Abstract
This review reflects a few of Mike Land's many and varied contributions to visual science. In it, we show for wood ants, as Mike has done for a variety of animals, including readers of this piece, what can be learnt from a detailed analysis of an animal's visually guided eye, head or body movements. In the case of wood ants, close examination of their body movements, as they follow visually guided routes, is starting to reveal how they perceive and respond to their visual world and negotiate a path within it. We describe first some of the mechanisms that underlie the visual control of their paths, emphasizing that vision is not the ant's only sense. In the second part, we discuss how remembered local shape-dependent and global shape-independent features of a visual scene may interact in guiding the ant's path.
Keywords: zigzag paths, multi-modal directional control, phase-dependent visual control, saccades, visual learning
1. Introduction
Insects have an array of visuo-motor control systems for moving effectively within cluttered surroundings [1,2]. Flies separate image motion caused by their own movements through a stationary environment from the image motion generated by other moving objects [3], and use the former for controlling their direction of flight [4] or for landing on surfaces [5] and the latter to pursue potential prey or mates [6,7] or to avoid possible threats [8]. Flying honeybees equalize the translational optic flow on their two eyes in order to pass through the centres of gaps [9]. They maintain the flow at a set value and so automatically slow down when gaps becomes narrow and hard to negotiate. Flies and locusts walking on a sequence of ‘stepping stones’ measure the gap between the stones to place their feet correctly [10,11]. Locusts [12–14] and praying mantises [15] peer from side to side to judge the distance of a nearby object before jumping on to it. Such examples show how well-crafted insects are to exploit visual information for safe navigation through three-dimensional environments. These tasks rely mostly on pre-wired circuitry for extracting generic visual information.
Some insects can, in addition, learn to take particular visually guided foraging paths through familiar surroundings [16]. This ability also requires elaborate innate computational mechanisms, but has the extra complication of extracting, storing and then using the specific visual information that is needed to follow a habitual route. Ants are turning out to be particularly informative, because the precision and stereotypy of their routes [17–20] make it possible to analyse the underlying strategies and mechanisms [21,22]. Ants are also interesting because of the multiple sensory cues that contribute to their navigation, particularly olfaction, with long range cues from wind-borne volatiles [23–25] and short-range cues from pheromone trails on the ground [26]. Many species of ants lay pheromone trails to mark foraging routes, and some of these species are also helped by remembered visual cues [27–30]. Once visual cues are acquired, they may dominate pheromone cues in experimental situations in which the two cues indicate different directions of travel [29,30]. Normally, the cues reinforce each other, so we can expect that the sensorimotor strategies for sampling and using visual and odour information are adapted to work together. We describe here, first, how wood ants (Formica rufa L.) set and maintain their direction towards a visually defined goal within a familiar scene and how visual control can operate without compromising olfactory sampling. Second, we consider what visual information ants extract from the scene in order to guide their route.
2. Intermittent visual control and the wood ants’ zigzag path
Hangartner [31,32], nearly five decades ago, illustrated the sinuous form of a naturally laid trail by the ant Solenopsis geminata (figure 1a), and the equally sinuous path of the ant Lasius fuliginosus as it keeps to an artificially laid straight trail (figure 1b). Zigzag paths are found in a variety of walking and flying insects either following pheromone trails or moving up odour plumes [34]. The paths of wood ants (F. rufa L.) often have a similar sinuous or zigzag shape (figure 1c,d) and are also guided both by olfactory cues [35] and by visual information [36–39]. How do the mechanisms of visual guidance in these ants operate so as to avoid disrupting the ants’ zigzag path?
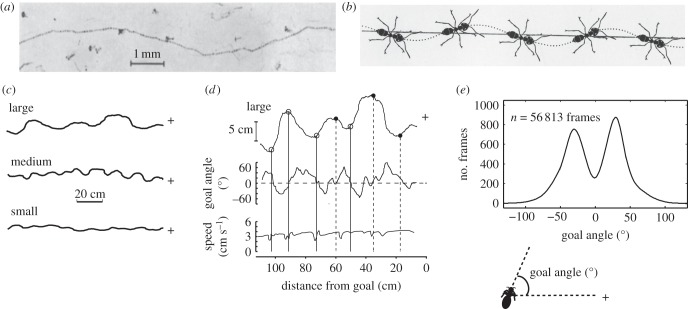
Figure 1.
(a) Pheromone trail laid by the fire ant Solenopsis geminata, while walking over smoked glass. Dots show when ant lowered its sting and marked the glass. Dots are more frequent when reward is stronger. (b) Schematic drawing of the ant Lasius fulginosis following an artificially laid trail. (c) The zizgag paths of wood ants to a visually defined goal (+). Zigzag amplitude can range from 0.8 to 11.1 cm. (d) Details of large zigzags. Top: scale of zigzags is expanded perpendicularly to the direct path to the goal. Middle: the angle between the ant's longitudinal axis and the goal (goal angle). Bottom: the ant's translational speed. Solid vertical lines indicate the occurrence of saccade-like turns (SLTs) and dashed vertical lines goal facing just after a turn. (e) Distribution of goal angles during 30 complete 1.2 m paths. Frequency is number of frames. Inset: diagram defining goal angle. (a,b) Reproduced from [31] and [32] respectively with permission from Springer Science + Business Media. (c–e) Adapted from [33].
In their pursuit of moving targets such as prey or potential mates, insects often engage control systems that bring and keep the target within the animal's frontal visual field [6,7,40]. The target may be fixated by means of intermittent saccades, but once the target is fixated these systems tend to act continuously and to dominate an insect's behaviour. The wood ant's zigzag path towards a stationary goal and the alignment of its body with its path mean that it spends little time facing the goal or moving directly towards it (figure 1d,e), and suggest the use of a more discontinuous control system.
