Abstract
Purpose
The purpose of this study was to evaluate the two-stage surgical technique combining induced membrane, spongy autograft and intramedullary fixation for the treatment of congenital pseudarthrosis of the tibia (CPT).
Methods
Three boys and two girls were treated by this technique between 2003 and 2008. All patients had type IV CPT in Crawford’s classification. Four of them had a limited dystrophic form, whereas one case presented an extensive tibia bone dystrophy. The average age of patients at the time of surgery was 23 months (range 10–30 months), with an average follow-up of 5.8 years (range 2.4–8.1 years).
Results
Satisfactory tibial bony union was achieved in all cases at the last follow-up. Bone healing was obtained in the four limited forms after an average term of 4 months. One patient suffered from a non-displaced fracture that healed by casting in a usual period of time. The patient with an extensive dystrophic bone had to undergo a secondary inter-tibiofibular bone graft to finally achieve bone union.
Conclusions
The preliminary results show that this technique is successful in CPT. It may be used even in young children and offers a good alternative to other treatments available, avoiding external fixation and the technical difficulties of microvascular surgery.
Keywords: Congenital pseudarthrosis of the tibia, Induced membrane
Introduction
Congenital pseudarthrosis of the tibia (CPT) is a rare condition and surgical treatment is one of the most challenging problems in pediatric orthopedics because of the difficulty in achieving healing, the residual deformities, and the limb-length discrepancies. Multiple operations are often necessary to obtain lasting union of the bone and amputation has not been entirely eliminated [1].
Masquelet and Begue [2] reported the use of an innovative two-stage technique that involves the insertion of a cement spacer, induction of a membrane and reconstruction of the defect with cancellous bone graft in the treatment of extensive diaphyseal bone defect. It is a simple two-stage technique enabling early surgery in children suffering from CPT [3, 4]. Moreover, although the etiology of CPT is unknown, recent reports suggest the periosteum as the primary site for the pathologic processes in this pathology [5]. The excision of the diseased periosteum with the induction of a new membrane responds to the latest etiopathogenic theories and could be a new method of treatment for CPT. The purpose of this study is to evaluate the two-stage surgical technique combining induced membrane, spongy autograft and intramedullary fixation for the treatment of CPT.
Patients and methods
Population data
We retrospectively reviewed the clinical and radiographic outcome in five patients with CPT treated between 2003 and 2008 in one center by the induced membrane technique. At the time of presentation, all the patients had established non-union with pseudarthrosis and could walk only with the aid of external supports. None of the patients had previous surgeries or other concomitant procedures. One patient had no associated disorder and four patients had NF1 based on criteria defined by the National Institutes of Health in 1987. CPT involved the right side in two cases, left side in two cases, and was bilateral in one case, with pseudarthrosis on the right side and anterolateral bowing on the left side without pseudarthrosis. According to Crawford’s classification [6], the five tibiae were type IV. The pseudarthrosis was localized in the distal third of the tibia in four cases (Figs. 1, 3, 4, and 5) and in the distal metaphysis of the tibia in one case (Fig. 2). Four cases had a limited dystrophic form and one presented an extensive tibia bone dystrophy. Associated pseudarthrosis of the fibula was present preoperatively in 3 of the 5 patients (Figs. 1, 2, and 3). The average age of patients at the time of surgery was 23 months (range 10–30 months). Two-stage surgical management was standardized for all patients. The first stage consisted of the excision of the dystrophic zone, replaced by a cement spacer and intramedullary pinning. Two months later, a second stage was conducted and the spacer was removed to make way for an autologous cancellous bone graft.
Fig. 1.
