Abstract
Purpose
The 2012 multistate fungal meningitis outbreak caused by contaminated methylprednisolone suggests that contaminated compounded drugs can pose a public health threat. The problem has not been well described. Our objective was to systematically review the literature to describe: a) features of infectious outbreaks associated with exposure to contaminated drugs produced by compounding pharmacies, b) sterile compounding procedures that caused microbial contamination, and c) outbreak features relevant for detection and investigation.
Methods
We searched PubMed (reviewing 850 citations) and the CDC and FDA Web sites to identify infectious outbreaks associated with compounding pharmacies outside the hospital setting between January 2000 and November 2012. We extracted information from peer-reviewed literature, FDA and CDC documents, meeting abstracts, and congressional testimony.
Results
Between 2000 and prior to the 2012 fungal meningitis outbreak, 11 infectious outbreaks from contaminated compounded drugs were reported involving 207 case-patients with 17 deaths (8.2% case fatality rate). The 2012 meningitis outbreak increased totals almost 5-fold. Half the outbreaks involved case-patients in more than 1 state. Three outbreaks involved ophthalmic drugs: trypan blue and Brilliant Blue-G ophthalmic solutions used during surgery, and triamcinolone and bevacizumab for intravitreal injection. Remaining outbreaks involved corticosteroids (n=2), heparin flush solutions (n=2), cardioplegia, intravenous magnesium sulfate, total parenteral nutrition, and fentanyl. The outbreaks were caused by pathogens commonly associated with healthcare associated infections (n=6), common skin commensals (n=1), and organisms that rarely cause infection (n=5). Morbidity was substantial, including vision loss; mortality rates during earlier outbreaks were similar to the 2012 meningitis outbreak. A variety of problems with sterile procedures were found. No single source reported all outbreaks.
Conclusion
Sporadic but serious infectious outbreaks associated with contaminated drugs from compounding pharmacies occurred before the 2012 fungal meningitis outbreak. These outbreaks illustrate root causes that could be addressed with preventive policies and practices.
Background
The 2012 multistate fungal meningitis outbreak caused by contaminated injectable methylprednisolone manufactured by New England Compounding Center (NECC) has raised concerns about the quality of compounded drugs.1 Within 2 months of initially detecting the outbreak, the Centers for Disease Control and Prevention (CDC) and state health agencies identified 490 case-patients with serious fungal infection, including meningitis, stroke or other central nervous system-related infection (n=478) and joint infection (n=12); 34 case-patients had died, and the outbreak involved 19 states.2 The CDC estimated that approximately 14,000 patients received injections from 3 lots of methylprednisolone.2 The number of patients exposed to this contaminated compounded drug is unprecedented, the clinical consequences severe, and many will undergo additional invasive procedures and treatments.3 This scenario is unusual, but may only be the tip of the iceberg. There is no single summary in the peer-reviewed literature describing infectious outbreaks linked to compounded drugs.
Under the traditional definition of pharmacy compounding, a pharmacist compounds a medication for a single patient after receipt of a prescription written by a single clinician.4 Traditional compounding practices are regulated by state boards of pharmacy to meet the needs of patients not served by FDA-approved drugs. Compounding pharmacies are not required to establish efficacy and safety, obtain FDA approval, or comply with manufacturing and labeling standards for compounded drugs.5 In contrast, pharmaceutical manufacturers are regulated by the FDA and must comply with Good Manufacturing Practices. Over the last few decades, the regulatory gap between state regulation of traditional compounding practices and FDA regulation of pharmaceutical manufacturing has allowed the proliferation of compounding pharmacies that distribute large quantities of compounded sterile products nationwide. According to the International Academy for Compounding Pharmacists, 1 to 3% of all prescriptions in the US are compounded.6 An estimated 7,500 pharmacies in the US provide advanced compounding services, including approximately 3,000 that provide sterile compounding services.6 Only about 2% of compounding pharmacies participate in the industry’s voluntary accreditation program.5
Since 2000, the FDA has issued numerous warnings about quality problems with compounded drugs,1 including problems with potency7-10 and sterility11, and the presence of particulates12, 13 or contaminants.14, 15 In 2001 and 2006, FDA investigators found that 30% of compounded drug samples they tested contained either too little or too much of the active ingredient.16, 17 This compared to a testing failure rate of less than 2% for FDA-approved prescription drugs.16
In the past, potential outbreaks have been averted before compounded drugs reached large numbers of patients.18 In 2003, a compounding pharmacy that specialized in respiratory drugs issued a partial recall of two batches of albuterol/ipratropium respiratory solution (more than 1 million doses) that were contaminated with B cepacia.11 A full recall was issued after the Missouri Board of Pharmacy obtained a restraining order to halt further dispensing of contaminated respiratory solutions. More than 19,000 patients throughout the US may have been exposed to the contaminated drug, but no known infections resulted from this event.
We questioned whether infectious outbreaks associated with contaminated compounded drugs are a new or recurring problem, and whether there are features associated with compounded drugs or surveillance systems that may hinder outbreak detection. Outbreak detection requires that a person or automated system recognizes unusual events, and systems are in place to enable reporting and investigation. Therefore, the objective of this analysis was to describe: a) features of infectious outbreaks associated with exposure to contaminated drugs produced by compounding pharmacies outside the hospital setting, b) sterile compounding procedures associated with microbial contamination, and c) outbreak features relevant for detection and investigation.
Methods
First, we searched the literature available in PubMed using MeSH term combinations of Drug Compounding and Drug Contamination, Drug Compounding and Disease Outbreaks, MMWR[ta] and Drug Contamination, Drug Compounding and Medication Errors, and Drug Compounding tagged with the subheading ‘adverse effects’. We reviewed 850 citations published between January 2000 and November 2012. Second, we manually reviewed content on the FDA ‘Pharmacy Compounding’ Web page1 to identify the name of potential pharmacies involved in outbreaks, then expanded our search of the FDA Web site for additional documents about the pharmacies identified. Third, we used terms associated with compounding pharmacies and the infectious organisms in the identified outbreaks to manually search CDC’s Morbidity and Mortality Weekly Reports (MMWR)19, and abstracts from CDC’s Epidemic Intelligence Service (EIS) annual meetings available on the CDC Web site for 2006 through 2012.20
We selected infectious outbreaks in the US that were determined after public health investigation to result from exposure to drugs that were likely contaminated during production by a compounding pharmacy. We limited our analysis to compounding pharmacies that prepare sterile products outside the hospital setting and to outbreaks identified since January 2000 with information available as of November 25, 2012.
