that all ants, except one (blue path in second row), irrespective of their status and their initial paths on the platform, eventually find their way home (open circles in fourth column panels).
4. Discussion
We have shown that foragers of M. croslandi when displaced to locations that they are very unlikely to have been before, are able to detect their home direction after briefly scanning the landmark panorama. They do not need to translate (move in space) significantly to access that information. Their scanning movements are executed by saccadic head and body rotations that at some stage result in a distinct decision to move rapidly into a certain direction. At this level of analysis, we find no indication that the ants’ scanning movements are influenced by salient or significant features of the visual panorama. We therefore suggest that rotational scanning allows ants to perform a global comparison between remembered, nest-directed snapshots and the panorama as seen from the different release sites with the aim of detecting a familiar heading direction, such as a minimum in image differences.
A wealth of experimental evidence suggests that insects learn panoramic snapshots along routes and close to the nest and are able to use the mismatch between what they currently see and these stored views to identify familiar heading directions along routes or to pinpoint the nest on their final approach ([1,2,14,16]; reviewed in [5,7,18,31]). The navigational information content of such panoramic views is twofold: first, image differences develop smoothly with distance from a reference location (translational image difference functions [5]), and second, they can serve as a visual compass and define heading directions (rotIDFs, see [5,18]). The simplest way in which ants can access this information when confronted with a new scene is by moving and comparing [5,18]. For a simple gradient descent in translational image differences, for instance, the direction in which image differences become smaller can only be determined by generating motion parallax through moving. However, it is important to note that there are alternative ways of accessing this information: animals may be able to replace test steps by predicting how views change through movement [23], or by identifying panorama features that appear lower or higher than expected in vertical extent, they could minimize image differences by moving towards lower and away from higher features [31], without the need for moving and comparing. In both cases, it is likely to help to first find the best alignment of views through rotational scanning.
Yet, given the large visual field of ant compound eyes, it is not entirely obvious why displaced ants have to use rotational scanning movements, especially because their compound eyes do not have a particularly well-developed acute zone such as a frontal area of increased resolution and sensitivity (for eye maps of M. croslandi, see [25]) that would need to be directed at features of interest [32]. So why do ants have to look around when released at unfamiliar locations? We suggest that these rotational scanning movements are a reflection of the way they store and recall navigational information: first, the fact that ants have to perform rotational scans before deciding on a heading direction is a strong indication that they cannot mentally rotate their remembered snapshots [2]. This leads us to the interesting question of whether it is indeed quite difficult to implement mental rotation in neural networks that store information in physically altered synaptic complexes [33]. The second reason why ants need to look around is that they may not have fully panoramic reference images. Although laterally placed compound eyes do tend to have nearly panoramic visual fields, there is always an area behind the insect, occupied by the body, that will not be covered. The only way of comparing not fully panoramic views is to shift the current scene across the remembered image. A last but not exclusive reason why ants have to scan the panorama is their possible need to generate image motion. This may be necessary and advantageous for two reasons: first, to break local adaptation and to generate an ‘image’ in the first place, and second, to effect image comparisons at the level of a very sparse code (image motion signal distributions) that will highlight the parts of the panorama with the highest contrast, such as the landmark skyline, and reduce the contribution of potentially corrupting visual information from cloud-texture in the sky.
The results of our comparatively coarse analysis of gaze directions in M. croslandi ants cannot be easily reconciled with the findings by Lent et al. [24] that wood ants perform very targeted and pre-calculated saccadic body rotations to minimize the angular difference between the retinal position of a salient edge that defines the location of a target feeder and the position of that edge in the memorized view. First, our ants tend to make many saccades into the same direction before reversing scanning direction (figures 3 and 7c), and thus do not appear to align individual features in the panorama with a memorized view. Second, we did not find common patterns of gaze directions for individual ants at different sites, nor of different ants at the same sites (figures 5 and 6), although all ants share very similar reference views that they must have learnt close to the nest. However, there is a need to extend our analysis to the level of detail achieved by Lent et al. [24], in particular with regard to saccade sizes and saccade targets in the panorama. The main problem here is that gaze direction changes in our ants involve quite significant head movements (up to 30°; figure 2b) that are difficult to accurately resolve even within the relatively small recording area of 40 × 40 cm that we used here. It will also be important to find ways of analysing gaze directions along the homing paths of ants beyond their initial heading after release to identify the information that makes them decide to correct their heading direction (figure 8).
At this stage, however, displaced M. croslandi foragers, when surfacing from their transport vials, give the distinct impression that they are not trying to identify and align individual features in the current scene with memorized ones, but that they are seeking information on heading direction in the shape of the compass bearing in which the scene looks most familiar (sensu Baddeley et al. [22]).