Case 1. a Anteroposterior radiograph showing congenital pseudarthrosis of the tibia (CPT) of the distal third of the tibia in a 30-month-old girl. b Postoperative radiograph of the first stage of the induced membrane technique with cement spacer on the tibia and osteosynthesis with two transplantar tibial K-wires and one fibular K-wire. c Postoperative radiograph of the second stage of the induced membrane technique after grafting in the biological chamber surrounded by the membrane. d Full-length standing anteroposterior radiograph of the lower limbs at follow-up of 6 years showing a discrepancy of 4 cm on the CPT side
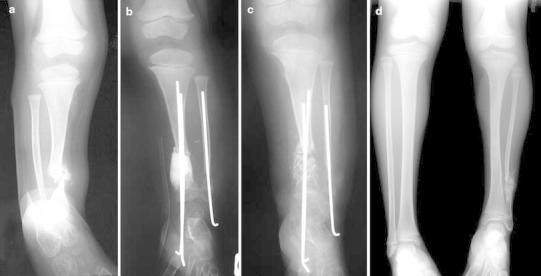
Fig. 3.

Case 3. a Anteroposterior radiograph showing CPT of the distal third of the tibia in a 26-month-old boy. b Graft appearance 2 months after removal of the cement spacer and osteosynthesis with tibial transplantar telescopic nail and fibular K-wire. c Full-length standing anteroposterior radiograph of the lower limbs at follow-up of 5.6 years showing a discrepancy of 2 cm on the CPT side
Fig. 4.

Case 4. a Anteroposterior radiograph showing CPT of the distal third of the tibia in a 30-month-old boy. b Postoperative radiograph of the first stage of the induced membrane technique with cement spacer on the tibia and the fibula, and osteosynthesis with tibial transplantar telescopic nail and fibular K-wire. c Postoperative radiograph of the second stage of the induced membrane technique after grafting in the biological chamber surrounded by the membrane. d Persistent non-union 11 months after the last procedure showing bone graft lysis on the tibia and fibular union. e Postoperative radiograph of the inter-tibiofibular graft. f Radiograph at the last follow-up (4.7 years)
Fig. 5.
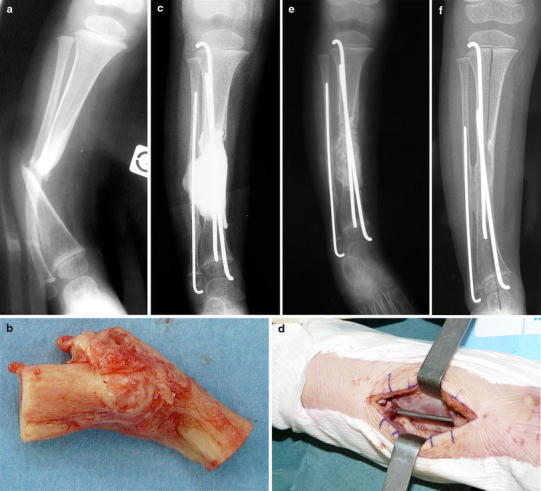
Case 5. a Anteroposterior radiograph showing CPT of the distal third of the tibia in a 20-month-old girl. b Operative specimen of the excision of the pseudarthrosis and the abnormal periosteum. c Postoperative radiograph of the first stage of the induced membrane technique. Cement spacer bridging both the tibial and the fibular gap, and intramedullary pinning of the tibia and the fibula. d Intraoperative view of the induced membrane after removal of the cement spacer opened in continuity with the periosteum of the proximal and distal remaining tibia. e Postoperative radiograph of the second stage of the induced membrane technique after grafting in the biological chamber surrounded by the membrane. f Radiograph at the last follow-up (2.4 years)
Fig. 2.