After identifying an outbreak that met our selection criteria, we extracted information (in order of precedence if content differed between sources) from government publications (including FDA Enforcement Letters and recall notices), peer-reviewed literature, congressional testimony, and meeting abstracts. The information was used to describe the a) triggers that led to outbreak detection, b) number and location of case-patients involved, c) drug name and route of administration, d) infectious organisms and clinical outcomes, e) name and location of the compounding pharmacy, and f) findings from the investigation. For one outbreak, the publication indicated that case-patients were located in 7 states but the states were not described. We assumed that the 7 state public health officials that authored the report represented the 7 states where case-patients were identified.21
Results
We identified and describe 12 outbreaks (including the most recent outbreak associated with NECC) that met the inclusion criteria (Table 1 and 2). These outbreaks specifically involving pharmacies that compound drugs outside the hospital setting. No single source provided information about all 12 outbreaks. Ten of the eligible outbreaks were identified using PubMed, including 6 outbreaks reported in peer-reviewed journals, 2 outbreaks reported in MMWR, and 2 outbreaks reported in both MMWR and a peer-reviewed journal. Two additional outbreaks were identified from EIS conference abstracts (outbreaks #7 and 9). Information about 5 of the pharmacies involved in the identified outbreaks were listed on the FDA ‘Pharmacy Compounding’ page as of November 28, 2012 (outbreak # 4,7,9-11) (Table 2). Information about outbreak #12, the NECC multistate fungal meningitis outbreak, was readily available on the FDA site. Once we identified the names of pharmacies involved, we found additional documents about 9 of the outbreaks using the FDA Web site search engine (outbreak #2, 4-7, 9-12). The outbreaks identified were investigated by state or federal public health authorities, or both.
Table 1.
Description of outbreaks associated with contaminated drugs produced by compounding pharmacies outside the hospital setting, January 2000 – November 2012
| # | Year | Infectious organism |
Drug name and route* | Morbidity and mortality | Total # of cases (deaths) |
Incubation period |
Initial presentation indicating outbreak |
Ref |
|---|---|---|---|---|---|---|---|---|
| 1 | 2001 |
Serratia
marcescens |
Betamethasone epidural, conjunctival,** or intra- articular injection |
Meningitis (n=5; 3 died), epidural abscess (n=5), septic arthritis (n=1) |
11 (3) | 6 hours to 15 days |
4 patients at 1 hospital |
45 |
| 2 | 2002 |
Exophiala
dermatitidis |
Methylprednisolone suspension for epidural injection |
Meningitis (n=4; 1 died) and septic arthritis (n=2) |
6 (1) | 34 to 152 days |
2 patient specimens at 1 lab noted by mycology staff (1 patient hospitalized elsewhere) |
42, 46 |
| 3 | 2004 |
Burkholderi
a cepacia |
Heparin-vancomycin IV flush |
Sepsis (with seizure activity) |
2 (0) | ~1 hour | 2 children within 1 week cared for by the same physician |
47 |
| 4 | 2005 |
Serratia
marcescens |
Magnesium Sulfate IV | Bloodstream infections. | 19 (1)*** | <24 to 72 hours |
6 patients in Cardiac Surgery Unit noted at 1 hospital by Infection Control |
32, 48 |
| 5 | 2005 |
Pseudomon
as fluorescens |
Heparin saline IV flush | Bloodstream infections | 80 (0) | Early onset: 8 hours Delayed onset: 84 to 421 days |
4 patients at 1 oncology clinic |
22, 49, 50 |
| 6 | 2005 |
Pseudomon as aeruginosa or Burkholderi a cepacia |
Trypan Blue ophthalmic solution |
Endophthalmitis (permanent vision loss. 2 patients underwent enucleation of the effected eye) |
6 (0) | 1 to 94 days |
2 patients following cataract surgery on same day at 1 hospital |
25 |
| 7 | 2005 | multiple gram- negative bacilli and endotoxin |
Cardioplegia solution | Systemic inflammatory response syndrome |
11 (3) | <24 hours | 3 patients over 8 days following cardiac bypass surgery at 1 hospital |
51, 52 |
| 8 | 2007 |
Sphingomo
nas paucimobili s |
Fentanyl IV | Bloodstream infections. | 8 (0) | 48 hours | 6 patient specimens over 2 weeks noted by 1 hospital laboratory |
53 |
| 9 | 2011 |
Serratia
marcescens |
Total parenteral nutrition IV |
Bloodstream infections | 19 (9) | Not reported |
5 patients at 1 hospital |
54, 55 |
| 10 | 2011 |
Streptococc
us mitis/oralis |
Bevacizumab intravitreal injection (preservative free) |
Endophthalmitis (permanent vision loss; 7 patients had globe loss) |
12 (0) | 1 to 6 days | 9 patients to 1 emergency room, as well as 3 consultations seen over 3 days. Patients injected at 4 clinics |
26, 56, 57 |
| 11 | 2012 |
Fusarium
incarnatum- equiseti |
Brilliant Blue-G (preservative free), |
Endophthalmitis (vision loss, repeat ophthalmic surgery) |
33 | Not reported |
9 patients at 1 ambulatory surgical center reported to public health |
21 |
|
Bipolaris
hawaiiensis |
Triamcinolone intravitreal injection (preservative free) |
|||||||
| 12 | 2012 | Fungi (Aspergillus spp, Exserohilu m spp |
Methylprednisolone acetate epidural or intra- articular injection (preservative free) |
Meningitis, basilar stroke, spinal osteomyelitis or epidural abscess, septic arthritis or osteomyelitis of peripheral joint |
720 (48****) (as of March 9, 2013) |
1 to 4 weeks, but maximum not yet established |
1 immunocompetent patient at an ambulatory surgery center was reported to Tennessee Department of Health by a physician |
2, 5, 28,24 |
The presence of preservatives in the drug was not indicated in the literature for the first nine outbreaks.
Patients with ophthalmic conditions who received drug by the conjunctival route did not get infected. They also received gentamicin prophylaxis.
18 case-patients were identified in the cluster reported by Susenshine et al.32, 48In the FDA investigation report, an additional case-patient that died was reported in another state. 49
On November 19, 2012, 34 deaths due to meningitis were reported by CDC. Subsequently, the CDC Web site reports deaths from all causes among persons who meet the case definition and may not be directly attributed to a fungal infection 24
Table 2.
