Abstract
In alpine species the classification of the various mechanisms underlying seed dormancy has been rather questionable and controversial. Thus, we investigated 28 alpine species to evaluate the prevailing types of dormancy. Embryo type and water impermeability of seed coats gave an indication of the potential seed dormancy class. To ascertain the actual dormancy class and level, we performed germination experiments comparing the behavior of seeds without storage, after cold-dry storage, after cold-wet storage, and scarification. We also tested the light requirement for germination in some species. Germination behavior was characterized using the final germination percentage and the mean germination time. Considering the effects of the pretreatments, a refined classification of the prevailing dormancy types was constructed based on the results of our pretreatments. Only two out of the 28 species that we evaluated had predominantly non-dormant seeds. Physiological dormancy was prevalent in 20 species, with deep physiological dormancy being the most abundant, followed by non-deep and intermediate physiological dormancy. Seeds of four species with underdeveloped embryos were assigned to the morphophysiologial dormancy class. An impermeable seed coat was identified in two species, with no additional physiological germination block. We defined these species as having physical dormancy. Light promoted the germination of seeds without storage in all but one species with physiological dormancy. In species with physical dormancy, light responses were of minor importance. We discuss our new classification in the context of former germination studies and draw implications for the timing of germination in the field.
Abbreviations: CDSfresh, cold-dry storage of seeds before incubation under long-day conditions; CDSsc, scarification of seeds following cold-dry storage before incubation under long-day conditions; CWSfresh, cold-wet storage of seeds before incubation under long-day conditions; CWSsubs, cold-wet storage subsequent to a germination experiment before incubation under long-day conditions; FGP, final germination percentage; FRESHdark, seeds without storage incubated in darkness; FRESHsc, scarification of seeds without storage before incubation under long-day conditions; FRESHLD, seeds without storage incubated under long-day conditions; GA3, gibberellic acid; MD, morphological dormancy; MGT, mean germination time; MPD, morphophysiological dormancy; ND, non-dormant; PD, physiological dormancy; PY, physical dormancy; PY + PD, combinational dormancy of PY and PD
Keywords: Cold-dry seed storage, Cold-wet seed storage, Dormancy classification, Embryo morphology, Light response, Scarification
Introduction
A dormant seed was defined by Baskin and Baskin (2004a) as ‘one that does not have the capacity to germinate in a specified period of time under any combination of normal physical environmental factors (temperature, light/dark, and so forth)’ that would otherwise be favorable for the germination of non-dormant seeds (ND). Dormancy is an innate mechanism of seeds that is determined by both the morphological and physiological properties of the seed (Finch-Savage and Leubner-Metzger, 2006), whereas the release of dormancy is triggered by environmental stimuli (Baskin and Baskin, 1998; Benech-Arnold et al., 2000; Fenner and Thompson, 2005; Finch-Savage and Leubner-Metzger, 2006; Vleeshouwers et al., 1995).
The study of these commonly accepted phenomena led to the comprehensive hierarchical system of seed dormancy classification proposed by Baskin and Baskin (1998, 2004a,b). It distinguishes five classes: (1) physiological dormancy (PD), (2) morphological dormancy (MD), (3) morphophysiological dormancy (MPD), (4) physical dormancy (PY), and (5) a combinational dormancy (PY + PD). Although endogenous and exogenous factors are responsible for the maintenance or release of dormancy (Baskin and Baskin, 1998, 2004a; Benech-Arnold et al., 2000; Fenner and Thompson, 2005; Finch-Savage and Leubner-Metzger, 2006; Vleeshouwers et al., 1995), a feasible key for the determination of dormancy status based on embryo morphology, the seed coat's permeability to water, and the capacity of fresh seeds to germinate within one month is available (Baskin and Baskin, 2004b).
Various physiological mechanisms, which may be present in the embryo itself and/or in surrounding structures, were reported to inhibit radicle emergence, all of which are classified as PD (reviewed in Finch-Savage and Leubner-Metzger, 2006). Three levels of dormancy are distinguished within PD (Baskin and Baskin, 1998, 2004a,b): non-deep, intermediate, and deep dormancy. Temperature is the main driving factor that releases or induces PD (Baskin and Baskin, 2004a; Vleeshouwers et al., 1995), although other environmental factors such as naturally occurring chemical signals may have roles as well (Finch-Savage and Leubner-Metzger, 2006). Therefore, exposing seeds to certain temperatures may provide an indication of the type of dormancy exhibited by a species (Baskin and Baskin, 2004b). Seeds with non-deep PD are released from dormancy, often at a slow rate, when they are stored dry at room temperature. This phenomenon is called ‘after-ripening’ (Finch-Savage and Leubner-Metzger, 2006), and this transitional stage was termed conditional dormancy by Baskin and Baskin (1998). Release from non-deep PD may occur also during dry-cold storage (Wang et al., 2010). Seeds with intermediate PD often require two to three months of cold stratification, i.e., storage under temperatures below 10 °C in an imbibed stage, to overcome dormancy, whereas seeds with a deep PD are released of dormancy only after longer periods of cold stratification (Baskin and Baskin, 1998, 2004b). Dormancy induced by the underdevelopment of embryos (i.e., in MD and MPD) requires a short period of favorable conditions for the embryo to grow to a species-specific critical size (Baskin and Baskin, 1998, 2004b; Finch-Savage and Leubner-Metzger, 2006). In MPD, an additional physiological component is involved (Baskin and Baskin, 1998). Seeds with PY or PY + PD remain dormant until something disintegrates the covering layers, which are otherwise impermeable to water (Baskin et al., 2000).
Whether light plays a role in the release of dormancy or merely promotes germination has been the subject of some debate (Baskin and Baskin, 2004a; Benech-Arnold et al., 2000; Fenner and Thompson, 2005; Finch-Savage and Leubner-Metzger, 2006; Pons, 2000; Vleeshouwers et al., 1995). Independent of the criteria used to determine when dormancy ends and germination begins, light affects germination in many species in the field (Pons, 2000). In the investigations reported here we considered light as an environmental cue that may influence germination in non-dormant seeds (Baskin and Baskin, 2004a).
In alpine species, dormancy is a long recognized phenomenon. As early as 1913, Braun had already reported that many species did not germinate at all under laboratory conditions, even after cold stratification. Other pioneer studies suggested that most alpine species are non-dormant (Amen, 1966). Low germination success of some alpine plants challenged botanists and growers of ornamental plants for decades (Favarger, 1953). In more recent reviews, Baskin and Baskin (1998, 2004b) reported that >70% of arctic-alpine species have dormant seeds, exhibiting mainly PD or, to a much lesser extent, PY. Despite some early comprehensive studies on seed germination in alpine environments in the beginning of the 20th century (reviewed in Körner, 2003) and Amen's early overview on seed dormancy in alpine plants (1966), there is still a lack of knowledge of the mechanisms that underlie dormancy in alpine species (Baskin and Baskin, 1998).
The goal of this study was to reconsider the types of dormancy exhibited by 28 species of alpine plants in light of the classification system proposed by Baskin and Baskin (1998, 2004a,b). We first considered embryo type and the water impermeability of seed coats, to determine the potential classes of seed dormancy. Furthermore, we applied several germination experiments comparing the germination of freshly collected seeds with germination following various pretreatments. Additionally, the light requirements for the germination of some species were tested. Germination behavior was characterized by final germination percentage (FGP) and mean germination time (MGT). With the data generated by our germination experiments, we refined the classification of dormancy types. According to Baskin and Baskin (1998), several alpine species previously classified as exhibiting ND may actually have PD. Based on that suggestion, we hypothesized that the majority of fresh seeds of our investigated species are dormant, i.e., few mature seeds would germinate readily within one month and without any pretreatments. More specifically, we asked which types of dormancy can be found in the alpine species and what preconditions do they require for overcoming dormancy. Furthermore, we hypothesized that light promotes germination in alpine species, as they should be adapted to the pronounced seasonal patterns of the light environment in the mainly open vegetation types.
Materials and methods
Study site and species
Seeds were collected in the Rotmoos Valley (11°02′E/46°49′N), a glacial valley in the central Alps of Austria. For a detailed description of the vegetation, see Raffl et al. (2006). Seeds were collected at altitudes ranging from 2250 to 3000 m.
We investigated 28 alpine species, representing 14 families (Table 1). The investigated species include some of the most abundant species in glacier forelands, such as Anthyllis alpicola, Poa alpina, Trifolium pallescens, Saxifraga aizoides and S. oppositifolia (Raffl et al., 2006) and some species occurring frequently on the adjacent slopes (Potentilla aurea, P. frigida, Ranunculus glacialis). Fully ripened seeds or fruits were collected in the late summer and autumn from 2000 to 2010 (see Appendix A). For simplicity, the term ‘seed’ is used to describe both seeds and fruits. Seeds were collected from randomly chosen individuals (mostly >50 individuals per species). They were mixed thoroughly to minimize the effects of single individuals on germination. Immediately after collection, the seeds were stored in a commercial refrigerator at 4 °C until the start of the pretreatments or germination experiments (Table 1).
Table 1.