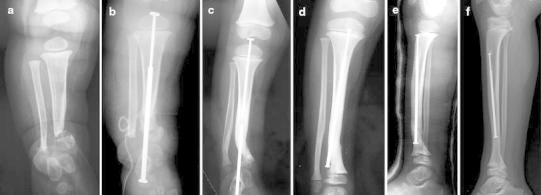
Case 2. a Anteroposterior radiograph showing CPT of the distal metaphysis of the tibia in a 10-month-old boy. b Postoperative radiograph of the first stage of the induced membrane technique with cement spacer on the tibia and osteosynthesis with a tibial transplantar telescopic nail. c Dissociation of the two parts of the telescopic nail lead to changing the nail in order to release the tibiotalar joint. d Anteroposterior radiograph after changing the telescopic nail 2.5 years after the induced membrane procedure. e Non-displaced fracture after a trauma 3.5 years after the induced membrane procedure treated by cast. f Radiograph at the last follow-up (8 years)
Operative technique
A sterile tourniquet was placed on the thigh and inflated after elevation of the lower limb. The tibia pseudarthrosis site was approached through an anteromedial incision and the fibula pseudarthrosis site through a lateral incision. The first stage consisted in the excision of the pseudarthrosis, the fibrous hamartoma and the diseased periosteum. The surgeon determines the extent of resection during surgery by macroscopic evaluation of the bone. The pseudarthrosis was completely excised until finding a normal bleeding bone in the medullary canal on both the proximal and distal sides. Intramedullary pinning was then conducted bridging the excised dystrophic zone, under radioscopic control. A polymethyl methacrylate cement spacer was placed in the gap around the pins to induce the formation of a pseudo-synovial membrane. Intramedullary pinning used two transplantar K-wires in one case (Fig. 1), two telescopic K-wires in one case (Fig. 5) and a transplantar Bailey–Dubow telescopic nail in three cases (Figs. 2, 3, and 4). Intramedullary pinning of the fibula by a K-wire was associated in four patients. The cement spacer was placed only on the tibia in the first two patients, two distinct spacers were placed on the fibula and the tibia for the next two patients, and, finally, the last patient had a unique spacer bridging both the tibial and the fibular gap (Fig. 5). The operative variants are resumed in Table 1. A long leg cast was applied between the two stages, and weight bearing as tolerated is permitted.
Table 1.
Demographic data of the patient series
| Case | Sex | Side | NF1 | Form | Age at surgery (months) | Operative procedure | Follow-up (years) | Complications | Reoperations for non-union | Results | Leg length discrepancy (cm) | Johnston classification |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 (Fig. 1) | F | L | Yes | 1/3 distal tibia and fibula | 30 | 1 tibial spacer, 2 transplantar K-wires, fibular K-wire | 8.1 | Rods migration, pseudarthrosis of the fibula | Inter-tibiofibular graft | Union | −4 | 1 |
| 2 (Fig. 2) | M | R | No | 1/4 distal tibia | 10 | 1 tibial spacer, transplantar telescopic nail | 8 | Refracture | No | Union | −3.5 | 1 |
| 3 (Fig. 3) | M | R | Yes | 1/3 distal tibia and fibula | 26 | 1 tibial and 1 fibular spacers, transplantar telescopic nail, fibular K-wire | 5.6 | None | No | Union | −2 | 1 |
| 4 (Fig. 4) | M | L | Yes | 1/3 distal tibia and fibula | 30 | 1 tibial and 1 fibular spacers, transplantar telescopic nail, fibular K-wire | 4.7 | Non-union | Inter-tibiofibular graft | Union | −3 | 2 |
| 5 (Fig. 5) | F | Bilat. | Yes | 1/3 distal tibia | 20 | 1 tibial and 1 fibular spacers, 2 telescopic K-wires, fibular K-wire | 2.4 | None | No | Union | 0.5 | 1 |
Two months later, the second stage of the reconstruction was performed. The patient was placed prone and a posterior cortico-cancellous iliac crest autograft was harvested. After the wound was closed, the patient was positioned supine. The cement spacer was easily approached with minimal dissection using the previous incision. The membrane covering the cement was opened all along the spacer and in continuity with the first centimeter on the proximal and distal part of the tibia (Fig. 5). The bone was decorticated at the junction with the spacer to facilitate removal of the cement and to produce autogenous bone chips. The cement was readily removed around the intramedullary pins. Care was taken to leave the surrounding soft tissues attached to the external side of the membrane. The medullary canal of both tibial fragments was reamed. The iliac autograft was used to fill the remaining defect throughout its length and was positioned on the proximal and distal part of the reconstruction. In one case, the iliac graft was mixed with a femoral head allogenous graft (case 5). The membrane was closed over the graft without a suction drain. Postoperative plain radiographs were satisfactory.