Description of the drugs, compounding pharmacies, and findings from the outbreak investigation
| # | Drug name and route |
Source – pharmacy name and location |
Lots involved and scope of recall | Factors contributing to outbreak | Re f |
|---|---|---|---|---|---|
| 1 | Betamethason e suspension for epidural, conjunctival, or intra- articular injection |
Doc’s Pharmacy; Walnut Grove, CA |
1 lot of 60 vials (5 mL per vial). 35 of 51 recovered vials grew Serratia marcescens. State of California forced recall of all ophthalmic and injectable drugs made by the pharmacy. |
Pharmacy technician omitted final autoclaving step for a single lot on a single day. California State Board of Pharmacy found improper segregation of sterile compounding area, inadequate staff training and supervision, and poor labeling practices. S marcescens cultured on compounding equipment. Information about outbreak was not found on FDA Web site. |
45, 58 |
| 2 | Methylpredni solone suspension for epidural injection |
Urgent Care Pharmacy; Spartanburg, SC |
Estimated 1000 vials dispensed from Feb to July 2002. Exophiala dermatitidis cultured from unopened vials in 3 lots. All products from the company recalled because sterility could not be assured. |
South Carolina Board of Pharmacy found improper autoclave performance, no sterility testing, inadequate clean-room practices. |
42, 46,59 |
| 3 | Heparin- vancomycin IV flush |
Pharmacy name unknown, FL |
1 lot of approximately 35 flush solutions made specifically for 2 patients. Burkholderia cepacia cultured in 1 of 21 flushes for patient 1 and 1 of 14 flushes for patient 2. |
Source of B. cepacia contamination in pharmacy not identified. FDA inspection did not identify specific deficiencies. Information about outbreak was not found on FDA Web site. |
47 |
| 4 | Magnesium sulfate IV injection |
PharMEDium Services LLC; Houston, TX |
240 units per lot (50 mL admixtures). S marcescens cultured from 3 of 20 units from lot A which was shipped to 5 hospitals in 5 states. Patients in CA received drug from another lot, but no samples available for testing. After multiple Gram-negative organisms cultured from units in an additional lot, company recalled all magnesium sulfate lots and stopped making the product. |
A definitive source of contamination was not found, but manipulation of small admixture bags by compounding pharmacist was suspected source. Samples from each lot of were not retained or tested for sterility. FDA inspection of facilities in several states found problems with environmental monitoring, product labeling, and quality assurance processes. |
32, 48 |
| 5 | Heparin- saline IV flush |
IV Flush, Rowlett TX. |
Number of doses and lots unclear. 7 of 9 lots of unopened prefilled syringes grew Pseudomonas fluorescens. Product distributed to facilities in up to 17 states in the year before outbreak detection. There was a nationwide Class I recall of this product. |
IV Flush contracted with a compounding pharmacy to make concentrated heparin solution that IV Flush then diluted and repackaged into syringes. CDC determined contamination occurred at IV Flush. Sterility testing was not performed for concentrated heparin solution or finished product. Contamination of raw material was unlikely. FDA inspection found global quality assurance and production problems. |
22, 49, 50 60, 61 |
| 6 | Trypan blue 0.06% ophthalmic solution |
Custom RX Compounding Pharmacy; Richfield, MN |
2 lots of 50 syringes each. Pseudomonas aeruginosa cultured from 12 syringes and B cepacia from 4 syringes in these lots. Recall involved 5 lots of trypan blue. |
Source of contamination in compounding pharmacy was not identified. Details of the FDA investigation could not be located. |
25, 62 |
| 7 | Cardioplegia solution |
Central Admixture Pharmacy Services, Inc.; Lanham, MD. |
Gram-negative bacilli and endotoxin found in 2 lots of cardioplegia. Outbreak led to recall of all of the company’s sterile compounding products. |
Compounding pharmacy was the only likely source of contamination. FDA inspection at 4 facilities found multiple deficiencies including inadequate environmental monitoring, training of personnel, and quality assurance, and failure to maintain equipment. |
51, 52, 63 64 |
| 8 | Fentanyl 10 mcg/mL in 250 mL normal saline for IV injection |
Illinois* | 16 of 26 unopened IV bags from implicated lot grew Spingomonas paucimobilis; 9 samples from 3 other lots produced no growth. No product was recalled. |
Evidence strongly suggests the compounding pharmacy was the source of outbreak. Information about outbreak was not found on FDA Web site. |
53 * |
| 9 | Total parenteral nutrition for IV injection |
Meds IV Pharmacy; Birmingham, AL |
Number of doses and lots unknown. Company recalled all IV compounded products. |
Alabama Dept of Public Health found deficiencies in mixing, filtration, and sterility testing. S marcescens contamination likely came from water faucet that was used to clean a container in which TPN was mixed. |
54, 55 |
| 1 0 |
Bevacizumab intravitreal injection (preservative free) |
Infupharma, Hollywood, FL |
2 single-use vials of Avastin (bevacizumab) repackaged into 65 syringes (0.1 mL each) in 4 lots. Streptococcus mitis/oralis cultured from 7 unused syringes. FDA found microbial growth in 3 of 21 syringes in 2 additional lots of bevacizumab. |
The most likely cause of this outbreak was contamination during syringe preparation by the compounding pharmacy. FDA found single-use vials had been used for days to weeks after initial vial puncture, inadequate environmental monitoring, use of non- sterile materials, inadequate personnel training, and other deficiencies. |
26, 56, 57 65, 66 |
| 1 1 |
Brilliant Blue-G (preservative free) |
Franck’s Compounding Laboratory; Ocala, FL |
4 lots recalled; multiple bacterial and fungal species, including Fusarium incarnatium-equesteti, cultured from unused syringes. |
FDA found bacterial and fungal growth in ISO Class 5 laminar flow hoods and ISO Class 7 clean room. Deficiencies included inadequate personnel training, environmental monitoring, and sealing of clean room. |
21, 67 68 |
| Triamcinolon e intravitreal injection (preservative free) |
2 lots of triamcinolone acetonide 80 mg/mL recalled; 8 prescriptions in 1 lot and 5 in the other. Microbial testing ongoing. Company recalled all sterile compounded products. |
||||
| 1 2 |
Methylpredni solone acetate (MPA) suspension for epidural or intra-articular injection (preservative free) |
New England Compounding Center, Framingham, MA |
Initial recall involved 17,676 vials from 3 lots of MPA 80 mg/mL. Unopened vials in 2 lots were contaminated with E rostratum. Testing of third lot is ongoing. After inspection of facility found that sterility could not be ensured, all products from NECC and sister company, Ameri-dose, were recalled. Three clinics in TN used 1663, 189, and 211 vials of MP during outbreak. In this case cluster, attack rate varied by lot involved and age of lot. |
FDA inspection found multiple deficiencies, including bacteria and mold in clean rooms used for sterile compounding, standing water near the clean room, airborne particulates resulting from close proximity to recycling facility. Investigation is ongoing. |
27, 69, 70 71 |
L. Maragakis, MD, e-mail communication, December 15, 2012
Outbreak descriptions
Between January 2000 and prior to the 2012 fungal meningitis outbreak associated with NECC, we identified 11 outbreaks involving 207 infected case-patients with 17 deaths following exposure to contaminated compounded drugs (Table 1). The overall case fatality rate was 8.2%. The 11 outbreaks involved from 2 to 80 patients each and occurred sporadically. When the 2012 fungal meningitis outbreak was included, the totals increased almost 5-fold (as of March 9, 2013), to 927 infected case-patients with 65 deaths (case fatality rate: 7%).
Including the 2012 fungal meningitis outbreak, the 12 outbreaks were associated with 13 drugs (Table 1). Three outbreaks involved 4 ophthalmic drugs: trypan blue ophthalmic solution used during cataract surgery, Brilliant Blue-G ophthalmic solution used during vitrectomy, and triamcinolone and bevacizumab for intravitreal injection. Three outbreaks involved methylprednisolone or betamethasone primarily for epidural injections. Two outbreaks involved heparin flush and heparin-vancomycin flush solutions for indwelling catheters. The remaining 4 outbreaks involved cardioplegia and intravenous magnesium sulfate, total parenteral nutrition, and fentanyl (Table 1).
The most recent 3 outbreaks involved drugs that were preservative-free formulations intended for intravitreal, intra-articular, or epidural injection (Tables 1 and 2). Information about preservatives was not available for earlier outbreaks.