Alpine species used in the germination experiments, their family affiliation, description of the dispersal units, and the germination experiments conducted for each species. Seeds without storage were tested under long-day conditions (FRESHLD) or in darkness (FRESHdark). Cold-wet storage was applied either on seeds after a short period (max. 10 weeks) of cold-dry storage (CWSfresh) or subsequent to a germination experiment on fresh seeds using any remaining viable seeds (CWSsubs). For cold-dry storage, fresh seeds were used (CDSfresh). The effect of scarification was tested on seeds without storage (FRESHSC) and following cold-dry storage (CDSsc). For details of collection year, storage period, starting time, and incubation period of germination experiments, number of replicates, and number of seeds per replicate see Appendix A.
| Species | Family | Dispersal units | Germination experiments |
||||||
|---|---|---|---|---|---|---|---|---|---|
| FRESHLD | FRESHdark | FRESHsc | CWSfresh | CWSsubs | CDSfresh | CDSsc | |||
| Achillea moschata | Asteraceae | Fruits (single-seeded achenes) | X | X | – | X | – | X | – |
| Anthyllis vulneraria subsp. alpicolaa | Fabaceae | Fruits (mostly single-seeded siliculae)b | X | X | X | X | – | X | X |
| Arabis caerulea | Brassicaceae | Winged seeds | X | – | – | – | X | X | – |
| Arenaria ciliata | Caryophyllaceae | Seeds | X | – | – | – | X | – | – |
| Artemisia genipi | Asteraceae | Fruits (single-seeded achenes) | X | X | – | X | – | X | – |
| Campanula scheuchzeri | Campanulaceae | Seeds | X | – | – | – | X | – | |
| Carex bicolor | Cyperaceae | Fruits (single-seeded nutlets with utriculus) | X | – | – | – | X | X | – |
| Cerastium uniflorum | Caryophyllaceae | Seeds | X | – | – | – | X | X | – |
| Comastoma tenellum | Gentianaceae | Seeds | X | – | – | – | X | – | – |
| Draba aizoides | Brassicaceae | Seeds | X | – | – | – | X | – | – |
| Draba dubia | Brassicaceae | Seeds | X | – | – | – | X | – | – |
| Draba hoppeana | Brassicaceae | Seeds | X | – | – | – | X | – | – |
| Epilobium fleischeri | Onagraceae | Plumed seeds | X | – | – | – | – | X | – |
| Erigeron uniflorus | Asteraceae | Fruits (single-seeded achenes with pappus) | X | – | – | – | – | X | – |
| Gentiana orbicularis | Gentianaceae | Seeds | X | – | – | – | X | – | – |
| Geum reptans | Rosaceae | Fruits (single-seeded nutlets with elongated hairy style) | X | X | – | X | – | X | – |
| Leontodon hispidus | Asteraceae | Fruits (single-seeded achenes with pappus) | X | – | – | – | – | X | – |
| Linaria alpina | Antirrhinaceae | Winged seeds | X | X | – | X | – | – | – |
| Minuartia gerardii | Caryophyllaceae | Seeds | X | – | – | – | X | – | – |
| Oxyria digyna | Polygonaceae | Fruits (single-seeded achenes with wings) | X | X | – | X | – | X | – |
| Poa alpina | Poaceae | Fruits (single-seeded caryopses, in part attached to glumes)c | X | X | – | – | – | X | – |
| Potentilla aurea | Rosaceae | Fruits (single-seeded nutlets) | X | – | – | – | X | – | – |
| Potentilla frigida | Rosaceae | Fruits (single-seeded nutlets) | X | – | – | – | X | – | – |
| Ranunculus glacialis | Ranunculaceae | Fruits (single-seeded nutlets) | X | – | – | – | X | – | – |
| Saxifraga aizoides | Saxifragaceae | Seeds | X | X | – | X | – | X | – |
| Saxifraga oppositifolia | Saxifragaceae | Seeds | X | X | – | X | – | – | – |
| Silene acaulis subsp. exscapad | Caryophyllaceae | Seeds | X | – | – | – | – | X | – |
| Trifolium pallescens | Fabaceae | Seeds | X | X | X | X | – | X | X |
Nomenclature follows Fischer et al. (2008).
To improve readability, we use Anthyllis alpicola.
To test the effect of scarification, fruit coat was removed and scarified seeds were used.
For germination experiments, all glumes were removed.
To improve readability, we use Silene exscapa.
Potential dormancy classes
Based on embryo morphology and the impermeability of the seed coat to water (Baskin and Baskin, 2004b), we assigned each species to one of the following potential seed dormancy classes (including ND): species with a water-impermeable seed coat (PY/PY + PD); species with a water-permeable seed coat and underdeveloped embryos (MD/MPD); species with a water-permeable seed coat and fully developed embryos (ND/PD). For the determination of the embryo type, we used the key provided by Baskin and Baskin (2007). Information on embryo and endosperm structures was available from studies by Martin (1946) and other authors (Akhalkatsi and Wagner, 1997; Baskin and Baskin, 2005; Wagner and Tengg, 1993; Wagner et al., 2010). For some species, we inferred embryo type from a reference species, i.e., a related taxon, as ‘their basic internal organization varies only slightly among related species and genera’ (Martin, 1946). We assumed a water-impermeable seed coat for those species belonging to families for which evidence of physical dormancy has been reported (Baskin et al., 2000).
Preconditions
Germination experiments with different preconditions were conducted over several years (Table 1). Germination of fresh seeds without storage under long-day conditions was tested for all investigated species (FRESHLD), whereas the germination without storage in darkness (FRESHdark) was measured for only a subset (Table 1). Seeds without storage were tested within one week of seed collection.
To test the effects of cold storage, the samples were placed in a commercial refrigerator at 4 °C. For cold-dry storage (CDSfresh), fresh seeds were placed, respectively, either in dry paper bags for 26–64 weeks or on moist filter paper in Petri dishes for cold-wet storage. Cold-wet storage was applied either for 10 weeks after a short period of cold-dry storage (CWSfresh) or for 15–20 weeks following a germination experiment with fresh seeds, using any remaining viable seeds (CWSsubs). Details of the storage periods for all experiments are provided in Appendix A. The results of CWSfresh have already been reported to some extent by Schwienbacher and Erschbamer (2002), but we re-analyzed these data for comparison with other treatments.
The effects of scarification were tested for the species of the Fabaceae family with seeds without storage (FRESHsc) and following cold-dry storage (CDSsc). Scarification was done with sandpaper immediately before incubation in the growth chamber using dry seeds of Anthyllis alpicola and Trifolium pallescens. The extent of scarification was monitored under a reflected-light microscope. The fruit coat of Anthyllis alpicola was removed, and the seeds were scarified for the germination experiments.
Germination experiments
For the germination experiments, batches of 25–115 undamaged seeds, each with four or five replicates per species and preconditions, were prepared. For some species, no replicates could be assembled due to an insufficient amount of seeds. Details on the number of replicates and the number of seeds per replicate are provided in Appendix A. We placed each batch of seeds in a regular grid on three layers of filter paper in Petri dishes (90 mm) and added 5 ml of deionized water. For the germination experiments in darkness, the Petri dishes were wrapped in two layers of aluminum foil to exclude light. All germination experiments were performed in growth chambers (Sanyo Growth Cabinet, Model MLR-350H) under long-day conditions (16 h light at 25 °C/8 h darkness at 10 °C). The maximum photosynthetically active photon flux density measured in the empty growth chamber was 180 μmol m−2 s−1, provided by 15 fluorescent tubes (OSRAM L 36W/840 Active 3350 lm). This temperature regime and the photoperiod simulate field conditions at the beginning of the growing season and have been proven to promote efficient germination in several alpine species (Schwienbacher and Erschbamer, 2002).
For the majority of the species and effects tested, an incubation period of approx. one month was chosen; however, the time in the growth chamber was prolonged up to two months for some species with low germination success. Details of the starting times and incubation periods are provided in Appendix A.
During incubation in the growth chamber, the samples were monitored regularly (one to three times per week) under a reflected-light microscope and 1 ml of deionized water was added when the filter paper started to dry out. Germination was defined as emergence of the radicle. To adhere to a commonly used germination criteria but to still account for the differences in seed size, we defined the critical size of the radicle to be at least half of the seed's minimum dimension or a 1 mm extension. Seedlings were removed when counted. Petri dishes used for the germination experiments in darkness were unwrapped in a dark room and monitoring was performed under a microscope. Illumination was provided by a flashlight with a green filter.
Refined classification of dormancy
Seed dormancy classes and levels of PD were inferred from the results of the germination experiments with different preconditions, based on preliminary assignment to potential dormancy classes. Therefore, we developed a key according to the suggestions made by Baskin and Baskin (1998, 2004b).
Statistical analyses
All of the data on FGP were arcsin-transformed. The comparison of the FGP of FRESHLD between groups of potential dormancy classes was done using a one-way analysis of variance (ANOVA).
For those species yielding a FGP >50% in any of the experiments, the Gompertz function was fitted to each set of cumulative germination data on time using SigmaPlot for Windows 10.0. The Gompertz function belongs to the class of sigmoidal equations and has been applied successfully to modeling the cumulative germination and emergence of several species (Brown and Mayer, 1988). Curve-fitting by non-linear regressions was processed for each replicate separately. Curve-fitting was successful for 85% of the data sets (each with P < 0.05 in the ANOVA; mean R2 = 0.97). The derived parameters of the curve function for those replicates were used to calculate the MGT, the time to 50% of the FGP. If the FGP was lower than 9% or less than three replicates could be processed, the MGT was omitted from the analyses and figures.
The effects of the preconditions on the FGP and the MGT were compared for each species separately. Comparisons were made between different storage conditions (FRESHLD, CWSfresh, CDSfresh), between the germination of seeds without storage in darkness and under long-day conditions (FRESHdark, FRESHLD), and between scarified seeds and non-scarified seeds (FRESHsc, FRESHLD and CDSsc, CDSfresh). For the illustration of the cumulative germination course in the figures, a regression line was calculated from the whole set of replicates as described for the single replicates of each species and precondition.
Differences in FGP and MGT between germination experiments with different preconditions were tested for each species using an ANOVA or t tests if the data met the conditions for using a parametric test; otherwise, the differences were tested with Kruskal–Wallis or Mann–Whitney tests. Groups were determined by Scheffé post hoc tests following ANOVA or by pairwise group comparisons using Mann–Whitney tests. All statistical analyses used to test for the effects of preconditions were performed using SPSS 15.0 for Windows.
For species with a generally low germination success, only the FGP is given for each precondition tested. For this group, no statistical analyses were performed as the incubation period differed between some preconditions and a low number of replicates constrained the power of statistical tests for some species.