Patients were immobilized by a long leg cast for 2 months without weight bearing. After 2 months, the cast was changed for another long leg cast, this time allowing weight bearing. Finally, after 4 months, the cast was removed and patients only wore a protective leg brace.
Radiographic follow-up was made at 2-month intervals until bone union and then every 6 months with anterior and lateral views. Clinical office notes were reviewed to determine functional outcome according to the criteria defined by Johnston [7]. Grade 1 outcome was characterized by unequivocal union with maintenance of alignment that required no additional surgical treatment. A grade 2 outcome was characterized by equivocal union and/or deformity for which additional surgery was required or anticipated and a grade 3 outcome was characterized by persistent non-union or refracture, pain or instability of the limb, and a need for full-time orthotic support.
Results
The average follow-up term was 5.8 years (range 2.4–8.1 years). The results are summarized in Table 1 and all five cases are illustrated in the figures. Tibia consolidation was obtained after 4 months in 4 of the 5 patients. These patients were the ones with a limited dystrophic form. Among these four patients, case 1 presented a persistent fibula non-union with progressive distal migration of the intramedullary rods. A secondary procedure was performed 1 year after the induced membrane technique to obtain consolidation of the fibula and consisted in the removal of the rods and in an inter-tibiofibular graft.
Case four presented an extensive dystrophic form associated with NF1. Bone resection of the pseudarthrosis and the abnormal periosteum was probably insufficient. We observed progressive bone graft lysis and persistent non-union 11 months after the last procedure (Fig. 4d). Revision surgery was necessary, involving decortication and inter-tibiofibular grafting with posterior cortico-cancellous iliac crest autograft. At the last follow-up, bone union had been obtained, but consolidation remained fragile and the patient still wore an orthotic support.
There were no septic complications in our series. After an average follow-up duration of 5.8 years (range 2.4–8.1 years), all patients were pain-free, with full knee motion, and feet were in a plantigrade position. A persistent bowing in varus was observed in two patients (cases 2 and 5) and related to a fault with the intramedullary fixation. Case 2 suffered from a non-displaced fracture following a trauma 3.5 years after the induced membrane procedure. This fracture healed without secondary surgery after 45 days of long leg casting with weight bearing (Fig. 2). In this case, the intramedullary transplantar nail was changed 14 months after the induced membrane procedure to release the tibiotalar joint.
The average limb-length discrepancy was 2.4 cm (ranging from +0.5 to −4 cm) and clinical results at the last follow-up following Johnston’s classification [7] found four cases of stage 1 and one of stage 2.
Discussion
Until today, no treatment of CPT has proven to be ideal to achieve and maintain bone union while providing a functional lower limb. Even if union has been achieved, many cases will need multiple procedures to correct residual deformities and amputation has not been entirely eliminated [1]. There is no gold standard surgical technique to successfully treat all types and presentations of CPT. The choice of technique should be adapted to the type of pseudarthrosis and especially to the extent of the bone defect. In normal and hypertrophic types, when there is little shortening, good results can be obtained with intramedullary nailing with a bone graft or with the Ilizarov technique. The debate usually involves atrophic forms in which resection results in significant loss of bone substance and when treatment becomes more challenging. The purpose of this study is to evaluate a new technique in the treatment of CPT adapted to atrophic types.
Today, the best results to achieve bone union have been reported using vascularized fibular grafting [8, 9] and Ilizarov intercalary bone transport [10, 11]. The transfer of healthy vascularized bone in the first case and increasing blood flow by proximal corticotomy and diaphyseal transfer in the second creates a favorable vascular environment for union while compensating for bone defects. The induced membrane technique is also appropriate for this indication by creating a biological chamber with osteogenic and vascular growth factors while filling large-volume bone defects. If bone substance loss is no more than 4 cm, these three techniques can be used depending on the experience of the surgical team. Beyond this or in case of extensive sclerotic forms which make diaphyseal transport impossible, a vascularized graft which would fill bone defects and result in faster union, or the induced membrane technique, can be considered.
