Abstract
Improgan, a non-opioid, antinociceptive drug, activates descending analgesic circuits following brain administration, but the improgan receptor remains unidentified. Since biotinylation of drugs can enhance drug potency or facilitate discovery of new drug targets, a biotinylated congener of improgan (CC44) and several related compounds were synthesized and tested for antinociceptive activity. In rats and mice, intracerebroventricular (i.c.v.) administration of CC44 produced dose-dependent reductions in thermal nociceptive (tail flick and hot plate) responses, with 5-fold greater potency than improgan. CC44 also robustly attenuated mechanical (tail pinch) nociception in normal rats and mechanical allodynia in a spinal nerve ligation model of neuropathic pain. Similar to the effects of improgan, CC44 antinociception was reversed by the GABAA agonist muscimol (consistent with activation of analgesic circuits), and was resistant to the opioid antagonist naltrexone (implying a non-opioid mechanism). Also like improgan, CC44 produced thermal antinociception when microinjected into the rostral ventromedial medulla (RVM). Unlike improgan, CC44 (i.c.v.) produced antinociception which was resistant to antagonism by the cannabinoid CB1 antagonist/inverse agonist rimonabant. CC44 was inactive in mice following systemic administration, indicating that CC44 does not penetrate the brain. Preliminary findings with other CC44 congeners suggest that the heteroaromatic nucleus (imidazole), but not the biotin moiety, is required for CC44’s antinociceptive activity. These findings demonstrate that CC44 is a potent analgesic compound with many improgan-like characteristics. Since powerful techniques are available to characterize and identify the binding partners for biotin-containing ligands, CC44 may be useful in searching for new receptors for analgesic drugs.
Keywords: improgan, analgesia, pain, neuropathic pain, biotin
1. Introduction
Despite the powerful, pain-relieving properties of opioids, opioid side effects and the epidemic rise in prescription opioid abuse continue to motivate the search for potent, non-opioid analgesics (Stannard, 2011). Improgan (Fig. 1), an imidazole-containing analgesic drug derived from the H2 receptor antagonist cimetidine, is one such drug (Li et al., 1996; Hough et al., 2001a). When administered directly into the CNS, improgan is highly effective in thermal (Li et al., 1996), mechanical (Li et al., 1997) and neuropathic pain tests (Albrecht et al., 2011). Like morphine (Basbaum and Fields, 1984), improgan acts in the periaqueductal gray and rostral ventromedial medulla (RVM) to stimulate descending analgesic pathways (Nalwalk et al., 2004; Phillips et al., 2012). However, unlike morphine (which targets μ opioid receptors), improgan does not act through known opioid receptor mechanisms (Hough et al., 2000b). Consistent with this non-opioid profile, improgan lacks morphine’s locomotor stimulant (Li et al., 1997), respiratory depressant (Phillips et al., 2012), and tolerance-producing (Bannoura et al., 1998) side effects.
Fig. 1.

Chemical structures of improgan, CC44, CC46, and CC47. Four structural regions of the drugs are illustrated.
Despite this favorable pre-clinical profile, two significant impediments have prevented the clinical development of improgan-like drugs: 1) the molecular site of action remains unknown despite screening at over 100 potential receptors, and 2) these drugs fail to cross the blood-brain barrier (thus requiring direct brain administration, Hough et al., 2000a; Hough et al., 2001a). To surmount these obstacles, the long-term goals of our laboratory are to 1) discover the improgan receptor and/or 2) develop more potent, brain-penetrating improgan-like analgesics.
One strategy for drug receptor identification has been chemical modification of that drug to create a receptor probe. For example, drug biotinylation has been used to identify or study receptor/binding partners for antibiotics (Ki et al., 2000), anti-spermatogenics (Tash et al., 2008), prostaglandins (Stamatakis et al., 2006), non-glycosaminoglycans (Harris and Weigel, 2008), and epoxyeicosanoids (Yang et al., 2007). Typically, the biotinylated drug is incubated with potential target molecules, treated with radiolabeled or matrix-tethered avidin/streptavidin, and characterized further (Green, 1990). Thus, biotinylation of improgan could facilitate discovery of the improgan receptor.
Chemical modification of drugs can also improve transcellular delivery and in vivo potency. For example, biotinylation of ganciclovir enhanced drug pharmacokinetics following injection into the vitreous fluid (Janoria et al., 2009). Covalent attachment of polyethylene glycol groups (PEGylation) has been used to improve drug pharmacokinetics while retaining or enhancing pharmacodynamic parameters (Pasut and Veronese, 2007). Furthermore, use of PEGylated nanoparticles improved blood-brain barrier penetration (Calvo et al., 2001). Thus, biotinylation/PEGylation of improgan could improve the brain penetration and/or potency of this drug.
Because biotinylated/ PEGylated improgan congeners could be important pain-related research tools, or have potent, brain-penetrating analgesic properties, we recently synthesized and tested several such compounds (Fig. 1). The goal of the present work was to evaluate the analgesic activity and brain-penetrating properties of the prototype, CC44.
2. Materials and methods
2.1. Materials
CC44 hydrochloride, CC46 (base), and CC47 (base) were synthesized as described in the Supplementary Materials. Improgan was available from laboratory stock. Rimonabant was synthesized according to literature methods (Lan et al., 1999). Except where noted otherwise, these drugs were dissolved in 100% DMSO. Muscimol hydrobromide, naltrexone hydrochloride, and biocytin were purchased from Sigma-Aldrich (St. Louis, MO) and dissolved in isotonic saline.
2.2. Avidin- and streptavidin-binding activity of CC44
A biotin quantitation assay kit was used to confirm the biotin-like (streptavidin-binding) properties of CC44 (FluoReporter Biotin Quantitation Assay Kit, F30751, Life Technologies, Grand Island, NY). The assay was performed according to kit instructions in opaque 96-well microplates with a fluorescence plate reader. Briefly, biotinylated reagents (biocytin, CC44, or phosphate-buffered saline vehicle, 25 μl/well) were added to streptavidin solution (or saline blank, 25 μl/well) in microplates. Following a 5 min room temperature incubation, kit components were added (including biotective fluorescent green reagent, 50 μl/well, total volume of 100 μl/well), and fluorescence was measured on a Victor X3 Multilabel Plate Reader (excitation: 485 nm; emission: 535 nm). Data are reported as relative fluorescence units without background subtracted.
2.3. Animals
Male Sprague-Dawley rats (220–360 g) or male Swiss-Webster mice (20–40 g) were housed in groups of two to five until surgery, after which they were housed singly. Animals were maintained on a 12-h light/dark cycle (on 0700 off 1900) with food and water provided ad libitum. All animals were supplied by Taconic Farms, Germantown, NY. Animal experiments were approved by the Institutional Animal Care and Use Committee of Albany Medical College. As described below, animals were subjected to one or more procedures in order to administer drugs and to assess the effects on nociceptive scores. In all cases, subjects were only used for a single experiment.