The outbreaks were caused by organisms that are commonly associated with healthcare associated infections (Serratia marcescens [3 outbreaks], Burkholderia cepacia [2 outbreaks], and Pseudomonas aeruginosa [1 outbreak]); organisms considered commensal but can be associated with infection (Streptococcus mitis/oralis[1 outbreak]); and organisms that rarely cause infection, (Exserohilum rostratum, Fusarium incarnatum-equiseti, Bipolaris hawaiiensis, Pseudomonas fluorescens, Exophiala dermatitidis and Sphingomonas paucimobilis). Time between exposure and onset or recognition of symptoms varied among the outbreaks by organism, route of exposure, and presence of indwelling catheters (Table 1). For example, in the outbreak of P fluorescens bloodstream infections, 59% of the 80 case-patients developed symptoms within 24 hours following exposure to contaminated heparin-saline flush while the remaining 41% were diagnosed from 84 to 421 days after the last potential exposure to a contaminated flush.22 Delayed onset of symptoms and recognition of infection has also been observed in the 2012 fungal meningitis outbreak. 23
Among the 65 case-patients that died in all 12 outbreaks (as of March 9, 2013), at least 38 died of meningitis (and 14 others died from meningitis or other causes24) following epidural injections of betamethasone or methylprednisolone, 10 died of bloodstream infections following intravenous administration of magnesium sulfate or total parenteral nutrition, and 3 died of systemic inflammatory response syndrome (SIRS) following administration of a cardioplegia solution during coronary artery bypass graft surgery. The case-fatality rate varied by route of administration, ranging from 27% for case-patients that received contaminated cardioplegia solution during heart surgery, 7.8% for drugs administered intravenously, and 7.0% for epidural or intra-articular administration.
Among the 51 case-patients who developed endophthalmitis after exposure to contaminated Trypan Blue or Brilliant Blue-G during ophthalmic surgery or intravitreal injection of bevacizumab or triamcinolone, none died. However, the patients with endophthalmitis required repeat surgery (rates of 80-83% reported for outbreaks #10 and 11), experienced high rates of vision loss (ranging from 77% to 100% in the 3 outbreaks) and 5 patients (10%) were reported to undergo evisceration or enucleation of the affected eye (14%).21, 25, 26
Implicated pharmacies and practices
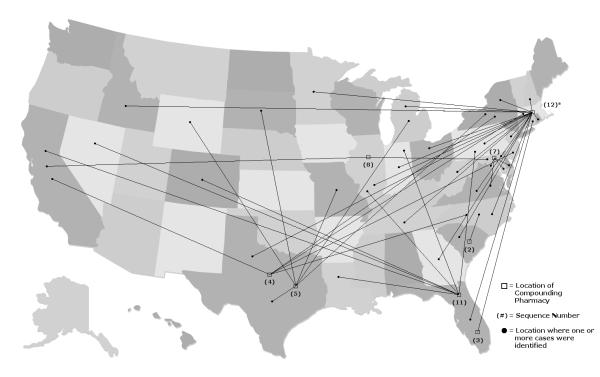
Drugs involved in the outbreaks were compounded by 12 pharmacies located in 10 states throughout the US (Table 2). Two-thirds (n=8) of the outbreaks involved case-patients exposed to drugs compounded by a pharmacy located in a different state (Figure 1), and half involved case-patients located in more than 1 state. In only 3 outbreaks, the compounding pharmacy and case-patients were located in the same state. One additional outbreak (#6) was likely limited to 1 state, but the location of the case-patients was not provided.25
Figure 1.
Location of implicated compounding pharmacies and associated case patients involved in eight multistate outbreaks identified between January 2000 and November 2012.
A source of contamination at the implicated pharmacy was not established in 5 outbreaks. However, the remaining investigations uncovered a myriad of problems including improper autoclave performance or failure to follow autoclave procedures, no sterility testing of finished product, inadequate clean room or environmental sampling practices, failure to follow recommended filter-sterilization processes, and inadequate staff training and quality assurance processes (Table 2). After investigation, sterility could not be assured for any products from the compounding facilities associated with 6 (50%) of the 12 outbreaks, leading to recall of all of the companies sterile products (including #1,2,7,9,11, and 12).
Outbreak detection
Scenarios that led to initial recognition of these outbreaks varied (Table 1). In 2 outbreaks (#2 and #12), an astute clinician or laboratorian reported 1 or 2 cases of rare infections that triggered active surveillance and a public health response. The remaining outbreaks were only identified when a cluster of case-patients from a common hospital or clinic presented with similar clinical characteristics to a common setting. The 2005 outbreak of S marcescens bloodstream infections was independently detected in 2 states simultaneously. Investigation of the 2012 outbreak of fungal endophthalmitis associated with Brilliant Blue-G led to the detection of a second contaminated drug (intravitreal triamcinolone) from the same compounding pharmacy associated with additional case-patients.
Discussion
To our knowledge, this is the first systematic summary in the literature of infectious outbreaks associated with drugs compounded by pharmacies outside the hospital setting. The reported infectious outbreaks were severe and half involved case-patients in multiple states. The 2012 fungal meningitis outbreak was the largest; however, the case morbidity and fatality rates were similar to earlier outbreaks. Case-patients lost their vision, required hospitalization, surgeries, or treatments, and about 7% lost their lives. Each outbreak illustrated one or more root causes that could have been addressed with preventive policies and practices (Figure 2). In particular, the literature described lapses in recommended processes for producing sterile compounded drugs and evidence that clinicians were sometimes unaware they were using compounded drugs. This lack of awareness may obfuscate links between adverse events and compounded drugs, and lead to under-recognition and under-reporting of problems. No single source included information about all the outbreaks.
Figure 2.
Description of factors that may contribute to infectious outbreaks associated with compounded drug
Several of the outbreaks involved suspensions or solutions that were were reported to be preservative-free or were likely preservative-free, including methylprednisolone suspension (preservative status unknown; outbreak #2), preservative-free bevacizumab solution (outbreak #10), preservative-free triamcinolone (probably a suspension; outbreak #11), and preservative-free methylprednisolone suspension (outbreak #12). Although initial publications about the 2012 fungal meningitis outbreak reported the methylprednisolone was a solution, congressional documents later identified it as a suspension.27, 28 The betamethasone formulation in outbreak #1 was also likely a suspension because it was compounded to replace an FDA-approved injectable betamethasone suspension that was not available at the time. Some compounding pharmacies market preservative-free corticosteroids for epidural injection as an alternative to FDA-approved products that contain a preservative. The rationale is that preservative-free formulations may avoid potential neurotoxicity (aseptic meningitis, arachnoiditis). Compounded drugs are also sometimes favored because of lower costs and ready availability during shortages of commercial products.5, 22 Clinicians who use compounded preservative-free solutions and suspensions need to weigh the risks and benefits of these competing concerns.