Results
Endosperm, embryo, and seed coat characteristics, based on literature information
About one-third of the investigated species belonged to genera lacking an endosperm, whereas 40% had a non-starchy endosperm, and the other quarter had a starchy endosperm, according to reference literature (Table 2). The investigated species were assigned to eight different embryo types (Table 2). The ‘spatulate fully developed’ seed type (Baskin and Baskin, 2007) was the most abundant, representing almost 30% of the studied species, followed by ‘peripheral’ and ‘bent’ types, which comprised approximately one-fifth of the species. Four species are described having ‘underdeveloped linear’ or ‘rudimentary’ embryos. Within the species belonging to the ‘fully developed linear’ embryo type, the Saxifraga species, with relatively small embryos, were borderline to the underdeveloped seed types. Other seed types were represented by only one species each (Table 2). The two species from the Fabaceae family presumably have water-impermeable seed coats and were therefore classified as PY/PY + PD. Four species with underdeveloped embryos were assigned to the MD/MPD group, and all other species, comprising almost 80% of the investigated species, fell into the ND/PD group (Table 2).
Table 2.
Potential seed dormancy classes of the investigated species based on the reference species with a literature-based description of endosperm characteristics, embryo type, and potential for water impermeability of the seed coat (PWI).
| Species | Reference speciesa | Endospermb | Embryoc | PWId | Dormancy classese |
|---|---|---|---|---|---|
| Achillea moschata | Achillea (3 sp.) | Not present | Spatulate fully developed | No | ND/PD |
| Anthyllis alpicola | A. vulneraria | Non-starchy | Bent | Yes | PY/PY + PD |
| Arabis caerulea | Arabis (6 sp.) | Not present | Bent | No | ND/PD |
| Arenaria ciliata | Arenaria (6 sp.) | Conspicuouslyf | Peripheral | No | ND/PD |
| Artemisia genipi | Artemisia (11 sp.) | Not present | Spatulate fully developed | No | ND/PD |
| Campanula scheuchzeri | C. americanag | Non-starchy | Linear underdeveloped | No | MD/MPD |
| Carex bicolor | Carex (139 sp.) | Starchy | Capitate | No | ND/PD |
| Cerastium uniflorum | C. uniflorumh | Conspicuouslyf | Peripheral | No | ND/PD |
| Comastoma tenellum | Gentiana (8 sp.), G. pyrenaicai | Non-starchy | Linear underdeveloped | No | MD/MPD |
| Draba aizoides | Draba (4 sp.) | Not present | Bent | No | ND/PD |
| Draba dubia | Draba (4 sp.) | Not present | Bent | No | ND/PD |
| Draba hoppeana | Draba (4 sp.) | Not present | Bent | No | ND/PD |
| Epilobium fleischeri | Epilobium (12 sp.) | Not present | Spatulate fully developed | No | ND/PD |
| Erigeron uniflorus | Erigeron (13 sp.) | Not present | Spatulate fully developed | No | ND/PD |
| Gentiana orbicularis | Gentiana (8 sp.), G. pyrenaicai | Non-starchy | Linear underdeveloped | No | MD/MPD |
| Geum reptans | Geum (8 sp.) | Not present | Spatulate fully developed | No | ND/PD |
| Leontodon hispidus | Leontodon sp. | Not present | Spatulate fully developed | No | ND/PD |
| Linaria alpina | Linaria (3 sp.) | Non-starchy | Linear fully developed | No | ND/PD |
| Minuartia gerardii | Arenaria (6 sp.) | Conspicuouslyf | Peripheral | No | ND/PD |
| Oxyria digyna | O. digyna | Conspicuouslyf | Peripheral | No | ND/PD |
| Poa alpina | Poa (16 sp.) | Starchy | Lateral | No | ND/PD |
| Potentilla aurea | Potentilla (23 sp.) | Non-starchy | Spatulate fully developed | No | ND/PD |
| Potentilla frigida | Potentilla (23 sp.) | Non-starchy | Spatulate fully developed | No | ND/PD |
| Ranunculus glacialis | R. glacialisj | Non-starchy | Rudimentary | No | MD/MPD |
| Saxifraga aizoides | S. oppositifoliah,k | Non-starchy | Linear fully developed | No | ND/PD |
| Saxifraga oppositifolia | S. oppositifoliah,k | Non-starchy | Linear fully developed | No | ND/PD |
| Silene exscapa | S. acaulis | Conspicuouslyf | Peripheral | No | ND/PD |
| Trifolium pallescens | Trifolium (18 sp.) | Non-starchy | Bent | Yes | PY/PY + PD |
ND = non-dormant; PD = physiological dormancy; MD = morphological dormancy; MPD = morphophysiological dormancy; PY = physical dormancy; PY + PD = combinational dormancy.
All from Martin (1946) if not otherwise indicated.
As in Martin (1946).
Determined from reference species according to Baskin and Baskin (2007).
Family comprises of species with water impermeable seed coats (Baskin et al., 2000).
Inferred from endosperm characteristics, embryo type, and water impermeability of seed coat (Baskin and Baskin, 2004b).
Actually perisperm.
Borderline case with embryo:seed length ratio = 0.5–0.6.
Germination of seeds without storage
Nine out of the 28 species reached a FGP between 42% and 88% for fresh seeds incubated under long-day conditions, and another five species yielded between 10% and 40%. Half of the 28 study species remained below 10%, with nine of those not germinating at all.
Germination of fresh seeds without storage was not significantly different in the three potential dormancy classes due to a high degree of variability within groups (Fig. 1). The ND/PD group showed the largest variability in FGP, ranging from 0% to 88%. Among the PY/PY + PD group, half of the fresh seeds germinated (Fig. 1), whereas the FGP of fresh seeds in the MD/MPD group was the lowest because three out of four species did not germinate at all (Table 3).
Fig. 1.
Final germination percentage (FGP) of seeds without storage under long-day conditions for the species groups defined by potential dormancy classes (for assignments of species see Table 2). The box plots illustrate the median, minimum, and maximum, and the box encompasses values from the 1st to the 3rd quartile. ND = non-dormant; PD = physiological dormancy; MD = morphological dormancy; MPD = morphophysiological dormancy; PY = physical dormancy; PY + PD = combinational dormancy.
Table 3.
Final germination percentage (FGP, mean ± s.e.) of ND/PD and MD/MPD species with an overall low germination success yielded in different germination experiments.
| Potential dormancy classes | FGP (%) |
|||
|---|---|---|---|---|
| Species | FRESHLD | FRESHdark | CWSa | CDSfresh |
| ND/PD | ||||
| Arabis caerulea | 6.0 ± 2.5 | – | 19.0 ± 3.7 | 5.8 ± 0.5 |
| Arenaria ciliata | 0 ± 0 | – | 0 ± 0 | – |
| Carex bicolor | 0 ± 0 | – | 2.0 ± 1.1 | 0 ± 0 |
| Cerastium uniflorum | 14.4 ± 1.6 | – | 33.0 ± 12.4 | 17.0 ± 3.4 |
| Draba aizoides | 0 | – | 2 | – |
| Draba dubia | 0 | – | 2 | – |
| Draba hoppeana | 2 | – | 0 | – |
| Linaria alpina | 0 ± 0 | 0 ± 0 | 0.0 ± 0 | – |
| Minuartia gerardii | 6.1 | – | 8.9 | – |
| Potentilla aurea | 4.0 | – | 23.4 | – |
| Potentilla frigida | 0 | – | 1.0 | – |
| Saxifraga oppositifolia | 0 ± 0 | 0 ± 0 | 1.4 ± 0.5 | – |
| Species | FRESHLD | CWSsubs |
|---|---|---|
| MD/MPD | ||
| Campanula scheuchzeri | 22.8 ± 1.1 | 27.0 ± 3.1 |
| Comastoma tenellum | 0 | 4 |
| Ranunculus glacialis | 0 | 0 |
| Gentiana orbicularis | 0.4 ± 0.4 | 0 ± 0 |
FRESHLD = seeds without storage under long-day conditions; FRESHdark = seeds without storage in darkness; CWS = cold-wet storage; CDSfresh = cold-dry storage. ND = non-dormant; PD = physiological dormancy; MD = morphological dormancy; MPD = morphophysiological dormancy.
CWS refers to CWSsubs, except for L. alpina and S. oppositifolia for which CWSfresh was conducted.
The onset of germination, the MGT and the FGP of fresh seeds under long-day conditions were strongly correlated in the ND/PD group. The 10 species with a FGP >15% started to germinate within two weeks (Fig. 2). The fastest germination of fresh seeds was observed for Silene exscapa, starting within a few days of incubation and reaching half of the FGP within one week (Fig. 2). All fast germinating species (MGT <14 days) yielded a FGP of >50%, except Silene exscapa which remained slightly below that (Fig. 2). Among the species with a FGP <15% (Table 3) only Arabis caerulea showed a main germination peak within one week. All other species germinated predominantly after the second week.
Fig. 2.
Germination course and mean germination time (MGT, vertical lines) of seeds without storage in comparison with seeds exposed to different storage conditions from selected species of the ND/PD group. ND = non-dormant; PD = physiological dormancy; FRESHLD = fresh seeds under long-day conditions (– ● –); CWSfresh = cold-wet storage (□); CDSfresh = cold-dry storage (▵). Groups revealed by post hoc group comparison at a significance level of P < 0.05 are indicated with different letters at the right end of the regression lines for final germination (%) and at the top of the vertical dashed lines for MGT, respectively. Symbols show means ± s.e.
Effect of storage
Storage had opposing effects depending on the species. In four species storage significantly decreased the FGP, whereas storage accelerated germination and increased the FGP in several other species, but 16 species still yielded a FGP <35% in any of the germination experiments. Among them, nine species remained below 5% (Table 3).
