Abstract
Previous studies on both human and mice livers showed MDB formation in both drug hepatitis and hepatocellular carcinoma. Using the drug hepatitis mouse model of MDB formation, numerous markers for progenitor cells were found in the cells forming MDBs. In current study, using the drug hepatitis mouse model, we found that the MDB forming cells expressed two additional progenitor cell markers. These markers were CD49f and TLR4.
Keywords: Mallory–Denk bodies (MDBs), Toll-like receptor 4 (TLR4), Integrin alpha 6 (CD49f), Progenitor cells, Tumor-initiating stem-like cells (TISC)
Introduction
Previous studies on both human (Nakanuma and Ohta, 1985) and mice (Tazawa et al., 1983) showed formed MDBs in hepatocellular carcinoma (HCC). Drug fed mice showed that liver cells over expressing gamma-glutamyl transferase (a marker for preneoplastic change in mice hepatocytes), formed Mallory–Denk bodies (MDBs) in both the cirrhotic liver and the associated hepatocellular carcinomas that developed (Tazawa et al., 1983). More recently, when mice were fed the carcinogen DDC (1,4-dihydro-2,4,6-trimethyl-3,5-pyridine carboxylate) for 10 weeks, withdrawn from it for 1 month and then refed DDC for 6 days, the liver cells that were forming MDBs showed a growth advantage compared to intervening normal hepatocytes (Nan et al., 2006a, Nan et al., 2006b and Oliva et al., 2008) indicating that they had developed progenitor characteristics.
The microarrays of the mouse livers forming MDBs showed upregulation of indicators of preneoplasia i.e. KLP6, alpha fetal protein and UBD (FAT 10) confirmed by PCR (Oliva et al., 2008). Other markers expressed in drug-primed mice forming MDBs were markers for cell proliferation. These markers were c-myc, c-jun and AP-1 (Nagao et al., 1998). Other markers of preneoplasia expressed by drug-primed mice livers forming MDBs include A2 macroglobulin, GSTmu2, fatty acid synthetase, glypican-3, p38 and AKT (Nagao et al., 1999, Nan et al., 2006a, Nan et al., 2006b and Roomi et al., 2006).
Stem cells and markers for progenitor cells are present in the livers in which MDBs are formed in both the DDC mouse model and human alcoholic liver disease. Humans with alcoholic liver disease and who have developed acute degeneration of liver function (alcoholic hepatitis) show balloon degeneration of hepatocytes with MDB formation (French et al., 1993 and Mookerjee et al., 2011). This change is associated with progenitor cell change identified by stem cell marker formation in drug-primed, HCV transgenic mice fed ethanol and in human patients who have alcoholic hepatitis with or without cirrhosis and hepatocellular carcinoma. The preneoplastic change markers identified are as follows: 1) AFP (Nan et al., 2006a and Nan et al., 2006b), 2) EZH2, (French et al., 2012a), 3) SOX2 and p27 (French et al., 2012b), and 4) FAT10 (French, 2010 and Oliva et al., 2008).
Recently Machida et al. (2012) reported that the stem cell marker CD49f was expressed in cells isolated by FACS from HCCs that developed in HCV core tg mice fed alcohol and diethylnitrosamine. CD47f was also expressed in alcoholic patients with or without HCV. CD49f enhances multipotency and maintains stemness through direct regulation of Oct 4 and SOX2 (Yu et al., 2012). In the present report we show that balloon cells forming MDBs in the liver biopsies from patients with alcoholic hepatitis stain positive for CD49f supporting the concept that cells forming MDBs are progenitor cells.
TLR-4 is a universal oncogene responsible for the genesis of TLR4-NANOG dependent tumor-initiating stem-like cells (TISC) (Machida et al., 2012). TLR4 silencing with shRNA attenuates the CD133/CD49f ability to induce liver tumors in vitro or in a xenograft model (Machida et al., 2012). The TLR4 induction pathway is activated by alcoholic liver disease (ALD) and NASH. ALD and NASH are important etiologies in the development of hepatocellular carcinoma. TLR4 expression is upregulated in the livers of rats fed alcohol intragastrically (Oliva et al., 2011). Mice fed DDC (Bardag-Gorce et al., 2010) also over express TLR4 and develop hepatocellular carcinoma (French et al., 2010a, French et al., 2010b and French et al., 2011). TLR4 or TLR2 KO mice fed DDC still formed MDBs (French et al., 2011). Only FAT10 KO mice fed DDC failed to develop MDBs. This indicated that the presence of FAT10 but not TLR4 or TLR2 was essential in the formation of MDBs (French et al., 2012c). However, the TLR4 signaling pathway is also involved in MDB pathogenesis (French et al., 2011). For this reason we report that balloon cells forming MDBs in alcoholic hepatitis, express TLR4.
