Abstract
The increasing incidence of bacterial infection and the appearance of Staphylococcus aureus (S. aureus) strains that are resistant to commonly used antibiotics has made it important to develop non-antibiotic approaches for infection prevention. The aim of this study was to develop local monocyte chemoattractant protein-1 (MCP-1) and interleukin-12 p70 (IL-12 p70) therapies to prevent S. aureus infection by enhancing the recruitment and activation of macrophages, which are believed to play an important role in infection prevention as the first line of defense against invading pathogens. Nanocoating systems for MCP-1 and IL-12 p70 deliveries were prepared and their release characteristics desirable for infection prevention in open fractures were explored. Local MCP-1 therapy reduced S. aureus infection and influenced white blood cell populations, and local IL-12 p70 treatment had a more profound effect on preventing S. aureus infection. No synergistic relationship in decreasing S. aureus infection was observed when MCP-1 and IL-12 p70 treatments were combined. This reported new approach may reduce antibiotic use and antibiotic resistance.
Keywords: Infection, trauma, cell-mediated immunity, antibiotic resistance, local drug delivery
INTRODUCTION
Open fracture-associated infection is a significant clinical complication affecting millions of people annually. Due to bacterial contamination and severe soft tissue damage, open fractures usually have high infection rates: Gustilo type I – 0 to 2%, type II – 2 to 10%, type IIIA – 7%, type IIIB – 10 to 50%, and type IIIC – 25 to 50%.1 Open fracture-associated infection has also attracted military attention; more than half of the injuries in Iraq and Afghanistan are orthopaedic injuries, and 2 to 15% of combat-related extremity injuries develop osteomyelitis, despite advances in sanitation and hygiene practices.2
The current primary approach for treating open fracture-associated infection or osteomyelitis is systemic antibiotic therapy, which has drawbacks such as systemic toxicity and poor penetration into ischemic or necrotic tissues. Due to extensive use of antibiotics, more and more bacteria are becoming drug resistant, and antibiotic resistance has become a worldwide issue.3,4 Also, bacteria such as S. aureus can invade human cells including osteoblasts and leukocytes,5,6 and undergo phenotypic changes that could lead to bacterial survival within infected cells. Therefore, in today’s era of emerging multidrug-resistant bacteria and increasing numbers of infection in contaminated war and civil traumas, the development of advanced strategies other than antibiotic therapies to prevent and treat infections is important.
Macrophages constitute the primary line of innate defense against most bacterial pathogens,7,8 and they play an essential role in cell-mediated immune response against bacterial infection.7 Their role in host defense is particularly important early in the host-pathogen encounter, when specific immunity has not yet developed.9 Activated macrophages play a significant role in wound healing and damaged tissue repair,10,11 and local injection of activated macrophages significantly reduces the mortality of infected patients.12 Macrophages are found in all tissues, and they detect infectious organisms via a plethora of receptors, phagocytose them, and orchestrate an appropriate host response. To perform these functions, macrophages must be recruited to a wound site; the preferential infiltration of monocytes and macrophages into wounds likely depends on a local chemoattractant gradient that favors their infiltration.13 MCP-1 is among the most potent macrophage-recruiting chemokines,14 and is essential for monocyte and macrophage recruitment;15 mice deficient in MCP-1 had reduced wound macrophages.13 Mice lacking the MCP-1 receptor, CCR2, could not recruit macrophages to the inflamed peritoneum and were unable to clear bacterial infection.16 Meanwhile, IL-12 p70 (usually designated as IL-12), a heterodimeric cytokine (p35+p40), is a potent inducer of interferon γ (IFN-γ) production; IFN-γ can stimulate the bactericidal activity of macrophages.17 Moreover, a reduced level of IL-12 is accompanied by decreased resistance to infection.18,19 Therefore, through local application of MCP-1 and/or IL-12 p70, more macrophages may be recruited and activated and become more potent in killing bacterial pathogens thereby preventing infection.