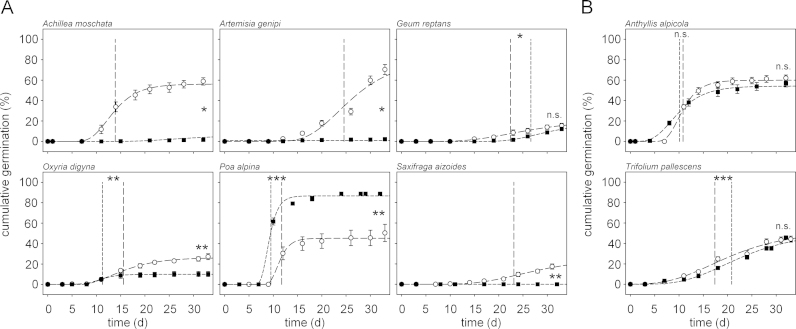
In half of the species, including in the CDSfresh treatment, the FGP increased significantly after storage when compared to the FRESHLD treatment (Fig. 2). The average increase in the FGP by 40% was correlated with a significant decrease in the MGT by approximately one week (range 6.8–8.5 days) in all of these species, with the exception of Achillea moschata. In Epilobium fleischeri only the MGT decreased significantly (Fig. 2).
Time to 50% germination decreased significantly in almost half of the species in the CWSfresh treatment in comparison to the FRESHLD treatment (Fig. 2). The magnitude of the decrease in MGT of Geum reptans and Oxyria digyna was significantly higher than in the CDSfresh treatment. For all other species, no significant difference in the MGT between CWSfresh and CDSfresh treatments was noticed (Fig. 2). Final germination in the CWSfresh treatment increased significantly only in the relatively slow germinating species of the series without storage, i.e., Geum reptans, Oxyria digyna, and Saxifraga aizoides (Fig. 2). However, the FGP of Geum reptans and Oxyria digyna was significantly lower than in the CDSfresh treatment.
A significantly lower FGP was detected for four species in the CDSfresh and the CWSfresh treatment. Final germination dropped by almost 20% in Leontodon hispidus and more than 50% in Erigeron uniflorus, along with a delayed germination onset and a higher MGT after cold-dry storage (Fig. 2). Such an inhibitory effect of storage was even more pronounced in the two Fabaceae species. Storage of any kind caused germination to nearly cease in Trifolium pallescens (CDSfresh: FGP = 12.5 ± 4.0%; CWSfresh: FGP = 7.8 ± 1.2%) and Anthyllis alpicola (CDSfresh: FGP = 14.0 ± 3.4%; CWSfresh: FGP = 9.2 ± 0.4%).
For species with a low FGP in the FRESHLD treatment, the FGP of only Arabis caerulea, Cerastium uniflorum, and Potentilla aurea increased substantially in the CWSsubs treatment (Table 3). However, the increase in Arabis caerulea was mainly caused by continuous, slow germination over a longer incubation period, and the difference in Cerastium uniflorum was statistically not significant due to very high variability between replicates.
Effect of scarification
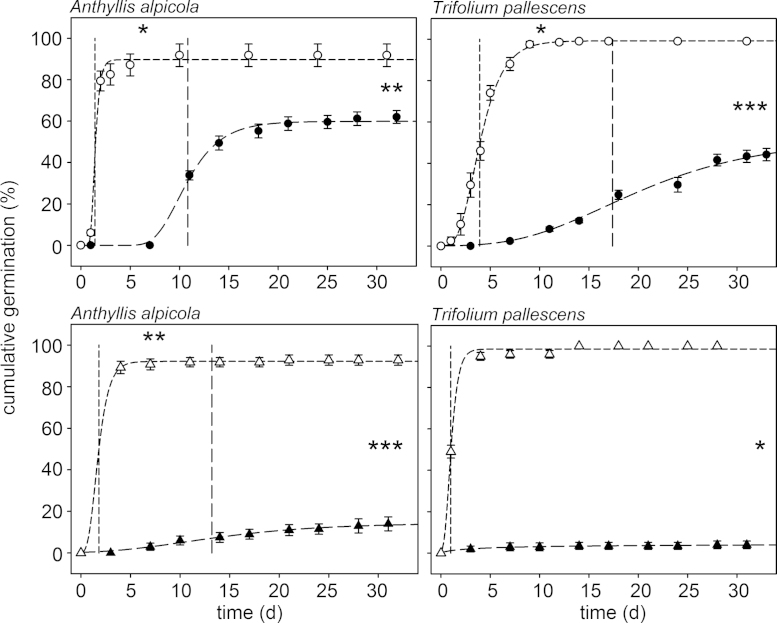
In both Fabaceae species, Anthyllis alpicola and Trifolium pallescens, scarification increased significantly the FGP and the MGT (FRESHsc and CDSsc, Fig. 3). Scarification led to imbibition within a few hours in almost all seeds. More than 90% of the seeds of Anthyllis alpicola germinated within four days in both storage treatments, and in Trifolium pallescens in the CDSsc treatment (Fig. 3).
Fig. 3.
Germination course and mean germination time (MGT, vertical lines) of seeds without scarification (closed symbols) and with scarification (open symbols) from species of the PY/PY + PD group. PY = physical dormancy; PY + PD = PY and physiological dormancy; upper panels (circles) = seeds without storage; lower panels (triangles) = cold-dry storage. Significant differences between both treatments are indicated for final germination (%) and MGT of each species (* P < 0.05, ** P < 0.01, *** P < 0.001). Symbols show means ± s.e.
Long-day conditions vs. permanent darkness
In half of the species tested, the long-day conditions yielded a significantly higher FGP of seeds without storage in comparison to permanent darkness (Fig. 4). However, some species showed different responses; Poa alpina germinated significantly better in darkness (Fig. 4A), and the FGPs of Anthyllis alpicola, Trifolium pallescens and Geum reptans were not affected by darkness (Fig. 4B).
Fig. 4.
Germination course and mean germination time (MGT, vertical lines) of seeds without storage in darkness (FRESHdark, – ■ –) or under long-day conditions (FRESHLD, – ○ –). (A) Selected species for which non-dormant or physiological dormant (PD) seeds were assumed and (B) species for which physical dormancy (PY) or a combinational dormancy (PY + PD) was assumed. Significant differences between both light conditions are indicated for final germination (%) and MGT of each species (n.s., P ≥ 0.05; * P < 0.05; ** P < 0.01; *** P < 0.001). Symbols show means ± s.e.
If the FGP was high enough to calculate a MGT in both conditions (as was the case for almost two-thirds of the species tested), a significant effect of light on the MGT was detected in all but one species (Anthyllis alpicola, Fig. 4). Long-day conditions significantly accelerated the MGT in Geum reptans and Trifolium pallescens, whereas the MGT was significantly delayed in Oxyria digyna and Poa alpina. The differences of MGT between the two light conditions ranged from 2.2 to 4.3 days (Fig. 4).
Assignment of species to dormancy class and level
Most species showed different dormancy mechanisms in varying proportions, impeding the simple classification of dormancy types. Thus, we considered all of our results and developed a key (Table 4) to refine the initial classification scheme (Table 2). Using this key, we assigned the prevailing dormancy class and level to each species. Only two species (Erigeron uniflorus, Leontodon hispidus) were found to have predominantly ND seeds (Table 5). The majority of species initially classified as ND/PD were found to exhibit different levels of PD: 12 out of 20 species were assigned to the deep level, six species to a non-deep level, and two species to an intermediate level (Table 5). None of the species were found to have mainly MD, but four species were assigned to MPD (Table 5). Both of the Fabaceae species were classified as PY (Table 5). A combinational dormancy of PY + PD was not detected.
Table 4.
Key used for the determination of prevailing seed dormancy class and level, based on germination experiments with different preconditions following preliminary assignment to potential classes.
| Potential dormancy classes | Class and level |
|---|---|
| 1. ND/PD | |
| 1.1. FGP (FRESHLD) ≥ FGP(CDSfresh) and FGP (FRESHLD) ≥ FGP (CWSfresh/CWSsubs) and MGT (FRESHLD) ≤ MGT (CDSfresh) and MGT (FRESHLD) ≤ MGT(CWSfresh/CWSsubs) and FGP (FRESHLD) ≥ 50% | |
| Yes | ND |
| No | 1.2 |
| 1.2. FGP (CDSfresh) ≥ FGP(CWSfresh/CWSsubs) and MGT (CDSfresh) ≤ MGT (CWSfresh/CWSsubs) and FGP (CDSfresh) ≥ 50% | |
| Yes | PD, non-deep level |
| No | 1.3 |
| 1.3 FGP (FRESHLD) ≥ 50% or FGP (CDSfresh) ≥ 50% or FGP (CWSfresh/CWSsubs) ≥ 50% | |
| Yes | PD, intermediate level |
| No | PD, deep level |
| 2. MD/MPD | |
| 2.1. FGP (FRESHLD) ≥ 50% | |
| Yes | MD |
| No | MPD |
| 3. PY/PY + PD | |
| 3.1. FGP (FRESHLD) ≥ FGP(FRESHsc) and MGT (FRESHLD) ≤ MGT (FRESHsc) and FGP (FRESHLD) ≥ 50% | |
| Yes (assumption of impermeable seed coat dismissed!) | ND |
| No | 3.2 |
| 3.2. FGP (FRESHsc) ≥ 50% | |
| Yes | PY |
| No | PY + PD |
For unknown values of FGP or MGT the truth-value of the functional operation was assumed to become TRUE, except for the comparison of FGP with 50%, where missing values resulted in a FALSE.
FGP = final germination percentage; MGT = mean germination time; ND = non-dormant; PD = physiological dormancy; MD = morphological dormancy; MPD = morphophysiological dormancy; PY = physical dormancy; PY + PD = combinational dormancy; FRESHLD = seeds without storage under long-day conditions; CWSfresh = cold-wet storage; CWSsubs = cold-wet storage subsequent to a germination experiment; CDSfresh = cold-dry storage; FRESHsc = scarification of seeds without storage.
Table 5.
