Abstract
An important component of the cone photoreceptors of bird eyes is the oil droplets located in front of the visual-pigment-containing outer segments. The droplets vary in colour and are transparent, clear, pale or rather intensely yellow or red owing to various concentrations of carotenoid pigments. Quantitative modelling of the filter characteristics using known carotenoid pigment spectra indicates that the pigments’ absorption spectra are modified by the high concentrations that are present in the yellow and red droplets. The high carotenoid concentrations not only cause strong spectral filtering but also a distinctly increased refractive index at longer wavelengths. The oil droplets therefore act as powerful spherical microlenses, effectively channelling the spectrally filtered light into the photoreceptor's outer segment, possibly thereby compensating for the light loss caused by the spectral filtering. The spectral filtering causes narrow-band photoreceptor spectral sensitivities, which are well suited for spectral discrimination, especially in birds that have feathers coloured by carotenoid pigments.
Keywords: carotenoids, dispersion, Kramers–Kronig, feather colour, spectral tuning
1. Introduction
Bird eyes have a rich set of photoreceptor cells, consisting of rods, double cones and single cones, which endows birds with visual capacities far beyond that of humans. The four classes of cone photoreceptor cells mediate a tetrachromatic colour vision system covering a wide range of wavelengths, from the ultraviolet to the red [1–3]. Each of the four participating cone photoreceptor classes has a different visual pigment, and in addition, each contains a small optical device, the so-called oil droplet. The cone oil droplets are variously coloured spherules positioned in the photoreceptor inner segment, optically immediately in front of the outer segment, where the visual pigment is concentrated [2]. As indicated by their name, lipids form the main constituent of the oil droplets, but they can also contain high concentrations of carotenoid pigments. The oil droplets act as long-pass filters, bathochromic shifting the sensitivity peaks of the photoreceptors to a wavelength longer than the peak wavelengths of their visual pigments [4]. The spectral filtering also narrows the spectral sensitivity functions and thus reduces the overlap with adjacent spectral classes of cone [5].
Oil droplets are not only found in bird photoreceptors but also found in some amphibians, fishes and reptiles [2]. Most of the scientific interest has been devoted to birds, but the first thorough analysis of the oil droplet pigments was, in fact, performed on turtle photoreceptors [6]. Because of their small size and high absorbance, it is very hard to measure accurately the absorbance spectrum of single oil droplets, but the contained pigments could be successfully characterized by injecting mineral oil into the spherules, resulting in much larger spheres and thus reduced absorbances. Liebman & Granda [6] thus demonstrated that the red oil droplets contain extremely high concentrations of the carotenoid astaxanthin, resulting in peak optical densities of 50–90; astoundingly high values given their diameter of only 6 µm. Lipetz [7,8] confirmed and extended this turtle study, showing that orange droplets contained a lower concentration of astaxanthin, and yellow droplets contained zeaxanthin.
Following the microinjection methods of Liebman & Granda [6], Goldsmith et al. [9] investigated the cone oil droplets of several bird species and characterized five oil droplet types: T (transparent), C (colourless or pale-yellow), P (pale or greenish), Y (yellow) and R (red). Bird oil droplets are slightly smaller than those of turtle, 2–4 µm, and according to their size, the T- and C-type oil droplets were subdivided in Ts, Tl and Cs, Cl (s, small; l, large). Applying additional extractions and chromatography, Goldsmith et al. [9] could identify at least five carotenoid pigments. Galloxanthin, absorbing in the UV and blue wavelength range, ɛ-carotene and zeaxanthin, absorbing at intermediate wavelengths, and astaxanthin, absorbing at green/orange wavelengths, were identified in C-, Y- and R-type droplets, respectively. Carotenoids were shown to be absent in T-type oil droplets. Some pigmented oil droplets harboured mixtures of carotenoids; for instance, the P droplets contained galloxanthin as well as a blue-absorbing carotenoid (carotene and/or zeaxanthin, presumably) and another UV-absorbing pigment. The latter was also encountered in C droplets. Further extensive comparative studies on bird photoreceptor oil droplets were performed by Bowmaker and co-workers [10–15], who established the term clear for the C-type droplets.
The carotenoid concentration may be closely related to the refractive index of the oil droplets as can be deduced from the study on turtle oil droplets by Ives et al. [16]. Using immersion matching, they measured the refractive index values with a refractometer using a broadband red filter, with maximum transmission at 685 nm, which blocked all wavelengths less than 580 nm, thus allowing only wavelengths above the absorption range of the carotenoid pigments. For the clear, orange, yellow and red oil droplets they found refractive indices of 1.48, 1.51, 1.55 and 1.69, respectively. This suggests that the carotenoid pigments directly affect the refractive index. Below, we analyse how the absorbance spectrum of the oil droplets may affect their wavelength-dependent refractive index, that is, the spectral dispersion. The refractive index of the oil droplets being higher than that of the surrounding cytoplasm causes the oil droplets to act as microlenses. If the presence of carotenoid pigment increases the refractive index of the oil droplet, then it also increases the optical power. The microlens function then is intimately linked to the spectral filter properties.
In each of the four cone photoreceptors, a specific oil droplet type is characteristically combined with a specific class of visual pigment. The four cone visual pigments (SWS1, SWS2, RH2 and long-wavelength sensitive (LWS)) have absorption maxima clustering around 410 (or 370), 450, 505 and 570 nm, respectively [2]. The SWS1 cone contains a T-type droplet, and the other combinations are SWS2–C, RH2–Y and LWS–R. The double cones contain LWS visual pigments with P-type droplets (figure 1). Because the oil droplets act as spectral filters for the visual pigments, they modify the spectral sensitivity of the photoreceptors. The aim of this paper is to analyse quantitatively the optical function of the droplets. Central questions are how the amount of carotenoid pigment determines the spectral filtering and how the pigment concentration can affect the refractive index of the oil droplets.
Figure 1.