Compounded sterile products that are preservative-free have a high risk of microbial contamination if standards in United States Pharmacopoeia chapter <797> Pharmaceutical Compounding–Sterile Preparations (USP <797>) are not strictly followed.29 Sterile suspensions in particular may have a high risk of microbial contamination if inappropriate sterilization methods are used. Sterilization of suspensions by heat may not adequately remove pyrogens and fungal contaminants.30 Likewise, sterilization by filtration may be problematic because suspensions can clog a 0.2 micron filter. Some experts recommend against the use of compounded injectable corticosteroid suspensions on the basis that sterilization methods available to compounding pharmacies are inadequate.30
In the bevacizumab outbreak (#10), preservative-free single-dose vials of bevacizumab solution were repackaged into multiple 0.1 mL syringes for multiple patients. A number of endophthalmitis outbreaks with compounded bevacizumab have been linked to poor aseptic technique and non-adherence to requirements for the safe repackaging of single-dose vials outlined in USP <797>.12 The CDC has taken the position that single-dose vials should only be used for 1 patient, and advises that repackaging of unopened single-dose vials for multiple patients should only occur in times of critical need and should be performed in accordance with USP <797>.31
The outbreaks we identified in the literature were those that were investigated and reported by public health authorities after they were identified by clinicians. Investigations of reported compounded drug outbreaks were triggered when they were associated with a cluster of unusual infections (e.g., postprocedural endophthalmitis) or when the identified organism was somehow atypical, because it rarely causes infection or was cultured from an unexpected site. For example, the initial report of a patient with culture-confirmed Aspergillus fumigatus meningitis 46 days after an epidural steroid injection was sufficient to trigger active surveillance and led to the rapid recognition of additional case-patients with similar exposures28. This finding heralded the 2012 fungal meningitis outbreak28. Outbreaks may be more difficult to detect when exposures and case-patients are scattered geographically, or when clinical outcomes are less severe and patients have underlying conditions or treatments that could explain their outcomes. For example, even perceptive clinicians may miss compounded drugs as a source in common and expected infections, such as with central line associated bloodstream infections (e.g., S marcescens sepsis in patients receiving TPN or magnesium sulfate). Lack of clinician awareness that patients even received a compounded product can be a factor that delays, or prevents, the recognition of the source of an outbreak. For example, in the S. marsescens bloodstream infections outbreak following cardiac surgery, neither of the hospital pharmacies was aware the product was compounded as “there was no specific indication on the package labeling, and the company’s website did not specify that the product was compounded.”32 In addition, redistribution of products from a compounding pharmacy before delivery to the final customer may limit the recognition of the potential role of compounded drugs in an infectious outbreak. For example, in the P fluorescens bloodstream infection outbreak, a company employed a compounding pharmacy to make a concentrated heparin solution which was then diluted and repackaged to make heparin-saline flush syringes.22 These syringes were “sold to distributors who redistributed to other medical distributors and hospitals.”33 Such redistribution can blur the recognition of a common source.
To identify and link unusual infections across settings, there is a need for robust surveillance systems that support detection and reporting. Existing systems have limitations. First, communicable disease and injury reporting systems that require reporting of selected diseases and conditions to public health departments34 may capture events, but underreporting is common.35, 36 In addition, the types of infections associated with contaminated compounded drugs would rely on general reporting requirements, such as “[Report] any unusual occurrence of infectious or communicable disease ….”37 Even when providers recognize and consider reporting unusual events, they can be stymied by concerns about potential Health Insurance Portability and Accountability Act (HIPAA) violations. Second, the CDC National Healthcare Safety Network (NHSN) includes a Patient Safety Component, but this component would be unlikely to detect events from compounded drugs, because it is focused on surveillance of a) infections associated with devices or common procedures, or multidrug-resistant organisms, and b) events associated with dialysis and blood products.38 NHSN is not designed to address surveillance of sporadic adverse drug events in outpatient settings, and does not provide real time reporting.
We focused our evaluation on compounding pharmacies in the community, rather than pharmacies that compound drugs in the hospital setting, because the former type of pharmacy may present unique public health risks. The large scale of production and multistate distribution of products from sterile compounding pharmacies may contribute to the size of outbreaks when they occur. In the 2012 fungal meningitis outbreak associated with NECC, approximately 17,600 doses of methylprednisolone were shipped to 76 facilities in 23 states and an estimated 14,000 patients were exposed.27 As the outbreak progressed, more than 4,000 drugs made by NECC and its sister company, Ameridose, were voluntarily recalled. The volume of recalled drugs was large enough to exacerbate drug shortages.39 The size of these companies does not appear to be an anomaly. Central Admixture Pharmacy Services (outbreak # 7) reports making 300,000 deliveries of intravenous admixtures each year.40 PharMEDium (outbreak # 4) employs over 700 people and provides sterile compounding services to over 1800 hospitals in 49 US states. 32, 41
Limitations
Our study has limitations. In particular, our strategy for identifying outbreaks missed outbreaks not reported in the literature or investigated by the FDA or CDC. For example, an EpiNotes publication by the North Carolina Department of Health commented on 2 patients that developed Chryseomonas meningitis from compounded epidural methylprednisolone in 2001, but the details are unpublished.42 Despite this limitation, this paper summarizes the current published literature describing outbreaks of infection associated with drugs compounded outside the hospital setting, which likely presents an underestimate of the problem.
Recommendations
We recommend addressing the root causes identified in these outbreaks (Figure 2), including the regulatory gap, compounding processes, awareness among clinicians, and the public health systems required to monitor compounding practices and identify and respond to outbreaks. Compounding pharmacies should fully comply with USP <797>, the standard for sterile compounding safety in the US29, 43 In 2011, state regulations had not consistently adopted USP <797> as the standard for sterile compounding in their jurisdiction (http://clinicaliq.com/797-state-survey), and compliance among compounding pharmacies was found to be 72% among 1,148 respondents from hospital settings and 82% among a limited sample of 40 respondents from community pharmacy settings.44 In addition, electronic health record systems should be enhanced to recognize links between compounded drugs and infections. This would require improvements in: a) real-time processing of triggers for unusual organisms, unusual organism/specimen source combinations, or rare diagnoses (e.g., endophthalmitis); b) improved coding of compounded drugs in the electronic health record to identify compounded drugs); and c) sharing of laboratory results not explicitly defined in public health communicable disease reporting rules, but indicative of unusual infections.
Conclusion
Prior to the nationwide 2012 fungal meningitis outbreak associated with NECC, compounded drugs produced by compounding pharmacies were associated with 11 other outbreaks that occurred sporadically over the past 12 years. These earlier outbreaks, though not as large, had substantial morbidity and a mortality rate that was similar to the NECC outbreak. Lapses in sterile compounding procedures led to contamination of compounded drugs, exposure to patients, and a threat to public health. Recognition of these outbreaks and subsequent public health investigation was usually triggered by the occurrence of multiple case-patients in a single healthcare setting. Given that drugs and patients are distributed widely, compounded drugs are not always produced using USP <797> standards, and surveillance systems are not optimized to detect these outbreaks in outpatient settings, public health authorities should address root causes to improve the safe use of compounded drugs.
Acknowledgements
Institutional review board approval was not necessary for this summary of publically available information. Authors were partially supported by funding from the Centers for Disease Control and Prevention (Center of Excellence in Public Health Informatics Grant # 5 P01HK000069) and the National Library of Medicine (NLM Training Grant No. T15LM007124).