Refined assignment of the 28 species to the prevailing dormancy class and level.
| Species | Dormancy |
|
|---|---|---|
| Class | Level of PD | |
| Achillea moschata | PD | Non-deep |
| Anthyllis alpicola | PY | – |
| Arabis caerulea | PD | Deep |
| Arenaria ciliata | PD | Deep |
| Artemisia genipi | PD | Non-deep |
| Campanula scheuchzeri | MPD | – |
| Carex bicolor | PD | Deep |
| Cerastium uniflorum | PD | Deep |
| Comastoma tenellum | MPD | – |
| Draba aizoides | PD | Deep |
| Draba dubia | PD | Deep |
| Draba hoppeana | PD | Deep |
| Epilobium fleischeri | PD | Non-deep |
| Erigeron uniflorus | ND | – |
| Gentiana orbicularis | MPD | – |
| Geum reptans | PD | Intermediate |
| Leontodon hispidus | ND | – |
| Linaria alpina | PD | Deep |
| Minuartia gerardii | PD | Deep |
| Oxyria digyna | PD | Intermediate |
| Poa alpina | PD | Non-deep |
| Potentilla aurea | PD | Deep |
| Potentilla frigida | PD | Deep |
| Ranunculus glacialis | MPD | – |
| Saxifraga aizoides | PD | Non-deep |
| Saxifraga oppositifolia | PD | Deep |
| Silene exscapa | PD | Non-deep |
| Trifolium pallescens | PY | – |
ND = non-dormant; PD = physiological dormancy; MPD = morphophysiological dormancy; PY = physical dormancy.
Discussion
Non-dormancy vs. dormancy in seeds without storage
The proportion of non-dormant seeds, as well as the kind of dormancy, may vary within a species and year, depending on the position of seeds in fruits and inflorescences as well the environment of the mother plant during seed development (Baskin and Baskin, 2004b). This variation was also observed in our study. At least some of the fresh seeds germinated in more than half of the species. However, germination of these seeds was characterized by a relatively long lag-phase. Germination continued slowly and only few species exceeded a FGP of 50%. According to Baskin and Baskin (2004a), the time to completion of germination is also relevant to dormancy status. Alvarado and Bradford (2005) showed that, with increasing loss of dormancy, the percentage of germination increases while the time to germination decreases progressively. Following this approach, we had to assume conditional dormancy rather than ND, given that storage or scarification led to an increase in FGP or a decrease in MGT.
Species with non-dormant seeds
Only two species showed predominantly non-dormant seeds. The classification of Erigeron uniflorus as ND seems to be robust, with a FGP of almost 90%. However, the CDSfresh treatment induced dormancy, resulting in a reduced FGP and a delayed MGT. Braun (1913) reported a similar reduction in germination percentage within three months of storage. Our results suggest, that the seeds of Erigeron uniflorus may undergo cycles from non-dormancy to dormancy and back to non-dormancy. According to Baskin and Baskin (1998), only seeds with non-deep PD are known to exhibit such dormancy cycles. Leontodon hispidus was the second species classified as ND, although a relatively large fraction of seeds did not germinate in the FRESHLD treatment. Contradictory to our classification, the seeds of Leontodon hispidus from lowland populations underwent a cycle of different dormancy levels in a burial experiment done by Pons (1991). Although intraspecific variation in germination characteristics between populations of different altitudes needs to be considered (Cavieres and Arroyo, 2000; Wang et al., 2010), we assume that the seeds of Leontodon hispidus used in our study were also conditionally dormant, because the temperatures required to gain a full-scale level of germination success were high (Schwienbacher et al., in prep.). Cold stratification could lower these requirements as reported for many other species (Baskin and Baskin, 1998; Chambers et al., 1987; Nishitani and Masuzawa, 1996). However, the CDSfresh treatment lowered FGP in our study, but the onset of germination and the MGT did not differ. The lower FGP in the CDSfresh treatment was probably caused by divergent seed selection. An average of 14% of the achenes turned out to be ‘empty’, i.e., embryo development was aborted.
Species with non-deep physiological dormancy
In agreement with Baskin and Baskin (1998), PD was the prevailing dormancy mechanism in our study. However, non-deep PD was less frequently observed than reported earlier (Baskin and Baskin, 1998, 2004a,b). The presence of non-deep PD was mainly determined from significant increases in FGP or decreases in MGT in the CDSfresh treatment. This after-ripening effect was found in more than half of the species, including in the CDSfresh treatment. In accordance with findings of Wang et al. (2010), cold temperatures efficiently released non-deep PD; thus, warm temperatures were not required for after-ripening. The behavior of these non-deep PD species was similar to results reported earlier (Achillea moschata: Gallmetzer, 1995; Artemisia genipi: Niederfriniger Schlag, 2001; Poa alpina: Acharya, 1989; Niederfriniger Schlag, 2001; Saxifraga aizoides: Meier and Holderegger, 1998; Silene exscapa: Weilenmann, 1981).
The reason for the lower germination success of Artemisia genipi caused by the CWSfresh treatment remains unclear, as almost all seeds were proven to be viable. A large amount of variation between replicates was observed in Artemisia genipi in both the CDSfresh treatment and the CWSfresh treatment. Large degrees of within-species variation in seed viability and germination have been reported for many alpine species (Acharya, 1989; Giménez-Benavides et al., 2005; Schütz, 1988). They could be caused by effects of the maternal environment, which vary strongly in heterogeneous environments (Baskin and Baskin, 1998; Fenner and Thompson, 2005). Such temporal variation in germination was interpreted as risk spreading strategy in environments with unpredictable climates (Jurado and Westoby, 1992).
Cold-dry storage resulted in a faster germination rate in Epilobium fleischeri compared with seeds without storage, justifying the classification of this species as non-deep PD. The relatively low FGP of Epilobium fleischeri in all germination experiments was caused by low seed quality. Seed selection in this species was very laborious due to the small size of the seeds and the attached plume; therefore, the proportion of inferior seeds was probably higher. In addition, seeds featuring a plume, a pappus or other kinds of appendages are more susceptible to infections by fungi or algae and face a higher risk of seed die-back during incubation in the growth chamber (data not shown).
Although non-deep PD was found to be the prevailing dormancy mechanism in Silene exscapa, a substantial part of the seeds are assumed to exhibit intermediate levels of PD. Cold-dry storage did not accelerate germination and increased the FGP only slightly, whereas subsequent cold-wet storage was shown to be more efficient but still incomplete (data not shown). In contrast, cold stratification was not effective in a study by Niederfriniger Schlag (2001), but the stratification period of four weeks used there may have been too short. Scarification or removal of the seed coat was found to promote germination in many species with non-deep PD, apparently by lowering resistance of the embryo covering layer to radicle penetration (Baskin and Baskin, 2004b). This effect was observed in the closely related Silene acaulis subsp. longiscapa (Erschbamer and Pfattner, 2002), for which scarification of seed coat substantially increased final germination. Excised embryos of Silene exscapa also produced normal seedlings in our study (data not shown). The addition of Gibberellic acid (GA3) was shown to be the most successful treatment for stimulating germination in Silene acaulis subsp. longiscapa (Erschbamer and Pfattner, 2002). Dormancy break by GA3 is typical in seeds with non-deep PD, but it was also reported for intermediate PD (Baskin and Baskin, 1998, 2004b).
Species with intermediate physiological dormancy
Only two species (Geum reptans, Oxyria digyna) were assigned to this level. Baskin and Baskin (1998) classified Oxyria digyna as ND based on the work of Mooney and Billings (1961), who concluded that ‘there does not seem to be any appreciable after-ripening requirement’ and that increased germination after cold stratification was of minor relevance. According to the guidelines set by Baskin and Baskin (1998, 2004b), cold stratification for one week is probably too short and storage in a freezer may be inappropriate for after-ripening. In our study FGP, onset of germination, and MGT, all were significantly affected by after-ripening, supporting the designation of a conditionally dormant status of fresh seeds. Furthermore, the CWSfresh treatment reduced the MGT further. A very similar pattern was obtained for Geum reptans. The lower FGP of both species after the CWSfresh treatment was caused by a substantial die-back of seeds due to heavy fungal infections. Storage under moist conditions promoted proliferation of fungi and algae and therefore affected seed viability more than dry storage (data not shown).
Species with deep physiological dormancy
Our germination study revealed that fully ripe seeds of more than half of the study species with PD had no reasonable germination success even after several months of cold-dry or cold-wet storage. The findings are in agreement with former studies covering 75% of the species we studied (Braun, 1913; Fossati, 1980; Gallmetzer, 1995; Niederfriniger Schlag, 2001; Schütz, 1988; Weilenmann, 1981; Zuur-Isler, 1982). Therefore, we conclude that deep levels of PD are much more common in alpine species than previously reported (Baskin and Baskin, 1998; Körner, 2003). However, the requirements to overcome dormancy remain unclear, as cold stratification alone seems to be insufficient to break deep PD, even if applied for more than four months. Probably, some additional preconditions have to be fulfilled to release seeds from dormancy, e.g., a sequence of different temperatures as reported for other species (Baskin and Baskin, 1998).
Species with physical dormancy
In accordance with most species of the Fabaceae (Baskin and Baskin, 1998; Baskin et al., 2000; Flüeler, 1992; Van Assche et al., 2003), PY was detected as the main dormancy mechanism of Anthyllis alpicola and Trifolium pallescens. In this family, PY typically develops during the maturation drying of the seed through a hardening of the seed coat that impedes water uptake (Baskin et al., 2000). Although detailed anatomical investigations of the seed coat are lacking in our study, we conclude that the water impermeability in Anthyllis alpicola and Trifolium pallescens was not yet fully developed in ripe, fresh seeds, although the seeds already had a ‘hard’ seed or fruit coat. The onset of germination required almost 10 days and proceeded relatively slowly. In this state, water-uptake seems to be delayed by the seed coat and, in case of Anthyllis alpicola, additionally by the fruit coat. However, in a large fraction of the seeds, imbibition was only delayed, not prevented. In contrast, storage of any kind caused germination to nearly cease, indicating a fully developed PY. Scarification was reported to release dormancy efficiently in alpine Fabaceae (Flüeler, 1992; Schütz, 1988). Consistently, scarification resulted in an immediate uptake of water by seeds, in both the FRESHsc treatment and the CDSsc treatment. The size of imbibed seeds almost doubled within a few hours, and they germinated within few days. Despite the higher MGT of Trifolium pallescens in the FRESHsc treatment compared with the CDSsc treatment, full germination was gained within two weeks in both treatments. Thus, no indication of a PY + PD class was found for either of the Fabaceae species.