Free fibular transfers require specialized surgery with the technical difficulties of microsurgery in young children. The need for secondary grafting at the bone–fibular junction to obtain final union has been reported in more than 30 % of cases of vascularized fibular transfers. The risk of recurrent fracture is high, usually at the bone–fibular junction or in the body of the fibular graft before hypertrophy [12]. This technique is also the source of residual angulation, occurring in more than half the cases, in particular valgus and recurvatum deformities, which do not resolve during growth. Valgus ankle deformities on the side of the harvested fibula in growing children may cause chronic pain to develop later on [9].
Studies after external fixator osteosynthesis [13, 14] showed a high rate of complications, with recurrent fractures linked to persistent axial deformities and pin infections. A second intervention is necessary in nearly half of the cases to correct a residual angular deformity or to treat a recurrent fracture or an infection. A valgus ankle deformity was found in 25 % of the cases because of persistent fibular pseudarthrosis or a distal tibial epiphyseal growth defect. Associating internal fixation with pins or telescopic nails with the external fixation has been proposed because of the high rate of recurrent fracture and axial deformities [15]. External fixators may be difficult to use in small children because of the size and difficulties fixing small osteoporotic bony fragments, especially in distally located dystrophic lesions [16]. For this reason, some authors [9, 17] and the European Pediatric Orthopaedic Society (EPOS) [10, 18] report better results in older patients. Conversely, other authors [5, 19] who combine external fixation to bone grafting have obtained higher rates of bone union in children under the age of 3 years. Early surgery avoids hypoplasia of the leg and foot, muscular atrophy, and increased risk of leg length discrepancies, but it can also lead to technical problems. The induced membrane technique is adapted to small children (10 months old for the youngest child of our series).
We believe that the quality and stability of the bone fixation is the key to the success of this technique. We have used intramedullary synthesis by pins or nails in all our patients. We used a transplantar intramedullary nail in our first four cases, but this material leads to significant ankle stiffness and muscle atrophy. To avoid this, the nail was removed in one patient (case 1) and changed for a tibia nail in another patient (case 2) to release ankle mobility. We prefer using telescopic tibia pinning to avoid interfering with ankle motion, as used in case 5 (Fig. 5). This intramedullary fixation has the advantage of providing long-lasting mechanical stability by internal splinting. It maintains the tibia axis during growth, avoiding angular deformities and protects from repeated fractures. Insertion of the intramedullary telescopic nails or rods requires a rigorous surgical technique with a perfect final mechanical axis. The material must be of sufficient diameter to fill the intramedullary canal to have primary stability. Postoperative axial deviation is likely to increase with the growth, thus being a source of refracture, as observed in case 2. Treating the fibula pseudarthrosis if it exists also appears to be indispensable [20]. Fibular union associated or not with internal fixation by intramedullary nailing reinforces stability by distributing loads and protects, in particular, from rotational trauma [11, 21]. In the induced membrane technique, the fibular surgery is an important step. In four cases, we performed excision of the fibula pseudarthrosis and used a spacer. The spacer used was in continuity with the tibia spacer or separate with intramedullary fibular pinning. Fibular union was obtained in all four cases. Case 1 was the only case where no spacer was used on the fibular side and bone union was not achieved without a reoperation.