Methods
Liver biopsies from patients diagnosed with alcoholic hepatitis, with or without cirrhosis were selected from archived files based on the presence of balloon cell degeneration with or without MDB formation. The balloon cells were identified by CAM5.2 and ubiquitin immunohistochemistry. To stain for the presence of CD49f expressed by the liver cell, 7 biopsies and 1 autopsy were used. Two cases with normal histology served as controls. To stain for the presence of TLR4 expression in the liver, 10 biopsies and 1 autopsy were used. Two cases of normal liver with normal histology served as controls.
Immunohistochemistry (IHC)
Double IHC stains were done on the liver biopsies. The primary antibodies used were: 1) Mouse anti-ubiquitin (Millipore, Temecula, CA), 2) rabbit anti-TLR4 (Lifespan Biosciences Inc. Seattle, WA), and 3) rabbit anti-CD49f (Abcam, Cambridge, MA). The secondary antibodies used were: 1) Donkey anti-mouse Alexa Fluor 594 (Jackson Immuno Research Labs. Inc. West Grove, PA.), used to detect ubiquitin and 2) donkey anti-rabbit Alexa Fluor 488 (Jackson Immuno Research Labs. Inc. West Grove, PA.), used to detect TLR4 and CD49f. The nuclei were stained with Dapi (Invitrogen, Eugene, OR). The immunofluorescent stain results were photographed using 3 filters (FITC, Texas Red and tricolor) using a Nikon fluorescent microscope.
Results
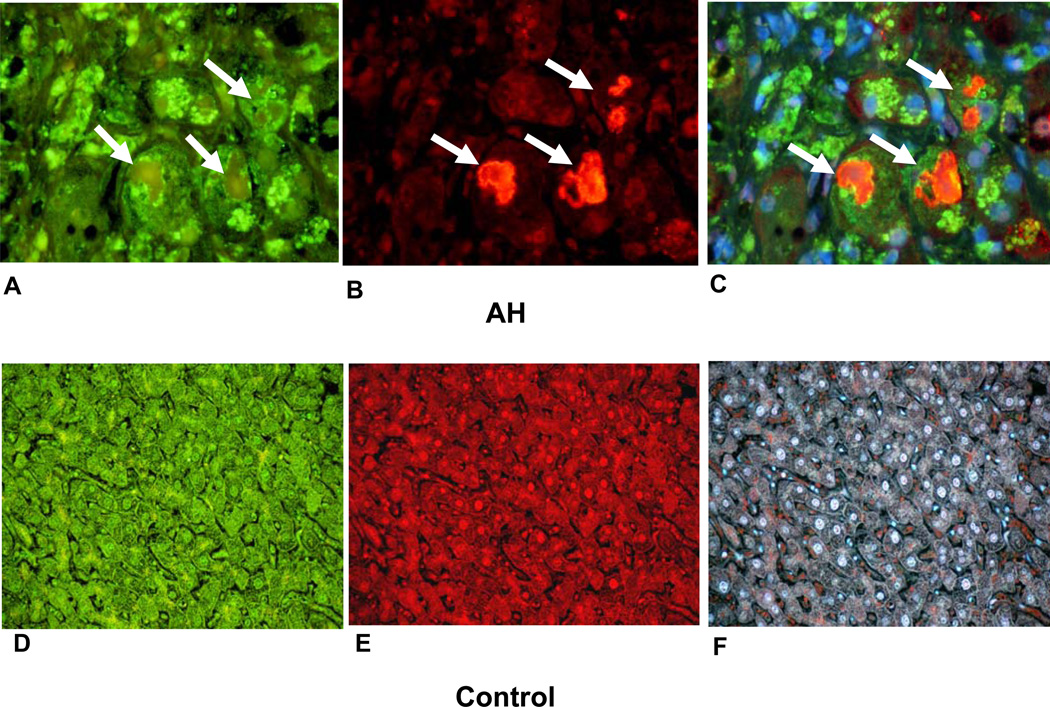
The antibody for CD49f clearly stained the cytoplasm of balloon cells, which had formed MDBs (Figs. 1(A, B, C)). The MDBS stained positive with the antibody to ubiquitin (arrows). The intervening normal hepatocytes stained slightly positive. Macrophage secondary lysosomes also stained positive. In contrast, the stain for CD49f was weak in control livers as was indicated by negative staining when the tricolor filter was used (Fig. 1F).