Species with morphophysiological dormancy
In agreement with the few reported cases of MD for temperate/arctic zones (Baskin and Baskin, 1998, 2004b), we found no evidence for MD, but we assumed MPD for four species. The fundamental cause of both classes MD and MPD is an underdeveloped embryo, which requires that an interval of embryo maturation occurs prior to germination (Baskin and Baskin, 1998, 2004b). Although embryo development was not directly investigated in our study, several observations of rudimentary or underdeveloped embryos in alpine species were reported. Wagner et al. (2010) found rudimentary embryos in ripe seeds of Ranunculus glacialis and suggested MPD. Our results support the assumptions of MPD rather than MD, as fresh seeds of Ranunculus glacialis did not germinate within one month. However, cold stratification was not effective in releasing seeds from dormancy. We assume they require a temporal sequence of different environmental conditions to break MPD. Such mechanisms were reported not only for species of the Ranunculaceae (Baskin and Baskin, 1998; Forbis and Diggle, 2001; Vandelook et al., 2009) but also for a few species of the Gentianaceae (Threadgill et al., 1981), and the Campanulaceae (Baskin et al., 2005). Cold stratification was reported to increase germination in some species of these families (review for Gentianaceae in Simpson and Webb, 1980; Campanula americana in Baskin and Baskin, 1988), but GA3 was the only treatment that promoted germination in other species of both families (Ægisdóttir and Thórhallsdóttir, 2006; Fossati, 1980; Gallmetzer, 1995; Schütz, 1988; Zuur-Isler, 1982). Hence we assume that MPD is more common in the alpine species of these two families, as previously reported. The MPD class was shown to comprise the most complex dormancy mechanisms and still needs more research to unravel all levels and the details of these mechanisms (Baskin and Baskin, 1998).
Limitations of dormancy classification methods
The classification of seed dormancy based on seed morphology data from published studies and certain pretreatments in germination experiments may have some shortcomings. Species assigned to deep PD in our study could probably have a larger proportion of seeds with intermediate and non-deep PD if additional methods had been applied. Scarification and the addition of GA3 were reported to be inefficient in releasing deep PD (Baskin and Baskin, 1998, 2004b), but either one or both of these treatments significantly increased the FGP in Arabis caerulea (Fossati, 1980; Weilenmann, 1981), Cerastium uniflorum (Schütz, 1988), Linaria alpina (Gallmetzer, 1995; Schütz, 1988), Minuartia gerardii (Zuur-Isler, 1982), and Potentilla aurea (Gallmetzer, 1995). In the Saxifragaceae, we would expect MPD to be more common than previously known, as underdeveloped embryos are documented in several genera of this family (Baskin and Baskin, 2007; Martin, 1946). Wagner and Tengg (1993) found an embryo index (embryo:seed length) of approximately 0.5 in mature seeds of Saxifraga oppositifolia, a value commonly used to differentiate between fully developed and underdeveloped embryos (Baskin and Baskin, 2007). However, more detailed investigations of embryo growth between dispersal and prior to germination are needed to support an assignment of MPD.
Ecological implications of dormancy mechanisms and germination requirements
Exposure of seeds to light under long-day conditions promoted germination in all species with PD, either in terms of germination onset, MGT or FGP, except in Poa alpina, for which light delayed germination of seeds without storage and decreased the FGP. The higher germination success of Poa alpina in darkness confirms the findings of Acharya (1989), although the differences were more pronounced in our study. After-ripening of seeds could have lowered the effect of light in the experiments of Acharya (1989). In the case of Poa alpina, a temporary delay in germination caused by light could prevent the disadvantageous germination of fresh seeds in autumn. The requirement of light for germination was shown to vary with seasons (Pons, 1991) in many species with non-deep PD that undergo dormancy cycles, or this requirement was reduced after cold stratification (Chambers et al., 1987; Shimono and Kudo, 2005). We would also expect such seasonal cycles of light responses for alpine species, as the light environment at high altitudes in temperate regions is strongly influenced by snow cover in winter and high solar radiation in summer (Körner, 2003). Densmore (1997) showed that the germination of arctic-alpine plants was significantly reduced under short-day conditions in autumn, delaying germination to spring. The light responses of seeds have considerable consequences. The ability of seeds to sense their light environment gives them a chance to have at least some control over the timing of germination (Fenner and Thompson, 2005). Light was found to inhibit germination in some species, whereas darkness prevents germination of light-requiring buried seeds (Pons, 2000). Both mechanisms were proposed to facilitate the formation of a persistent soil seed bank (Fenner and Thompson, 2005; Pons, 2000). However, in other species, the role of light remains unclear or seems to be negligible. The two Fabaceae species in our study did not require light for germination. Species of this family tend to germinate readily in darkness (Fenner and Thompson, 2005).
According to our results, a slow onset of germination and a low FGP of fresh seeds seem to be common phenomena in alpine species, but the causes of this behavior are manifold and sometimes difficult to disentangle. Diverse seed dormancy mechanisms, that were proposed by several authors for arctic and alpine species (Baskin and Baskin, 1998; Shimono and Kudo, 2005) seem to be the main cause of the prevention or the delay of germination in autumn for most seeds. Dormancy was suggested to prevent germination when conditions are favorable for germination but are preceding subsequent periods of unfavorable conditions that are likely to cause low survival of the seedlings (Vleeshouwers et al., 1995). In alpine species, germination in late summer or autumn may result in a high loss of seedlings (Shimono and Kudo, 2003), because these youngest stages in a plants life cycle are very sensitive to disturbances and stress (Leck et al., 2008). Severe frosts, often causing cryoturbation by cycles of freezing and thawing, are common in late autumn before an adequate snow cover provides certain protection against harsh environmental conditions (Körner, 2003). Although a substantial fraction of fresh seeds may be non-dormant or only conditionally dormant, the prevailing low temperatures in autumn, combined with short day lengths, are likely to delay and prevent germination. Low temperatures in winter and the moist but still cold environment during snow melt provide after-ripening and cold stratification conditions to break dormancy in seeds with PD and MPD. Van Assche et al. (2003) showed that chilling followed by a period of alternating temperatures triggered spring germination of several lowland Fabaceae species. We suggest the same environmental cues may drive release of PY in alpine Fabaceae. Seed responses to the seasonal changes of the light environment do complete the complex mechanisms that trigger germination (Densmore, 1997; Pons, 2000; Shimono and Kudo, 2005).
Our study confirms the diversity of dormancy mechanisms and germination requirements already reported (Amen, 1966; Baskin and Baskin, 1998; Körner, 2003). It gives clear evidence that alpine species do not feature a common ‘alpine’ dormancy mechanism.
Acknowledgments
This study was funded by the Austrian Science Fund (FWF P19090-B16). Additional financial support provided by the Austrian Academy of Science within the KIÖS-project ‘Gletschervorfeld’ (Rüdiger Kaufmann, Univ. Innsbruck) is gratefully appreciated. We thank Silvia Marcante and many helping hands for assistance in seed collection and laboratory work.
Contributor Information
Erich Schwienbacher, Email: Erich.Schwienbacher@uibk.ac.at.
Jose Antonio Navarro-Cano, Email: Jose-Antonio.Navarro@botan.su.se.
Gilbert Neuner, Email: Gilbert.Neuner@uibk.ac.at.
Brigitta Erschbamer, Email: Brigitta.Erschbamer@uibk.ac.at.
Appendix A.
Study species and details of the germination experiments, with collection time (CT: month-year), storage period (SP: weeks), starting time (ST: month-year), and incubation period (IP: weeks) of germination experiments, number of replicates (n), and number of seeds per replicate (seeds) for each species. All germination experiments were performed under alternating temperature regimes and long-day conditions (16 h light at 25 °C/8 h darkness at 10 °C), except for experiments in darkness in which only temperature was varied. Seeds without storage were tested under long-day conditions (FRESHLD) or in darkness (FRESHdark) within 1 week of seed collection. Storage of seeds: CWSfresh – cold-wet storage after a short period (max. 10 weeks) of cold-dry storage; CWSsubs – cold-wet storage subsequent to a germination experiment with fresh seeds using the remaining viable seeds; CDSfresh – cold-dry storage of fresh seeds. The effect of scarification was tested on seeds without storage (FRESHsc) and following cold-dry storage (CDSsc).