(a). Saccade-like turns and their occurrence within a zigzag
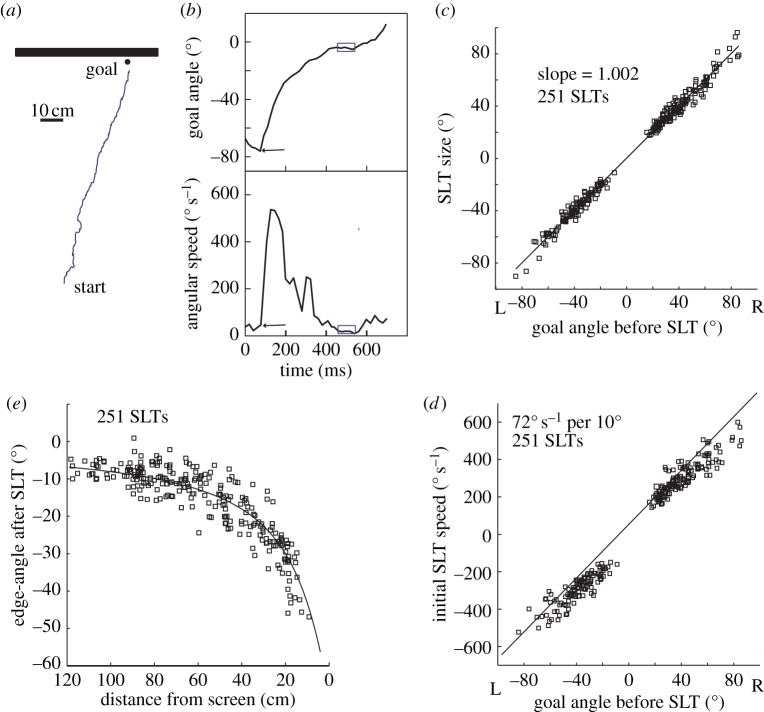
Wood ants correct their direction relative to a visual scene (figure 2a) by means of rapid saccade-like turns (SLTs) (figure 2b). An ant will set the amplitude of each SLT so that at the end of the turn it faces the panorama at a point that is in line with the goal (figure 2c). The ant's speed of rotation at the start of an SLT is related linearly to the size of the turn needed to face the goal (the goal angle in figure 1). This relationship (figure 2d) indicates that before turning ants compute the approximate size of the SLT, although the precision of the pre-computed turn may well be improved by visual feedback during its execution [41]. These saccades resemble those of male hoverflies tracking a potential mate [42], but with a major difference. Whereas the saccades of a fly function to keep a high contrast, black blob at the front of its eye, the learnt visual features of a scene that define a direction to the goal can be at relatively large angles from the goal. The ant's SLTs thus serve to place these features in whatever retinal position results in the ant facing the goal.
Figure 2.
Saccade-like turns on an ant's path towards a goal inset from a black–white edge. (a) Representative path from start to goal. (b) Time course of SLT shown as plots of goal angle and of rotational speed against time. Start of SLT (arrow) is defined by a sudden increase in speed and endpoint (box) by when angular speed drops to less than 50° s−1 for at least 60 ms. (c) Plot of SLT size against goal angle. (d) Plot of initial speed of SLT against goal angle. (e) Plot of the angle between the edge and the goal at the end of each SLT. Solid line shows predicted endpoints of SLTs, assuming that turns end with ants facing the goal. (a–e) Adapted from [41].
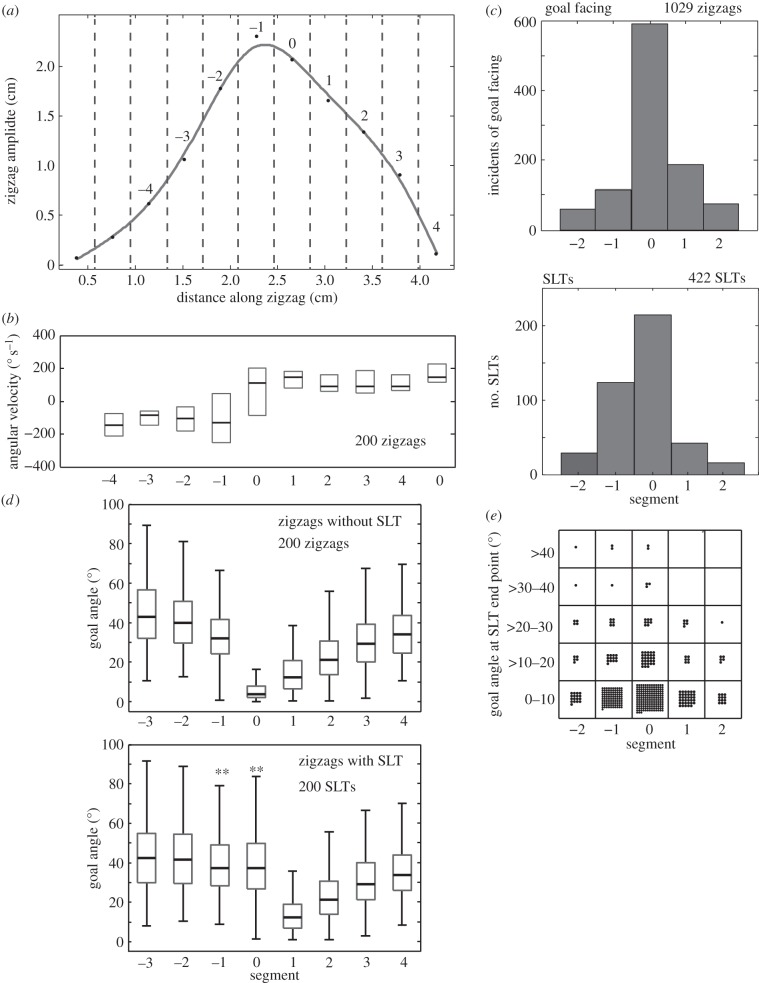
Ants avoid disturbing the zigzag pattern of their path by tending to insert SLTs just after changing their direction of rotation at the end of a zig or zag (figure 3a–c), which is when they normally face the goal (figure 3c) but for some reason fail to do so (figure 3d). SLTs are preceded by a segment of a zig or zag in which goal angles (figure 3d bottom) are larger than they are in the corresponding segments of zigs and zags without SLTs (figure 3d top). In segments of the zig or zag immediately after an SLT, the goal angles do not differ from those of zigs or zags without SLTs [33]. SLTs, thus, occur at particular phases, when goal angles are typically small. They can, by this means, act rapidly to correct the direction of a zigzag without altering the zigzag's subsequent form or phase (figure 3d).
Figure 3.