Figure 1.
Double IHC stains of balloon cells forming MDB green (white arrows) macrophages and pale staining hepatocytes (A, B, C). A. 49f stained balloon cells. B. Ubiquitin stained MDBs red. C. Tricolor filter stained red, green and blue × 780. Normal liver biopsy stained for 49f (green), D, ubiquitin (red), E, and tricolor, F, × 260.
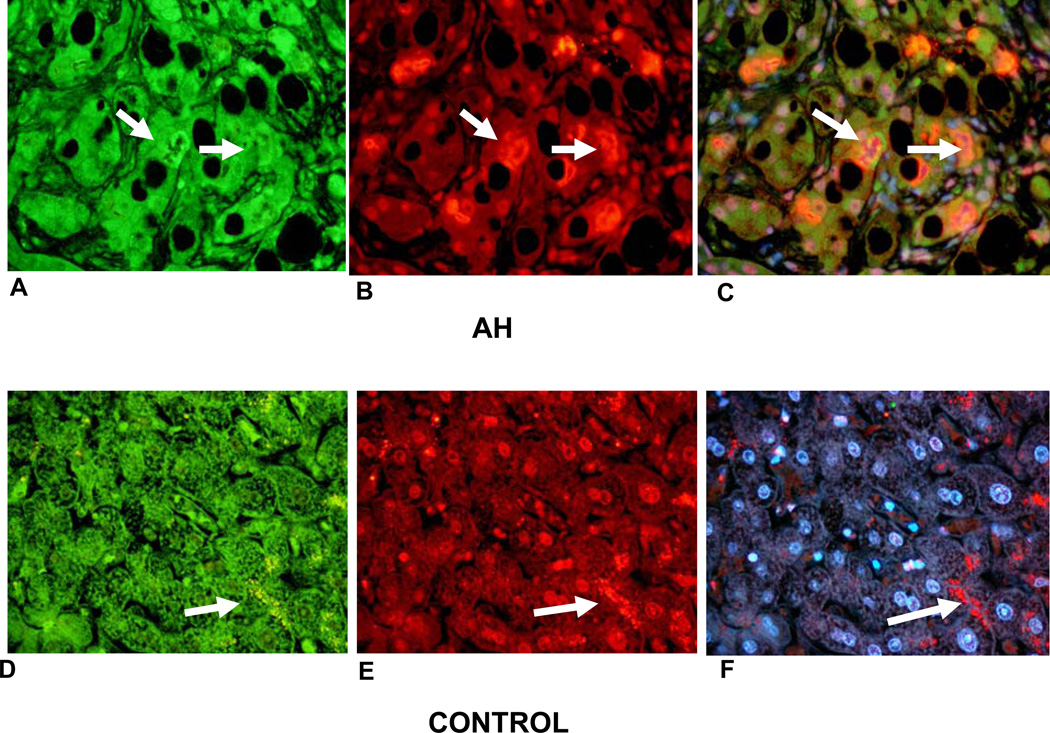
The antibody stain for TLR4 was positive in both MDB forming balloon cells and intervening hepatocytes (Figs. 2(A, B, C)). In control livers the hepatocytes also stained positive (Fig. 2D but not F) indicating that the normal hepatocytes stained weaker than the normal liver cells in livers with alcoholic hepatocytes. The normal liver cells formed ubiquitin positive secondary lysosomes focally (Fig. 2F).
Figure 2.
Double IHC stains of balloon cells forming MDBs (A, B, C). A. TLR4 stained balloon cells green, forming MDBs (arrows), B. ubiquitin stained MDBS red (white arrows), C. tricolor filter (alcoholic hepatitis) × 520. Normal liver biopsy (D, E, F) showing hepatocytes staining green for TLR4, (D), ubiquitin antibody showing lysosomes staining red (arrow), (E), and tricolor showing lysosomes stained red (arrow), (F), × 520.