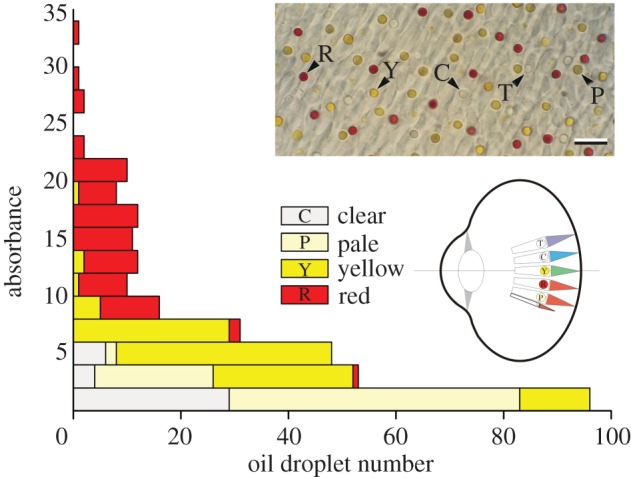
Distribution of the different oil droplet types of birds (adapted from fig. 9 of [9]). The diagram shows a schematic bird eye with the four single cone classes with their oil droplet types (T, transparent; C, clear (or colourless); Y, yellow; R, red), and the double cones with P (pale) droplets (after fig. 8 of [2]). Inset: section of the retina of an ostrich (Struthio camelus); scale bar, 10 µm (adapted from [12]).
2. Material and methods
(a). Optical parameters of the oil droplets
Crucial parameters in our analysis are the transmittance, absorbance, absorption coefficient and refractive index, which are linked as follows. If an object has thickness d and contains a pigment with absorption coefficient κ(λ), i.e. absorption per unit length, then its transmittance spectrum is T(λ) = exp[−κ(λ)d], where λ is the wavelength. The object's absorbance spectrum then is D(λ) = −log10[T(λ)]. The absorbance spectrum thus is proportional to the absorption coefficient: D(λ) = 0.43dκ(λ), and the normalized absorbance and absorption coefficient spectra are identical. When the reflectance of the object is negligible, its absorptance becomes A(λ) = 1−T(λ) = 1–10−D(λ).
Generally, the absorption coefficient of a medium can be considered to be the sum of several bands
| 2.1 |
Each band contributes to the refractive index of the medium an amount given by the Kramers–Kronig dispersion relation [17,18]:
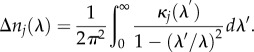 |
2.2 |
Calculations of these refractive index contributions were performed for four carotenoids: the (normalized) absorption spectra of which were derived from the literature, i.e. galloxanthin [19]; ɛ-carotene [9]; zeaxanthin [20,21]; and astaxanthin [9,22]. We also calculated the absorptance, A(λ), connected to various peak absorbance values, Dmax, for an object containing galloxanthin, zeaxanthin or astaxanthin.
(b). Spectral sensitivities of the cone photoreceptors
The spectral sensitivities of the four cone photoreceptor types filtered by their oil droplets were calculated by assuming a cone length d = 16 µm, and a visual pigment with peak absorption coefficient κmax = 0.035 µm−1 (equivalent to a decadic absorbance coefficient of 0.014 µm−1; see [15]). The T-, C-, Y- and R-type oil droplets were assumed to be filled with no carotenoid, galloxanthin, zeaxanthin and astaxanthin, respectively; in the latter three cases, the density of the carotenoids was varied. The cone spectral sensitivities were calculated with S(λ) = T(λ)(1 − exp[−dκmaxP(λ)]), where T(λ) = 1 − A(λ) is the oil droplet transmittance, and P(λ) is the normalized visual pigment absorption spectrum, calculated with a rhodopsin template [23] using average, rounded peak wavelength values: λmax = 390, 450, 505 and 565 nm, respectively [4]. Applying the same procedure, we also calculated the spectral sensitivities of the cone photoreceptor set of the blue tit, Cyanistes caeruleus (previously Parus caeruleus), using the visual pigment peak wavelengths λmax = 372, 449, 502 and 563 nm, whereas the transmittance of the transparent oil droplet was T(λ) = 1, and the other oil droplet transmittances were approximated by T(λ) = exp(−exp[−b(λ − λ0)]), with b = 0.106, 0.068, 0.059 and λ0 = 425, 524, 592, respectively (table 1 of [15]). We have additionally taken into account that the light flux reaches the visual pigments after having passed the ocular media by taking the average eye transmittance spectrum of finches (fig. 7 of [14]).
(c). Finite-difference time-domain modelling
The optical focusing properties of the oil droplets were investigated by finite-difference time-domain (FDTD) modelling by using TDME3D and MEEP [24,25]. We considered homogeneous spheres with diameter 3.0 µm, and compared a transparent oil droplet (refractive index 1.48) with a droplet containing astaxanthin with peak absorption coefficient 16 µm−1, causing an axial peak absorbance of 21, surrounded by water (refractive index 1.34). The simulation was performed with perfectly matched-layer boundaries in a boundary box of 4 × 4 × 8 µm3 with a mesh size of 20 nm on the IBM BlueGene/P of the University of Groningen.
(d). Reflectance spectra of bird feathers
Feather reflectance spectra of passerine birds (siskin, Carduelis spinus; bullfinch, Pyrrhula pyrrhula; European goldfinch, Carduelis carduelis) were measured on single feathers (obtained from Dr J.M. Tinbergen, University of Groningen) as well as on mounted birds (in the Groningen University Museum collection, curator S.L. Ackermann) with a bifurcated reflection probe connected to a spectrophotometer (AvaSpec-2048-2; Avantes, Eerbeek, The Netherlands), using a deuterium/halogen light source (AvaLight-D(H)-S). A white diffusing reflectance standard (Avantes WS-2) served as the reference.
3. Results
(a). Dependence of oil droplet refractive index on carotenoid absorption
A bird photoreceptor contains one of the five principal types of oil droplets: T (transparent), C (colourless or clear), P (pale), Y (yellow) and R (red). Goldsmith et al. [9] studied the distribution of the pigmented droplet types in 19 bird species and categorized the oil droplets according to their peak absorbance in two-unit-sized bins (figure 1). They found that the absorbance can reach extremely high values; for the yellow and red droplets up to 20 and 34, respectively. On average, the absorbance of the C-, P-, Y- and R-type was about 2, 2, 5 and 15, respectively.
Assuming for a red droplet an effective diameter of d = 3.1 µm [13], an absorbance of 15 means an absorption coefficient of 11 µm−1. For a yellow droplet with diameter d = 2.2 µm [13], a peak absorption coefficient of 5.2 µm−1 follows. We have investigated whether such huge values can have consequences for the refractive index by considering the absorption spectra of four principal carotenoids identified from measured transmittance spectra as well as from chromatography of retinal extracts [9]. Figure 2a shows the carotenoid absorption spectra, assuming a medium with peak absorption coefficient 1 µm−1. The carotenoid's contribution to the medium's refractive index, as calculated with the Kramers–Kronig dispersion relation (equation (2.2)) using wavelengths greater than 300 nm, is as shown in figure 2b. The calculated spectra feature classical anomalous dispersion, with the refractive index contribution being negative (positive) in the wavelength range below (above) the absorption peak wavelength.