References
- 1.Pharmacy Compounding. US Food and Drug Administration; Silver Spring: [updated November 28, 2012; cited 2012 November 2]. 2012. Available from: http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/PharmacyCompounding/default.htm. [Google Scholar]
- 2.Multistate Fungal Meningitis Outbreak Investigation. Centers for Disease Control and Prevention; Atlanta: [updated November 14, 2012; cited 2012 November 25]. 2012. Available from: http://www.cdc.gov/hai/outbreaks/meningitis.html. [Google Scholar]
- 3.Centers for Disease Control and Prevention (CDC) Multistate fungal meningitis outbreak - interim guidance for treatment. Morb Mortal Wkly Rep. 2012 Oct 19;61:842. [PubMed] [Google Scholar]
- 4.Meadows M. Warnings for makers of compounded pain products. FDA Consum. 2007 Mar-Apr;41(2):20–2. [PubMed] [Google Scholar]
- 5.Wilson LE, Blythe D, Sharfstein JM. Fungal Meningitis From Injection of Contaminated Steroids: A Compounding Problem. JAMA. 2012 Oct;26:1–2. doi: 10.1001/jama.2012.47932. [DOI] [PubMed] [Google Scholar]
- 6.Press release: International Academy of Compounding Pharmacists Responds to Meningitis Outbreak Tied to Compounding Pharmacy. International Association for Compounding Pharmacists; Houston, Tx: 2012. [Google Scholar]
- 7.Romano MJ, Dinh A. A 1000-fold overdose of clonidine caused by a compounding error in a 5-year-old child with attention-deficit/hyperactivity disorder. Pediatrics. 2001 Aug;108(2):471–2. doi: 10.1542/peds.108.2.471. [DOI] [PubMed] [Google Scholar]
- 8.Whelan GJ, Spahn JD, Brown EE, Szefler SJ. Subpotentcy of a compounded budesonide for nebulization product in a patient with poorly controlled asthma. J Allergy Clin Immunol. 2006;117(2 suppl):S12. [Google Scholar]
- 9.Burton JM, Bell CM, Walker SE, O’Connor PW. 4-aminopyridine toxicity with unintentional overdose in four patients with multiple sclerosis. Neurology. 2008 Nov 25;71(22):1833–4. doi: 10.1212/01.wnl.0000339380.23073.58. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention (CDC) Deaths from intravenous colchicine resulting from a compounding pharmacy error--Oregon and Washington, 2007. Morb Mortal Wkly Rep. 2007 Oct 12;56(40):1050–2. [PubMed] [Google Scholar]
- 11.Hundley K. Lincare pharmacy runs afoul of Missouri regulators. St Petersburg Times Online Business. 2003 Apr;18:2003. [Google Scholar]
- 12.Gonzalez S, Rosenfeld PJ, Stewart MW, Brown J, Murphy SP. Avastin doesn’t blind people, people blind people. Am J Ophthalmol. 2012 Feb;153(2):196–203. e1. doi: 10.1016/j.ajo.2011.11.023. [DOI] [PubMed] [Google Scholar]
- 13.Chollet JL, Jozwiakowski MJ. Quality investigation of hydroxyprogesterone caproate active pharmaceutical ingredient and injection. Drug Dev Ind Pharm. 2012 May;38(5):540–9. doi: 10.3109/03639045.2012.662511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones TF, Feler CA, Simmons BP, Melton K, Craig AS, Moore WL, et al. Neurologic complications including paralysis after a medication error involving implanted intrathecal catheters. Am J Med. 2002 Jan;112(1):31–6. doi: 10.1016/s0002-9343(01)01032-4. [DOI] [PubMed] [Google Scholar]
- 15.Goldman MP. Sodium tetradecyl sulfate for sclerotherapy treatment of veins: is compounding pharmacy solution safe? Dermatol Surg. 2004 Dec;30(12 Pt 1):1454–6. doi: 10.1111/j.1524-4725.2004.30502.x. [DOI] [PubMed] [Google Scholar]
- 16.Report: Limited FDA Survey of Compounded Drug Products. US Food and Drug Administration; [updated June 18, 2009; cited 2012 November 25]. 2009. Available from: http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/PharmacyCompounding/ucm155725.htm. [Google Scholar]
- 17.Inspections, Compliance, Enforcement, and Criminal Investigations: Med-South Pharmacy Inc. U.S. Food and Drug Administration (FDA); [updated September 28, 2007; cited November 25, 2012]. Sep 28, 2007. 07. Available from: http://www.fda.gov/ICECI/EnforcementActions/WarningLetters/2007/ucm076516.htm. [Google Scholar]
- 18.Sellers S, Utian WH. Pharmacy compounding primer for physicians: prescriber beware. Drugs. 2012 Nov 12;72(16):2043–50. doi: 10.2165/11640850-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morbidity and Mortality Weekly Reports (MMWR) Centers for Disease Control and Prevention; [updated December 13, 2012; cited 2012 December 14]. 2012. Available from: http://www.cdc.gov/mmwr/mmwr_wk/wk_cvol.html. [Google Scholar]
- 20.EIS Conference - Program archives for 2006- 2012. Centers for Disease Control and Prevention; Atlanta: [updated April 6, 2012; cited 2012 November 10]. 2012. Available from: http://www.cdc.gov/EIS/Conference/Archive.html. [Google Scholar]
- 21.Centers for Disease Control and Prevention (CDC) Notes from the field: Multistate outbreak of postprocedural fungal endophthalmitis associated with a single compounding pharmacy - United States, March-April 2012. Morb Mortal Wkly Rep. 2012 May 4;61(17):310–1. [PubMed] [Google Scholar]
- 22.Gershman MD, Kennedy DJ, Noble-Wang J, Kim C, Gullion J, Kacica M, et al. Multistate outbreak of Pseudomonas fluorescens bloodstream infection after exposure to contaminated heparinized saline flush prepared by a compounding pharmacy. Clin Infect Dis. 2008 Dec 1;47(11):1372–9. doi: 10.1086/592968. [DOI] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention [updated December 20, 2012; cited 2013 January 21];Health alert Network Update: Multistate Outbreak of Fungal Infections among Persons Who Received Injections with Contaminated Medication. 2012 Available from: http://emergency.cdc.gov/HAN/han00338.asp.