| Species | CT | SP | ST | IP | n | Seeds |
|---|---|---|---|---|---|---|
| Achillea moschata | ||||||
| FRESHSTD | 10-2004 | ≤1 | 10-2004 | 32 | 5 | 100 |
| FRESHdark | 10-2004 | ≤1 | 10-2004 | 32 | 5 | 100 |
| CWSfresh | 09-2000 | 10 | 01-2001 | 33 | 5 | 100 |
| CDSfresh | 09-2007 | 37 | 06-2008 | 31 | 4 | 50 |
| Anthyllis vulneraria subsp. alpicolaa | ||||||
| FRESHSTD | 09-2004 | ≤1 | 09-2004 | 32 | 5 | 100 |
| FRESHdark | 09-2004 | ≤1 | 09-2004 | 32 | 5 | 100 |
| FRESHsc | 08-2010 | ≤1 | 08-2010 | 31 | 4 | 50 |
| CWSfresh | 09-2000 | 10 | 01-2001 | 33 | 5 | 100 |
| CDSfresh | 09-2006 | 45 | 07-2007 | 31 | 4 | 50 |
| CDSsc | 09-2006 | 72 | 01-2008 | 32 | 4 | 50 |
| Arabis caerulea | ||||||
| FRESHSTD | 08-2007 | ≤1 | 08-2007 | 33 | 5 | 50 |
| CWSsubs | 08-2007 | 19 | 02-2008 | 62 | 5 | 45.0b |
| CDSfresh | 09-2006 | 45 | 07-2007 | 31 | 4 | 50 |
| Arenaria ciliata | ||||||
| FRESHSTD | 09-2007 | ≤1 | 09-2007 | 58 | 5 | 50 |
| CWSsubs | 09-2007 | 15 | 03-2008 | 60 | 5 | 49.6b |
| Artemisia genipi | ||||||
| FRESHSTD | 09-2004 | ≤1 | 09-2004 | 33 | 5 | 100 |
| FRESHdark | 09-2004 | ≤1 | 09-2004 | 33 | 5 | 100 |
| CWSfresh | 08-2000 | 10 | 01-2001 | 33 | 5 | 100 |
| CDSfresh | 09-2007 | 37 | 06-2008 | 31 | 4 | 50 |
| Campanula scheuchzeri | ||||||
| FRESHSTD | 09-2007 | ≤1 | 09-2007 | 58 | 5 | 50 |
| CWSsubs | 09-2007 | 15 | 03-2008 | 60 | 5 | 35.8b |
| Carex bicolor | ||||||
| FRESHSTD | 08-2007 | ≤1 | 08-2007 | 36 | 5 | 50 |
| CWSsubs | 08-2007 | 19 | 03-2008 | 63 | 5 | 49.0b |
| CDSfresh | 09-2007 | 26 | 02-2008 | 59 | 5 | 50 |
| Cerastium uniflorum | ||||||
| FRESHSTD | 08-2007 | ≤1 | 08-2007 | 33 | 5 | 50 |
| CWSsubs | 08-2007 | 19 | 02-2008 | 62 | 5 | 30.6b |
| CDSfresh | 09-2006 | 64 | 12-2007 | 32 | 4 | 25 |
| Comastoma tenellum | ||||||
| FRESHSTD | 08-2009 | ≤1 | 08-2009 | 29 | 1 | 50 |
| CWSsubs | 08-2009 | 19 | 02-2010 | 32 | 1 | 50b |
| Draba aizoides | ||||||
| FRESHSTD | 08-2009 | ≤1 | 08-2009 | 29 | 1 | 50 |
| CWSsubs | 08-2009 | 19 | 02-2010 | 32 | 1 | 49b |
| Draba dubia | ||||||
| FRESHSTD | 08-2009 | ≤1 | 08-2009 | 29 | 1 | 50 |
| CWSsubs | 08-2009 | 19 | 02-2010 | 32 | 1 | 50b |
| Draba hoppeana | ||||||
| FRESHSTD | 08-2009 | ≤1 | 08-2009 | 29 | 1 | 50 |
| CWSsubs | 08-2009 | 19 | 02-2010 | 32 | 1 | 49b |
| Epilobium fleischeri | ||||||
| FRESHSTD | 09-2007 | ≤1 | 09-2007 | 32 | 5 | 50 |
| CDSfresh | 09-2007 | 37 | 06-2008 | 31 | 4 | 50 |
| Erigeron uniflorus | ||||||
| FRESHSTD | 08-2007 | ≤1 | 08-2007 | 32 | 5 | 50 |
| CDSfresh | 08-2007 | 37 | 06-2008 | 31 | 4 | 50 |
| Gentiana orbicularis | ||||||
| FRESHSTD | 09-2007 | ≤1 | 09-2007 | 58 | 5 | 50 |
| CWSsubs | 09-2007 | 15 | 03-2008 | 60 | 5 | 47.0b |
| Geum reptans | ||||||
| FRESHSTD | 09-2004 | ≤1 | 09-2004 | 33 | 5 | 100 |
| FRESHdark | 09-2004 | ≤1 | 09-2004 | 33 | 5 | 100 |
| CWSfresh | 08-2000 | 10 | 01-2001 | 33 | 5 | 100 |
| CDSfresh | 08-2007 | 42 | 06-2008 | 31 | 4 | 50 |
| Leontodon hispidus | ||||||
| FRESHSTD | 09-2007 | ≤1 | 09-2007 | 32 | 5 | 30 |
| CDSfresh | 09-2006 | 45 | 07-2008 | 31 | 4 | 50 |
| Linaria alpina | ||||||
| FRESHSTD | 09-2004 | ≤1 | 09-2004 | 33 | 5 | 100 |
| FRESHdark | 09-2004 | ≤1 | 09-2004 | 33 | 5 | 100 |
| CWSfresh | 08-2000 | 10 | 01-2001 | 33 | 5 | 100 |
| Minuartia gerardii | ||||||
| FRESHSTD | 08-2007 | ≤1 | 08-2007 | 33 | 1 | 115 |
| CWSsubs | 08-2007 | 19 | 03-2008 | 63 | 1 | 79b |
| Oxyria digyna | ||||||
| FRESHSTD | 08-2004 | ≤1 | 08-2004 | 33 | 5 | 100 |
| FRESHdark | 08-2004 | ≤1 | 08-2004 | 33 | 5 | 100 |
| CWSfresh | 08-2000 | 10 | 01-2001 | 33 | 5 | 90 |
| CDSfresh | 09-2006 | 45 | 07-2007 | 31 | 4 | 50 |
| Poa alpina | ||||||
| FRESHSTD | 09-2004 | ≤1 | 08-2004 | 33 | 5 | 25 |
| FRESHdark | 09-2004 | ≤1 | 08-2004 | 32 | 5 | 25 |
| CDSfresh | 09-2006 | 45 | 07-2007 | 31 | 4 | 50 |
| Potentilla aurea | ||||||
| FRESHSTD | 08-2009 | ≤1 | 08-2009 | 31 | 1 | 50 |
| CWSsubs | 08-2009 | 20 | 02-2010 | 32 | 1 | 47b |
| Potentilla frigida | ||||||
| FRESHSTD | 08-2009 | ≤1 | 08-2009 | 31 | 2 | 50 |
| CWSsubs | 08-2009 | 20 | 02-2010 | 32 | 2 | 50b |
| Ranunculus glacialis | ||||||
| FRESHSTD | 08-2009 | ≤1 | 09-2009 | 29 | 1 | 50 |
| CWSsubs | 08-2009 | 19 | 02-2010 | 32 | 1 | 48b |
| Saxifraga aizoides | ||||||
| FRESHSTD | 10-2004 | ≤1 | 10-2004 | 31 | 5 | 100 |
| FRESHdark | 10-2004 | ≤1 | 10-2004 | 32 | 5 | 100 |
| CWSfresh | 09-2000 | 10 | 01-2001 | 33 | 5 | 100 |
| CDSfresh | 10-2006 | 40 | 07-2007 | 31 | 4 | 50 |
| Saxifraga oppositifolia | ||||||
| FRESHSTD | 09-2004 | ≤1 | 09-2004 | 33 | 5 | 100 |
| FRESHdark | 09-2004 | ≤1 | 09-2004 | 31 | 5 | 100 |
| CWSfresh | 08-2000 | 10 | 01-2001 | 33 | 5 | 100 |
| Silene acaulis subsp. exscapa | ||||||
| FRESHSTD | 09-2007 | ≤1 | 09-2007 | 32 | 5 | 50 |
| CDSfresh | 09-2006 | 45 | 07-2007 | 31 | 4 | 50 |
| Trifolium pallescensa | ||||||
| FRESHSTD | 09-2004 | ≤1 | 09-2004 | 33 | 5 | 100 |
| FRESHdark | 09-2004 | ≤1 | 09-2004 | 32 | 5 | 100 |
| FRESHsc | 09-2010 | ≤1 | 09-2010 | 31 | 4 | 50 |
| CWSfresh | 09-2000 | 10 | 01-2001 | 33 | 5 | 100 |
| CDSfresh | 09-2006 | 42 | 07-2007 | 31 | 4 | 50 |
| CDSsc | 09-2006 | 79 | 04-2008 | 28 | 4 | 50 |
Seeds were pinched with tweezers and soft seeds were discarded.
Mean number of remaining viable seeds per replicate following the germination experiment with fresh seeds.