The occurrence of saccade-like turns (SLTs) within a zigzag. (a) Typical zigzag simulated from the changing values of goal angles and of angular velocities during zigzags. Dotted lines show division into segments. (b) Box plot of median angular velocity and inter-quartile range (IQR) over the course of a zigzag. Ants tend to change direction of angular rotation at the peak and trough of each zigzag. Because the distance between peaks and troughs is variable, data from different zigzags are pooled by dividing each zig or zag into five equal segments, from 0 to 4, and averaging parameter values within a segment. (c) SLTs and incidents of facing the goal (within ±5°) occur mostly in segment 0, shortly after a change in the direction of rotation. These events are less frequent in later segments (+1 or +2) or in earlier segments (−1 or −2, counting back into the previous zig or zag). (d) Top: box plot (median, IQR and range) of the distribution of goal angles in each segment of 200 zigs or zags containing no SLTs; bottom: similar plot for 200 zigs or zags with an SLT in segment 0. Segments in which the median goal angle is significantly (p < 0.01) larger than in the top plot are indicated by double asterisks. Zigs or zags with SLTs have larger goal angles than those without in segments −1 and 0. By segment 1, goal angles are similar whether or not that zig or zag contained an SLT. (e) Grid in which 422 SLTs are each shown as a dot in one of the cells. Horizontal axis: segment in which SLT occurs. Vertical axis: goal angle at the endpoint of each SLT. (a–e) Adapted from [33].
(b). Saccade-like turns and the adjustment of travel direction
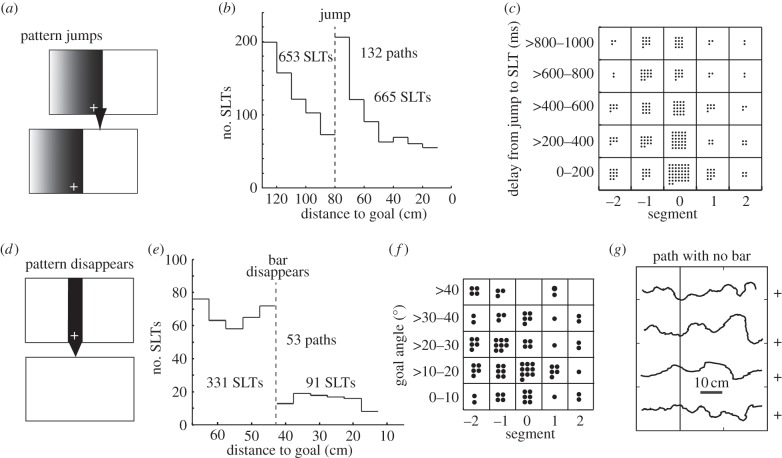
Evidence that SLTs lead to an overall adjustment of the direction of the ant's path comes from the sequence of events that happens after a visual pattern, which is displayed on an LCD screen and defines the position of the goal, is made to jump unexpectedly, so shifting the goal's horizontal position on the retina (figure 4a). The jump induces a transient increase in the frequency of SLTs (figure 4b). SLTs evoked this way have similar properties to spontaneous SLTs [41] and are delayed until ants reach the point of a zig or zag in which they normally face the goal (figure 4c). This delay makes it possible to show that the ant's direction of travel, as given by its mean body orientation over a complete zigzag cycle, is linked more tightly to the performance of SLTs than to the jump of the visual pattern [33]. SLTs thus appear to have a causal role in the control of the ant's direction of travel relative to a familiar visual scene.
Figure 4.
How SLTs are affected by the jump of a pattern or its disappearance during an ant's approach to a visually defined goal (+). (a) Edge that ant approached jumped to left or right. (b) The frequency of SLTs before and after pattern jump (vertical dashed line) plotted against ant's distance from goal. Pattern jump is associated with brief increase in frequency of SLTs. Bins are 5 cm wide. (c) Grid in which each of 258 SLTs following a jump is shown as a dot in one of its cells. Horizontal axis indicates segment in which each SLT occurs. Vertical axis gives delay between jump of pattern and start of SLT. Segment 0 has the most SLTs. (d) Bar defining the position of goal (+) disappears. (e) Bar's disappearance (vertical dashed line) is associated with drop in frequency of SLTs. (f) Grid in which each of 91 SLTs following the bar's disappearance is shown as a dot in one of its cells. Horizontal axis shows segment in which SLT occurs and vertical axis shows the goal angle at end of the SLT. (g) The zigzag path to the goal (+) persists after the visual stimulus defining the goal disappears. (a) Adapted from [41]. (b–g) Adapted from [33].
(c). An absence of saccade-like turns after a goal defining pattern disappears
SLTs are error driven in that the amplitude and the initial speed of the turn are correlated with the magnitude of the goal angle just before the turn (figure 2c,d). What happens when the pattern defining the position of a goal is made to vanish? Do SLTs vanish as well? Ants were trained to approach a goal at the base of a black vertical bar against a white background (figure 4d) displayed on an LCD screen [43]. The bar was shifted to a new position on the screen, and the ant's starting position changed before every training trial to ensure that other visual features did not cue the goal's position. In tests, the bar vanished during the ant's approach (figure 4d). SLTs became rarer after the bar's disappearance (figure 4e). When they did occur, they were both more broadly distributed over a zigzag than usual and they were also less accurate (figure 4f). It is unclear what drives the imprecise SLTs when the bar is not there; it could be a short-term memory of target position and direction [44], or possibly rough information from other visual cues in the room which are not linked tightly to the direction of the goal. Nonetheless, and despite the missing bar, 47% of approaches continued straight to the former position of the bar on the screen and the paths maintained their zigzag form (figure 4g).
Two separate systems seem to control direction during these approaches. The first involves a remembered direction relative to visual features in the scene that, from trial to trial, bear a consistent relationship to the direction of the goal. In this experiment, the bar on the screen was the only feature to meet these requirements. Other visual features, such as the frame of the LCD screen, that were fixed in the room cannot act as reliable substitutes. The ants’ ability to maintain a straight trajectory in the absence of the bar is probably the result of intrinsic motor patterns and control systems that rely on proprioceptive input or optic flow [43,45]. Path control through SLTs that are governed by the retinal position of learnt visual features is distinct from stabilizing reflexes that provide ‘inertial’ control to enable ants to continue on their current direction along a zigzag path. This result also tells us that, when considering how an ant's path is controlled by learnt visual features of a scene, it is safer to examine the endpoints of SLTs, which are an outward expression of a comparison between an ant's current view of a scene and its memory of that scene, than it is to make deductions from the ant's overall travel direction.