Our objective was to develop new approaches to enhance the body’s natural defense systems to combat pathogens, thereby enhancing resistance to infection. We studied S. aureus, a prototypical gram-positive microorganism and an important cause of life-threatening bacterial infections.20 We hypothesized that local MCP-1 and IL-12 p70 therapies would lead to altered infection rates in S. aureus contaminated open fractures. We developed MCP-1 and IL-12 p70 nanocoatings on orthopaedic implants and determined their release characteristics. We also evaluated the infection rates among rats with MCP-1 and IL-12 p70 coated implants using an open fracture infection model and studied the systemic responses and gene expression in muscle tissue. In addition, the effect of a combination of MCP-1 and IL-12 p70 was investigated.
MATERIALS AND METHODS
MCP-1 and IL-12 p70 nanocoating preparation and their in vitro release
MCP-1 and IL-12 p70 nanocoatings were prepared on stainless steel Kirschner wires (K-wires) using electrostatic layer-by-layer self-assembly nanotechnology, and their release was studied (MCP-1 and IL-12 p70 quantities are shown in Table 1; for details see Supplementary Material). The thickness of the nanocoatings was examined under scanning electron microscopy (SEM) after scratches were made through the coatings. The stability of the nanocoatings was evaluated in an open fracture rat model by comparing the thickness of the coating before implantation and after explantation.
Table 1.
Amounts of MCP-1 and IL-12 p70 in the nanocoating, infection rates, and gross observations.
| Animal Group | Drugs in nanocoating | Infection Rate, % | Observation | |
|---|---|---|---|---|
| MCP-1, pg | IL-12 p70, pg | |||
| #1 | 0 | 0 | 90 | Good callus (0/10*) Fracture not healed (10/10) |
| #2 | 770±68 | 0 | 67 | Good callus (2/6) Fracture not healed (6/6) |
| #3 | 1168±172 | 0 | 83 | Good callus (1/6) Fracture not healed (6/6) |
| #4 | 621±214 | 9549±458 | 80 | Good callus (0/5^) Fracture not healed (5/5^) |
| #5 | 0 | 10608±450 | 20 | Good callus (3/5^) Well healed (3/5^) |
One animal died post-operatively.
This group was repeated, and one died at each time.
Animals and operative procedures
Approval for in vivo studies was obtained from our Institutional Animal Care and Use Committee. 36 male Sprague-Dawley rats were used. The hindlimb to be fractured was shaved. A setup (Fig. 1a) was used to produce an open midshaft femur fracture. The thigh was incised through a lateral approach, and the fracture ends were exposed. The rats were infected by injecting 100 µL of bacterial suspension containing 102 CFU/0.1mL S. aureus with a sterile pipette directly at the fracture site (Fig. 1b). The bacterial concentration resulted in 90 to 100% infection in our previous studies. The fracture was left open for one hour to mimic the “golden hour” of a trauma patient. The fracture was fixed (Figs. 1c & d) using a K-wire as an intramedullary nail. The protruding portion of the K-wire was cut off, and the incision closed (Fig. 1e). A post-operative radiograph verified placement of the K-wire and fracture fixation (Fig. 1f). After 21 days, the animals were euthanized.
Fig. 1.
(a) A custom designed setup for creating a femur fracture, (b) bacterial challenge, (c)-(d) fixation by intramedullary K-wire nailing, (e) rat after closing the incision using skin staples, and (f) a post-operative radiograph.
Quantitative culturing of tissue homogenates
S. aureus was provided by West Virginia University Hospital Clinical Laboratory; it was a clinical isolate from an operative wound infection. Infection rates were determined by culturing bone tissue homogenates.21,22 Briefly, the surgical femur was excised post mortem and, for each specimen, about 500 mg of femur was homogenized in brain heart infusion broth (BHI); the ratio was kept at 100 mg of bone to 1 mL of broth. Then 0.1 mL of homogenized broth was plated and cultured at 37°C for 48 h. Infection was defined as the presence of >2 to 5 bacterial colonies per plate (corresponding to 200 to 500 CFU/gram of tissue); the infected rats had numerous colonies.