References
- Acharya S.N. Germination response of two alpine grasses from the Rocky Mountains of Alberta. Can. J. Plant Sci. 1989;69:1165–1178. [Google Scholar]
- Ægisdóttir H.H., Thórhallsdóttir T.E. Breeding system evolution in the Arctic: a comparative study of Campanula uniflora in Greenland and Iceland. Arct. Antarct. Alp. Res. 2006;38:305–312. [Google Scholar]
- Akhalkatsi M., Wagner J. Comparative embryology of three Gentianaceae species from the Central Caucasus and the European Alps. Pl. Syst. Evol. 1997;204:39–48. [Google Scholar]
- Alvarado V., Bradford K.J. Hydrothermal time analysis of seed dormancy in true (botanical) potato seeds. Seed Sci. Res. 2005;15:77–88. [Google Scholar]
- Amen R.D. Extent and role of seed dormancy in alpine plants. Q. Rev. Biol. 1966;41:271–281. [Google Scholar]
- Baskin C.C., Baskin J.M. Germination ecophysiology of herbaceous plant species in a temperate region. Am. J. Bot. 1988;75:286–305. [Google Scholar]
- Baskin C.C., Baskin J.M. Academic Press; San Diego: 1998. Seeds. Ecology, Biogeography and Evolution of Dormancy and Germination. [Google Scholar]
- Baskin C.C., Baskin J.M. Underdeveloped embryos in dwarf seeds and implications for assignment to dormancy class. Seed Sci. Res. 2005;15:357–360. [Google Scholar]
- Baskin C.C., Baskin J.M. A revision of Martin's seed classification system, with particular reference to his dwarf-seed type. Seed Sci. Res. 2007;17:11–20. [Google Scholar]
- Baskin C.C., Baskin J.M., Yoshinaga A. Morphophysiological dormancy in seeds of six endemic lobelioid shrubs (Campanulaceae) from the montane zone in Hawaii. Can. J. Bot. 2005;83:1630–1637. [Google Scholar]
- Baskin J.M., Baskin C.C. Classification, biogeography, and phylogenetic relationships of seed dormancy. In: Smith R.D., Dickie J.B., Linington S.H., Pritchard H.W., Probert R.J., editors. Seed Conservation: Turning Science Into Practice. Kew Publishing; London: 2004. pp. 517–544. [Google Scholar]
- Baskin J.M., Baskin C.C. A classification system for seed dormancy. Seed Sci. Res. 2004;14:1–16. [Google Scholar]
- Baskin J.M., Baskin C.C., Li X. Taxonomy, anatomy and evolution of physical dormancy in seeds. Plant Spec. Biol. 2000;15:139–152. [Google Scholar]
- Benech-Arnold R.L., Sanchez R.A., Forcella F., Kruk B.C., Ghersa C.M. Environmental control of dormancy in weed seed banks in soil. Field Crop. Res. 2000;67:105–122. [Google Scholar]
- Braun J. Die Vegetationsverhältnisse der Schneestufe in den Rätisch-Lepontischen Alpen. Neue Denkschr. Schweiz Naturforsch. Ges. 1913;48:1–347. [Google Scholar]
- Brown R.F., Mayer D.G. Representing cumulative germination. 2. The use of the Weibull function and other empirically derived curves. Ann. Bot. 1988;61:127–138. [Google Scholar]
- Cavieres L.A., Arroyo M.T.K. Seed germination response to cold stratification period and thermal regime in Phacelia secunda (Hydrophyllaceae): altitudinal variation in the mediterranean Andes of Central Chile. Plant Ecol. 2000;149:1–8. [Google Scholar]
- Chambers J.C., MacMahon J.A., Brown R.W. Germination characteristics of alpine grasses and forbs: a comparison of early and late seral dominants with reclamation potential. Reclam. Reveg. Res. 1987;6:235–249. [Google Scholar]
- Densmore R.V. Effect of day length on germination of seeds collected in Alaska. Am. J. Bot. 1997;84:274–278. [PubMed] [Google Scholar]
- Erschbamer B., Pfattner M. Das Keimverhalten von alpinen Arten in der Klimakammer und im Gelände. Ber. Nat-Med. Ver. Innsbruck. 2002;89:87–97. [Google Scholar]
- Favarger C. Sur la germination des gentianes. Phyton (Horn) 1953;4:275–289. [Google Scholar]
- Fenner M., Thompson K. University Press; Cambridge: 2005. The Ecology of Seeds. [Google Scholar]
- Finch-Savage W.E., Leubner-Metzger G. Seed dormancy and the control of germination. New Phytol. 2006;171:501–523. doi: 10.1111/j.1469-8137.2006.01787.x. [DOI] [PubMed] [Google Scholar]
- Fischer, M.A., Oswald, K., Adler, W., 2008. Exkursionsflora für Österreich, Liechtenstein und Südtirol, 3. Aufl., Land Oberösterreich, Biologiezentrum der Oberösterr. Landesmuseen, Linz.
- Flüeler R.P. Untersuchungen über Keimung und Etablierung von alpinen Leguminosen. Veröff. Geobot. Inst. der ETH Stiftung Rübel Zürich. 1992;110:1–149. [Google Scholar]
- Forbis T.A., Diggle P.K. Subnivean embryo development in the alpine herb Caltha leptosepala (Ranunculaceae) Can. J. Bot. 2001;79:635–642. [Google Scholar]
- Fossati A. Keimverhalten und frühe Entwicklungsphasen einiger Alpenpflanzen. Veröff. Geobot. Inst. der ETH Stiftung Rübel Zürich. 1980;73:1–193. [Google Scholar]
- Gallmetzer, W., 1995. Keimverhalten alpiner Kräuter. Diplomarbeit, Univ. Innsbruck.
- Giménez-Benavides L., Escudero A., Pérez-García F. Seed germination of high mountain Mediterranean species: altitudinal, interpopulation and interannual variability. Ecol. Res. 2005;20:433–444. [Google Scholar]
- Jurado E., Westoby M. Germination biology of selected central Australian plants. Austral. J. Ecol. 1992;17:341–348. [Google Scholar]
- Körner C. 2nd ed. Springer; Berlin-Heidelberg: 2003. Alpin Plant Life: Functional Plant Ecology of High Mountain Ecosystems. [Google Scholar]
- Leck A.M., Parker V.T., Simpson R.L. Why seedlings? In: Leck A.M., Parker V.T., Simpson R.L., editors. Seedling Ecology and Evolution. Cambridge University Press; Cambridge: 2008. pp. 3–13. [Google Scholar]
- Martin A.C. The comparative internal morphology of seeds. Am. Midl. Nat. 1946;36:513–660. [Google Scholar]
- Meier C., Holderegger R. Breeding system, germination, and phenotypic differences among populations of Saxifraga aizoides (Saxifragaceae) at the periphery of its alpine distribution. Nord. J. Bot. 1998;18:681–688. [Google Scholar]
- Mooney H.A., Billings W.D. Comparative physiological ecology of arctic and alpine populations of Oxyria digyna. Ecol. Monogr. 1961;31:1–29. [Google Scholar]
- Niederfriniger Schlag, R., 2001. Keim- und Wachstumsraten von Gletschervorfeldarten. In: Niederfriniger Schlag, R. (Hrsg.) Primärsukzession im Gletschervorfeld. Keimung, Etablierung, Wachstum und Interaktionen im Gletschervorfeld des Rotmoosferners (Ötztal, Tirol). Dissertation, Univ. Innsbruck, pp. 34–58.
- Nishitani S., Masuzawa T. Germination characteristics of two species of Polygonum in relation to their altitudinal distribution on Mt. Fuji, Japan. Arct. Alp. Res. 1996;28:104–110. [Google Scholar]
- Pons T.L. Dormancy, germination and mortality of seeds in a chalk-grassland flora. J. Ecol. 1991;79:765–780. [Google Scholar]
- Pons T.L. Seed responses to light. In: Fenner M., editor. The Ecology of Regeneration in Plant Communities. 2nd ed. CAB International; Wallingford: 2000. pp. 237–260. [Google Scholar]
- Raffl C., Mallaun M., Mayer R., Erschbamer B. Vegetation succession pattern and diversity changes in a glacier valley, Central Alps, Austria. Arct. Antarct. Alp. Res. 2006;38:421–428. [Google Scholar]
- Schwienbacher E., Erschbamer B. Longevity of seeds in a glacier foreland of the Central Alps—a burial experiment. Bull. Geobot. Inst. ETH. 2002;68:63–71. [Google Scholar]
- Schütz M. Genetisch-ökologische Untersuchungen an alpinen Pflanzenarten auf verschiedenen Gesteinsunterlagen: Keimungs- und Aussaatversuche. Veröff. Geobot. Inst. der ETH Stiftung Rübel Zürich. 1988;99:1–153. [Google Scholar]
- Shimono Y., Kudo G. Intraspecific variations in seedling emergence and survival of Potentilla matsumurae (Rosaceae) between alpine fellfield and snowbed habitats. Ann. Bot. 2003;91:21–29. doi: 10.1093/aob/mcg002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimono Y., Kudo G. Comparisons of germination traits of alpine plants between fellfield and snowbed habitats. Ecol. Res. 2005;20:189–197. [Google Scholar]
- Simpson M.J.A., Webb C.J. Germination in some New Zealand species of Gentiana: a preliminary report. N. Z. J. Bot. 1980;18:495–501. [Google Scholar]
- Threadgill P.F., Baskin J.M., Baskin C.C. Dormancy in seeds of Frasera caroliniensis (Gentianaceae) Am. J. Bot. 1981;68:80–86. [Google Scholar]
- Van Assche J.A., Debucquoy K.L.A., Rommens W.A.F. Seasonal cycles in the germination capacity of buried seeds of some Leguminosae (Fabaceae) New Phytol. 2003;158:315–323. [Google Scholar]
- Vandelook F., Lenaerts J., van Assche J. The role of temperature in post-dispersal embryo growth and dormancy break in seeds of Aconitum lycoctonum L. Flora. 2009;204:536–542. [Google Scholar]
- Vleeshouwers L.M., Bouwmeester H.J., Karssen C.M. Redefining seed dormancy: an attempt to integrate physiology and ecology. J. Ecol. 1995;83:1031–1037. [Google Scholar]
- Wagner J., Steinacher G., Ladinig U. Ranunculus glacialis L.: successful reproduction at the altitudinal limits of higher plant life. Protoplasma. 2010;243:117–128. doi: 10.1007/s00709-009-0104-1. [DOI] [PubMed] [Google Scholar]
- Wagner J., Tengg G. Embryology of two high-mountain plants, Saxifraga oppositifolia and Cerastium uniflorum, in relation to phenology. Flora. 1993;188:203–212. [Google Scholar]
- Wang J.H., Baskin C.C., Chen W., Du G.Z. Variation in seed germination between populations of five sub-alpine woody species from eastern Qinghai-Tibet Plateau following dry storage at low temperatures. Ecol. Res. 2010;25:195–203. [Google Scholar]
- Weilenmann K. Bedeutung der Keim- und Jungpflanzenphase für alpine Taxa verschiedener Standorte. Ber. Geobot. Inst. ETH Stiftung Rübel. 1981;48:68–119. [Google Scholar]
- Zuur-Isler D. Germination behaviour and early life phases of some species from alpine serpentine soils. Ber. Geobot. Inst. ETH Stiftung Rübel. 1982;49:76–107. [Google Scholar]