- 24.Centers for Disease Control and Prevention . Multi-state meningitis outbreak - Current case count. Atlanta: [updated March 6, 2013; cited 2013 March 9]. 2013. Available from: http://www.cdc.gov/hai/outbreaks/meningitis-map-large_2013-01-07.html. [Google Scholar]
- 25.Sunenshine R, Schultz M, Lawrence MG, Shin S, Jensen B, Zubairi S, et al. An outbreak of postoperative gram-negative bacterial endophthalmitis associated with contaminated trypan blue ophthalmic solution. Clin Infect Dis. 2009 Jun 1;48(11):1580–3. doi: 10.1086/598938. [DOI] [PubMed] [Google Scholar]
- 26.Goldberg RA, Flynn HW, Jr., Isom RF, Miller D, Gonzalez S. An outbreak of streptococcus endophthalmitis after intravitreal injection of bevacizumab. Am J Ophthalmol. 2012 Feb;153(2):204–8. e1. doi: 10.1016/j.ajo.2011.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. [updated November 12, 2012; cited 2012 December 8];Majority Memorandum: Hearing on “The Fungal Meningitis Outbreak: Could It Have Been Prevented?”. The Committee on Energy and Commerce. 2012 Available from: http://energycommerce.house.gov/sites/republicans.energycommerce.house.gov/files/Hearings/OI/20121114/HMTG-IF02-20121114-SD001.pdf.
- 28.Centers for Disease Control and Prevent (CDC) Multistate outbreak of fungal infection associated with injection of methylprednisolone acetate solution from a single compounding pharmacy - United States, 2012. Morb Mortal Wkly Rep. 2012 Oct 19;61:839–42. [PubMed] [Google Scholar]
- 29.United States Pharmacopeia . The United States Pharmacopeia. 35th rev. The National Formulary. 30th ed The United States Pharmacopeial Convention; Rockville, Maryland: 2012. Pharmaceutical Compounding: Sterile Preparations (General information chapter 797) [Google Scholar]
- 30.Bronstein D, Kandel S. Meningitis Outbreak Fallout: Whom Can You Trust? Pain Medicine News. 2012;10(11) [Google Scholar]
- 31.Centers for Disease Control and Prevention [updated May 2, 2012; cited 2013 January 28];Protect Patients Against Preventable Harm from Improper Use of Single–Dose/Single–Use Vials. Available from: http://www.cdc.gov/injectionsafety/cdcposition-singleusevial.html.
- 32.Sunenshine RH, Tan ET, Terashita DM, Jensen BJ, Kacica MA, Sickbert-Bennett EE, et al. A multistate outbreak of Serratia marcescens bloodstream infection associated with contaminated intravenous magnesium sulfate from a compounding pharmacy. Clin Infect Dis. 2007 Sep 1;45(5):527–33. doi: 10.1086/520664. [DOI] [PubMed] [Google Scholar]
- 33.FDA news release: FDA renews nationwide alert on IV flush brand of heparin or sodium chloride intraveious catheter flushes in light of new contamination reports. US Food and Drug Administration; [updated February 4, 2005; cited 2012 November 12]. 2005. Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2005/ucm108409.htm. [Google Scholar]
- 34.Roush S, Birkhead G, Koo D, Cobb A, Fleming D. Mandatory reporting of diseases and conditions by health care professionals and laboratories. JAMA. 1999;282(2):164–70. doi: 10.1001/jama.282.2.164. [DOI] [PubMed] [Google Scholar]
- 35.Doyle TJ, Glynn MK, Groseclose SL. Completeness of Notifiable Infectious Disease Reporting in the United States: An Analytical Literature Review. Am J Epidemiol. 2002;155(9):866–74. doi: 10.1093/aje/155.9.866. [DOI] [PubMed] [Google Scholar]
- 36.Overhage JM, Grannis S, McDonald CJ. A Comparison of the Completeness and Timeliness of Automated Electronic Laboratory Reporting and Spontaneous Reporting of Notifiable Conditions. Am J Public Health. 2008;98(2):344–50. doi: 10.2105/AJPH.2006.092700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Disease Reporting. Utah Department of Health; Salt Lake City: [updated August 17, 2012; cited 2012 December 15]. 2012. Available from: http://health.utah.gov/epi/report.html. [Google Scholar]
- 38.Monitoring healthcare associated infections. Centers for Disease Control and Prevention; Atlanta: [updated May 23, 2012; cited 2012 November 3]. 2012. Available from: http://www.cdc.gov/HAI/surveillance/index.html. [Google Scholar]
- 39.CDC Health Advisory: Voluntary Recall of All Ameridose Medical Products. Centers for Disease Control and Prevention; [updated November 1, 2012; cited 2012 November 12]. 2012. Available from: http://emergency.cdc.gov/HAN/han00332.asp. [Google Scholar]
- 40.Central Admixture Pharmacy Services, Inc. (CAPS) Central Admixture Pharmacy Services, Inc. (CAPS); [updated 2012; cited 2012 November 19]. 2010. Available from: http://www.capspharmacy.com/Products.html. [Google Scholar]
- 41.Harris M, Wong W. Chicago technology venture capital scorecard for 2011. Chicago Tribune. 2012 Jun;16:2012. [Google Scholar]
- 42.Engel J. Exophiala (Wangiella) dermatitidis fungal infection outbreak. North Carolina Department of Health and Human Services Epi Notes. 2002;4:4–6. [Google Scholar]
- 43.Kastango ES. Compounding USP <797>: inspection, regulation, and oversight of sterile compounding pharmacies. J Parenter Enteral Nutr. 2012 Mar;36(2 Suppl):38S–9S. doi: 10.1177/0148607111434779. [DOI] [PubMed] [Google Scholar]
- 44.Douglass K, Kastango ES, Cantor P, Shevitz M. 2011 USP <797> compliance study. Pharmacy Purchasing & Products. 2011 Oct;8:8. [Google Scholar]
- 45.Civen R, Vugia DJ, Alexander R, Brunner W, Taylor S, Parris N, et al. Outbreak of Serratia marcescens infections following injection of betamethasone compounded at a community pharmacy. Clin Infect Dis. 2006 Oct 1;43(7):831–7. doi: 10.1086/507336. [DOI] [PubMed] [Google Scholar]
- 46.Centers for Disease Control and Prevention (CDC) Exophiala infection from contaminated injectable steroids prepared by a compounding pharmacy--United States, July-November 2002. Morb Mortal Wkly Rep. 2002 Dec 13;51(49):1109–12. [PubMed] [Google Scholar]
- 47.Held MR, Begier EM, Beardsley DS, Browne FA, Martinello RA, Baltimore RS, et al. Life-threatening sepsis caused by Burkholderia cepacia from contaminated intravenous flush solutions prepared by a compounding pharmacy in another state. Pediatrics. 2006 Jul;118(1):e212–5. doi: 10.1542/peds.2005-2617. [DOI] [PubMed] [Google Scholar]
- 48.Inspections, Compliance, Enforcement, and Criminal Investigations: PharMEDium Services, LLC 13-Apr-07 (Warning letter) US Food and Drug Administration; [updated July 7, 2009; cited 2012 November 25]. 2007. Available from: http://www.fda.gov/ICECI/EnforcementActions/WarningLetters/2007/ucm076357.htm. [Google Scholar]
- 49.Centers for Disease Control and Prevention (CDC) Pseudomonas bloodstream infections associated with a heparin/saline flush--Missouri, New York, Texas, and Michigan, 2004-2005. Morb Mortal Wkly Rep. 2005 Mar 25;54(11):269–72. [PubMed] [Google Scholar]
- 50.Centers for Disease Control and Prevention (CDC) Update: Delayed onset Pseudomonas fluorescens bloodstream infections after exposure to contaminated heparin flush--Michigan and South Dakota, 2005-2006. Morb Mortal Wkly Rep. 2006 Sep 8;55(35):961–3. [PubMed] [Google Scholar]
- 51.Patel AS, Woolard D, McDonald LC, Dewey L, Noble-Wang J, Forster T, et al., editors. Outbreak of Systemic Inflammatory Response Syndrome Linked to a Compounding Pharmacy — Virginia. 55th Annual Epidemic Intelligence Service (EIS) Conference; Atlanta, GA. 2006 April 24-28; Centers for Disease Control and Prevention; 2005. [Google Scholar]
- 52.Compounded Menopausal Hormone Therapy Questions and Answers. US Food and Drug Administration; [updated February 5, 2010; cited 2012 November 11]. 2010. Available from: http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/PharmacyCompounding/ucm183088.htm. [Google Scholar]
- 53.Maragakis LL, Chaiwarith R, Srinivasan A, Torriani FJ, Avdic E, Lee A, et al. Sphingomonas paucimobilis bloodstream infections associated with contaminated intravenous fentanyl. Emerging infectious diseases. 2009 Jan;15(1):12–8. doi: 10.3201/eid1501.081054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gupta N, Hocevar S, O’Cannell H, Stenvens K, Massingale S, McIntyre M, et al. Serratia marcescens Bloodstream infections in patients receiving total parenteral nutrition - Alabama. 61st Annual Epidemic Intelligence Service (EIS) Conference; Atlanta GA. 2012 April 16-20; Centers for Disease Control and Prevention (CDC); 2011. [Google Scholar]
- 55. [updated March 30, 2011; cited 2012 Oct 28];CDC and ADPH Investigate Outbreak At Alabama Hospitals; Products Recalled. US Food and Drug Administration Web site. 2011 Available from: http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm249099.htm.