Figure 2.
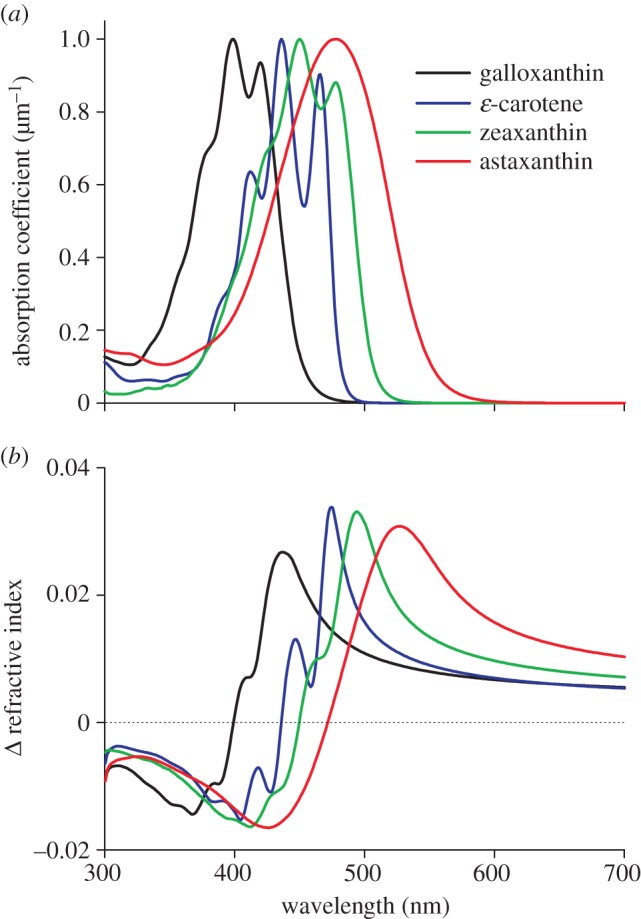
(a) Absorption coefficients of four carotenoids demonstrated in bird oil droplets, normalized to 1 µm−1 and (b) the corresponding contribution to the refractive index of the oil droplets.
For media-containing zeaxanthin or astaxanthin with 1 µm−1 peak absorption coefficient, the refractive index increase at 600 nm, where the absorption is negligible, is 0.010 and 0.016 (figure 2b). Accordingly, with average peak absorption coefficients 5.2 and 11 µm−1, the refractive index increases at 600 nm are 0.05 and 0.18, respectively. These values can be related to the measurements on turtle eyes by Ives et al. [16]. They estimated that in the red, at around 650 nm, the refractive index of clear, yellow and red oil droplets is 1.48, 1.55 and 1.69, respectively. This means that the carotenoids increased the refractive index of the yellow and red oil droplets by 0.07 and 0.21, values similar to the 0.05 and 0.18 of the carotenoid contributions calculated for the yellow and red oil droplets of birds. The oil droplets are surrounded by the watery photoreceptor cytoplasm, the refractive index of which will be around 1.34. Assuming that the transparent oil droplets of birds, such as those of turtle [16], have in the red a refractive index 1.48, the refractive indices of the yellow and red oil droplets will be 1.48 + 0.05 = 1.53 and 1.48 + 0.18 = 1.66, so that the refractive index contrast with the cytoplasm for the T-, Y- and R-droplets becomes 0.14, 0.19 and 0.32. All oil droplets thus will act as a spherical lens, but their optical power will be quite different.
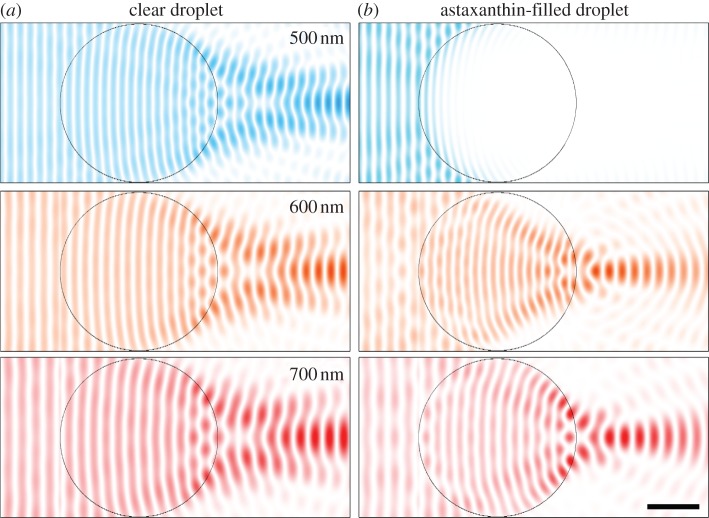
Considering a sphere with diameter 3 µm with refractive index 1.48 or 1.66 in water, geometrical optics predicts powers of 1.7 × 105 and 3.5 × 105 dioptre. The back focal lengths are 6.5 and 2.4 µm, respectively. Geometrical optics is of course no longer well suited to a sphere of a few micrometres. To investigate the effect of the carotenoid-based change in refractive index more effectively, we performed a computational study of the light propagation through a small sphere, applying FDTD modelling. We considered two cases, a transparent, non-absorbing 3 µm sphere with refractive index 1.48 and a sphere containing astaxanthin, with peak absorption coefficient 11 µm−1 and refractive index 1.66. The examples of figure 3a show that the transparent sphere focuses 500, 600 and 700 nm light quite similarly. The astaxanthin-filled sphere fully blocks 500 nm light, and it strongly focuses the 600 and 700 nm light (figure 3b). The modelling clearly confirms that the higher refractive index of the pigmented oil droplet means that it considerably increases the lens focusing power and that the light wave is concentrated within a few micrometre of the oil droplet, near the entrance of the cone's outer segment.
Figure 3.
Snapshots of light intensity of wavelengths 500, 600 and 700 nm after propagation through oil droplets with diameter 3 µm calculated by FDTD modelling [24,25]. (a) A transparent droplet, with refractive index at all wavelengths n = 1.48 (k = 0). The light passing the droplet is slightly focused. (b) An oil droplet containing astaxanthin with real part of the refractive index n = 1.82, 1.76 and 1.66, respectively, and imaginary part of the refractive index k = 0.55, 0.005 and 0.0, respectively. The pigment in the oil droplet absorbs short-wavelength light (top row), whereas the droplet strongly focuses light of longer wavelengths. The intensity is normalized to the maximal intensity in the simulation box (scale bar, 1 µm).