Another issue concerns the limits of resection of the dystrophic bone and pathological periosteum. We think that incomplete resection leads to non-union in case 4. At present, the extent of resection is determined by the surgeon during surgery by macroscopic evaluation of the bone and the reappearance of a permeable intramedullary canal. Magnetic resonance imaging (MRI) could play an important role in the preoperative evaluation of the fibrous hamartoma, the periosteum, and bone lesions [22] to improve identification of the extent of bone and soft tissue lesions to be excised. New bone perfusion sequences can show bone vascularization defects, and help define the limits of resection and increase understanding of the physiopathological mechanisms of this disease. The periosteal cuff surrounding the pseudarthrosis may play a major role in the advent of a bone lesion. It is markedly thickened in the region where the pseudarthrosis develops with disturbance of blood circulation. The resulting impaired oxygen and nutrient supply of the subperiosteal bone could explain subsequent fracture and recalcitrant non-union [23]. In our technique, complete excision of the diseased periosteum at the same time as the pseudarthrosis is important because the induced membrane may act as a substitute for the pathologic periosteum. In agreement with these findings, the reported CPT healing rates were higher when the pathologic periosteum and fibromatous tissue were entirely removed [12]. Recently, periosteal grafting combined with bone grafting has been described to treat CPT, with good results [5, 24].
The induced membrane creates a biological chamber for the bone graft. Animal studies have shown that the membrane is vascularized by numerous small capillaries associated with positive cells to the runt-related transcription factor 2 (Runx2), a specific transcription factor of osteoblasts and necessary for their differentiation [25]. High concentration rates of growth factors have been found in the membrane’s cells, especially transforming growth factor β (TGFβ), vascular endothelial growth factor (VEGF), and specific bone growth factors such as BMP-2. The membrane promotes human mesenchymal cell proliferation and differentiation into bone-forming cells [26]. In addition to this biological function, the induced membrane also prevents soft tissue protrusion in the defect, retains the bone graft in place and protects it against resorption, thus creating a favorable environment for bone engraftment. The use of the induced membrane has been reported previously for the reconstruction of large bony defects following trauma [27] in adults or bone tumors in children [28] and recently in congenital pseudarthrosis of the tibia [3, 4]. One limit of the technique is the quantity of autogenous bone graft available in young children. In the original technique, the use of allograft or bone graft substitute combining the autogenous bone grafts is possible as long as the ratio is not greater than one-third. This allows to expand the volume of the graft or to preserve a posterior iliac crest in patients with bilateral involvement. We expanded the graft volume with a femoral head allogenous graft in case 5 with a good result.
In our series, successive radiographs showed rapid integration of the autograft with excellent bone union and cortical reconstruction in four of the five patients. In the case of repeated fracture (case 2), the patient presented a varus deformity of the distal tibia secondary to a fault in the mechanical quality of the osteosynthesis. We observed a progressive correction of this deformity with growth. This is important because it shows that the dystrophic bone has been replaced by a bone capable of remodeling and spontaneous axis correction. It would be interesting in the future to study this induced membrane specifically in CPT to understand its origin and function by mean of biopsies made during the second-stage surgery.
Leg length discrepancies remained limited, with an average of 2.4 cm. The rigid cement spacer that avoids compression and length loss in the gap left by the resection can explain this good result. A contralateral epiphysiodesis may be proposed during growth to equalize the lower limbs.
Conclusion
Early results show that the induced membrane technique offers a new solution to treat congenital pseudarthrosis of the tibia (CPT). It is a simple and reliable technique without microvascular surgery or external fixation, and may be used in young children. The induced membrane technique responds to both the mechanical and biological aspects of this disease, including complete excision of pathological periosteum, induction of a vascularized biological membrane and bone grafting combined with intramedullary fixation to achieve and maintain union. Long-term follow-up until skeletal maturity is required in order to secure treatment success.