Discussion
Balloon cells forming MDBs are sometimes regarded as liver cells undergoing degenerative change leading to an early demise (Zatloukal et al., 2007). But the expression of CD49f, SOX2 and p27 would suggest that balloon cells are changed hepatocytes which express progenitor cells potentially destined to form HCCs. CD49f (integrin subunit alpha 6) regulates signaling pathways in a variety of cellular activities (Yu et al., 2012). CD49f is upregulated in human embryonic stem cells. Knock down of CD49f downregulates P13K/AKT signaling and upregulates p53, inducing differentiation of the 3 germ layers (Yu et al., 2012). CD133 +/CD49f cells isolated from animal models and patients are tumorigenic both in vitro and in a xenograph model (Machida et al., 2012). Induction of MDB formation using liver cells derived from the mouse DDC feeding model, upregulated integrin alpha 6 in the MDB forming cells. MDB formation required integrin alpha 6 induction in vitro (Wu et al., 2005). Laminin–integrin signaling activated ERK, which triggered MDB formation in this model in vitro (Wu et al., 2005).
The role of TLR4 in transformation of progenitor cells (tumor-initiating stem-like cells, TISC) to form tumors in the mouse model where alcohol and diethylnitrosamine were fed to HCV core Tg mice, showed that either TLR4 or NANOG silencing with shRNA attenuated the CD133/CD49f induced tumor initiation. This led to the conclusion that TLR4 is a universal proto-oncogene responsible for the genesis of the TLR4-NANOG dependent TISC, which leads to the development of HCC (Machida et al., 2012).
In conclusion, TLR4 and CD49f expression by balloon cells forming MDBs in alcoholic hepatitis provides a mechanism for the initiation of HCC development in patients who suffer from ALD.
Highlights.
Balloon cells making MDBs express many genes expressed in progenitor cells.
CD49f up regulated in embryonic stem cells is expressed by balloon cells forming MDBs.
TLR4 is expressed by TISC cells expressed in balloon cells forming MDBs.
Acknowledgments
We thank Adriana Flores for typing the manuscript. The study was supported by NIH/NIAAAR01020585-01 and Morphology CoreP50-011999-14.
Footnotes
Conflict of interest statement
The authors declare that there are no conflicts of interest.
References
- 1.Bardag-Gorce F, Oliva J, Lin A, Li J, French BA, French SW. SAMe prevents the up regulation of toll-like receptor signaling in Mallory–Denk body formatting hepatocytes. Experimental and Molecular Pathology. 2010;88:376–379. doi: 10.1016/j.yexmp.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.French SW. Molecular events in hepatic preneoplasia: a review. Experimental and Molecular Pathology. 2010;88:219–224. doi: 10.1016/j.yexmp.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.French SW, Nash J, Shitabata P, Kachi K, Chedid A, Mendenhall CL. Pathology of alcoholic liver disease. Seminars in Liver Disease. 1993;13:154–169. doi: 10.1055/s-2007-1007346. [DOI] [PubMed] [Google Scholar]
- 4.French SW, Bardag-Gorce F, Li J, French BA, Oliva J. Mallory–Denk body pathogenesis revisited. World Journal of Hepatology. 2010a;2:295–301. doi: 10.4254/wjh.v2.i8.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.French SW, Oliva J, French BA, Li J, Bardag-Gorce F. Alcohol, nutrition and liver cancer: toll-like receptor signaling. World Journal of Gastroenterology. 2010b;16:1344–1348. doi: 10.3748/wjg.v16.i11.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.French SW, Bardag-Gorce, French BA, Li J, Oliva J. The role of innate immunity in the pathogenesis of preneoplasia in drug-induced chronic hepatitis based on a mouse model. Experimental and Molecular Pathology. 2011;91:653–659. doi: 10.1016/j.yexmp.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.French BA, Oliva J, Bardag-Gorce F, Li J, Zhong J, Buslon V, French SW. Mallory–Denk bodies form when EZH2/H3K27me3 fails to methylate DNA in the nuclei of human and mice liver cells. Experimental and Molecular Pathology. 2012a;92:318–326. doi: 10.1016/j.yexmp.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.French SW, Lee J, Zhong J, Morgan TR, Buslon V, Lungo W, French BA. Alcohol liver disease-hepatocellular carcinoma transformation. Journal of Gastrointestinal Oncology. 