(d). The strategy of phase-dependent control
In summary, wood ants set their travel direction towards a goal that is specified with respect to a familiar panorama by turning rapidly to place the panorama in the appropriate position on their retina. The SLTs, by which an ant faces the goal, are timed so that they do not disrupt the ant's normal zigzag path. When an ant is on course, it will face the goal briefly after each zigzag turn and has no need of additional SLTs. SLTs occur just after a zigzag turn, when goal angles are larger than expected. That the initial speeds of SLTs are correlated with their amplitude suggests that the ant may always have implicit knowledge of its orientation relative to the goal. But the phase-dependent occurrence of SLTs indicates that, except for brief windows within the zigzag pattern, the ant's saccadic control system mostly ignores the error signal that can drive it.
The more elaborate possibility that ants monitor and correct differences between the current and expected goal angle along the whole zigzag would imply a much broader distribution of SLTs across the zigzag cycle than is the case (figure 3). It is also contradicted by the properties of the relatively rare SLTs that occur at unusual phases of zigs or zags. These ectopic SLTs eliminate the goal angle rather than set it to the expected value for the phase in which the SLT occurs (figure 3e). Over the remainder of the zigzag, the visual system can be engaged in other tasks, as the ant scans from side to side across its immediate surroundings. In laboratory studies, SLTs are frequent at the start of a route, when ants are establishing their direction of travel. They become less frequent as the route progresses and direction becomes fixed (figures 4b and 6a). It seems that as well as limiting when SLTs occur, ants have additional stabilizing mechanisms that reduce the need for SLTs and also give the visual system an opportunity to multi-task.
Figure 6.
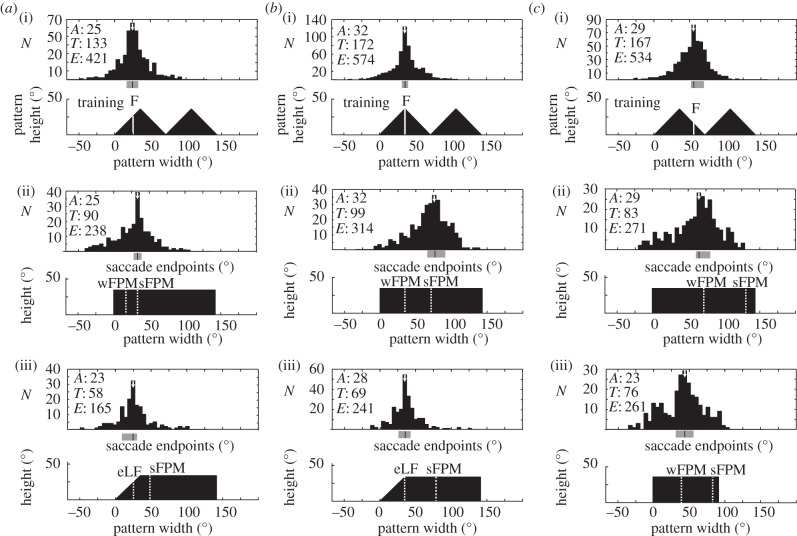
The use of fractional position of mass (FPM) as a visual cue to direction. (a) Facing directions at the end of SLTs during training to a feeder (F) that is inset 30° from a 160°-wide and 38°-high rectangle Stimulus angles are measured from the centre of the arena floor. (a)(i) Individual training trajectories to the feeder within a cylinder. Ants are released from the centre of the arena and approach a feeder 60 cm away. Black portions highlight the initial 30 cm of the path that are analysed. (a)(ii) Facing directions at the end of SLTs during initial 30 cm to the feeder. Bottom: rectangle with white bar marking the feeder position. Middle: contour plot of facing directions along the initial 30 cm (white, no SLTs; black, maximum frequency of SLTs). Dashed lines mark facing directions predicted by the use of FPM or of centre of mass (CoM) as computed at the start of the trajectory. Top: histogram of facing directions pooled over initial path. Bin width is 5°. The white arrow marks modal value. The grey horizontal bar below the histogram shows the mode and its 95% confidence interval. Sample sizes: A, number of ants; T, number of tracks; E, number of endpoints. (b,c) Facing directions during tests with 80° wide and 38° high rectangle (b) and wedge (c). The dashed bar on test patterns marks FPM corresponding to FPM of feeder on training pattern. The use of CoM predicts initial facing directions that lie significantly to the left of the mode of the test distributions as shown by labelled arrow. (a–c) Adapted from [57].
Perhaps more significantly, this phase-dependent control means that an ant's path can be under visual control without disrupting its zigzag form or its potential use in sampling and following pheromone trails. In the experiments summarized here, we have taken pains to minimize guidance by olfactory cues. But wood ants can use these cues, although how they do so has not been studied. If wood ants are like other ants [31], then it is plausible that olfactory sampling and control involve turning inwards at each edge of a trail. In this case, the organization of the wood ant's zigzags and their visual control is well suited to combining visual and olfactory information in guidance. SLTs would tend to occur soon after ants have responded to an olfactory stimulus and they would add a precise visually defined direction to an olfactory driven response. A role of zigzags in olfactory guidance does not, of course, exclude the possibility that the visual consequences of zigzags give ants additional information about their surroundings. Lastly, it is tempting to extrapolate and suggest that this kind of phase-dependent visual control may in addition apply to the fluttering flight of butterflies with their seemingly haphazard flight path, but which over a larger scale turns out to be well-directed.
3. Visual features that control the ant's saccade-like turns
What visual cues do wood ants extract from a panoramic scene and use to guide their path? Visual information for controlling an ant's direction can come both from celestial compass cues and from the surrounding panorama. Ants resemble honeybees [46] in that they can focus on directional signals supplied by the panorama and ignore discrepant compass cues [47,48]. An experiment [48] on the Australian honey ant, Melophorus bagoti, reveals how little of the information that is potentially available in a panoramic scene is needed for controlling direction (figure 5). Foragers familiar with a stable route between their nest and an artificially provisioned feeding site were caught near the nest at the end of a foraging trip and released in the centre of a small arena surrounded by a cylinder of black plastic (figure 5b). The top of the cylinder mimicked the skyline (figure 5c) that the ants would normally view when at the feeding site (figure 5d). Ants ran from the centre of the arena in the homeward direction as defined by the facsimile of the skyline. They were equally precise, whether or not the compass orientation of the artificial skyline was aligned with the real skyline (figure 5f,g).