2.4. Cannulations for brain microinjections
Prior to antinociceptive drug testing, anesthetized animals (pentobarbital 50 mg/kg, i.p.; supplemented with isoflurane) were positioned in a stereotaxic apparatus and the skull exposed. For rats, a single guide cannula (Crane and Glick, 1979) was implanted into either the left lateral ventricle (i.c.v.) or the RVM and anchored to the skull using three stainless steel screws and dental cement. Stereotaxic coordinates, in mm from bregma (Paxinos and Watson, 1998), were (i.c.v.): AP −0.8, ML +1.5, and DV −4.3; or (RVM): AP −11.0, ML 0.0, and DV −7.5. For mice, i.c.v. cannula placement was performed as described (Conroy et al., 2010). Mouse coordinates, in mm from bregma (Paxinos and Franklin, 2001), were: AP −0.5, ML +1.0, and DV −2.0 mm. Nociceptive testing occurred at least 6 days after cannulation surgery.
2.5. Nociceptive testing
Thermal nociceptive responses were assessed in rats with a combination of the hot plate (Eddy and Leimbach, 1953) and tail flick (D’Amour and Smith, 1941) tests. For the hot plate test, rats were placed on a 52°C metal surface, and the latency to hind paw lift or lick was measured with a maximal (cut-off) exposure of 60 s; baseline latencies were 10 to 15 s. For tail flick testing, the ventral surface of the tail was exposed to radiant heat, and the latency to movement of the tail was recorded. Baseline latencies were 3 to 4 s, with a maximal exposure of 15 s. The heat sources were not adjusted for individual animals. At the beginning of all drug testing experiments, baseline latencies were measured in rats with a single hot plate test, followed by three tail flick tests at one min intervals, with the third measurement taken as baseline latency. In mice, thermal nociception was tested with the hot water tail immersion assay (Sewell and Spencer, 1976). Mice were restrained in a conical polypropylene tube, the tail was immersed 2–3 cm in a 55°C water bath, and latency to sudden tail movement or tail removal was recorded, with a cutoff latency of 10 s. A single baseline test was made in mice.
Mechanical nociception was separately assessed in rats with the tail pinch test (Bianchi and Franceshini, 1954). Rats were placed in a clean cage and an alligator clip (Fine Science Tools, Foster City, CA) with spring tension equal to 625 g was applied 2.5 cm from the tip of the tail. The latency to vocalize, or to flick or bite the tail was recorded. Baseline latencies were 15 to 25 s. The clip was removed as soon as a response occurred or after a maximal exposure of 90 s. A single baseline test was made in each subject.
2.6. Drug treatment and testing
Following baseline testing, animals were gently secured in a laboratory pad and received i.c.v., RVM, or systemic drug injections. For i.c.v. and RVM injections, the stylet was removed from the guide cannula and a 32 g injection cannula inserted. I.c.v. and RVM cannulas extended 1 mm or 2 mm, respectively, below the tip of the guide cannula. Except where specified otherwise, single injections were made in total volumes of 5 μl over 5 min (rat i.c.v.), 2 μl over one min (mouse i.c.v.), or 0.5 μl over one min (rat RVM). One min after the end of the injection, wire cutters were used to cut and seal the injection cannula. To study the effects of i.c.v. pretreatments with muscimol, rimonabant, or biocytin, subjects first received an i.c.v. injection of one of these agents, followed by post-testing, and a second i.c.v. injection of CC44, as described in the figure legends. The success of injections was assured by following the movement of a small air bubble in the tubing between the cannula and syringe. If leakage was observed during injections, or following removal of the first injection cannula, the animal was excluded from the study. Subjects were observed in their home cages following drug administration for the presence of normal locomotion and the absence of any abnormal movement or tremor.
For mouse systemic experiments, subjects were baseline tested (tail immersion), restrained in a laboratory pad and received s.c. injections into the dorsal neck or flank, followed by re-testing at the specified intervals. In mouse dual treatment experiments, following baseline testing, subjects were injected with biocytin or saline (s.c. flank), post-tested at 30 min, injected with CC44 (s.c. neck), and re-tested 30, 60, and 90 min later. For all studies, each animal was only used in a single experiment. After testing, animals were euthanized with CO2 (mice) or pentobarbital sodium (rats, 100 mg/kg, i.p.). Cannula placements for brain injections were histologically verified by injection of India Ink.
2.7. Spinal nerve ligation (SNL)
In addition to nociceptive testing with mechanical and thermal stimuli in normal animals (Figs. 3 – 5), the effects of CC44 were studied in a model of neuropathic pain employing SNL. Rats were anesthetized and unilateral tight ligations of the L5 and L6 spinal nerves were performed (Kim and Chung, 1992; Lee et al., 2003). A midline incision was made over the lumbar area, and the back musculature was opened to reveal the L6 transverse process. This process was removed, and the L4, L5, and L6 spinal nerves were exposed using a pulled glass hook. The L5 and L6 spinal nerves were tightly ligated, and the L4 nerve root was gently irritated by light rubbing and stretching using the glass hook. The deep back musculature was sutured, the wound was closed with clips, and antibacterial ointment was applied. Animals were observed daily for general health and allowed to recover for at least 5 days prior to nociceptive testing. Animals displaying mechanical allodynia (i.e. von Frey scores less than 6 g; see below) after SNL surgery were subjected to a second surgery for insertion of an i.c.v. guide cannula (section 2.4). A minimum of 7 days elapsed between SNL surgery and i.c.v. cannulation. At least 14 days elapsed between SNL surgery and drug testing.
Fig. 3.

Antinociceptive activity of CC44. Subjects were tested (baseline [BL]), received an i.c.v. injection of drug or vehicle, and were then re-tested at the post-i.c.v. times shown (abscissa). Thermal (A, B, tail flick; C, tail immersion) or mechanical (D, tail pinch) nociceptive latencies (s, mean ± S.E.M.) are shown as left ordinate values. (A) Time course of antinociceptive effect for specified doses of CC44 in rats. (B) Dose-response curve for CC44 (data from Fig. 3A, filled squares) and improgan (triangles, n=3 to 8 per dose) at 5 min post-i.c.v. injection. Abscissa is dose of drug (nmol, log scale). Unfilled squares show the percentage (right ordinate) of rats presenting with motor side effects (head tremor or hind limb weakness) following the specified dose of CC44. (C) Time course of antinociceptive effect of CC44 (dissolved in saline) on the tail immersion test in mice. (D) Time course of antinociceptive effect for CC44 (77 nmol) on tail pinch test in rats. For all plots, bracketed values represent number of animals per group. *,**P < 0.05, 0.01 vs. vehicle, respectively.
Fig. 5.