(b). Oil droplet absorbance and absorptance
To investigate the spectral filtering properties into more detail, we have calculated the absorptance spectra of three objects, containing galloxanthin, zeaxanthin and astaxanthin, for a number of peak absorbance values: 0.5, 1, 2, …, 32 (figure 4). At low densities, the objects act as band filters, absorbing strongly in the violet (figure 4a), blue (figure 4b) and green (figure 4c) wavelength ranges. With increasing density, the pigmented objects gradually change into long-pass filters. The so-called mid-wavelength, λmid, where the absorptance (and transmittance) is 0.5, indicated by the small circles in figure 4, shifts bathochromically with an approximately constant step when doubling the absorbance.
Figure 4.
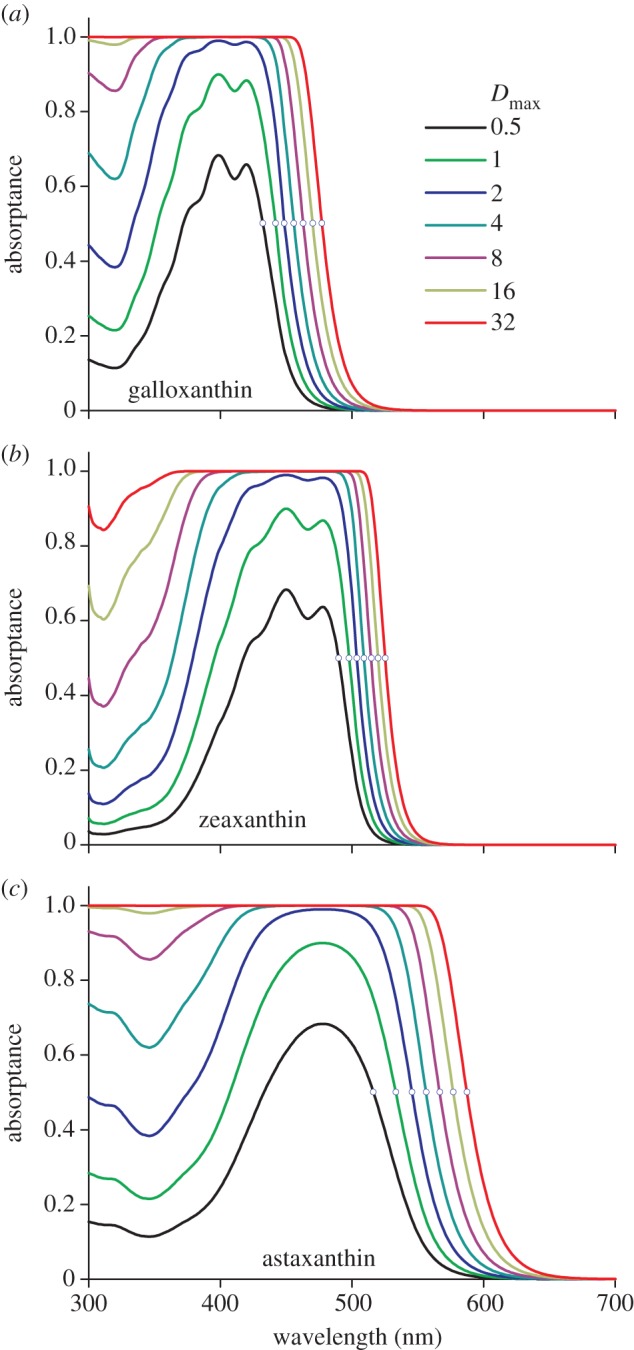
Absorptances of an object containing the carotenoids (a) galloxanthin, (b) zeaxanthin and (c) astaxanthin, with peak absorbance increasing twofold, from 0.5, 1, 2, … to 32. The mid-wavelength of each spectrum, λmid, is where the absorptance equals 0.5 (indicated with a small circle). The value of Dmax is the absorbance of the oil droplet at the peak wavelength.
The three cases of figure 4 represent distinctly different long-pass filters. For the C-oil droplet, which contains galloxanthin with an average absorbance of 2, λmid = 450 nm, whereas for the Y- and R-oil droplets, which contain zeaxanthin and astaxanthin with average absorbances of 5 and 15, respectively, the mid-wavelength values are λmid = 510 and 576 nm.
(c). Effect of oil droplets on the spectral sensitivity of the cone photoreceptors
Figure 5 investigates the effect of the oil droplets on the spectral sensitivity of the cones. We assumed four photoreceptors with T-, C-, Y- and R-type oil droplets, filled with no carotenoid, galloxanthin, zeaxanthin and astaxanthin, in front of an outer segment 15 µm long, containing a visual pigment with peak absorption coefficient 0.035 µm−1, absorbing maximally at 390, 450, 505 and 565 nm [4], respectively. Except for the UV/violet receptor, which has a transparent oil droplet, the effective absorbance of the oil droplets was assumed to change in steps, increasing twofold, as in figure 4. Figure 5 shows that the short-wavelength part of the photoreceptor sensitivity spectrum is progressively reduced, shifting the sensitivity peak wavelength bathochromically. The bold spectra in figure 5 are obtained with the average oil droplet absorbances. The smaller halfwidth of the sensitivity spectra, owing to the presence of the oil droplets, reduces the overlap of the sensitivity spectra, which will improve spectral discrimination by the photoreceptors.
Figure 5.