Figure 5.
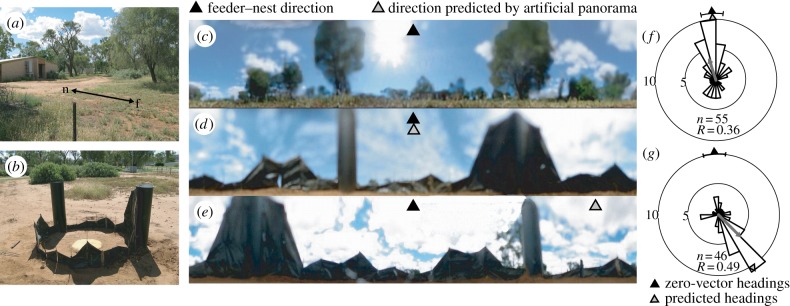
Travel direction of the ant, Melophorus bagoti, in an artificial panoramic skyline. (a) 5 m route between nest and feeder. (b) Plastic replica of skyline. (c) Panorama viewed from feeder. (d) Replica oriented as the natural panorama. (e) Replica rotated clockwise by 150°. (f) Heading directions with replica as in (d) are measured when ant has walked 15 cm from the centre and displayed in a circular histogram. (g) Similar distribution of heading directions with replica oriented as in (e) Adapted from [48]. (Online version in colour.)
This finding implies that travel direction can be extracted from an artificial skyline without normal chromatic cues and potentially with erroneous distance information. It reinforces the suggestion of Möller [49] that the skyline silhouette is likely to be of special significance in directional guidance. More practically, it encourages laboratory experiments with black and white patterns to explore what visual properties of a scene drive SLTs, on the assumption that such simple stimuli will engage the same perceptual processes that ants use for navigation in natural surroundings. Despite the artificial nature of the experimental surroundings—a lack of celestial cues, a panoramic scene that is unnaturally close—this methodology has the great advantage of allowing quick changes of scene. Ants that have learnt a route in one scene can be tested in new scenes that contain just some of the visual features of the training pattern, making it possible to isolate the influence of different features of a scene in their control of direction.
(a). The global and local features of scenes: using global features for guidance
Studies of pattern vision in a variety of insects show that insects extract local visual features from shapes and can identify patterns by them. Oriented edges are the best understood of these features [50–52]. Indeed, a recent calcium imaging study on the blowfly, Calliphora vicina, indicates that edge orientation is extracted very early in the visual pathway—in the medulla of the optic lobe [53]. Honeybees and fruitflies learn the positions of oriented bars within a pattern [51,54], and bees fail to distinguish between patterns when oriented features are displaced from their usual positions [51,52]. Bees can also learn to distinguish between two patterns, each of which is composed of the same four bars in different orientations but placed in different arrangements in the two patterns [55]. In insects, just as in mammals, the orientation of contours may be fundamental to pattern perception.
In addition to such local features, honeybees and fruitflies learn global features of patterns extracted over large areas of the retina. Thus, they learn the vertical position of the centre of mass (CoM) of a pattern, and, after training with one pattern, will accept a differently shaped pattern as similar, so long as the vertical CoMs of the test and training patterns are in the same vertical position [54,56].
Similarly, wood ants aiming towards a feeding site that is defined by its position with respect to a black shape, as in figure 6, extract local and global features of the shape [36,57]. The ants learn the approach to the feeding site in terms of the retinal positions taken up by those features when they face the feeding site. They then control their direction to the feeding site by generating SLTs that restore the features to their learnt retinal positions. The global property of a shape that ants extract for guidance to the feeder is more appropriate than its CoM. Instead of the CoM, they estimate the proportions of the mass of the shape that lie to the left and right of their learnt heading. This property, a shape's fractional position of mass (FPM), is thus not a function of the shape alone, but of an interaction between the shape and the ant's familiar route. It provides a signal derived from the visual scene that is related directly to the ant's desired heading.
In the example of figure 6, an ant is trained to approach a rectangle (figure 6a) and then tested with a rectangle of half the width (figure 6b), chosen so that the shape was of a size that they had not previously encountered. The ants’ facing direction in these tests was towards the FPM that they acquired during training and which they computed over the unfamiliar test rectangle. To find out whether the FPM is estimated over the width and height of the rectangle, ants were tested with a wedge of the same width as the test rectangle (figure 6c). The FPM shifted rightwards in accord with a prediction derived from a FPM computed using height and width. The ants’ facing directions in these tests differ significantly from the predictions of a strategy involving the CoM (figure 6b,c). This strategy supposes that ants learn the retinal position of the CoM when facing the feeder in training trials and that in test trials they place the CoM of test shapes in the same retinal position.
(b). Scene segmentation and the stability of fractional position of mass
The above results imply that ants are learning some value resulting from a computation over a scene rather than an obvious visual feature of it. In a natural 360° scene, the direction associated with a remembered FPM will depend on where in the scene the ant starts and ends its computation. To head in a consistent direction on successive approaches, the ant has to use the same start and endpoints on each occasion. With the simple quadrilaterals in figure 6, the ant just computes the FPM over the whole shape.
Whether and how a shape should be segmented becomes a problem when shapes have several parts. Thus, ants that were trained to a goal specified by two abutting triangles segmented the triangles in different ways depending upon the position of the goal along the base of the triangles (figure 7) [57]. One group of ants was trained to approach a feeder placed left of the peak of the left-hand triangle (figure 7a(i)), a second group was trained with the feeder below the peak of that triangle (figure 7b(i)) and the last group had the feeder to the right of that triangle's peak (figure 7c(i)). In tests with rectangles, the first two groups approached the test rectangles as though ants had segmented the triangles at the most obvious break point—the trough between the triangles—and computed the FPM over the left-hand triangle (figure 7a(ii),b(ii)). The third group faced the rectangle as if the FPM were computed over both triangles with no segmentation (figure 7c(ii)(iii)). Note the ants seemed to have learnt the FPM computed over one shape—a triangle, and in tests computed the same FPM over a different shape—a rectangle.
Figure 7.