Body weight measurements, complete blood counting, flow cytometry, and serum cytokine evaluation
Rat body weights were determined before fracture and at euthanasia. Upon euthanasia, blood samples (~ 8 mL) were collected; 0.5 mL was analyzed for complete blood cell count. 2 mL of the blood was collected for flow cytometry analysis, where cells were labeled with purified anti-rat mononuclear phagocyte antibody (1C7) followed by Cy-5 labeled goat anti-mouse IgG and FITC labeled anti-rat MHC class II (OX-6). The remaining blood sample was centrifuged twice to extract serum for ELISA tests.
Real time polymerase chain reaction (RT-PCR)
The local mRNA levels of proinflammatory cytokines (IFN-γ, tissue necrosis factor α, i.e. TNF-α, and IL-12 p40) and macrophage products (inducible nitric oxide synthase, i.e. iNOS and macrophage inflammatory protein-2, i.e. MIP-2), and MCP-1, were determined using RT-PCR on muscle tissue samples from the fracture site. The levels in MCP-1 and IL-12 p70 treated groups were compared with those of the control group (Group #1 in Table 1; Supplementary Material).
Sample size and statistical analysis
Sample size was determined using the JMP statistical software package (Cary, NC). The procedure employs an algorithm based on the Noncentral F-distribution to determine the minimum sample size needed to detect, as significant, a specified difference among treatment groups. The power analysis was conducted specifying a significance level of 0.05 and a minimum power of 0.80, assuming a one-sided alternate hypothesis (decrease in infection rate of the treatment groups). Under these conditions, a minimum of 6 rats were required for each group; a total of 36 rats were used. Experimental data were expressed as mean ± standard deviation. Statistical analysis was conducted using one-way ANOVA and compared to the control; p<0.05 was considered significant. SPSS 11.0 software was used (Chicago, IL).
RESULTS
Preparation of MCP-1 and IL-12 p70 multilayer nanocoatings and their release characteristics
Electrostatic layer-by-layer self-assembly nanotechnology was applied to form multilayer coatings on K-wires. The technology was modified to facilitate the incorporation of MCP-1 and IL-12 p70 into the coatings by introducing bovine serum albumin as a carrier protein of MCP-1 and IL-12 p70 (Fig. 2). The average thickness was 6 nm, and the coating was uniform. The nanocoating sustained the implantation process in the fracture model; 70 to 95% of the nanocoating withstood the implantation process based on thickness measurements before implantation and after explantation.
Fig. 2.