Acknowledgements
We acknowledge the supply of aerial photographs by the ACT Planning Authority (ACTPLA). We are grateful to Chloé Raderschall, Eliza Middleton, Fiorella Ramirez Esquivel, Johannes Zanker, Piyankarie Jayatilaka and Zóltan Kócsi for the discussions we have had on this work.
Funding statement
We acknowledge funding support through the Australian Research Council's (ARC) Centre of Excellence Scheme (CE0561903), an ARC Discovery Project and Australian Postdoctoral Fellowship (DP0986606), an ARC Discovery Early Career Award (DE120100019), a grant from the Hermon Slade Foundation, from the Go8-DAAD Germany Australia Joint Research Cooperation Scheme and support from the Defence Science and Technology Organisation and the German Aerospace Centre (DLR).
References
- 1.Land MF, Collett TS. 1975. Visual spatial memory in a hoverfly. J. Comp. Physiol. 100, 59–84. ( 10.1007/BF00623930) [DOI] [Google Scholar]
- 2.Cartwright BA, Collett TS. 1987. Landmark maps for honeybees. Biol. Cybernet. 57, 85–93. ( 10.1007/BF00318718) [DOI] [Google Scholar]
- 3.Vardy A, Möller R. 2005. Biologically plausible visual homing methods based on optical flow techniques. Connect. Sci. 17, 47–89. ( 10.1080/09540090500140958) [DOI] [Google Scholar]
- 4.Zeil J, Boeddeker N, Stürzl W. 2009. Visual homing in insects and robots. In Flying insects and robots (eds Floreano D, Zufferey J-C, Srinivasan MV, Ellington C.), pp. 87–100. Berlin, Germany: Springer. [Google Scholar]
- 5.Zeil J, Hofmann MI, Chahl JS. 2003. Catchment areas of panoramic snapshots in outdoor scenes. J. Opt. Soc. Am. A 20, 450–469. ( 10.1364/JOSAA.20.000450) [DOI] [PubMed] [Google Scholar]
- 6.Stürzl W, Zeil J. 2007. Depth, contrast and view-based homing in outdoor scenes. Biol. Cybernet. 96, 519–531. ( 10.1007/s00422-007-0147-3) [DOI] [PubMed] [Google Scholar]
- 7.Zeil J. 2012. Visual homing – an insect perspective. Curr. Opin. Neurobiol. 22, 285–293. ( 10.1016/j.conb.2011.12.008) [DOI] [PubMed] [Google Scholar]
- 8.Andrew Philippides A, Hempel de lbarra N, Olena Riabinina O, Collett TS. 2013. Bumblebee calligraphy: the design and control of flight motifs in the learning and return flights of Bombus terrestris. J. Exp. Biol. 216, 1093–1104. ( 10.1242/jeb.081455) [DOI] [PubMed] [Google Scholar]
- 9.Collett TS, Hempel de lbarra N, Riabinina O, Philippides A. 2013. Coordinating compass-based and nest-based flight directions during bumblebee learning and return flights. J. Exp. Biol. 216, 1105–1113. ( 10.1242/jeb.081463) [DOI] [PubMed] [Google Scholar]
- 10.Land MF, Collett TS. 1974. Chasing behaviour of houseflies (Fannia canicularis). A description and analysis. J. Comp. Physiol. 89, 331–357. ( 10.1007/BF00695351) [DOI] [Google Scholar]
- 11.Collett TS, Land MF. 1975. Visual control of flight behaviour in the hoverfly, Syritta pipiens L. J. Comp. Physiol. 100, 1–66. ( 10.1007/BF01464710) [DOI] [Google Scholar]
- 12.Collett TS, Lent DD, Graham P. 2014. Scene perception and the visual control of travel direction in navigating wood ants. Phil. Trans. R. Soc. B 369, 20130035. ( 10.1098/rstb.2013.0035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeil J, Boeddeker N, Hemmi JM, Stürzl W. 2007. Going wild: toward an ecology of visual information processing. In Invertebrate neurobiology (eds North G, Greenspan R.), pp. 381–403. New York, NY: Cold Spring Harbor Press. [Google Scholar]
- 14.Wystrach A, Beugnon G, Cheng K. 2012. Ants might use different view-matching strategies on and off the route. J. Exp. Biol. 215, 44–55. ( 10.1242/jeb.059584) [DOI] [PubMed] [Google Scholar]