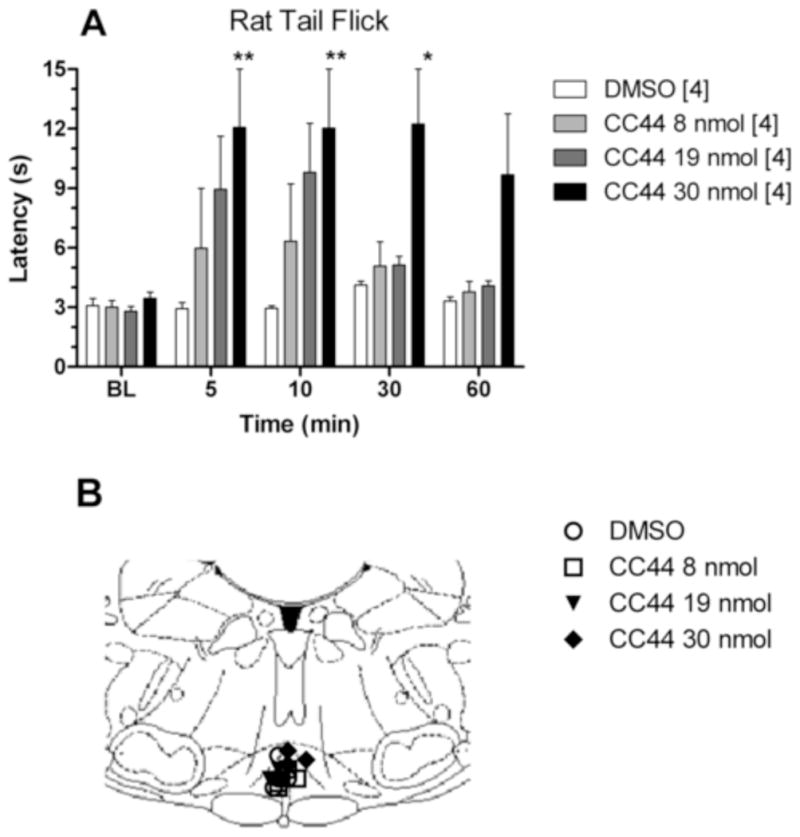
Antinociceptive effects of CC44 microinjected into the RVM. Rats were tested (baseline [BL]), received an RVM injection of drug or vehicle (100% DMSO), and were then re-tested at post-injection times shown (A; abscissa). Tail flick latencies (s, mean ± S.EM, n in brackets) are shown as ordinate values. (A) Time course of antinociceptive effect for RVM-administered CC44. *,**P < 0.05, 0.01 vs. vehicle, respectively. (B) Locations for intracerebral microinjections in the RVM from the experiment in Fig. 5A are depicted in the AP coronal plane (Paxinos and Watson, 1998); example is 11.0 mm posterior to bregma).
2.8. Mechanical allodynia testing
SNL rats were placed on a raised wire mesh floor in a Plexiglass cylinder, allowed to acclimate for five min, and baseline tested. Mechanical sensitivity (up to 15 g) was assessed with von Frey filaments (Stoelting, Inc., Wood Dale, IL) applied to the rat hind paw plantar surface following the “up-down method” (Chaplan, Bach et al., 1994). The presence of allodynia in post-SNL rats was defined as a mechanical threshold below 6 g. Eighty-four percent of surgical subjects displayed allodynia. Of these, 75% remained allodynic after the i.c.v. surgery. Animals exhibiting allodynia after both SNL and i.c.v. surgeries (63% overall) were used for testing with CC44. Following baseline testing, rats received i.c.v. injections as described and were tested at specified intervals (Fig. 7). Cannula placements were verified as described.
Fig. 7.
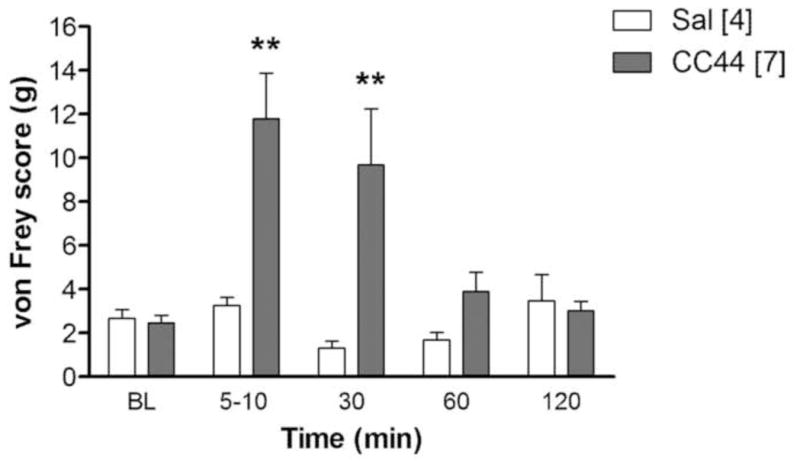
Anti-allodynic effects of CC44. SNL, i.c.v.-cannulated rats were baseline tested [BL] with von Frey filament pressure (ordinate, g), received CC44 (77 nmol, i.c.v.) or saline (Sal), and were re-tested at the times specified (abscissa). Values are mean ± S.E.M. (n in brackets). **P < 0.01 vs. saline.
2.9. Statistical analysis
Nociceptive data are expressed as latencies (s, mean ± S.E.M.). Data from mechanical allodynia testing are given as force equated to mass (g, mean ± S.E.M.). Data were subjected to analysis of variance (ANOVA) with repeated measures along with Bonferroni post-hoc testing where appropriate (Prism 5.0, GraphPad Software Inc., San Diego, CA). In all cases, a resultant P value less than 0.05 was deemed significant. Dose-response data were analyzed by non-linear regression (sigmoidal dose-response curves with variable slope, Prism 5.0). Tops of all curves were constrained to cutoff latencies. Converged fits gave ED50 values along with 95% confidence intervals.
3. Results
3.1. Avidin/streptavidin binding of CC44
The avidin-binding properties of CC44 were confirmed with a sensitive, quantitative biotin assay (Fig. 2). Levels of avidin-bound biotin increased proportionately with amounts of CC44 added (0 to 40 pmol). Co-incubation of CC44 with free streptavidin (5 pmol or 20 pmol/well) attenuated the formation of avidin-bound CC44, confirming CC44’s affinity for both avidin and streptavidin. As expected, the assay of biocytin (biotinyl-L-lysine, included as a positive control) detected biotin.
Fig. 2.

Avidin/streptavidin binding properties of CC44. Increasing concentrations (abscissa) of biocytin (filled triangles) or CC44 (open circles) were incubated in the presence or absence of the indicated amounts of streptavidin (SA) in 96-well plates and the samples processed with a biotin-detection kit. Biotin-containing substances were detected by fluorescence (ordinate, RFU). Duplicates from a single experiment are shown.