Coated K-wire with scratches. The K-wire was 1.1 mm in diameter and 63.5 mm in length. The inset shows the nanocoating and thickness; each layer was about 6 nm.
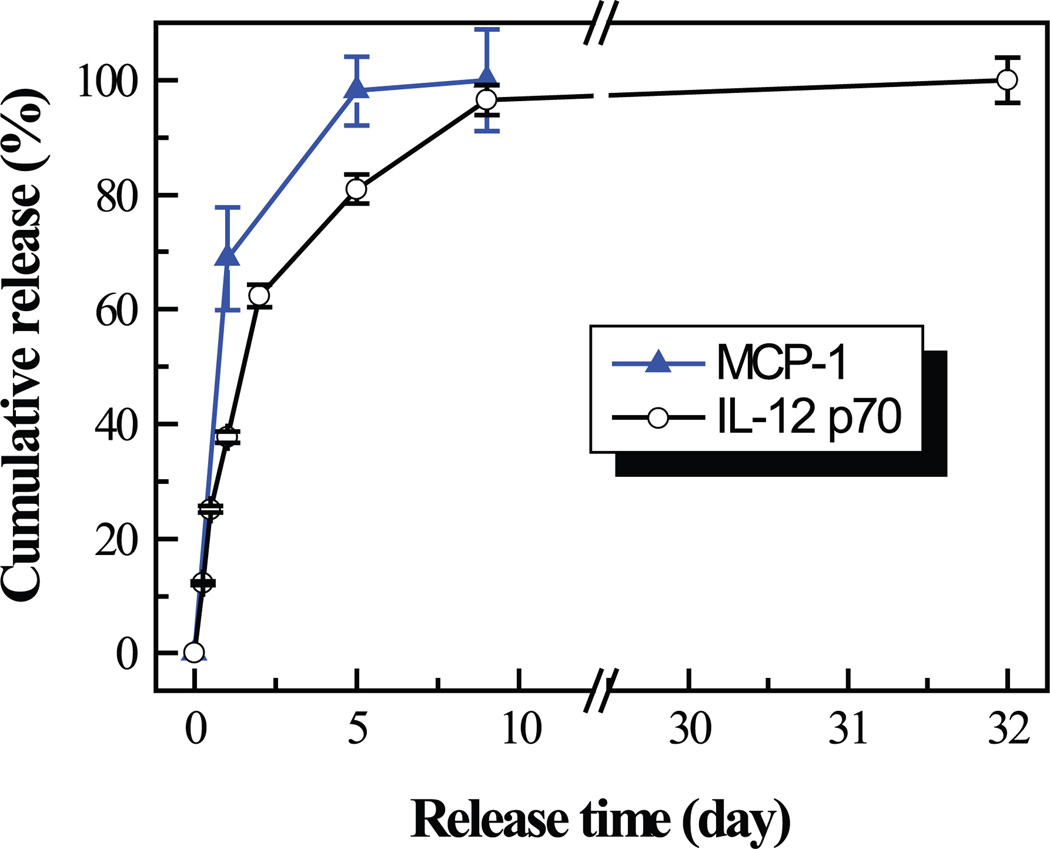
A burst release occurred in the beginning and almost all the MCP-1 and IL-12 p70 were released within 5 and 9 days, respectively (Fig. 3). MCP-1 had faster release kinetics than IL-12 p70.
Fig. 3.
Release profiles of MCP-1 and IL-12 p70 from nanocoatings on K-wires.
Infection evaluation
Local MCP-1 and IL-12 p70 applications had different impacts on prevention of open fracture-associated infection (Table 1). Compared to the control group (Group #1), MCP-1 treatments decreased infection; a relatively lower quantity of MCP-1 (Group #2 of 770 pg) decreased the infection rate from 90% to 67%, while a higher quantity (1168 pg) of MCP-1 or a combination of MCP-1 and IL-12 p70 had less impact on preventing infection, with infection rates of 83% and 80%, respectively. IL-12 p70 alone (Group #5) had a more profound effect on infection prevention, decreasing the infection rate from 90% to 20%. Gross observations of callus formation and bone healing upon animal euthanasia showed that better healing was generally observed in groups with lower infection rates and that rats treated with IL-12 p70 alone had the best callus formation.
No significant difference in weight change in rats before fracture and after sacrifice was found in Groups #1, #2, #3, and #4 (Fig. 4). Group #5 had significant weight gain at post-operative day 21.
Fig. 4.
Weight change in rats at post-operative day 21. Group #1 (control); Group #2 (MCP-1: 770 pg); Group #3 (MCP-1: 1168 pg); Group #4 (MCP-1: 621 pg; IL-12 p70: 9549 pg); Group #5 (IL-12 p70: 10608 pg). *Significantly greater than the other groups, p < 0.05.
Systemic responses and macrophage activation
No significant difference in MCP-1 or IL-12 p40 was observed in blood samples among the groups (Fig. 5), and no obvious difference in the number of white blood cells in serum was found (Fig. 6a). In contrast, the groups had different cell populations of lymphocytes, neutrophils, and monocytes (Fig. 6b). The percentage of lymphocytes was not correlated to infection rates; instead, the rats treated with IL-12 p70 alone had a similar percentage of lymphocytes as the controls, and the groups treated with MCP-1 (Groups #2, 3, and 4) had lower percentages of lymphocytes compared to the control. Correspondingly, the rats treated with IL-12 p70 alone had a similar percentage of neutrophils at post-operative day 21 as the control group, and the groups treated with MCP-1 had a higher percentage of neutrophils compared to the controls. No significant difference in monocytes was observed among the groups.