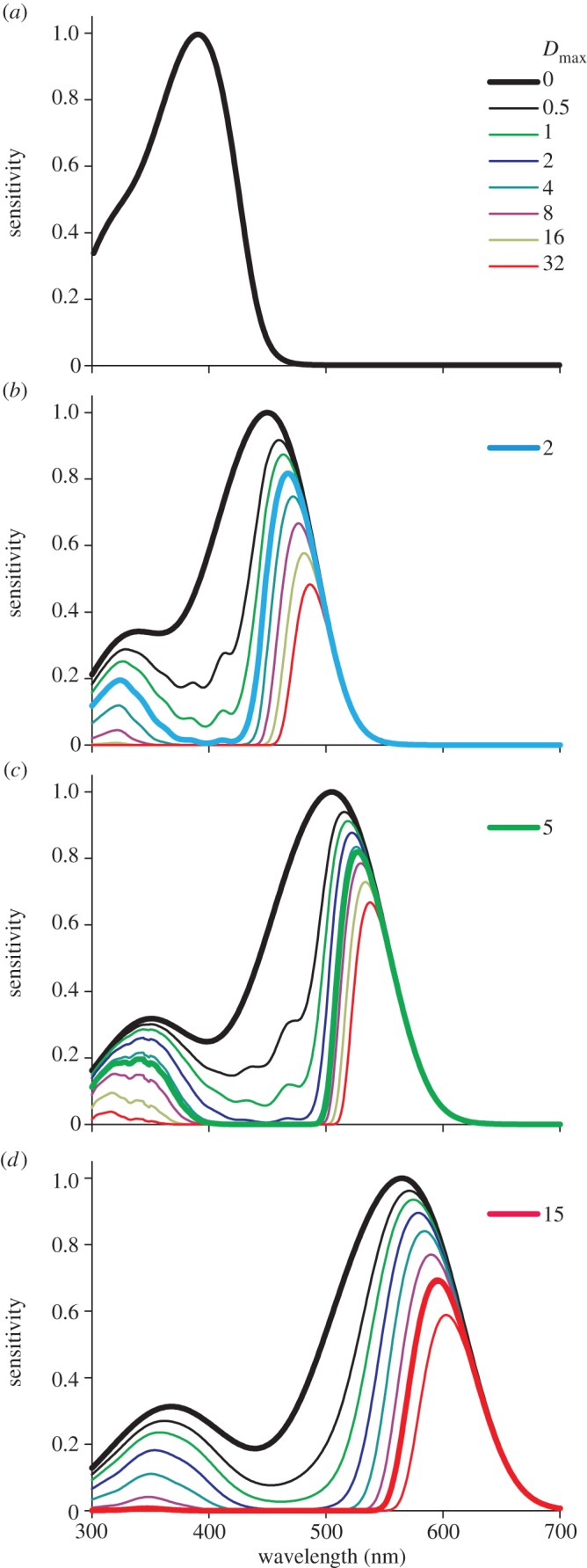
Dependence of the spectral sensitivity of four cone photoreceptors on oil droplet filtering. (a) An (ultra)violet-wavelength-sensitive (UVS) cone, containing R390, i.e. a visual pigment peaking at 390 nm, with a transparent droplet. (b) A short-wavelength-sensitive (SWS) cone, containing R450, with the clear droplet of figure 4a. (c) A middle-wavelength-sensitive (MWS) cone, containing R505, with the yellow droplet of figure 4b. (d) A long-wavelength-sensitive (LWS) cone, containing R565, with the red droplet of figure 4c. Dmax is the peak absorbance of the oil droplet (figure 4). The coloured bold curves result for oil droplets with the average peak absorbance (for C, Y and R: 2, 5 and 15, respectively).
4. Discussion
(a). Oil droplet characteristics and function
The four cone photoreceptor classes of bird eyes each have a specific type of oil droplet. The absorbance of the oil droplets, most notably in the LWS photoreceptors, can become very high. As suggested by measurements on turtle oil droplets, and in agreement with the Kramers–Kronig dispersion theory, the high absorbance of the red oil droplets more than doubles the refractive index contrast at longer wavelengths when compared with transparent oil droplets. The carotenoid pigment absorption not only raises the refractive index at long wavelengths but also causes a refractive index decrease at the shorter wavelengths (figure 2b). Yet, the only effect, if any, will be degraded light focusing, which will however be inconsequential in the presence of the considerable light absorption.
FDTD modelling showed that the increased power indeed results in much stronger light focusing, presumably to enhance light capture by the visual pigment in the outer segment, thus compensating for the light loss owing to spectral filtering. Whether this is, indeed, the case will be studied in a follow-up study with FDTD modelling of the integrated optical system of oil droplet and cone outer segment (in preparation).
We have based our analysis of the function of bird oil droplets mainly on the data of Goldsmith et al. [9], particularly the absorbance characteristics of the various droplet types (figure 1) and the identity of the carotenoids that are concentrated in the droplets. Goldsmith et al. [9] listed the carotenoids galloxanthin, ɛ-carotene, zeaxanthin and astaxanthin, as well as an unidentified UV-absorbing member, but other carotenoids may well be used. The oil droplets of the jungle crow, Corvus macrorhynchos, for instance, contain astaxanthin, galloxanthin and lutein (together with an unknown carotenoid) [26]. Zeaxanthin and lutein are also present in the human retina and may function as antioxidants [27]. Possibly, the high concentrations of carotenoids in the bird retina have a similar additional role.
The filter characteristics of the oil droplets are suitably characterized by the so-called mid-wavelength, that is, where the absorptance is 0.5 [7,15]. Figure 6 presents the mid-wavelength values calculated for various absorbances (figure 4) together with values derived from measured transmittance spectra [15]. For the C-type droplets, the maximal absorbance value found experimentally was about 6 ([9]; figure 1), which corresponds to a calculated λmid-value of about 460 nm. The maximal experimental λmid-value was 470 nm [15]. The maximum absorbance measured for Y-type oil droplets was 20 (figure 1), which corresponds to a calculated λmid-value of about 520 nm. However, a maximum λmid-value of 544 nm was measured ([15]; figure 6). This large difference can be understood as oil droplets often contain a mixture of different carotenoids [9]. When we assume that the Y-droplets contain zeaxanthin with a peak absorbance of 4.0 and add small amounts of astaxanthin, with peak absorbance 0.5, 1.0, 1.5 and 2.0, this causes in addition to a minor change in peak absorbance, from 4.0 to 5.6, a considerable shift in λmid, from 509 to 546 nm (Y+; figure 6). Such a shift could never be realized by zeaxanthin alone. Similarly, the range of λmid-values reachable with galloxanthin in C-type oil droplets may be easily expanded by a mixture of carotenoids. Adding short-wavelength-absorbing carotenoids will have the beneficial effect of suppressing the remaining sensitivity side bands in the ultraviolet, thus further improving spectral discrimination (figure 5b–d).
Figure 6.