3.2. Antinociceptive activity of CC44
In rats, i.c.v. administration of CC44 (10–155 nmol) attenuated thermal nociceptive responses on the tail flick (Fig. 3A) and hot plate (not shown) tests. Maximal antinociception occurred 5–10 min after administration. Latencies tended to return toward baseline levels 30 min later. Analysis of variance (ANOVA) of Fig. 3A (between groups: dose of CC44; within groups: time) found significant main effects for dose of drug (F4, 20 = 17.8, P < 0.0001) and time (F3, 60 = 26.0, P < 0.0001); and a significant dose-by-time interaction (F12, 60 = 6.9, P <0.0001). Analysis of the five-min tail flick data revealed dose-related antinociception (Fig. 3B), with an ED50 of 42.2 nmol for CC44. By way of comparison, improgan produced dose-dependent antinociception on the tail flick, with an estimated ED50 of 234 nmol (Fig. 3B). The same figure shows behavioral/motor toxicity of CC44 assessed over a range of doses. Three samples of CC44 were synthesized and provided for testing (Lots 1 – 3). Initial experiments with Lot 1 of the compound found no discernible motor or behavioral abnormalities with CC44 at doses between 10 – 77 nmol. After the highest dose (155 nmol, a supra-maximal analgesic dose), two out of six rats showed head tremor and hind limb weakness. With subsequent lots of CC44, occasional mild motor effects at the 77 nmol dose were observed (not shown). In mice, CC44 (8.5 and 17 nmol, i.c.v.) caused dose-related thermal antinociception (Fig. 3C). Maximal effects were observed 10–20 min after injection. Significant main effects (between groups: dose of CC44; within groups: time) were observed for drug dose (F2, 14 = 3.78, P < 0.05), time (F4, 56 = 18.3, P < 0.0001), and dose-by-time interaction (F8, 56 = 3.90, P < 0.01). Tail immersion latencies returned to baseline 60 min post-injection, with no behavioral or motor abnormalities noted in mice. Finally, in rats, CC44 (77 nmol, i.c.v.) produced mechanical antinociception (Fig. 3D). Similar to the results with the thermal testing, large antinociceptive effects were observed at 5 and 10 min after administration; these effects diminished by 30 min. ANOVA of Fig. 3D (between groups: CC44; within groups: time) found significant main effects for treatment (F1, 6 = 10.4, P < 0.05); time (F3, 18 = 13.9, P < 0.0001); and a significant treatment-by-time interaction (F3, 18 = 8.13, P < 0.01). Mechanical antinociception was not tested in mice.
3.3. Pharmacological characteristics of CC44 antinociception
The effects of naltrexone, muscimol, rimonabant, and biocytin were determined on CC44 (i.c.v.) antinociception in rats. Systemic pretreatment with a large dose of naltrexone (5 mg/kg, i.p.) had no effect on CC44 (77 nmol) antinociception (Fig. 4A). In contrast, pretreatment with muscimol (500 ng, i.c.v.) almost completely antagonized CC44 (77 nmol) antinociception (Fig. 4B). ANOVA of Fig. 4B (between groups: muscimol; within groups: time) found significant effects for: treatment (F1, 10 = 11.8, P < 0.01); time (F4, 40 = 16.2, P < 0.0001); and treatment-by-time interaction (F4, 40 = 6.08, P < 0.001). Pretreatment with rimonabant (50 μg, i.c.v.) did not affect CC44 (155 nmol, i.c.v.) antinociception (Fig. 4C). Biocytin (155 nmol, i.c.v.) neither caused antinociception itself, nor changed CC44 (77 nmol) antinociception (Fig. 3D). Separate ANOVAs of Figs. 4A, 4C, and 4D (experiments in which the pretreatment lacked effects) confirmed significant CC44 antinociception (time: P <0.0001, in all cases). The hot plate test, performed during experiments with naltrexone and biocytin, gave results similar to those from the tail flick (data not shown).
Fig. 4.
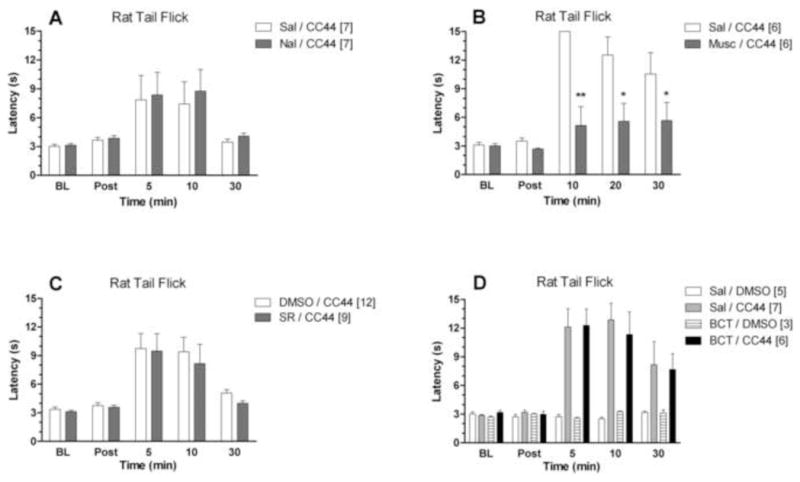
Pharmacology of CC44 antinociception in rats. Subjects were baseline (BL) tested on the tail flick test (ordinate, latencies in s, mean ± S.E.M., n in brackets), immediately received an injection of antagonist or vehicle, and were then re-tested (Post) at the specified intervals given below. Subjects then received CC44, and testing resumed at the post-CC44 times indicated (abscissa). (A) Effect of naltrexone (Nal, 5 mg/kg, i.p.) or saline vehicle (Sal) on CC44 (77 nmol, 5 μl in DMSO, i.c.v.) antinociception. Post-testing occurred 11 min after naltrexone. (B) Effect of muscimol (Musc, 500 ng, 2 μl, in saline, i.c.v.) on CC44 (77 nmol, 2 μl in DMSO, i.c.v.) antinociception. Post-testing occurred 23.5 min after muscimol. (C) Effect of rimonabant (SR, 50 μg, 2 μl in DMSO, i.c.v.) on CC44 (155 nmol, 10 μl in 60% DMSO, i.c.v.) antinociception. Post-testing occurred 5.5 min after rimonabant. (D) Effect of biocytin (BCT, 155 nmol, 2.5 μl saline, i.c.v.) on CC44 (77 nmol, 2 μl in DMSO, i.c.v.) antinociception. Post-testing occurred 5 min after biocytin *,**P < 0.05, 0.01 vs. vehicle, respectively.
3.4. Antinociceptive activity of CC44 in the RVM
In rats, RVM injections of CC44 (8–30 nmol) produced dose-dependent antinociception on both the tail flick (Fig. 5A) and hot plate (not shown) tests. Vehicle injections produced no such effects. As with i.c.v. administration, maximal antinociception occurred 5–10 min after injection. The effects of the 8 nmol and 19 nmol doses were fully reversed by 30 and 60 min. Following administration of the 19 nmol and 30 nmol doses, 50% of rats demonstrated mild head tremor and hind limb weakness, similar to the effects reported following the highest doses of i.c.v. CC44. ANOVA of Fig. 5A (between groups: dose of CC44; within groups: time) found significant main effects for dose (F3, 12 = 6.30, P < 0.01) and time (F4, 48 = 5.59, P < 0.001). RVM cannula placements, ranging from 10.5 to 11.3 mm posterior to bregma, are shown in Fig. 5B.