Fig. 5.
Serum concentrations of MCP-1 and IL-12 p40 in rats euthanized at post-operative day 21. Group #1 (control); Group #2 (MCP-1: 770 pg); Group #3 (MCP-1: 1168 pg); Group #4 (MCP-1: 621 pg; IL-12 p70: 9549 pg); Group #5 (IL-12 p70: 10608 pg).
Fig. 6.
(a) Total white blood cells, and (b) Percentages of the major white blood cells (neutrophils, lymphocytes, and monocytes) in blood of rats euthanized at post-operative day 21. Group #1 (control); Group #2 (MCP-1: 770 pg); Group #3 (MCP-1: 1168 pg); Group #4 (MCP-1: 621 pg; IL-12 p70: 9549 pg); Group #5 (IL-12 p70: 10608 pg). *, #Significantly different from the control group, p < 0.05.
Fig. 7 presents the macrophages and related macrophage activation at post-operative day 21. Macrophage percentages were not significantly different among the groups, while significantly higher activated macrophages were found in the IL-12 p70 alone treated group (Group #5) compared to the control group (Group #1).
Fig. 7.
Macrophages in blood of rats and normalized intensity of MHC II on 1C7+ cells, where the intensity of the control group (Group #1) was set at 100. Group #1 (control); Group #2 (MCP-1: 770 pg); Group #3 (MCP-1: 1168 pg); Group #4 (MCP-1: 621 pg; IL-12 p70: 9549 pg); Group #5 (IL-12 p70: 10608 pg). *Significantly greater than the other groups, p < 0.05.
Gene expression in the muscle tissues at the fracture/infection site
IFN-γ, TNF-α, iNOS, MIP-2, and MCP-1 did not show any significant difference in expression among the groups at post-operative day 21 (Fig. 8). However, compared to the control group (Group #1), the application of MCP-1 and IL-12 p70 increased the expression of IL-12 p40, and a significantly higher level of IL-12 p40 was found in the tissue samples of relatively higher MCP-1 application (Group #3).
Fig. 8.
mRNA levels of IFN-γ, TNF-α, IL-12 p40, MIP-2, iNOS, and MCP-1 in muscle tissues from the fracture/infection site at post-operative day 21. mRNA levels were expressed in relation to those of the control group (Group #1). Group #1 (control); Group #2 (MCP-1: 770 pg); Group #3 (MCP-1: 1168 pg); Group #4 (MCP-1: 621 pg; IL-12 p70: 9549 pg); Group #5 (IL-12 p70: 10608 pg). Bars represent fold increase above control (mean ± SD). *Significantly greater than control, p < 0.05.