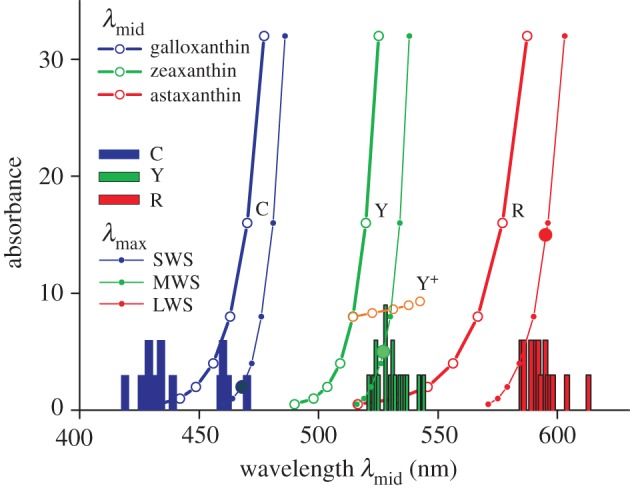
Mid-wavelengths of the absorptance spectra (λmid) of the three oil droplet types (from figure 4; bold symbols and curves), together with the histogram of measured λmid-values (bars; from table 1 of [12]), and the corresponding spectral sensitivity peak wavelengths, λmax, resulting for the three cone photoreceptors, SWS, MWS and LWS (from figure 5; small symbols and thin curves). The three droplet types, C, Y and R, were assumed to contain the carotenoids galloxanthin, zeaxanthin and astaxanthin, respectively. Y+ represents an oil droplet containing zeaxanthin with a peak absorbance of 4.0 as well as astaxanthin in increasing amounts, with peak absorbance 0.5, 1.0, 1.5 and 2.0 (orange symbols). The bold symbols on the λmax-curves indicate the λmax-values resulting with the average oil droplet absorbances.
Yet, the situation must be more complicated, because the absorbance range measured in R-type droplets, 2–34 (figure 1), corresponds to a λmid-range of 545–560 nm, whereas the measured range was 585–613 nm ([15]; figure 6). The carotenoid identified in oil droplets with the longest peak wavelength is astaxanthin, and we therefore might tentatively conclude that another pigment exists with an even longer peak wavelength, which has so far escaped identification. A possible candidate could be rhodoxanthin, which has been extracted from bird feathers [28]. A more likely, alternative explanation of the difference between the calculated and measured λmid-values is that the used carotenoid spectra were obtained in extractions, and that the carotenoids in the oil droplets may have a modified chemical structure. At the highest absorbance values, astaxanthin can take up 30–50% of the R-oil droplet volume [6,8], which may facilitate the formation of heteromeres or electronic interactions with the oil droplet's lipids, thus causing red-shifted absorbance spectra.
(b). Spectral sensitivity of the photoreceptors
The spectral range of bird vision extends from the ultraviolet to red and is based on a set of four visual pigments, absorbing maximally in the ultraviolet, blue, green and red. The absorption spectrum of the visual pigment principally determines the spectral sensitivity of the individual cone photoreceptors, but the actual spectral sensitivity can be greatly affected by the oil droplet acting as a spectral filter. The spectral sensitivities of the cone photoreceptors filtered by the pigmented oil droplets (figure 5) have peak wavelengths, λmax, that bathochromically shift proportional to the increase of oil droplet absorbance (plotted in figure 6 as small symbols on thin lines).
We have to consider, however, that actual photoreceptor spectral sensitivities may deviate somewhat from those calculated in figure 5, because the differences between calculated λmid-values and those derived from microspectrophotometric measurements are not negligible (figure 6). To assess the possible variation, we therefore have considered, in addition to a general bird with average, rounded peak wavelength values for the visual pigments, a particular case of a bird whose visual pigments as well as oil droplet characteristics is well-known, viz. the blue tit, P. caeruleus [15]. Figure 7a shows the resulting spectral sensitivities of the set of photoreceptors (solid curves). For comparison, the spectral sensitivities obtained for the average visual pigments and oil droplet absorbances (bold curves in figure 5) are also presented in figure 7a (dotted curves). All resulting spectra have narrow-band spectral sensitivities; not only the filtered LWS, MWS and short-wavelength-sensitive (SWS) receptors, but also the UVS receptor, which has a transparent oil droplet. Like in the other receptors, the short-wavelength flank of the UVS receptor falls off steeply. In this case, the cause is the filtering by the ocular media. The spectra of figure 7a emphasize that the photoreceptor sensitivities of a particular bird species represents a slight modulation on a general theme, namely to more or less evenly sample the spectral space with a sharply tuned set of narrow-band sensitive receptors.
Figure 7.
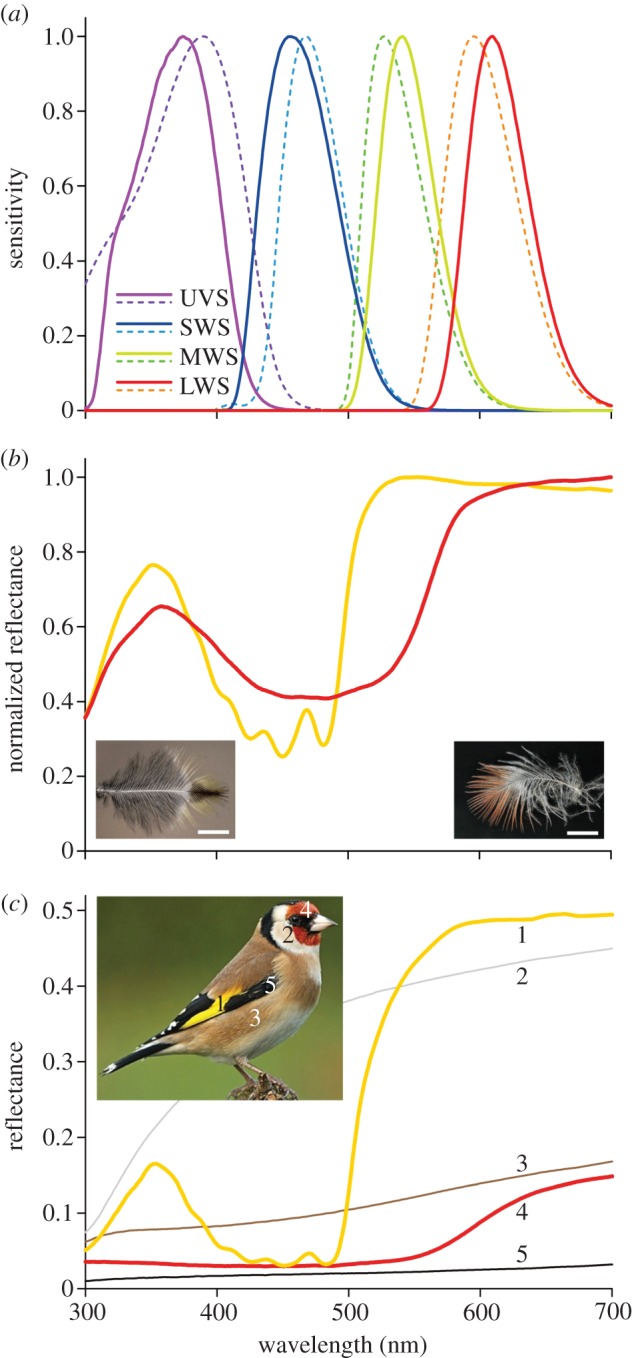
Photoreceptor spectral sensitivities and feather reflectance spectra. (a) Normalized spectral sensitivities of the four cone photoreceptors with oil droplets having average absorbances (dashed curves; from figure 5) and spectral sensitivities for cones of the blue tit (solid curves). (b) Reflectance spectra (normalized) measured from the distal ends of single breast feathers of a siskin (left inset), and a male bullfinch (right inset). (c) Reflectance spectra of different feather areas of a European goldfinch. The numbers near the spectra correspond to the numbered areas in the inset. The low reflectance for wavelengths <350 nm, even in the unpigmented white feathers (curve 2) is due to far-UV absorption by the main component of the feathers, keratin. The black (5) and brown (3) feather parts presumably contain mainly broadband absorbing eumelanin and phaeomelanin, respectively. The yellow feathers (1) have blue-absorbing carotenes and/or zeaxanthin and lutein, and the red head feathers (4) contain predominantly concentrated astaxanthin.