3.5. Assessment of antinociceptive activity of CC46 and CC47
CC46 (a CC44 congener lacking the imidazole group, Fig. 1) and CC47 (a CC44 congener lacking the biotin moiety, Fig. 1) were tested in rats. CC46 (155 nmol, i.c.v.) did not produce antinociception on either tail flick (Fig. 6) or hot plate (data not shown) tests, and also showed no abnormal motor effects. However, CC47 (155 nmol, i.c.v.) resulted in robust tail flick (Fig. 6) and hot plate (not shown) antinociception, which reversed gradually after 5 min. ANOVA of Fig. 6 (between groups: CC47; within groups: time) found significant main effects for: treatment (F1, 7 = 34.9, P < 0.001); time (F3, 21 = 7.1, P < 0.01); and treatment-by-time interaction (F3, 21 = 7.52, P < 0.01). Following CC47 administration, three out of four rats tested at the 155 nmol dose exhibited modest hind limb weakness and head tremor. Higher doses of CC47 (310 nmol and 620 nmol, 1 rat per group) gave motor rigidity and tremor which prevented reliable nociceptive testing.
Fig. 6.

Antinociceptive activity assays for CC46 and CC47. Rats were tested (baseline [BL]), received an i.c.v. injection of 155 nmol drug or vehicle (100% DMSO), and were then re-tested at the post-i.c.v. times shown (abscissa). Tail flick nociceptive latencies (ordinate, s, mean ± S.E.M., n in brackets) are shown. Data for the vehicle group are taken from Fig. 3A. **P < 0.01 vs. DMSO.
3.6. Effect of CC44 on mechanical allodynia
CC44 was tested in SNL rats to determine the effect of this drug on mechanical allodynia. Following an i.c.v. dose of 77 nmol, CC44 almost completely reversed allodynic responses up to 30 min after administration (Fig. 7). ANOVA of Fig. 7 (between groups:CC44; within groups: time) found significant effects for: treatment (F1, 9 = 5.8, P < 0.05); time (F4, 36 = 6.4, P <0.001); and treatment-by-time interaction (F4, 36 = 7.0, P < 0.001).
3.7. Assessment of systemic activity of CC44 in mice
To determine if CC44 has brain-penetrating characteristics, mice received CC44 (171 μmol/kg, s.c. neck) and were tested with the tail immersion nociceptive assay. At 20, 40, and 60 min after administration, this treatment did not significantly alter nociceptive latencies, and did not produce observable behavioral or motor side effects (n = 3, data not shown). Finally, there were no nociceptive latency/behavioral changes 30 min following pretreatment with biocytin (268 μmol/kg, s.c. flank), or 30–90 min following subsequent treatment with CC44 (171 μmol/kg, s.c. neck) (n = 3, data not shown). Mice (and not rats) were used in the systemic dosing studies with CC44 due to the limited amount of drug available.
4. Discussion
Significant information on the analgesic activity of improgan has been amassed (Hough et al., 2000a; Hough et al., 2001a; Heinricher et al., 2010). Robust antinociception is evident after administration into the lateral ventricle, the periaqueductal gray, or the RVM (Nalwalk et al., 2004). Recent studies found that improgan (i.c.v.) stimulates firing of pain-relieving, RVM OFF-cells, with subsequent activation of descending analgesic circuits (Heinricher et al., 2010). These and other findings led to an explicit model for improgan antinociception (Heinricher et al., 2010). Direct activation of RVM OFF-cells via an undiscovered receptor was proposed to evoke endocannabinoid-mediated retrograde inhibition of pre-synaptic GABA terminals. Withdrawal of GABA tone would then lead to further OFF-cell excitation. The model is consistent with the cannabinoid, but not opioid, character of this analgesia, and the lack of direct actions on known cannabinoid receptors (Gehani, et al., 2007). Finally, a cytochrome P450-epoxygenase mechanism has been proposed to mediate the improgan-activated endocannabinoid effects on GABA terminals within the RVM (Heinricher et al., 2010; Hough et al., 2011). A brain stem epoxygenase mechanism was also recently proposed for opioid analgesics (Conroy et al., 2010). Although activation of OFF-cells is thought to mediate acute analgesic properties, improgan (i.c.v.) also suppresses the firing of pain-enhancing RVM ON-cells, which may account for the attenuation of neuropathic allodynia by this drug (Heinricher et al., 2010; Albrecht et al., 2011).
The lack of brain penetration by improgan and failure to identify this receptor have limited the progress of this research. Development of congeners that help solve one or both of these problems is of significant interest. CC44 is a PEGylated, biotinylated improgan congener with the potential to help discover the receptor, improve drug potency, and/or enhance brain penetration. The goals of the present study were to examine the analgesic properties of the prototype CC44, and to compare them with those of improgan.
For CC44 to function as a receptor probe, the compound should bind to relevant targets and either mimic or block improgan analgesia. Like improgan, CC44 was active on four tests in rats, and one test in mice. CC44 also mirrored improgan in two out of three pharmacological tests. Likewise, both drugs are active following intra-RVM microinjection. Finally, CC44 is five times more potent than improgan. Thus, CC44 may serve as a new research tool, but could also function as an important lead in analgesic drug development.
DMSO remains in wide use to dissolve highly lipid soluble drugs for brain administration (e.g. Bellocchio et al., 2013; Rashidy-Pour et al., 2013), but results showing that this solvent can alter the analgesic activity of intracerebral morphine (Fossum et al., 2008) suggest caution in this use of DMSO. Presently, DMSO was used to dissolve CC44 in some experiments, but it is unlikely that use of this solvent invalidated the present conclusions. For example, published (Hough et al., 2009 vs. Nalwalk et al., 2004; Hough et al., 2002 vs. Hough et al., 2006) and unpublished studies from our lab found no differences in analgesic potency for improgan dissolved in saline as compared with DMSO. In addition, CC44 (i.c.v.) produced antinociception dissolved in DMSO (rats, Fig. 3B), or in saline (mice, Fig. 3C). CC44’s solubility in saline was sufficient for i.c.v. testing in mice, but not in rats.
The antinociceptive characteristics of CC44 revealed presently bear strong resemblance to those of improgan. Thus, the effects of both compounds are resistant to opioid antagonists, (indicating a non-opioid mechanism, [Hough et al., 2000b]), but strongly inhibited by pretreatment with the GABAA agonist muscimol, consistent with excitatory analgesic mechanisms for these drugs. (Hough et al., 2001b). The dose of muscimol (500 ng, i.c.v.) used presently is active against improgan (Hough et al., 2001b) and morphine (Mantegazza et al., 1979). Furthermore, like improgan (Nalwalk et al., 2004), CC44 produces antinociception following intra-RVM administration, suggesting that RVM OFF-cells may mediate the acute analgesic properties of CC44 (Heinricher et al., 2010). I.c.v. improgan also suppresses the firing of pain-enhancing RVM ON-cells (Heinricher et al., 2010), an effect suggested to account for the attenuation of SNL allodynia by this drug (Albrecht et al., 2011). CC44’s activity in the same model suggests a similar mechanism, but electrophysiological studies are needed to validate this hypothesis.
