Abstract
Epidemiologic studies of co-infection with tuberculosis (TB) and intestinal parasites in humans have not been extensively investigated in China. A cross-section study was conducted in a rural county of Henan Province, China. Pulmonary TB (PTB) case-patients receiving treatment for infection with Mycobacterium tuberculosis and healthy controls matched for geographic area, age, and sex were surveyed by using questionnaires. Fecal and blood specimens were collected for detection of intestinal parasites, routine blood examination, and infection with human immunodeficiency virus. The chi-square test was used for univariate analysis and multivariate logistic regression models were used to adjust for potential confounding factors. A total of 369 persons with PTB and 366 healthy controls were included; all participants were negative for human immunodeficiency virus. The overall prevalence of intestinal parasites in persons with PTB was 14.9%, including intestinal protozoa (7.9%) and helminthes (7.6%). The infection spectrum of intestinal parasites was Entamoeba spp. (1.4%), Blastocystis hominis (6.2%), Trichomonas hominis (0.3%), Clonorchis sinensis (0.3%), Ascaris lumbricoides (0.5%), Trichuris trichiura (2.2%), and hookworm (4.6%). The prevalence of intestinal parasites showed no significant difference between persons with PTB and healthy controls after adjusting for potential confounding factors. There was no factor that affected infection rates for intestinal parasites between the two groups. Infection with intestinal parasites of persons with PTB was associated with female sex (adjusted odds ratio [AOR] = 2.05, 95% confidence interval [CI] = 1.01–4.17), body mass index ≤ 19 (AOR = 3.02, 95% CI = 1.47–6.20), and anemia (AOR = 2.43, 95% CI = 1.17–5.03). Infection of healthy controls was only associated with an annual labor time in farmlands > 2 months (AOR = 4.50, 95% CI = 2.03–10.00). In addition, there was no significant trend between rates of infection with intestinal parasites and duration of receiving treatment for infection with M. tuberculosis in persons with PTB. The prevalence of intestinal parasites was not higher in persons with PTB, and there was no evidence that PTB increased susceptibility to intestinal parasites in this study. However, for patients with PTB, women and patients with comorbidities were more likely to be infected with intestinal parasites.
Introduction
Co-infection with tuberculosis (TB) and intestinal parasites in humans is one of the important public problems in co-endemic areas, especially in developing countries. For instance, 32% of hospitalized TB patients had intestinal parasites and 29% of TB patients from the community had intestinal helminths in Ethiopia.1,2
In China, many areas co-endemic for TB and parasitic diseases have been identified, particularly in rural areas. Approximately 0.92 million TB cases were reported in China in 2010, which accounted for approximately 15% of global TB cases,3 and the prevalence of active pulmonary TB (PTB) was higher in rural areas than in urban areas.4 A high burden of parasitic diseases has also been reported in rural areas of China. The infection rate for Blastocystis hominis was approximately 22% in rural central China,5,6 and the overall prevalence of soil-transmitted helminths was approximately 40–68% in rural southwestern and western China.7,8 However, there is a paucity of epidemiologic studies on co-infection with TB and intestinal parasites in China.
A study of co-infection with TB and intestinal helminths suggested that compared with TB patients or healthy controls (HCs), the immune response of co-infected patients to Mycobacterium tuberculosis (MTB) was decreased.10 Clinical and laboratory-based studies showed that when the course of TB was aggravated by opisthorchiasis, clinical signs of TB became more pronounced, disorders in functions of the liver and pancreas became increased, antibacterial therapy intolerance increased, and prognosis of the disease decreased.10,11 Two studies found that intestinal parasitic infections might also significantly alter the protective immune response to Bacillus Calmette-Guerin vaccination.12,13 Therefore, it is worthwhile to explore co-infection with TB and intestinal parasites because co-infection increases the complexity of control and prevention of TB and parasitic diseases in co-endemic areas.
The present study was conducted by using a cross-section survey to evaluate the prevalence of intestinal parasites and to explore possible factors associated with infection of intestinal parasites among patients with PTB compared with the general population. For this purpose, multiple fecal specimens were examined with a series of diagnostic approaches for intestinal protozoa and helminths in a rural county of central China. At the same time, blood specimens were collected for routine examination and detection of antibodies against human immunodeficiency virus (HIV). The ultimate goal of this study was to provide some guidance on control and prevention of co-infection with TB and parasitic diseases in central China where TB and parasitic diseases are transmitted.