(c). Spectral discrimination and feather reflectance spectra
Carotenoids play a key role in bird vision, but, interestingly, many passerine birds have feathers with carotenoid-based colours, at least more commonly than do birds from ancient avian lineages such as Galliformes, and the songbirds especially capitalize on carotenoid pigments for colour production [29]. As an example, figure 7b shows reflectance spectra of the distal ends of single breast feathers of a siskin, C. spinus and a male bullfinch, P. pyrrhula. Only the exposed, non-overlapping distal parts are coloured. The spectra show that the yellow siskin feather parts contain a mixture of blue-absorbing carotenoids, whereas the pinkish colour of the bullfinch feather appears to be dominantly determined by astaxanthin. (The reduced reflectance at wavelengths less than 350 nm is due to keratin absorbance.) The main reflectance difference between the siskin and bullfinch feathers occurs in the 500–600 nm range. Comparing the feather reflectance spectra with the photoreceptor spectra of the closely related blue tit shows that this wavelength range corresponds precisely to that covered by the MWS receptor. Perhaps not surprisingly, the distinct colour differences created by the carotenoids in the feathers are well discriminated by the photoreceptors with spectral sensitivities that are tuned by the carotenoids in the oil droplets.
The European goldfinch (C. carduelis) features feathers with similar reflectances (figure 7c). The white, brown and black feathers with broadband reflectance spectra of various magnitudes are not tuned to the photoreceptor set, but may serve a general contrast function. The blue tit has yellow chest feathers with a very similar carotenoid-dominated reflectance spectrum to those of the siskin and goldfinch [30]. The reflectance spectrum of the blue crest peaks actually in the ultraviolet [30] and predominantly activates the blue tit's UVS and SWS receptors (figure 7a). The origin of the blue colour is not pigmentary but structural, as it is created by spongy-structured barbs, causing a slight iridescence [31,32].
The spectral set of bird photoreceptors is surprisingly similar between different species. Slight changes may reflect tuning to slightly different visual discrimination tasks. Tuning like that suggested by the carotenoid-coloured passerines will certainly not be the case in birds with structurally coloured feathers that are strongly iridescent, such as the breast feathers of the bird of paradise Parotia lawesii [33]. Its behavioural movements evoke temporally changing signals in different receptors, quite different from the spectrally more static colour signalling of the songbird feathers.
Acknowledgements
We thank Niels Jelsma for the help with figure 3. Dr Ron Douglas read the manuscript and kindly offered linguistic suggestions.
Funding statement
This study was financially supported by the Air Force Office of Scientific Research/European Office of Aerospace Research and Development AFOSR/EOARD (grant no. FA8655-08-1-3012).
References
- 1.Hart NS. 2001. The visual ecology of avian photoreceptors. Prog. Retin. Eye Res. 20, 675–703. ( 10.1016/S1350-9462(01)00009-X) [DOI] [PubMed] [Google Scholar]
- 2.Bowmaker JK. 2008. Evolution of vertebrate visual pigments. Vision Res. 48, 2022–2041. ( 10.1016/j.visres.2008.03.025) [DOI] [PubMed] [Google Scholar]
- 3.Cuthill IC. 2006. Color perception. In Bird coloration, vol. I (eds Hill GE, McGraw KJ.), pp. 3–40. Cambridge, MA: Harvard University Press. [Google Scholar]
- 4.Bowmaker JK. 1977. The visual pigments, oil droplets and spectral sensitivity of the pigeon. Vision Res. 17, 1129–1138. ( 10.1016/0042-6989(77)90147-X) [DOI] [PubMed] [Google Scholar]
- 5.Govardovskii VI. 1983. On the role of oil drops in colour vision. Vision Res. 23, 1739–1740. ( 10.1016/0042-6989(83)90192-X) [DOI] [PubMed] [Google Scholar]
- 6.Liebman P, Granda A. 1975. Super dense carotenoid spectra resolved in single cone oil droplets. Nature 253, 370–372. ( 10.1038/253370a0) [DOI] [PubMed] [Google Scholar]
- 7.Lipetz LE. 1984. A new method for determining peak absorbance of dense pigment samples and its application to the cone oil droplets of Emydoidea blandingii. Vision Res. 24, 597–604. ( 10.1016/0042-6989(84)90114-7) [DOI] [PubMed] [Google Scholar]
- 8.Lipetz LE. 1984. Pigment types, densities and concentrations in cone oil droplets of Emydoidea blandingii. Vision Res. 24, 605–612. ( 10.1016/0042-6989(84)90115-9) [DOI] [PubMed] [Google Scholar]
- 9.Goldsmith TH, Collins JS, Licht S. 1984. The cone oil droplets of avian retinas. Vision Res. 24, 1661–1671. ( 10.1016/0042-6989(84)90324-9) [DOI] [PubMed] [Google Scholar]
- 10.Bowmaker JK, Kovach JK, Whitmore AV, Loew ER. 1993. Visual pigments and oil droplets in genetically manipulated and carotenoid deprived quail: a microspectrophotometric study. Vision Res. 33, 571–578. ( 10.1016/0042-6989(93)90180-5) [DOI] [PubMed] [Google Scholar]