Inhibition of improgan antinociception by the cannabinoid antagonist/inverse agonist rimonabant supports the proposed endocannabinoid mechanism for this compound (Hough et al., 2002; Nalwalk et al., 2006; Gehani et al., 2007; Heinricher et al., 2010). However, the present results showed that CC44 antinociception (155 nmol) is not blocked by pretreatment with rimonabant. Parameters for this CC44 experiment (vehicles, volumes, pre-injection intervals, and dose of antagonist) were identical with those previously used with improgan. Rimonabant also failed to antagonize the effects of a lower dose (77 nmol) of CC44 (pilot data not shown). The most parsimonious explanation for these results is that improgan and CC44 produce analgesia through multiple, non-opioid mechanisms. Alternatively, both drugs could target the same receptor, but use differential analgesic transduction mechanisms, a possibility consistent with the theory of functional selectivity (Urban et al., 2007). Either possibility requires substantial, additional study.
Biotin-related transporters exist in the CNS (Park and Sinko, 2005) and participate in the brain penetration by some compounds (Spector and Mock, 1988). Because biotinylated drugs can be substrates for these transporters (Janoria et al., 2009), we investigated their significance in CC44 action. The finding that i.c.v. pretreatment with biocytin had no effect on CC44 antinociception suggests that these transporters do not participate in CC44 action. The lack of antinociceptive effects of biocytin alone further implies that biotin-containing amides in general do not attenuate thermal nociception. The notable antinociceptive activity of CC47 (a CC44-like congener lacking the biotin tail) suggests that the biotin tail is not needed for CC44-like antinociceptive activity.
The present results show that CC44 is 5 to 8.5-fold more potent than improgan on the rat tail flick test (Hough et al., 2006), the mouse tail immersion test (Hough et al., 2002), and on rat anti-allodynic activity (Albrecht et al., 2011). Similarly, intra-RVM injections of the two drugs in rats found that 30 nmol of CC44 gave responses equivalent to 145 nmol of improgan (Nalwalk et al., 2004), a 4.8 fold difference. CC44’s greater potency could result from greater tissue penetration, or from enhanced potency at CNS drug targets. Chemically, CC44’s higher potency could result from the longer N-alkyl chain compared to that of improgan. Increasing the chain length of improgan’s propylene linker (to 4 or more carbons) led to substantial increases in analgesic potency (Hough et al., 2006). Alternatively, increased potency could arise from the presence of the PEG and/or amide moieties. PEGylation has been used to prolong circulation times of drug-conjugated liposomes and to increase water solubility of drugs (Pasut and Veronese, 2007).
Improgan does not penetrate the blood-brain barrier, but the incorporation of PEG- and biotin-containing groups into CC44 could possibly have improved brain penetration. Since CC44 was fully active after i.c.v. administration in mice, the lack of activity of systemically-administered CC44 in these subjects suggests negligible brain penetration by this drug. Insufficient amounts of available drug prevented systemic dosing studies in rats. In the systemic experiment, plasma CC44 might have been rapidly degraded by plasma biotinidase (Zempleni et al., 2009). However, following inhibition of biotinidase by a large dose of biocytin, systemically-administered CC44 remained inactive, excluding a role for this enzyme. Since the water-soluble biotin tail of CC44 seems not to be required for activity (see CC47), incorporation of lipophilic substituents in the place of biotin could lead to potent, brain-penetrating analgesics.
Since improgan and CC44 both contain imidazole, CC46 was synthesized to resemble CC44 but lacking the imidazolyl group. The finding that CC46 has no antinociceptive activity implies that an imidazolyl-like group is necessary for activity. Such a result is not surprising, since this group, or an isostere, is required for histamine H2 antagonism (Cooper et al., 1990), histamine H3 antagonism (Bongers et al., 2010), and improgan-like analgesia (Hough et al., 2007). Further studies are needed to establish the nature of such requirements for CC44-like drugs.
The present findings show that CC44 stimulates CNS pain-relieving circuits, but the receptors for improgan and CC44 remain unknown. CC44 may be an important new tool for discovering these unknown analgesic receptors, since this new compound is a potent analgesic which contains biotin. As mentioned, biotinylated forms of several drugs have been used to identify receptor/binding partners for these substances (see Introduction). For example, incubations of CC44 with brain membrane fractions, followed by the sensitive detection of protein-bound drug (with labeled or tethered streptavidin, see Green, 1990) could lead to the identification of CC44 receptors. The present results, which confirm the detection of very low levels of CC44 by avidin binding techniques, show the feasibility of such binding studies. In vitro exploration of the membrane-binding properties of CC44 and related congeners are currently in progress.
Supplementary Material
Acknowledgments
We thank Rachel Cleary for excellent technical assistance. This work was supported by U.S. National Institutes of Health Grants (DA03816, DA027835, LBH). The authors declare no competing financial interests.