Materials and Methods
Study design.
The study was conducted during July–September 2012 in Gushi County in Henan Province, which is an agricultural county in central China. All persons with PTB who were registered in the TB surveillance system during February–July 2012 and were undergoing anti-MTB treatment during July–September 2012 composed the PTB case (PC) group in this study. A PC was randomly matched with an HC individually through the local population management system. The HC had to be from the neighborhood of each PC (i.e., living in same village). The PC and HC were matched by age (±5 years) and sex. All HCs composed the HC group.
All potential participants were recruited by the local Center for Disease Control and Prevention (CDC). The participants were included in the study if they had no other disease, no severe disease of the immune system, and, if female, were not pregnant. Additionally, the HCs were confirmed not to have TB according to diagnostic criteria of National Tuberculosis Program.14 If any persons did not satisfy the inclusion criteria, those participants were excluded from the study.
Field survey procedures.
All participants were given two fecal collection containers for fecal specimen collection (at least 30 grams every day) for two consecutive mornings. On the first morning when they delivered the fecal specimen, they were administered questionnaires about sociodemographic characteristics, health conditions, hygienic habits, and labor in farmlands, and blood (10 mL) was obtained for routine examination and detection of antibodies against HIV. All investigations and specimen collections were conducted by the staff of the local CDC in the township hospitals near participants' villages. Specimens were sent to the laboratory of the local CDC for examination as soon as possible after they were collected every morning.
Laboratory procedures.
Blood specimens were tested by the staff of the local CDC within 2 hours of collection for antibodies against HIV by using the diagnostic kit for antibodies against HIV (colloidal gold) (ZhuHai Livzon Diagnostics Inc., Zhuhai, China) and for routine characteristics by using a MC-600 hematology analyzer (Shenzhen Maxcom Electronic Co., Ltd., Shenzhen, China). The diagnostic threshold for anemia was a hemoglobin level < 120 g/L for adult men and < 110 g/L for non-pregnant women and children in China.15
Fecal specimens were processed within 8 hours post-collection by using four standard fecal examination methods: simple saline smear for intestinal protozoa trophozoites, iodine-stained smear for protozoal intestinal cysts, in vitro cultivation for B. hominis,16 and a modified Kato-Katz thick smear (semi-quantitative fecal examination technique for detection of helminthic ova).17 Three smears of each fecal specimen were prepared in each method. Every smear was initially read by two examiners who were not aware of each others readings, and was reviewed by a third examiner if there was disagreement. Fecal specimen examinations were conducted by staff from the National Institute of Parasitic Diseases of the China CDC and staff from Henan Province CDC and the Anhui Province Institute of Parasitic Diseases Control.
Statistical analysis.
Data were double-entered and cross-checked by using the EpiData software version 3.1 (The EpiData Association, Odense, Denmark). Infection rates with 95% confidence intervals (CIs) of intestinal parasites were calculated by using binomial distribution. Infection rates between the PC group and the HC group were compared and stratified by characteristics of participants to determine whether some related factors had any impact on the infection rates of two groups.
In addition, we further examined risk factors related to the infection rate of each group and the impact of anti-MTB treatment on infections in the PC group. The Wilcoxon rank-sum test was used for the analysis of quantitative data that did not show a normal distribution. Univariate analysis using the chi-square test was conducted for computing odds ratios (ORs) with 95% CIs, and multivariate logistic regression was used to estimate the adjusted ORs with 95% CIs. Factors included in multivariate logistic regression were sociodemographic characteristics, concomitant medical conditions, hygienic habits when cooking, eating, walking, washing hands, raising animals, and labor in farmlands. A two-sided P value < 0.05 was regarded as significant. Statistical analyses were performed by using the SAS statistical package version 9.2 (SAS Institute, Inc., Cary, NC).
Ethical statement.