- 15.Narendra A, Reid SF, Raderschall CA. 2013. Navigational efficiency of nocturnal Myrmecia ants suffers at low light levels. PLoS ONE 8, e58801 ( 10.1371/journal.pone.0058801) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Narendra A, Gourmaud S, Zeil J. 2013. Mapping the navigational knowledge of individually foraging ants Myrmecia croslandi. Proc. R. Soc. B 280, 20130683 ( 10.1098/rspb.2013.0683) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jayatilaka P, Raderschall CA, Narendra A, Zeil J. 2014. Individual foraging patterns of the jack jumper ant, Myrmecia croslandi. Myrmecol. News 19, 75–83. [Google Scholar]
- 18.Graham P, Philippides A, Baddeley B. 2010. Animal cognition: multi-modal interactions in ant learning. Curr. Biol. 20, R639–R640. ( 10.1016/j.cub.2010.06.018) [DOI] [PubMed] [Google Scholar]
- 19.Müller M, Wehner R. 2010. Path integration provides a scaffold for landmark learning in desert ants. Curr. Biol. 20, 1368–1371. ( 10.1016/j.cub.2010.06.035) [DOI] [PubMed] [Google Scholar]
- 20.Nicholson DJ, Judd SPD, Cartwright BA, Collett TS. 1999. Learning walks and landmark guidance in wood ants (Formica rufa). J. Exp. Biol. 202, 1831–1838. [DOI] [PubMed] [Google Scholar]
- 21.Jayatilaka P, Raderschall C, Zeil J, Narendra A. 2013. Learning to forage: the learning walks of Australian jack jumper ants. In Front. Physiol. Conf. Abstract: Int. Conf. Invertebrate Vision ( 10.3389/conf.fphys.2013.25.00081) [DOI] [Google Scholar]
- 22.Baddeley B, Graham P, Philippides A, Husbands P. 2011. Holistic visual encoding of ant-like routes: Navigation without waypoints. Adapt. Behav. 19, 3–15. ( 10.1177/1059712310395410) [DOI] [Google Scholar]
- 23.Möller R. 2012. A model of ant navigation based on visual prediction. J. Theor. Biol. 305, 118–130. ( 10.1016/j.jtbi.2012.04.022) [DOI] [PubMed] [Google Scholar]
- 24.Lent DD, Graham P, Collett TS. 2010. Image-matching during ant navigation occurs through saccade-like body turns controlled by learned visual features. Proc. Natl Acad. Sci. USA 107, 16 348–16 353. ( 10.1073/pnas.1006021107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Narendra A, Reid SF, Greiner B, Peters RA, Hemmi JM, Ribi WA, Zeil J. 2011. Caste-specific visual adaptations to distinct daily activity schedules in Australian Myrmecia ants. Proc. R. Soc. B 278, 1141–1149. ( 10.1098/rspb.2010.1378) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown SGA, van Eeden P, Wiese MD, Mullins RJ, Solley GO, Puy R, Bleasel K, Taylor RW, Heddle RJ. 2011. Causes of ant sting anaphylaxis in Australia – the Australian Ant Venom Allergy Study. Med. J. Aust. 195, 69–73. [DOI] [PubMed] [Google Scholar]
- 27.Stürzl W, Mair E, Hirschmüller H, Zeil J. 2013. Mapping the navigational information content of insect habitats. In Front. Physiol. Conf. Abstract: Int. Conf. on Invertebrate Vision ( 10.3389/conf.fphys.2013.25.00085) [DOI] [Google Scholar]
- 28.Mair E, Stürzl W, Zeil J. 2013. Benchmark 3D Models of natural navigation environments at www.insectvision.org. In Front. Physiol. Conf. Abstract: Int. Conf. on Invertebrate Vision ( 10.3389/conf.fphys.2013.25.00084) [DOI] [Google Scholar]
- 29.Graham P, Cheng K. 2009. Ants use the panoramic skyline as a visual cue during navigation. Curr. Biol. 19, R935–R937. ( 10.1016/j.cub.2009.08.015) [DOI] [PubMed] [Google Scholar]
- 30.Graham P, Cheng K. 2009. Which portion of the natural panorama is used for view-based navigation in the Australian desert ant? J. Comp. Physiol. A 195, 681–689. ( 10.1007/s00359-009-0443-6) [DOI] [PubMed] [Google Scholar]
- 31.Wystrach A, Graham P. 2012. What can we learn from studies of insect navigation? Anim. Behav. 84, 13–20. ( 10.1016/j.anbehav.2012.04.017) [DOI] [Google Scholar]
- 32.Land MF. 1999. Motion and vision: why animals move their eyes. J. Comp. Physiol. A 185, 341–352. ( 10.1007/s003590050393) [DOI] [PubMed] [Google Scholar]
- 33.Stieb SM, Muenz TS, Wehner R, Rössler W. 2010. Visual experience and age affect synaptic organization in the mushroom bodies of the desert ant Cataglyphis fortis. Dev. Neurobiol. 70, 408–423. ( 10.1002/dneu.20785) [DOI] [PubMed] [Google Scholar]