DISCUSSION
Incidences of osteomyelitis caused by S. aureus have increased dramatically in the last decade. In the present study, we investigated the effects of local applications of MCP-1, which may play a key role in recruiting macrophages, in preventing S. aureus induced infection in an open rat fracture model. MCP-1 showed some effect in reducing infection and changed the populations of white blood cells. Fewer lymphocytes and more neutrophils were observed in MCP-1 treated groups than in the controls and the group treated with IL-12 p70 alone (Fig. 6). In addition, the application of a relatively high amount of MCP-1 significantly increased the IL-12 p40 level in the tissue at the infection site compared to control. No obvious difference in MCP-1 expression in muscle tissue was observed at post-operative day 21, probably because changes in secretion of MCP-1 occurred in the early stage; for instance, the expression of mRNA encoding MCP-1 was elevated in S. aureus infected murine bone tissues in the first 48 h.23
We also compared the effects of exogenous application of IL-12 p70, which may stimulate the activation of macrophages and enhance cell-mediated immune response with MCP-1 in preventing S. aureus induced infection. IL-12 p70 alone played a significant role in infection prevention, while MCP-1 combined with IL-12 p70 was less effective, possibly because of the suppression of lymphocytes by MCP-1 (Fig. 6b). The significant decrease in S. aureus infection by IL-12 p70 treatment alone was accompanied by weight gain (Fig. 4). Different from MCP-1, IL-12 p70 mediates some of its physiological activities by acting as a potent inducer of IFN-γ production by T and natural killer cells. One main target of IFN-γ activity is stimulating the bactericidal activity of macrophages;17 IFN-γ-activated macrophages have increased ability to kill bacterial or protozoan pathogens.24 In our study, IL-12 p70 application led to more activated macrophages compared to the other groups (Fig. 7). As a result, IL-12 p70 may be more effective in activating and building the body’s natural defenses, thereby tuning the macrophages’ activities toward favoring bacterial clearance and effectively preventing infections. The benefit of our approach may be that it enhances the body’s natural response so that infection can be reduced and prevented without risk of offending bacteria developing resistance to the treatment.
Another important issue related to infection prevention in open fractures is healing, though the role of inflammation in tissue healing and repair is not fully understood. On one hand, macrophages are believed to be the critical inflammatory cells required for wound healing,25 and macrophage function in the early healing phase is important for effective wound debridement, angiogenesis, and collagen synthesis.26 At therapeutic levels, agents that increase the number of macrophages within wounds could benefit nonhealing or poorly healing wounds, and the addition of macrophages, or macrophage stimulating agents, could augment wound repair.27 Local injection of activated macrophages significantly decreased the mortality of infected patients,12 accelerated vascularization and tissue repair, and improved cardiac remodeling and function.10 On the other hand, macrophages synthesize and release proinflammatory cytokines that may lead to tissue destruction. We did not observe severe tissue damage in MCP-1 or IL-12 p70 treated groups compared to the control group. This may be consistent with our observation that among the groups, no differences in systemic levels of MCP-1 and IL-12 p40, and mRNA levels of TNF-α, IFN-γ, MIP-2, iNOS, and MCP-1 in tissue were observed. Also, the group treated with IL-12 p70 alone had better healing (based on gross observation) and weight gain, which may be related to the higher activation of macrophages in the IL-12 alone treated animals (Fig. 7).
In the present study, MCP-1 and IL-12 p70 were released within 5 and 9 days, respectively. Such release characteristics of our nanocoating systems could be desirable because infection resistance is maximally suppressed during the first 10 days in major injuries,18 and the macrophage numbers may not reach the maximum prior to post-injury day 7 in wound patients.28 Meanwhile, the shortage of macrophages prior to or during the first 24 h of infection decreased animal survival dramatically,9 suggesting that sufficient macrophage presence in the early stage of infection is critical for dictating the fate of the infection: either bacterial clearance or spreading from a local infection to a progressively invasive disease. Therefore, our nanocoating systems are ideal for delivering high concentrations of IL-12 p70 and MCP-1 within desired release periods (i.e. a few days).
Limitations of this study may include that the changes in blood cell populations, proinflammatory cytokines, as well as macrophage recruitment and activation at the early stage of infection and healing were not studied. In future studies, we will address the short- and long-term impacts of local cytokine applications.
In conclusion, local applications of cytokines (MCP-1, IL-12 p70) can be effective in preventing infection, and the use of cytokines may ultimately have therapeutic relevance to circumstances of impaired wound/fracture healing in which macrophage function is suboptimal.
Supplementary Material
Acknowledgements
This work was supported in part by the AO Foundation, NSF (Grant OISE-0737735), NASA WV EPSCoR, and WVU. Project S-07-43L was supported by the AO Research Fund. The Flow Cytometry Core Facility is supported by NIH Grant RR16440. Any opinions, findings, conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the funding agencies or NIOSH. We thank Terence Meighan for assistance in RT/PCR studies, John Thomas, PhD, for consultation on bacterial studies, John Barnett, PhD, for mentoring BL on immunology consultation, Sanford Emery, MD, and Brock Lindsey, MD, for consultation on animal models, Jabeen Noore, PhD, for ELISA tests, Vincent Kish, ASEE, for building the fracture device, and Stanley Wearden, PhD, for assistance in statistical analysis.