References
- Albrecht PJ, Nalwalk JW, Hough LB. Efficacy of improgan, a non-opioid analgesic, in neuropathic pain. Brain Res. 2011;1424:32–37. doi: 10.1016/j.brainres.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannoura MD, Nalwalk JW, Tang Y, Carlile M, Leurs R, Menge WMPB, Timmerman H, Hough LB. Absence of antinociceptive tolerance to improgan, a cimetidine analog, in rats. Brain Res. 1998;814:218–221. doi: 10.1016/s0006-8993(98)01024-5. [DOI] [PubMed] [Google Scholar]
- Basbaum AI, Fields HL. Endogenous pain control systems: Brainstem spinal pathways and endorphin circuitry. Annu Rev Neurosci. 1984;7:309–338. doi: 10.1146/annurev.ne.07.030184.001521. [DOI] [PubMed] [Google Scholar]
- Bellocchio L, Soria-Gomez E, Quarta C, Metna-Laurent M, Cardinal P, Binder E, Cannich A, Delamarre A, Haring M, Martin-Fontecha M, Vega D, Leste-Lasserre T, Bartsch D, Monory K, Lutz B, Chaouloff F, Pagotto U, Guzman M, Cota D, Marsicano G. Activation of the sympathetic nervous system mediates hypophagic and anxiety-like effects of CB(1) receptor blockade. Proc Natl Acad Sci USA. 2013;110:4786–4791. doi: 10.1073/pnas.1218573110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi C, Franceshini J. Experimental observations on Haffner’s method for testing analgesic drugs. Br J Pharmacol. 1954;9:280–284. doi: 10.1111/j.1476-5381.1954.tb01681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bongers G, De Esch IJ, Leurs R. Molecular pharmacology of the four histamine receptors. Adv Exp Med Biol. 2010;709:11–19. doi: 10.1007/978-1-4419-8056-4_2. [DOI] [PubMed] [Google Scholar]
- Calvo P, Gouritin B, Chacun H, Desmaele D, D’Angelo J, Noel JP, Georgin D, Fattal E, Andreux JP, Couvreur P. Long-circulating PEGylated polycyanoacrylate nanoparticles as new drug carrier for brain delivery. Pharm Res. 2001;18:1157–1166. doi: 10.1023/a:1010931127745. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Conroy JL, Fang C, Gu J, Zeitlin SO, Yang W, VanAlstine MA, Nalwalk JW, Albrecht PJ, Mazurkiewicz JE, Snyder-Keller A, Shan Z, Zhang S, Wentland MP, Behr M, Knapp BI, Bidlack JM, Zuiderveld OP, Leurs R, Ding X, Hough LB. Opioids activate brain analgesic circuits through cytochrome P450/epoxygenase signaling. Nature Neuroscience. 2010;13:284–286. doi: 10.1038/nn.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper DG, Young RC, Durant GJ, Ganellin CR. Histamine receptors. Comp Med Chem. 1990;3:323–421. [Google Scholar]
- Crane LA, Glick SD. Simple cannula for repeated intracerebral drug administration in rats. Pharmacol Biochem Behav. 1979;10:799–800. doi: 10.1016/0091-3057(79)90336-8. [DOI] [PubMed] [Google Scholar]
- D’Amour FE, Smith DL. A method for determining loss of pain sensation. J Pharmacol Exp Ther. 1941;72:74–79. [Google Scholar]
- Eddy NB, Leimbach D. Synthetic analgesics, II Dithienylbutenyl and dithienylbutylamines. J Pharmacol Exp Ther. 1953;107:385–393. [PubMed] [Google Scholar]
- Fossum EN, Lisowski MJ, Macey TA, Ingram SL, Morgan MM. Microinjection of the vehicle dimethyl sulfoxide (DMSO) into the periaqueductal gray modulates morphine antinociception. Brain Res. 2008;1204:53–58. doi: 10.1016/j.brainres.2008.02.022. [DOI] [PubMed] [Google Scholar]
- Gehani NC, Nalwalk JW, Razdan RK, Martin BR, Sun X, Wentland M, Abood ME, Hough LB. Significance of cannabinoid CB1 receptors in improgan antinociception. J Pain. 2007;8:850–860. doi: 10.1016/j.jpain.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green NM. Avidin and streptavidin. Methods Enzymol. 1990;184:51–67. doi: 10.1016/0076-6879(90)84259-j. [DOI] [PubMed] [Google Scholar]
- Harris EN, Weigel PH. The ligand-binding profile of HARE: hyaluronan and chondroitin sulfates A, C, and D bind to overlapping sites distinct from the sites for heparin, acetylated low-density lipoprotein, dermatan sulfate, and CS-E. Glycobiology. 2008;18:638–648. doi: 10.1093/glycob/cwn045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinricher MM, Maire JJ, Lee D, Nalwalk JW, Hough LB. Physiological basis for inhibition of morphine and improgan antinociception by CC12, a P450 epoxygenase inhibitor. J Neurophysiol. 2010;104:3222–3230. doi: 10.1152/jn.00681.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinricher MM, Martenson ME, Nalwalk JW, Hough LB. Neural basis for improgan antinociception. Neurosci. 2010;169:1414–1420. doi: 10.1016/j.neuroscience.2010.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hough LB, De Esch IJ, Janssen E, Phillips J, Svokos K, Kern B, Trachler J, Abood ME, Leurs R, Nalwalk JW. Antinociceptive activity of chemical congeners of improgan: Optimization of side chain length leads to the discovery of a new, potent, non-opioid analgesic. Neuropharmacology. 2006;51:447–456. doi: 10.1016/j.neuropharm.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Hough LB, Menge WM, van de Stolpe AC, Nalwalk JW, Leurs R, De Esch IJ. Antinociceptive activity of furan-containing congeners of improgan and ranitidine. Bioorg Med Chem Lett. 2007;17:5715–5719. doi: 10.1016/j.bmcl.2007.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hough LB, Nalwalk JW, Barnes WG, Leurs R, Menge WM, Timmerman H. A Third Legacy for Burimamide: Discovery and Characterization of Improgan and a New Class of Non-Opioid Analgesics Derived from Histamine Antagonists. In: Watanabe T, Timmerman H, Yanai K, editors. Histamine Research in the New Millenium. Elsevier; Amsterdam: 2001a. pp. 237–242. [Google Scholar]
- Hough LB, Nalwalk JW, Barnes WG, Warner LM, Leurs R, Menge WMPB, Timmerman H, Wentland M. A Third Life for Burimamide: Discovery and Characterization of a Novel Class of Non-Opioid Analgesics Derived from Histamine Antagonists. In: Glick SD, Maisonneuve IM, editors. New Medications for Drug Abuse. New York Acad. Sci; New York: 2000a. pp. 25–40. [DOI] [PubMed] [Google Scholar]
- Hough LB, Nalwalk JW, Chen Y, Schuller A, Zhu Y, Zhang J, Menge WMPB, Leurs R, Timmerman H, Pintar JE. Improgan, a cimetidine analog, induces morphine-like antinociception in opioid receptor-knockout mice. Brain Res. 2000b;880:102–108. doi: 10.1016/s0006-8993(00)02776-1. [DOI] [PubMed] [Google Scholar]
- Hough LB, Nalwalk JW, Menge WM, Leurs R, Timmerman H. Significance of GABAergic systems in the action of improgan, a non-opioid analgesic. Life Sci. 2001b;68:2751–2757. doi: 10.1016/s0024-3205(01)01080-3. [DOI] [PubMed] [Google Scholar]