Facing directions of ants trained with a pattern of two abutting triangles. (a)(i)–(c)(i) Three groups of ants; each is trained to a different point (F) on the base of the left-hand triangle. a(ii),b(ii),c(ii)(iii) Facing directions in tests with rectangles. Dashed bars on patterns show the FPM of F when triangles are segmented and FPM is computed over the left-hand triangle (sFPM) or not segmented and computed over both triangles (wFPM). Modal facing directions in a(ii),b(ii) are aligned with sFPM. Modal facing directions in c(ii)(iii) are best predicted by wFPM. a(iii),b(iii) Tests with left-hand edge of rectangle slanted to match left-hand edge of triangle. Facing directions suggests ants are guided by the retinal position of the slanted edge (eLF). (a–c) Adapted from [57].
Why might ants segment the triangles in this variable manner? A possible answer, which still needs proper testing, comes from considering the distances of objects composing a scene. If the objects are all far away, such as distant mountains, and the ant's straight route is relatively short, then the FPM of a given segment will remain roughly constant over the whole route. If objects are closer, then the FPM will change along the route, but it will change less when computed over a narrow segment than over a wide one. It is also likely to be more constant if the segment is chosen so that the FPM at the start of the route is close to 0.5. The outcome of the ants’ decision, whether or not to segment the triangles, placed the FPM, in each of the three cases, closer to the CoM of the shape than if the ants had decided to do the reverse (figure 7).
When routes demand it, ants probably learn a changing FPM. The data in figures 6 and 7 consist of the first 30 cm of a 60 cm route with the pattern 150 cm from the start. The FPM does not change greatly over this initial segment, and SLTs are most frequent at the beginning of the route, the point where ants first choose their direction. The initial segment of the route can be sustained without much need for further SLTs. In contrast, the ants in the experiment of figure 2 were trained over a 1.2 m route to approach a feeder that lay very close to a black rectangle on an LCD screen and inset 15 cm from one edge [41]. Ants tended to take a straight path and generated SLTs almost to the feeder, indicating that they are guided actively over the whole of this route. The facing direction at the end of each SLT is plotted in figure 2e as the angle between the edge and the feeder position (the edge-angle) against the ant's distance from the feeder. The solid line shows the expected edge-angle on the assumption that the ant faces the feeder during a straight approach and the data cluster closely around this line. The FPM will also change over this distance. The SLT endpoints would cluster around the predicted changing FPM, but the complex luminance profile of the pattern (illustrated in figure 4a) makes calculations of the FPM uncertain.
(c). Local features and segment recognition
Because ants will learn the appropriate FPM for heading to a point on a segment of one shape and then compute that FPM over an unfamiliar test shape, segment recognition and guidance by FPM are likely to be separate processes. Local visual features, such as oriented edges, contribute to the recognition of a segment. The evidence for this statement is somewhat paradoxical. It is that oriented edges are able to control the size of SLTs. Thus, when ants trained to the two abutting triangles are tested with a trapezium in which the left edge of the rectangle is slanted to match the left edge of the triangles (figure 7a(iii),b(iii)), the ants' facing direction is no longer set by the FPM. Instead, SLTs bring the slanted edge to the same retinal position as the left edge of the left-most triangle in training. Ants place this edge in the appropriate retinal position to reach the goal and ignore the divergent route signalled by the FPM. That the slanted edge is a powerful guidance cue, dominating FPM, suggests that recognition of a segment and guidance by the FPM might be a sequential process. First, the appropriate segment is placed in the frontal visual field through an SLT that is driven by local features, and only then is the FPM computed and used for guidance.
4. Conclusion and questions
Much of an insect's behaviour is innately programmed, but there are also empty slots that need information that cannot be encoded genetically and must be acquired. The SLTs of wood ants provide an instructive example. The mechanisms that determine when SLTs are performed mesh closely with the circuitry that governs the ants’ zigzag path, whereas the magnitude of SLTs depends on what ants have learnt about the expected retinal position of specific features of the scene during their approach and on the difference between the current and expected retinal positions of these features. The mechanism for assessing this error and turning it into a motor command is again inbuilt, as is the transformation of the endpoint of an SLT into an overall adjustment of an ant's zigzag path. There is no intent to underestimate the complexity and significance of insect learning and memory, but rather to emphasize the strong constraints within which learning and memory must operate.
The phylogeny of these intrinsic components and their cooption by SLTs is quite unknown. But what is clear is that SLTs reflect an ant's active decisions about its choice of travel direction within learnt visual scenes. The somewhat surprising precision of SLTs makes them a window through which we can begin to interrogate ants about the ways in which they perceive and encode familiar scenes for their navigation. This job has only just started and there are many questions to answer; for instance: how do ants compute FPMs? What is the alphabet of features available for scene recognition and navigation and how does scene recognition by ants compare with what is known about honeybee [50,56] and fruitfly [54,58] pattern vision? How much of a scene is used in controlling a given direction? How do shape-dependent and shape-independent visual features interact in guiding an ant's path? Do the ant's specific responses to specific errors mean that ants learn particular views in particular places, or can this behaviour also be explained by more continuous or holistic encodings of familiar routes [59]?