This study was reviewed and approved by the Ethics Review Committee of the National Institute of Parasitic Diseases of the China CDC. According to arrangements made by the local CDC, authorities of township hospitals informed health workers at clinics of villages about the study procedures. Health workers then informed all potential participants and carefully explained the objectives, procedures, and potential risks of the study. Persons who agreed to participate in the study were asked to sign a written informed consent form by the staff of the local CDC, and were then included in the study if they met the inclusion criteria. If participants were less than 18 years of age, their parents were asked to sign a written parental permission form. All participants were offered professional counseling by the staff of the local CDC before and during the study, and all diagnostic test results were kept strictly confidential. At completion of the study and in accordance with local treatment policies, anti-parasitic treatment was offered through the local CDC at no charge to all participants who were found to be infected with intestinal parasites.
Results
Study cohort.
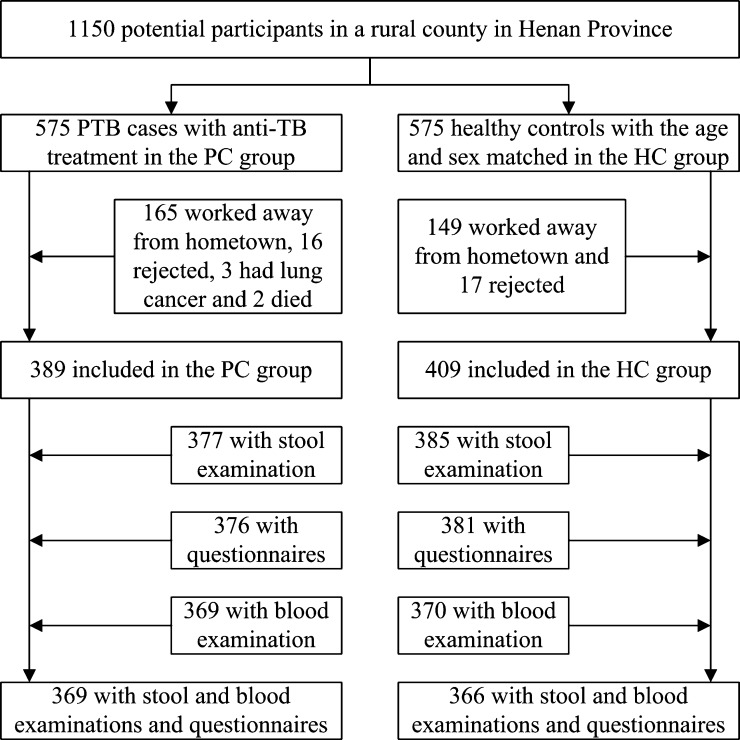
There were 1,150 potential participants (575 PCs and 575 HCs). Among PCs, 165 worked outside their home villages, 16 refused to participate in the study, 3 had lung cancer, and 2 died. Among HCs, 149 worked outside their home villages and 17 refused to participate in the study. Therefore, 389 PCs and 409 HCs participated in the study. After exclusion of participants whose fecal or blood specimens were not collected or who had not completed the questionnaires, 369 PCs and 366 HCs were included in the study (Figure 1). Of 575 PCs, all those excluded (median age = 53 years, interquartile range [IQR] = 31–66 years) were significantly younger (P < 0.0001) than those included in the study (median age = 62 years, IQR = 50–70 years), which was similar to age of the HCs.
Figure 1.
Study profile of intestinal parasite co-infection among pulmonary tuberculosis (PTB) cases without human immunodeficiency virus infection in a rural county in China. HC = healthy control.
Characteristics of participants.
Although most characteristics of participants in the two groups were similar in terms of sociodemographic characteristics, health conditions, hygienic habits, and labor in farmlands, there were some notable differences (Table 1). Body mass index (BMI) (median = 19, IQR = 18–20 versus median = 20, IQR = 18–23) and annual labor time in farmlands (median = 2 months, IQR = 0–3 months versus median = 3 months, IQR = 1–4 months) were generally lower (P < 0.001) in the PC group than in the HC group. However, proportions of persons never or occasionally washing fruits and vegetables before eating raw (51.0% versus 42.1%), ever walking barefoot (56.1% versus 45.4%), never or occasionally washing hands before meals (39.8% versus 28.4%), and never or occasionally washing hands after defecating (45.5% versus 29.0%) were significantly higher (P < 0.05) in the PC group than in the HC group. All participants were HIV negative.
Table 1.