- Hough LB, Nalwalk JW, Stadel R, Timmerman H, Leurs R, Paria BC, Wang X, Dey SK. Inhibition of improgan antinociception by the cannabinoid (CB)(1) antagonist N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-p yrazole-3-carboxamide (SR141716A): lack of obligatory role for endocannabinoids acting at CB(1) receptors. J Pharmacol Exp Ther. 2002;303:314–322. doi: 10.1124/jpet.102.036251. [DOI] [PubMed] [Google Scholar]
- Hough LB, Nalwalk JW, Yang J, Conroy JL, VanAlstine MA, Yang W, Gargano J, Shan Z, Zhang SZ, Wentland MP, Phillips JG, Knapp BI, Bidlack JM, Zuiderveld OP, Leurs R, Ding X. Brain P450 epoxygenase activity is required for the antinociceptive effects of improgan, a nonopioid analgesic. Pain. 2011;152:878–887. doi: 10.1016/j.pain.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hough LB, Svokos K, Nalwalk JW. Non-opioid antinociception produced by brain stem injections of improgan: significance of local, but not cross-regional, cannabinoid mechanisms. Brain Res. 2009;1247:62–70. doi: 10.1016/j.brainres.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janoria KG, Boddu SH, Wang Z, Paturi DK, Samanta S, Pal D, Mitra AK. Vitreal pharmacokinetics of biotinylated ganciclovir: role of sodium-dependent multivitamin transporter expressed on retina. J Ocul Pharmacol Ther. 2009;25:39–49. doi: 10.1089/jop.2008.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ki SW, Ishigami K, Kitahara T, Kasahara K, Yoshida M, Horinouchi S. Radicicol binds and inhibits mammalian ATP citrate lyase. J Biol Chem. 2000;275:39231–39236. doi: 10.1074/jbc.M006192200. [DOI] [PubMed] [Google Scholar]
- Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- Lan R, Liu Q, Fan P, Lin S, Fernando SR, McCallion D, Pertwee R, Makriyannis A. Structure-activity relationships of pyrazole derivatives as cannabinoid receptor antagonists. J Med Chem. 1999;42:769–776. doi: 10.1021/jm980363y. [DOI] [PubMed] [Google Scholar]
- Lee DH, Iyengar S, Lodge D. The role of uninjured nerve in spinal nerve ligated rats points to an improved animal model of neuropathic pain. Eur J Pain. 2003;7:473–479. doi: 10.1016/S1090-3801(03)00019-3. [DOI] [PubMed] [Google Scholar]
- Li BY, Nalwalk JW, Barker LA, Cumming P, Parsons ME, Hough LB. Characterization of the antinociceptive properties of cimetidine and a structural analog. J Pharmacol Exp Ther. 1996;276:500–508. [PubMed] [Google Scholar]
- Li BY, Nalwalk JW, Finkel JM, Glick SD, Hough LB. SKF92374, a cimetidine analog, produces mechanical and thermal antinociception in the absence of motor impairment. Analgesia. 1997;3:15–20. [Google Scholar]
- Mantegazza P, Tammiso R, Vicentini L, Zambotti F, Zonta N. Muscimol antagonism of morphine analgesia in rats. Br J Pharmacol. 1979;67:103–107. [PMC free article] [PubMed] [Google Scholar]
- Nalwalk JW, Svokos K, Hough LB. Cannabinoid-improgan cross-tolerance: Improgan is a cannabinomimetic analgesic lacking affinity at the cannabinoid CB(1) receptor. Eur J Pharmacol. 2006;549:79–83. doi: 10.1016/j.ejphar.2006.08.035. [DOI] [PubMed] [Google Scholar]
- Nalwalk JW, Svokos K, Taraschenko O, Leurs R, Timmerman H, Hough LB. Activation of brain stem nuclei by improgan, a non-opioid analgesic. Brain Res. 2004;1021:248–255. doi: 10.1016/j.brainres.2004.06.066. [DOI] [PubMed] [Google Scholar]
- Park S, Sinko PJ. The blood-brain barrier sodium-dependent multivitamin transporter: a molecular functional in vitro-in situ correlation. Drug Metab Dispos. 2005;33:1547–1554. doi: 10.1124/dmd.105.005231. [DOI] [PubMed] [Google Scholar]
- Pasut G, Veronese FM. Polymer-drug conjugation, recent achievements and general strategies. Progress in Polymer Science. 2007;32:933–961. [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. 2. Academic Press; San Diego: 2001. [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4. Academic Press; Sydney: 1998. [Google Scholar]
- Phillips RS, Cleary DR, Nalwalk JW, Arttamangkul S, Hough LB, Heinricher MM. Pain-facilitating medullary neurons contribute to opioid-induced respiratory depression. J Neurophysiol. 2012;108:2393–2404. doi: 10.1152/jn.00563.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashidy-Pour A, Pahlevani P, Vaziri A, Shaigani P, Zarepour L, Vafaei AA, Haghparast A. Involvement of CB1 receptors in the ventral tegmental area in the potentiation of morphine rewarding properties in acquisition but not expression in the conditioned place preference model. Behav Brain Res. 2013;247:259–267. doi: 10.1016/j.bbr.2013.03.015. [DOI] [PubMed] [Google Scholar]
- Sewell RDE, Spencer PSJ. Antinociceptive activity of narcotic agonist and partial agonist analgesics and other agents in the tail-immersion test in mice and rats. Neuropharmacol. 1976;15:683–688. doi: 10.1016/0028-3908(76)90037-x. [DOI] [PubMed] [Google Scholar]
- Spector R, Mock DM. Biotin transport and metabolism in the central nervous system. Neurochem Res. 1988;13:213–219. doi: 10.1007/BF00971535. [DOI] [PubMed] [Google Scholar]
- Stamatakis K, Sanchez-Gomez FJ, Perez-Sala D. Identification of novel protein targets for modification by 15-deoxy-Delta12,14-prostaglandin J2 in mesangial cells reveals multiple interactions with the cytoskeleton. J Am Soc Nephrol. 2006;17:89–98. doi: 10.1681/ASN.2005030329. [DOI] [PubMed] [Google Scholar]
- Stannard CF. Opioids for chronic pain: promise and pitfalls. Curr Opin Support Palliat Care. 2011;5:150–157. doi: 10.1097/SPC.0b013e3283458fbc. [DOI] [PubMed] [Google Scholar]
- Tash JS, Chakrasali R, Jakkaraj SR, Hughes J, Smith SK, Hornbaker K, Heckert LL, Ozturk SB, Hadden MK, Kinzy TG, Blagg BS, Georg GI. Gamendazole, an orally active indazole carboxylic acid male contraceptive agent, targets HSP90AB1 (HSP90BETA) and EEF1A1 (eEF1A), and stimulates Il1a transcription in rat Sertoli cells. Biol Reprod. 2008;78:1139–1152. doi: 10.1095/biolreprod.107.062679. [DOI] [PubMed] [Google Scholar]
- Urban JD, Clarke WP, von ZM, Nichols DE, Kobilka B, Weinstein H, Javitch JA, Roth BL, Christopoulos A, Sexton PM, Miller KJ, Spedding M, Mailman RB. Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther. 2007;320:1–13. doi: 10.1124/jpet.106.104463. [DOI] [PubMed] [Google Scholar]
- Yang W, Holmes BB, Gopal VR, Kishore RV, Sangras B, Yi XY, Falck JR, Campbell WB. Characterization of 14,15-epoxyeicosatrienoyl-sulfonamides as 14,15-epoxyeicosatrienoic acid agonists: use for studies of metabolism and ligand binding. J Pharmacol Exp Ther. 2007;321:1023–1031. doi: 10.1124/jpet.107.119651. [DOI] [PubMed] [Google Scholar]
- Zempleni J, Wijeratne SS, Hassan YI. Biotin. Biofactors. 2009;35:36–46. doi: 10.1002/biof.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