References
- 1.Egelhaaf M, Boeddeker N, Kern R, Kurtz R, Lindemann JP. 2012. Spatial vision in insects is facilitated by shaping the dynamics of visual input through behavioral action. Front. Neural Circuits 6, 108 ( 10.3389/fncir.2012.00108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Srinivasan MV. 2001. Honeybees as a model for the study of visually guided flight, navigation, and biologically inspired robotics. Physiol. Rev. 91, 389–411. [DOI] [PubMed] [Google Scholar]
- 3.Zabala F, Polidoro P, Robie A, Branson K, Perona P, Dickinson MH. 2012. A simple strategy for detecting moving objects during locomotion revealed by animal–robot interactions. Curr. Biol. 22, 1344–1350. ( 10.1016/j.cub.2012.05.024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taylor GK, Krapp HG. 2007. Sensory systems and flight stability: what do insects measure and why? Adv. Insect Physiol. 34, 231–316. [Google Scholar]
- 5.Borst A. 1990. How do flies land? BioScience 40, 292–299. ( 10.2307/1311266) [DOI] [Google Scholar]
- 6.Land MF, Collett T. 1974. Chasing behaviour of houseflies (Fannia canicularis). J. Comp. Physiol. A 89, 331–357. ( 10.1007/BF00695351) [DOI] [Google Scholar]
- 7.Wagner H. 1986. Flight performance and visual control of flight of the free-flying house-fly (Musca domestica). II. Pursuit of targets. Phil. Trans. R. Soc. Lond. B 312, 581–595. ( 10.1098/rstb.1986.0019) [DOI] [Google Scholar]
- 8.Maimon G, Straw AD, Dickinson MH. 2008. A simple vision-based algorithm for decision making in flying Drosophila. Curr. Biol. 18, 464–470. ( 10.1016/j.cub.2008.02.054) [DOI] [PubMed] [Google Scholar]
- 9.Srinivasan MV, Lehrer M, Kirchner WH, Zhang SW. 1991. Range perception through apparent image speed in freely flying honeybees. Vis. Neurosci. 6, 519–535. ( 10.1017/S095252380000136X) [DOI] [PubMed] [Google Scholar]
- 10.Pick S, Strauss R. 2005. Goal-driven behavioral adaptations in gap-climbing Drosophila. Curr. Biol. 15, 1473–1478. ( 10.1016/j.cub.2005.07.022) [DOI] [PubMed] [Google Scholar]
- 11.Niven JE, Buckingham CJ, Lumley S, Cuttle MF, Laughlin SB. 2010. Visual targeting of forelimbs in ladder-walking locusts. Curr. Biol. 20, 86–91. ( 10.1016/j.cub.2009.10.079) [DOI] [PubMed] [Google Scholar]
- 12.Wallace G. 1959. Visual scanning in the desert locust Schistocerca gregaria Forskål. J. Exp. Biol. 36, 512–525. [Google Scholar]
- 13.Collett T. 1978. Peering: a locust behaviour pattern for obtaining motion parallax information. J. Exp. Biol. 76, 237–241. [Google Scholar]
- 14.Sobel EC. 1990. The locust's use of motion parallax to measure distance. J. Comp. Physiol. A 167, 579–588. ( 10.1007/BF00192653) [DOI] [PubMed] [Google Scholar]
- 15.Kral K, Poteser M. 1997. Motion parallax as a source of distance information in locusts and mantids. J. Insect Behav. 10, 145–163. ( 10.1007/BF02765480) [DOI] [Google Scholar]
- 16.Cheng K. 2012. How to navigate without maps: the power of taxon-like navigation in ants. Comp. Cogn. Behav. Rev. 7, 1–22. ( 10.3819/ccbr.2012.70001) [DOI] [Google Scholar]
- 17.Collett TS, Dillmann E, Giger A, Wehner R. 1992. Visual landmarks and route following in desert ants. J. Comp. Physiol. A 170, 435–442. ( 10.1007/BF00191460) [DOI] [Google Scholar]
- 18.Wehner R, Michel B, Antonsen P. 1996. Visual navigation in insects: coupling of egocentric and geocentric information. J. Exp. Biol. 199, 129–140. [DOI] [PubMed] [Google Scholar]
- 19.Kohler M, Wehner R. 2005. Idiosyncratic route-based memories in desert ants, Melophorus bagoti: how do they interact with path-integration vectors? Neurobiol. Learn. Mem. 83, 1–12. ( 10.1016/j.nlm.2004.05.011) [DOI] [PubMed] [Google Scholar]
- 20.Mangan M, Webb B. 2012. Spontaneous formation of multiple routes in individual desert ants (Cataglyphis velox). Behav. Ecol. 23, 944–954. ( 10.1093/beheco/ars051) [DOI] [Google Scholar]
- 21.Collett M. 2010. How desert ants use a visual landmark for guidance along a habitual route. Proc. Natl Acad. Sci. USA 107, 11 638–11 643. ( 10.1073/pnas.1001401107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wystrach A, Beugnon G, Cheng K. 2012. Ants might use different view-matching strategies on and off the route. J. Exp. Biol. 215, 44–55. ( 10.1242/jeb.059584) [DOI] [PubMed] [Google Scholar]
- 23.Wolf H, Wehner R. 2000. Pinpointing food sources: Olfactory and anemotactic orientation in desert ants, Cataglyphis fortis. J. Exp. Biol. 203, 857–868. [DOI] [PubMed] [Google Scholar]
- 24.Steck K, Hansson BS, Knaden M. 2011. Desert ants benefit from combining visual and olfactory landmarks. J. Exp. Biol. 214, 1307–1312. ( 10.1242/jeb.053579) [DOI] [PubMed] [Google Scholar]
- 25.Buehlmann C, Hansson WS, Knaden M. 2012. Path integration controls nest-plume following in desert ants. Curr. Biol. 22, 645–649. ( 10.1016/j.cub.2012.02.029) [DOI] [PubMed] [Google Scholar]
- 26.Hölldobler B, Wilson EO. 1990. The ants. Cambridge, MA: Harvard University Press. [Google Scholar]
- 27.Hölldobler B. 1971. Homing in the harvester ant Pogonomyrmex badius. Science 171, 1149–1151. ( 10.1126/science.171.3976.1149) [DOI] [PubMed] [Google Scholar]
- 28.Aron S, Deneubourg JL, Pasteels JM. 1988. Visual cues and trail-following idiosyncrasy in Leptothorax unifasciatus: an orientation process during foraging. Insect. Soc. 35, 355–366. ( 10.1007/BF02225811) [DOI] [Google Scholar]
- 29.Aron S, Beckers R, Deneubourg JL, Pasteels JM. 1993. Memory and chemical communication in the orientation of two mass-recruiting ant species. Insect. Soc. 40, 369–380. ( 10.1007/BF01253900) [DOI] [Google Scholar]
- 30.Harrison JF, Fewell JH, Stiller TM, Breed MD. 1989. Effects of experience on use of orientation cues in the giant tropical ant. Anim. Behav. 37, 869–871. ( 10.1016/0003-3472(89)90076-6) [DOI] [Google Scholar]