- 56.Matthews J, Dubovy S, Goldberg RA, Flynn HW. American Academy of Ophthalmology - Asia-Pacific Academy of Ophthalmology annual meeting. American Academy of Ophthalmology; Chicago, IL: 2012. Histopathology of Streptococcal Endophthalmitis Following Intravitreal Injection of Bevacizumab: A Series of Seven Cases (Poster) [Google Scholar]
- 57.Beau De Rochars VM, Schmitz A, Pritchard P, Deall B, Noble-Wang J, O’Connell H, et al., editors. Streptococcal Endophthalmitis Associated with Contaminated Bevacizumab from a Compounding Pharmacy — Florida. 61st Annual Epidemic Intelligence Service (EIS) conference; Atlanta, GA. 2012; Centers for Disease Control and Prevention (CDC); 2011. [Google Scholar]
- 58.Kastango ES. The cost of quality in pharmacy. Int J Pharmaceut Compounding. 2002;6(6):404–7. [PubMed] [Google Scholar]
- 59. [updated July 8, 2009; cited 2012 October 28];Injectable drugs prepared by Urgent Care Pharmacy. US Food and Drug Administration Web site. 2009 Available from: http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm154503.htm.
- 60. [updated June 22, 2009; cited 2012 October 28];FDA Issued Nationwide Alert on IV Flush Brand of Heparin or Sodium Chloride Intravenous Catheter Flushes. US Food and Drug Administration Web site. 2009 Available from: http://www.fda.gov/downloads/ICECI/EnforcementActions/EnforcementStory/EnforcementStoryArchive/UCM091066.pdf.
- 61. [updated July 8, 2009; cited 2012 December 16];Inspections, Compliance, Enforcement, and Criminal Investigations: IV Flush, LLC 04-August-05. US Food and Drug Administration Web site. 2005 Available from: http://www.fda.gov/ICECI/EnforcementActions/WarningLetters/2005/ucm075511.htm.
- 62.Recall --firm press release: Custom-Rx Compounding Pharmacy Issues Nationwide Recall of Trypan Blue 0.06% Ophthalmic Solution. U.S. Food and Drug Administration (FDA); [updated June 18, 2009; cited 2012 November 11]. 2005. Available from: http://www.fda.gov/Safety/Recalls/ArchiveRecalls/2005/ucm111889.htm. [Google Scholar]
- 63.Central Admixture Pharmacy Services, Inc: Urgent drug recall: all injectable products. US Food and Drug Administration; [updated September 16, 2005; cited 2012 November 11]. 2005. Available from: http://www.fda.gov/downloads/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/UCM164417.pdf. [Google Scholar]
- 64. [updated July 8, 2009; cited 2012 October 28];Inspections, Compliance, Enforcement, and Criminal Investigations: B. Braun Medical Inc 15-Mar-06. US Food and Drug Administration Web site. 2006 Available from: http://www.fda.gov/ICECI/EnforcementActions/WarningLetters/2006/ucm075828.htm.
- 65. [updated August 29, 2012; cited 2012 October 28];Inspections, Compliance, Enforcement, and Criminal Investigations: Infupharma, LLC 7/30/12. US Food and Drug Administration Web site. 2012 Available from: http://www.fda.gov/ICECI/EnforcementActions/WarningLetters/2012/ucm317190.htm.
- 66.FDA Form 483 for Infupharma, LLC. US Food and Drug Administration; [updated September 27, 2011; cited 2012 December 21]. 2011. Available from: http://www.fda.gov/downloads/AboutFDA/CentersOffices/OfficeofGlobalRegulatoryOperationsandPolicy/ORA/ORAElectronicReadingRoom/UCM304256.pdf. [Google Scholar]
- 67.Inspections, Compliance, Enforcement, and Criminal Investigations: Franck’s Lab Inc., d.b.a. Franck’s Compounding Lab 7/9/12. US Food and Drug Administration; [updated July 18, 2012; cited 2012 November 10]. 2007. Available from: http://www.fda.gov/ICECI/EnforcementActions/WarningLetters/2012/ucm312645.htm. [Google Scholar]
- 68. [updated May 24, 2012; cited 2012 October 28];Franck’s Compounding Pharmacy Sterile Preparations: Reports of Fungal Endophthalmitis, Expanded Recall. US Food and Drug Administration Web site. 2012 Available from: http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm305592.htm.
- 69.FDA Form 483 for New England Compounding Center. US Food and Drug Administration; [updated October 26, 2012; cited 2012 December 8]. 2012. Available from: http://www.fda.gov/downloads/AboutFDA/CentersOffices/OfficeofGlobalRegulatoryOperationsandPolicy/ORA/ORAElectronicReadingRoom/UCM325980.pdf. [Google Scholar]
- 70.Kainer MA, Reagan DR, Nguyen DB, Wiese AD, Wise ME, Ward J, et al. Fungal Infections Associated with Contaminated Methylprednisolone in Tennessee. N Engl J Med. 2012 Nov 6;367(23):2194–203. doi: 10.1056/NEJMoa1212972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. [updated December 11, 2012; cited 2012 December 16];Multistate Fungal Meningitis Outbreak Investigation: Laboratory Testing and Results from the Outbreak. Centers for Disease Control and Prevention Web site. 2012 Available from: http://www.cdc.gov/hai/outbreaks/laboratory/lab_testing_results.html.