References
- 1.Lehman WB, Atar D, Feldman DS, Gordon JC, Grant AD. Congenital pseudoarthrosis of the tibia. J Pediatr Orthop B. 2000;9:103–107. doi: 10.1097/01202412-200004000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Masquelet AC, Begue T. The concept of induced membrane for reconstruction of long bone defects. Orthop Clin North Am. 2010;41:27–37. doi: 10.1016/j.ocl.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 3.Pannier S, Bourgeois A, Topouchian V, Pouliquen J-C, Finidori G, Glorion C. Membrane induite et greffe spongieuse dans le traitement de la pseudarthrose congénitale de jambe chez l’enfant: résultats préliminaires à propos de 3 cas. Rev Chir Orthop Reparatrice Appar Mot. 2007;93:106. doi: 10.1016/S0035-1040(07)79531-4. [DOI] [Google Scholar]
- 4.Gouron R, Deroussen F, Juvet M, Ursu C, Plancq MC, Collet LM. Early resection of congenital pseudarthrosis of the tibia and successful reconstruction using the Masquelet technique. J Bone Joint Surg Br. 2011;93:552–554. doi: 10.1302/0301-620X.93B4.25826. [DOI] [PubMed] [Google Scholar]
- 5.Thabet AM, Paley D, Kocaoglu M, Eralp L, Herzenberg JE, Ergin ON. Periosteal grafting for congenital pseudarthrosis of the tibia: a preliminary report. Clin Orthop Relat Res. 2008;466:2981–2994. doi: 10.1007/s11999-008-0556-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crawford AH. Neurofibromatosis in the pediatric patient. Orthop Clin North Am. 1978;9:11–23. [PubMed] [Google Scholar]
- 7.Johnston CE., 2nd Congenital pseudarthrosis of the tibia: results of technical variations in the Charnley–Williams procedure. J Bone Joint Surg Am. 2002;84:1799–1810. [PubMed] [Google Scholar]
- 8.Gilbert A, Brockman R. Congenital pseudarthrosis of the tibia. Long-term followup of 29 cases treated by microvascular bone transfer. Clin Orthop Relat Res. 1995;314:37–44. [PubMed] [Google Scholar]
- 9.Sakamoto A, Yoshida T, Uchida Y, Kojima T, Kubota H, Iwamoto Y. Long-term follow-up on the use of vascularized fibular graft for the treatment of congenital pseudarthrosis of the tibia. J Orthop Surg Res. 2008;3:13. doi: 10.1186/1749-799X-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grill F, Bollini G, Dungl P, Fixsen J, Hefti F, Ippolito E, Romanus B, Tudisco C, Wientroub S. Treatment approaches for congenital pseudarthrosis of tibia: results of the EPOS multicenter study. European Paediatric Orthopaedic Society (EPOS) J Pediatr Orthop B. 2000;9:75–89. doi: 10.1097/01202412-200004000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Paley D, Catagni M, Argnani F, Prevot J, Bell D, Armstrong P. Treatment of congenital pseudoarthrosis of the tibia using the Ilizarov technique. Clin Orthop Relat Res. 1992;280:81–93. [PubMed] [Google Scholar]
- 12.Weiland AJ, Weiss AP, Moore JR, Tolo VT. Vascularized fibular grafts in the treatment of congenital pseudarthrosis of the tibia. J Bone Joint Surg Am. 1990;72:654–662. [PubMed] [Google Scholar]
- 13.Cho TJ, Choi IH, Lee SM, Chung CY, Yoo WJ, Lee DY, Lee JW. Refracture after Ilizarov osteosynthesis in atrophic-type congenital pseudarthrosis of the tibia. J Bone Joint Surg Br. 2008;90:488–493. doi: 10.1302/0301-620X.90B4.20153. [DOI] [PubMed] [Google Scholar]
- 14.Kristiansen LP, Steen H, Terjesen T. Residual challenges after healing of congenital pseudarthrosis in the tibia. Clin Orthop Relat Res. 2003;414:228–237. doi: 10.1097/01.blo.0000076800.53006.c9. [DOI] [PubMed] [Google Scholar]