- 31.Hangartner W. 1967. Spezifität und Inaktivierung des Spurpheromons von Lasius fuliginosus Latr. und Orientierung der Arbeiterinnen im Duftfeld. Z. Vergl. Physiol. 57, 103–136. ( 10.1007/BF00303068) [DOI] [Google Scholar]
- 32.Hangartner W. 1969. Structure and variability of the individual odor trail in Solenopsis geminata Fabr. (Hymenoptera, Formicidae). Z. Vergl. Physiol. 62, 111–120. ( 10.1007/BF00298046) [DOI] [Google Scholar]
- 33.Lent DD, Graham P, Collett TS. Phase-dependent visual control of the zigzag paths of navigating wood ants. Curr. Biol. 23, 2393–2399. ( 10.1016/j.cub.2013.10.014) [DOI] [PubMed] [Google Scholar]
- 34.Cardé RT, Willis MA. 2008. Navigational strategies used by insects to find distant, wind-borne sources of odor. J. Chem. Ecol. 34, 854–866. ( 10.1007/s10886-008-9484-5) [DOI] [PubMed] [Google Scholar]
- 35.Rosengren R, Fortelius W. 1986. Ortstreue in foraging ants of the Formica rufa group: hierarchy of orienting cues and long-term memory. Insect. Soc. 33, 306–337. ( 10.1007/BF02224248) [DOI] [Google Scholar]
- 36.Judd SPD, Collett TS. 1998. Multiple stored views and landmark guidance in ants. Nature 392, 710–714. ( 10.1038/33681) [DOI] [Google Scholar]
- 37.Graham P, Collett TS. 2002. View-based navigation in insects: how wood ants (Formica rufa L.) look at and are guided by extended landmarks. J. Exp. Biol. 205, 2499–2509. [DOI] [PubMed] [Google Scholar]
- 38.Durier V, Graham P, Collett TS. 2003. Snapshot memories and landmark guidance in wood ants. Curr. Biol. 13, 1614–1618. ( 10.1016/j.cub.2003.08.024) [DOI] [PubMed] [Google Scholar]
- 39.Harris RA, Graham P, Collett TS. 2007. Visual cues for the retrieval of landmark memories by navigating wood ants. Curr. Biol. 17, 93–102. ( 10.1016/j.cub.2006.10.068) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Olberg RM, Worthington AH, Venator KR. 2000. Prey pursuit and interception in dragonflies. J. Comp. Physiol. A 186, 155–162. ( 10.1007/s003590050015) [DOI] [PubMed] [Google Scholar]
- 41.Lent DD, Graham P, Collett TS. 2010. Image-matching during ant navigation occurs through saccade-like body turns controlled by learned visual features. Proc. Natl Acad. Sci. USA 107, 16 348–16 353. ( 10.1073/pnas.0912091107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Collett T, Land M. 1975. Visual control of flight behaviour in the hoverfly Syritta pipiens L. J. Comp. Physiol. A 99, 1–66. ( 10.1007/BF01464710) [DOI] [Google Scholar]
- 43.Lent DD, Graham P, Collett TS. 2009. A motor component to the memories of habitual foraging routes in wood ants? Curr. Biol. 19, 115–121. ( 10.1016/j.cub.2008.11.060) [DOI] [PubMed] [Google Scholar]
- 44.Neuser K, Triphan T, Mronz M, Poeck B, Strauss R. 2008. Analysis of a spatial orientation memory in Drosophila. Nature 453, 1244–1247. ( 10.1038/nature07003) [DOI] [PubMed] [Google Scholar]
- 45.Strauss R, Pichler J. 1998. Persistence of orientation toward a temporarily invisible landmark in Drosophila melanogaster. J. Comp. Physiol. A 182, 411–423. ( 10.1007/s003590050190) [DOI] [PubMed] [Google Scholar]
- 46.von Frisch K, Lindauer M. 1954. Himmel und Erde in Konkurrenz bei der Orientierung der Bienen. Naturwissenschaften 41, 245–253. ( 10.1007/BF00634944) [DOI] [Google Scholar]
- 47.Collett TS, Collett M, Wehner R. 2001. The guidance of desert ants by extended landmarks. J. Exp. Biol. 204, 1635–1639. [DOI] [PubMed] [Google Scholar]
- 48.Graham P, Cheng K. 2009. Ants use the panoramic skyline as a visual cue during navigation. Curr. Biol. 19, R935–R937. ( 10.1016/j.cub.2009.08.015) [DOI] [PubMed] [Google Scholar]
- 49.Möller R. 2002. Insects could exploit UV-green contrast for landmark navigation. J. Theor. Biol. 214, 619–631. ( 10.1006/jtbi.2001.2484) [DOI] [PubMed] [Google Scholar]
- 50.van Hateren JH, Srinivasan MV, Wait PB. 1990. Pattern recognition in bees: orientation discrimination. J. Comp. Physiol. A 167, 649–654. [Google Scholar]
- 51.Horridge GA. 2003. Discrimination of single bars by the honeybee (Apis mellifera). Vis. Res. 43, 1257–1271. ( 10.1016/S0042-6989(03)00087-7) [DOI] [PubMed] [Google Scholar]
- 52.Stach S, Benard J, Giurfa M. 2004. Local-feature assembling in visual pattern recognition and generalization in honeybees. Nature 429, 758–761. ( 10.1038/nature02594) [DOI] [PubMed] [Google Scholar]
- 53.Spalthoff C, Gerdes R, Kurtz R. 2012. Neuronal representation of visual motion and orientation in the fly medulla. Front. Neural Circuits 6, 72 ( 10.3389/fncir.2012.00072) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ernst R, Heisenberg M. 1999. The memory template in Drosophila pattern vision at the flight simulator. Vis. Res. 39, 3920–3933. ( 10.1016/S0042-6989(99)00114-5) [DOI] [PubMed] [Google Scholar]
- 55.Stach S, Giurfa M. 2005. The influence of training length on generalization of visual feature assemblies in honeybees. Behav. Brain Res. 161, 8–17. ( 10.1016/j.bbr.2005.02.008) [DOI] [PubMed] [Google Scholar]
- 56.Horridge GA. 2009. What does the honeybee see and how do we know? A critique of scientific reason. Canberra, Australia: ANU E Press. [Google Scholar]
- 57.Lent DD, Graham P, Collett TS. 2013. Visual scene perception in navigating wood ants. Curr. Biol. 23, 684–690. ( 10.1016/j.cub.2013.03.016) [DOI] [PubMed] [Google Scholar]
- 58.Liu G, Seiler H, Wen A, Zars T, Ito K, Wolf R, Heisenberg M, Liu L. 2006. Distinct memory traces for two visual features in the Drosophila brain. Nature 439, 551–556. ( 10.1038/nature04381) [DOI] [PubMed] [Google Scholar]
- 59.Baddeley B, Graham P, Husbands P, Philippides A. 2012. A model of ant route navigation driven by scene familiarity. PLoS Comput. Biol. 8, e1002336 ( 10.1371/journal.pcbi.1002336) [DOI] [PMC free article] [PubMed] [Google Scholar]