Footnotes
Part of the work was presented at the 54th Orthopaedic Research Society (ORS) Annual Meeting, San Francisco, CA, March 2008 (oral presentation, paper #208).
References
- 1.Zalavras CG, Marcus RE, Levin LS, et al. Management of open fractures and subsequent complications. J Bone Joint Surg Am. 2007;89(4):884–895. doi: 10.2106/00004623-200704000-00027. [DOI] [PubMed] [Google Scholar]
- 2.Murray CK, Hsu JR, Solomkin JS, et al. Prevention and management of infections associated with combat-related extremity injuries. J Trauma. 2008;64(3 Suppl):S239–S251. doi: 10.1097/TA.0b013e318163cd14. [DOI] [PubMed] [Google Scholar]
- 3.Trnobranski P. Are we facing a ‘post-antibiotic era’? -a review of the literature regarding antimicrobial drug resistance. J Clin Nurs. 1998;7(5):392–400. doi: 10.1046/j.1365-2702.1998.00181.x. [DOI] [PubMed] [Google Scholar]
- 4.Nixon M, Jackson B, Varghese P, et al. Methicillin-resistant Staphylococcus aureus on orthopaedic wards: incidence, spread, mortality, cost and control. J Bone Joint Surg Br. 2006;88:812–817. doi: 10.1302/0301-620X.88B6.17544. [DOI] [PubMed] [Google Scholar]
- 5.Marriott I. Osteoblast responses to bacterial pathogens: a previously unappreciated role for bone-forming cells in host defense and disease progression. Immunol Res. 2004;30(3):291–308. doi: 10.1385/IR:30:3:291. [DOI] [PubMed] [Google Scholar]
- 6.Rogers DE, Tompsett R. The survival of staphylococci within human leukocytes. J Exp Med. 1952;95:209–230. doi: 10.1084/jem.95.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aderem A. Phagocytosis and the inflammatory response. J Infect Dis. 2003;187(Suppl. 2):S340–S345. doi: 10.1086/374747. [DOI] [PubMed] [Google Scholar]
- 8.Muller WA. Leukocyte-endothelial-cell interactions in leukocyte transmigration and the inflammatory response. Trends Immunol. 2003;24:327–334. doi: 10.1016/s1471-4906(03)00117-0. [DOI] [PubMed] [Google Scholar]
- 9.Goldmann O, Rohde M, Chhatwal GS, et al. Role of macrophages in host resistance to group A streptococci. Infect Immun. 2004;72(5):2956–2963. doi: 10.1128/IAI.72.5.2956-2963.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leor J, Rozen L, Zuloff-Shani A, et al. Ex vivo activated human macrophages improve healing, remodeling, and function of the infarcted heart. Circulation. 2006;114(1 Suppl):I94–I100. doi: 10.1161/CIRCULATIONAHA.105.000331. [DOI] [PubMed] [Google Scholar]
- 11.Danon D, Kowatch MA, Roth GS. Promotion of wound repair in old mice by local injection of macrophages. Proc Natl Acad Sci USA. 1989;86(6):2018–2020. doi: 10.1073/pnas.86.6.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Orenstein A, Kachel E, Zuloff-Shani A, et al. Treatment of deep sternal wound infections post-open heart surgery by application of activated macrophage suspension. Wound Repair Regen. 2005;13(3):237–242. doi: 10.1111/j.1067-1927.2005.130304.x. [DOI] [PubMed] [Google Scholar]
- 13.Dipietro LA, Reintjes MG, Low QE, et al. Modulation of macrophage recruitment into wounds by monocyte chemoattractant protein-1. Wound Repair Regen. 2001;9(1):28–33. doi: 10.1046/j.1524-475x.2001.00028.x. [DOI] [PubMed] [Google Scholar]
- 14.Fuentes ME, Durham SK, Swerdel MR, et al. Controlled recruitment of monocytes and macrophages to specific organs through transgenic expression of monocyte chemoattractant protein-1. J Immunol. 1995;155(12):5769–5776. [PubMed] [Google Scholar]