- 15.Mathieu L, Vialle R, Thevenin-Lemoine C, Mary P, Damsin JP. Association of Ilizarov’s technique and intramedullary rodding in the treatment of congenital pseudarthrosis of the tibia. J Child Orthop. 2008;2:449–455. doi: 10.1007/s11832-008-0139-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim HW, Weinstein SL. Intramedullary fixation and bone grafting for congenital pseudarthrosis of the tibia. Clin Orthop Relat Res. 2002;405:250–257. doi: 10.1097/00003086-200212000-00032. [DOI] [PubMed] [Google Scholar]
- 17.Ohnishi I, Sato W, Matsuyama J, Yajima H, Haga N, Kamegaya M, Minami A, Sato M, Yoshino S, Oki T, Nakamura K. Treatment of congenital pseudarthrosis of the tibia: a multicenter study in Japan. J Pediatr Orthop. 2005;25:219–224. doi: 10.1097/01.bpo.0000151054.54732.0b. [DOI] [PubMed] [Google Scholar]
- 18.Wientroub S, Grill F. Congenital pseudarthrosis of the tibia: Part 1. European Pediatric Orthopaedic Society multicenter study of congenital pseudoarthrosis. J Pediatr Orthop B. 2000;9:1–2. doi: 10.1097/01202412-200001000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Joseph B, Somaraju VV, Shetty SK. Management of congenital pseudarthrosis of the tibia in children under 3 years of age: effect of early surgery on union of the pseudarthrosis and growth of the limb. J Pediatr Orthop. 2003;23:740–746. doi: 10.1097/01241398-200311000-00011. [DOI] [PubMed] [Google Scholar]
- 20.Choi IH, Lee SJ, Moon HJ, Cho TJ, Yoo WJ, Chung CY, Park MS. “4-in-1 osteosynthesis” for atrophic-type congenital pseudarthrosis of the tibia. J Pediatr Orthop. 2011;31:697–704. doi: 10.1097/BPO.0b013e318221ebce. [DOI] [PubMed] [Google Scholar]
- 21.Dobbs MB, Rich MM, Gordon JE, Szymanski DA, Schoenecker PL. Use of an intramedullary rod for treatment of congenital pseudarthrosis of the tibia. A long-term follow-up study. J Bone Joint Surg Am. 2004;86-A:1186–1197. doi: 10.2106/00004623-200406000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Mahnken AH, Staatz G, Hermanns B, Gunther RW, Weber M. Congenital pseudarthrosis of the tibia in pediatric patients: MR imaging. AJR Am J Roentgenol. 2001;177:1025–1029. doi: 10.2214/ajr.177.5.1771025. [DOI] [PubMed] [Google Scholar]
- 23.Hermanns-Sachweh B, Senderek J, Alfer J, Klosterhalfen B, Büttner R, Füzesi L, Weber M. Vascular changes in the periosteum of congenital pseudarthrosis of the tibia. Pathol Res Pract. 2005;201:305–312. doi: 10.1016/j.prp.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 24.El-Rosasy MA, Paley D, Herzenberg JE. Congenital pseudarthrosis of the tibia. In: Rozbruch SR, Ilizarov S, editors. Limb lengthening and reconstruction surgery. New York: Informa Healthcare; 2007. pp. 485–493. [Google Scholar]
- 25.Viateau V, Guillemin G, Calando Y, Logeart D, Oudina K, Sedel L, Hannouche D, Bousson V, Petite H. Induction of a barrier membrane to facilitate reconstruction of massive segmental diaphyseal bone defects: an ovine model. Vet Surg. 2006;35:445–452. doi: 10.1111/j.1532-950X.2006.00173.x. [DOI] [PubMed] [Google Scholar]
- 26.Pelissier P, Masquelet AC, Bareille R, Pelissier SM, Amedee J. Induced membranes secrete growth factors including vascular and osteoinductive factors and could stimulate bone regeneration. J Orthop Res. 2004;22:73–79. doi: 10.1016/S0736-0266(03)00165-7. [DOI] [PubMed] [Google Scholar]
- 27.Masquelet AC, Fitoussi F, Begue T, Muller GP. Reconstruction des os longs par membrane induite et autogreffe spongieuse. Ann Chir Plast Esthet. 2000;45:346–353. [PubMed] [Google Scholar]
- 28.Biau DJ, Pannier S, Masquelet AC, Glorion C. Case report: reconstruction of a 16-cm diaphyseal defect after Ewing’s resection in a child. Clin Orthop Relat Res. 2009;467:572–577. doi: 10.1007/s11999-008-0605-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