- 11.Bowmaker JK, Heath LA, Wilkie SE, Hunt DM. 1997. Visual pigments and oil droplets from six classes of photoreceptor in the retinas of birds. Vision Res. 37, 2183–2194. ( 10.1016/S0042-6989(97)00026-6) [DOI] [PubMed] [Google Scholar]
- 12.Wright MW, Bowmaker JK. 2001. Retinal photoreceptors of paleognathous birds: the ostrich (Struthio camelus) and rhea (Rhea americana). Vision Res. 41, 1–12. ( 10.1016/S0042-6989(00)00227-3) [DOI] [PubMed] [Google Scholar]
- 13.Hart NS, Partridge JC, Cuthill IC. 1998. Visual pigments, oil droplets and cone photoreceptor distribution in the European starling (Sturnus vulgaris). J. Exp. Biol. 201, 1433–1446. [DOI] [PubMed] [Google Scholar]
- 14.Hart NS, Partridge JC, Cuthill IC, Bennett ATD. 2000. Visual pigments, oil droplets, ocular media and cone photoreceptor distribution in two species of passerine bird: the blue tit (Parus caeruleus L.) and the blackbird (Turdus merula L.). J. Comp. Physiol. A 186, 375–387. ( 10.1007/s003590050437) [DOI] [PubMed] [Google Scholar]
- 15.Hart NS, Vorobyev M. 2005. Modelling oil droplet absorption spectra and spectral sensitivities of bird cone photoreceptors. J. Comp. Physiol. A 191, 381–392. ( 10.1007/s00359-004-0595-3) [DOI] [PubMed] [Google Scholar]
- 16.Ives JT, Normann RA, Barber PW. 1983. Light intensification by cone oil droplets: electromagnetic considerations. J. Opt. Soc. Am. 73, 1725–1731. ( 10.1364/JOSA.73.001725) [DOI] [Google Scholar]
- 17.Bell EE. 1967. Optical constants and their measurements. In Handbuch der Physik, vol. XXV/2a (ed. Genzel L.), pp. 1–58. Berlin, Germany: Springer. [Google Scholar]
- 18.Stavenga DG, Barneveld HHv. 1975. On dispersion in visual photoreceptors. Vision Res. 15, 1091–1095. ( 10.1016/0042-6989(75)90006-1) [DOI] [PubMed] [Google Scholar]
- 19.Wald G. 1948. Galloxanthin, a carotenoid from the chicken retina. J. Gen. Physiol. 31, 377–383. ( 10.1085/jgp.31.5.377) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shafaa MWI, Diehl HA, Socaciu C. 2007. The solubilisation pattern of lutein, zeaxanthin, canthaxanthin and β-carotene differ characteristically in liposomes, liver microsomes and retinal epithelial cells. Biophys. Chem. 129, 111–119. ( 10.1016/j.bpc.2007.05.007) [DOI] [PubMed] [Google Scholar]
- 21.Rasmussen HM, Muzhingi T, Eggert EM, Johnson EJ. 2012. Lutein, zeaxanthin, meso-zeaxanthin content in egg yolk and their absence in fish and seafood. J. Food Comp. Anal. 27, 139–144. ( 10.1016/j.jfca.2012.04.009) [DOI] [Google Scholar]
- 22.Yuan J, Chen F, Liu X, Li X. 2002. Carotenoid composition in the green microalga Chlorococcum. Food Chem. 76, 319–325. ( 10.1016/S0308-8146(01)00279-5) [DOI] [Google Scholar]
- 23.Govardovskii VI, Fyhrquist N, Reuter T, Kuzmin DG, Donner K. 2000. In search of the visual pigment template. Vis. Neurosci. 17, 509–528. ( 10.1017/S0952523800174036) [DOI] [PubMed] [Google Scholar]
- 24.Wilts BD, Michielsen K, Kuipers J, De Raedt H, Stavenga DG. 2012. Brilliant camouflage: photonic crystals in the diamond weevil, Entimus imperialis. Proc. R. Soc. B 279, 2524–2530. ( 10.1098/rspb.2011.2651) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oskooi AF, Roundy D, Ibanescu M, Bermel P, Joannopoulos JD, Johnson SG. 2010. MEEP: a flexible free-software package for electromagnetic simulations by the FDTD method. Comp. Phys. Commun. 181, 687–702. ( 10.1016/j.cpc.2009.11.008) [DOI] [Google Scholar]
- 26.Rahman ML, Yoshida K, Maeda I, Tanaka H, Sugita S. 2010. Distribution of retinal cone photoreceptor oil droplets, and identification of associated carotenoids in crow (Corvus macrorhynchos). Zool. Sci. 27, 514–521. ( 10.2108/zsj.27.514) [DOI] [PubMed] [Google Scholar]
- 27.Kirschfeld K. 1982. Carotenoid pigments: their possible role in protecting against photooxidation in eyes and photoreceptor cells. Proc. R. Soc. Lond. B 216, 71–85. ( 10.1098/rspb.1982.0061) [DOI] [PubMed] [Google Scholar]
- 28.Hudon J, Storni A, Pini E, Anciães M, Stradi R. 2012. Rhodoxanthin as a characteristic keto-carotenoid of manakins (Pipridae). Auk 129, 491–499. ( 10.1525/auk.2012.11235) [DOI] [Google Scholar]
- 29.McGraw KJ. 2005. Interspecific variation in dietary carotenoid assimilation in birds: links to phylogeny and color ornamentation. Comp. Biochem. Physiol. B, Biochem. Mol. Biol. 142, 245–250. ( 10.1016/j.cbpb.2005.07.012) [DOI] [PubMed] [Google Scholar]
- 30.Hunt S, Bennett ATD, Cuthill IC, Griffiths R. 1998. Blue tits are ultraviolet tits. Proc. R. Soc. Lond. B. 265, 451–455. ( 10.1098/rspb.1998.0316) [DOI] [Google Scholar]
- 31.Osorio D, Ham AD. 2002. Spectral reflectance and directional properties of structural coloration in bird plumage. J. Exp. Biol. 205, 2017–2027. [DOI] [PubMed] [Google Scholar]
- 32.Stavenga DG, Tinbergen J, Leertouwer HL, Wilts BD. 2011. Kingfisher feathers: colouration by pigments, spongy nanostructures and thin films. J. Exp. Biol. 214, 3960–3967. ( 10.1242/jeb.062620) [DOI] [PubMed] [Google Scholar]
- 33.Stavenga DG, Leertouwer HL, Marshall NJ, Osorio D. 2010. Dramatic colour changes in a bird of paradise caused by uniquely structured breast feather barbules. Proc. R. Soc. B 278, 2098–2104. ( 10.1098/rspb.2010.2293) [DOI] [PMC free article] [PubMed] [Google Scholar]