- 15.Lu B, Rutledge BJ, Gu L, et al. Abnormalities in monocyte recruitment and cytokine expression in monocyte chemoattractant protein 1-deficient mice. J Exp Med. 1998;187(4):601–608. doi: 10.1084/jem.187.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kurihara T, Warr G, Loy J, et al. Defects in macrophage recruitment and host defense in mice lacking the CCR2 chemokine receptor. J Exp Med. 1997;186:1757–1762. doi: 10.1084/jem.186.10.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nauciel C, Espinasse-Maes F. Role of gamma interferon and tumor necrosis factor alpha in resistance to Salmonella typhimurium infection. Infect Immun. 1992;60(2):450–454. doi: 10.1128/iai.60.2.450-454.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Sullivan ST, Lederer JA, Horgan AF, et al. Major injury leads to predominance of the T helper-2 lymphocyte phenotype and diminished interleukin-12 production associated with decreased resistance to infection. Ann Surg. 1995;222(4):482–490. doi: 10.1097/00000658-199522240-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spolarics Z, Siddiqi M, Siegel JH, et al. Depressed interleukin-12-producing activity by monocytes correlates with adverse clinical course and a shift toward Th2-type lymphocyte pattern in severely injured male trauma patients. Crit Care Med. 2003;31(6):1722–1729. doi: 10.1097/01.CCM.0000063579.43470.AA. [DOI] [PubMed] [Google Scholar]
- 20.Sands KE, Bates DW, Lanken PN, et al. Epidemiology of sepsis syndrome in 8 academic medical centers. Academic Medical Center Consortium Sepsis Project Working Group. JAMA. 1997;278:234–240. [PubMed] [Google Scholar]
- 21.Kalicke T, Schierholz J, Schlegel U, et al. Effect on infection resistance of a local antiseptic and antibiotic coating on osteosynthesis implants: an in vitro and in vivo study. J Orthop Res. 2006;24(8):1622–1640. doi: 10.1002/jor.20193. [DOI] [PubMed] [Google Scholar]
- 22.Petty W, Spanier S, Shuster JJ, et al. The influence of skeletal implants on incidence of infection. J Bone Joint Surg Am. 1985;67(8):1236–1244. [PubMed] [Google Scholar]
- 23.Marriott I, Gray DL, Rati DM, et al. Osteoblasts produce monocyte chemoattractant protein-1 in a murine model of Staphylococcus aureus osteomyelitis and infected human bone tissue. Bone. 2005;37(4):504–512. doi: 10.1016/j.bone.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 24.Murray HW. The interferons, macrophage activation, and host defense against nonviral pathogens. J Interferon Res. 1992;12(5):319–322. doi: 10.1089/jir.1992.12.319. [DOI] [PubMed] [Google Scholar]
- 25.Langer R, Tirrell DA. Designing materials for biology and medicine. Nature. 2004;428(6982):487–492. doi: 10.1038/nature02388. [DOI] [PubMed] [Google Scholar]
- 26.Browder W, Williams D, Lucore P, et al. Effect of enhanced macrophage function on early wound healing. Surgery. 1988;104(2):224–230. [PubMed] [Google Scholar]
- 27.Leibovich SJ, Danon D. Promotion of wound repair in mice by application of glucan. J Reticuloendothel Soc. 1980;27(1):1–11. [PubMed] [Google Scholar]
- 28.Ashcroft GS, Horan MA, Ferguson MW. Aging alters the inflammatory and endothelial cell adhesion molecule profiles during human cutaneous wound healing. Lab Invest. 1998;78(1):47–58. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.