2012b;3:174–181. doi: 10.3978/j.issn.2078-6891.2012.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.French SW, French BA, Oliva J, Li JJ, Bardag-Gorce F, Tillman B, Canaan A. FAT 10 to knockout mice fail to develop Mallory–Denk bodies in the DDC mouse model. Experimental and Molecular Pathology. 2012c;93:309–314. doi: 10.1016/j.yexmp.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Machida K, Chen C-L, Liu J-C, Kashiwabara C, Feldman D, French SW, Sher L, Hyeongnan JJ, Tsukamoto H. Cancer stem cells generated by alcohol, diabetes, and HCV. Journal of Gastroenterology and Hepatology. 2012;27(Suppl. 2):19–22. doi: 10.1111/j.1440-1746.2011.07010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mookerjee RP, Lachner C, Stauber R, Stadbauer V, Deheragoda M, Aigelsreiter A, Jalan R. The role of liver biopsy in the diagnosis and prognosis of patients with acute deterioration of alcoholic cirrhosis. Journal of Hepatology. 2011;55:1103–1111. doi: 10.1016/j.jhep.2011.02.021. [DOI] [PubMed] [Google Scholar]
- 12.Nagao Y, Yuan QX, Wan Y-J, French BA, French SW. Pathogensis of Mallory body formation studies using the drug-primed mouse model. Hepatology Research. 1998;13:42–54. [Google Scholar]
- 13.Nagao Y, Wan YJY, Yuan QX, Kachi K, Marceau N, French SW. Mouse model of hyperplastic module formation. Characterization of mRNA expression. Hepatology Research. 1999;15:110–123. [Google Scholar]
- 14.Nakanuma Y, Ohta G. Is Mallory body formation a preneoplastic change? A study of 181 cases of liver bearing hepatocellular carcinoma and 82 cases of cirrhosis. Cancer. 1985;55:2400–2404. doi: 10.1002/1097-0142(19850515)55:10<2400::aid-cncr2820551017>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 15.Nan L, Bardag-Gorce F, Wu Y, Li J, French SW. Mallory body formation cells express the preneoplastic hepatocytic phenotype. Experimental and Molecular Pathology. 2006a;80:109–118. doi: 10.1016/j.yexmp.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Nan L, Dedes J, French BA, Bardag-Gorce F, Li J, Wu J, French SW. Mallory body (cytokeratin aggresome) formation is prevented in vitro by p38 inhibition. Experimental and Molecular Pathology. 2006b;80:228–240. doi: 10.1016/j.yexmp.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Oliva J, Bardag-Gorce F, French BA, Li J, McPhaul L, Amidi F, Dedes JJ, Habbi A, Nguyen S, French SW. FAT 10 is an epigenetic marker for liver preneoplasia in a drug-primed mouse model of tumorigenesis. Experimental and Molecular Pathology. 2008;84:102–112. doi: 10.1016/j.yexmp.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oliva J, Bardag-Gorce F, Li J, French BA, French SW. S-adenosylmethionine prevents the up regulation of toll-like receptor (TLR) signaling caused by ethanol feeding in rats. Experimental and Molecular Pathology. 2011;90:239–243. doi: 10.1016/j.yexmp.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roomi MW, Gaal K, Yuan QX, French BA, Fu P, Bardag-Gorce F, French SW. Preneoplastic liver foci expression induced by thioacetomide toxicity in drug-primed mice. Experimental and Molecular Pathology. 2006;81:8–14. doi: 10.1016/j.yexmp.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 20.Tazawa J, Irie T, French SW. Mallory body formation runs parallel to γ-glutamyl transferase induction in hepatocytes of griseofulvin-fed mice. Hepatology. 1983;3:989–1001. doi: 10.1002/hep.1840030617. [DOI] [PubMed] [Google Scholar]
- 1.Wu Y, Nan L, Bardag-Gorce F, Li J, French BA, Wilson LT, Dedes J, French SW. The role of laminin-integrin signaling in triggering MB formation. An in vivo and in vitro study. Experimental and Molecular Pathology. 2005;79:1–8. doi: 10.1016/j.yexmp.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 2.Yu KR, Yang SR, Jung JW, Kim H, Ko K, Han DW, Park SB, Choi W, Kang SK, Scholer H, Kang KS. CD49f enhancer multipotency and maintains stemness through the direct regulation of Oct4 and SOX2. Stem Cells. 2012;30:876–887. doi: 10.1002/stem.1052. [DOI] [PubMed] [Google Scholar]
- 3.Zatloukal R, French SW, Stumptner C, Stmad P, Harada M, Toivola DM, Cadrin M, Omary MB. From Mallory to Mallory–Denk bodies: what, how and why? Experimental Cell Research. 2007;313:2033–2049. doi: 10.1016/j.yexcr.2007.04.024. [DOI] [PubMed] [Google Scholar]