Characteristics of participants in a study of intestinal parasite co-infection among pulmonary tuberculosis cases without HIV infection in a rural county in China*
| Variable | PC group (n = 369) | HC group (n = 366) | P |
|---|---|---|---|
| Sex | 0.2435 | ||
| M | 249 (67.5) | 232 (63.4) | |
| F | 120 (32.5) | 134 (36.6) | |
| Age (years) | 62 (50–70) | 60 (48–68) | 0.0895† |
| Han nationality | 364 (98.6) | 364 (99.4) | 0.4509 |
| Education | 0.0948 | ||
| Illiterate | 185 (50.1) | 206 (56.3) | |
| Elementary school and above | 184 (49.9) | 160 (43.7) | |
| Married | 288 (78.0) | 299 (81.7) | 0.2179 |
| Body mass index | 19 (18–20) | 20 (18–23) | < 0.0001† |
| Anemia | 82 (22.2) | 83 (22.7) | 0.8824 |
| Only one cutting board in kitchen | 349 (94.6) | 341 (93.2) | 0.4251 |
| Only one cooking knife in kitchen | 253 (68.6) | 249 (68.0) | 0.8771 |
| Never or occasionally washed fruits and vegetables before eating raw | 188 (51.0) | 154 (42.1) | 0.0159 |
| Ever walked barefoot | 207 (56.1) | 166 (45.4) | 0.0036 |
| Never or occasionally washed hands before meals | 147 (39.8) | 104 (28.4) | 0.0011 |
| Never or occasionally washed hands after defecating | 168 (45.5) | 106 (29.0) | < 0.0001 |
| Raised pets (e.g., cats, dogs) | 224 (60.7) | 220 (60.1) | 0.8689 |
| Raised poultry or livestock (e.g., chickens, ducks, pigs) | 285 (77.2) | 277 (75.7) | 0.6198 |
| Annual labor time in farmlands (months) | 2 (0–3) | 3 (1–4) | < 0.0001† |
| Ever labored barefoot in farmlands | 128 (34.7) | 145 (39.6) | 0.1667 |
| HIV negative | 369 (100.0) | 366 (100.0) | NA |
Values are median (interquartile range) or no. (%). HIV = human immunodeficiency virus; PC = pulmonary tuberculosis case; HC = healthy control; NA = not applicable.
By Wilcoxon rank-sum test.
Clinical information for PCs.
In the PC group, 46.1% (170 of 369) of the patients had PTB involving only one lobe, 39.8% (147 of 369) had PTB involving two lobes, and 14.1% (52 of 369) had PTB involving more than two lobes. Of the PCs, 9.5% (35 of 369) had pulmonary cavitations and 49.6% (183 of 369) were sputum smear positive. All PCs had no extra-pulmonary disease.
Parasitic infections.
Because some characteristics differed between the PC group and the HC group, we compared infection rates of intestinal protozoa and helminths between two groups adjusted by characteristics to control for confounding factors (Table 2). Among the PC group, the overall prevalence of intestinal parasites was 14.9% (95% CI = 11.4–19.0%), including 7.9% (95% CI = 5.3–11.1%) with intestinal protozoa, 7.6% (95% = CI 5.1–10.8%) with helminthes, 1.4% (95% CI = 0.4–3.1%) with Entamoeba spp., 6.2% (95% CI = 4.0–9.2%) with B. hominis, 0.3% (95% CI = 0.3–1.5%) with Trichomonas hominis, 0.3% (95% CI = 0.3–1.5%) with Clonorchis sinensis, 0.5% (95% CI = 0.1–1.9%) with Ascaris lumbricoides, 2.2% (95% CI = 0.9–4.2%) with Trichuris trichiura, and 4.6% (95% CI = 2.7–7.3%) with hookworm. Although the absolute infection rate for hookworm was higher and those of other parasites were lower in the PC group than in the HC group, the infection rates of all intestinal parasites between two groups were similar.
Table 2.
Parasitic infection of participants in a study of intestinal parasite co-infection among pulmonary tuberculosis cases without HIV infection in a rural county in China*
| Parasite | PC group (n = 369) | HC group (n = 366) | OR (95% CI) | Adjusted OR (95% CI)† | ||
|---|---|---|---|---|---|---|
| No. infections | Infection rate (95% CI) | No. infections | Infection rate (95% CI) | |||
| Intestinal protozoa | 29 | 7.9% (5.3–11.1%) | 35 | 9.6% (6.8–13.0%) | 0.81 (0.48–1.35) | 0.78 (0.45–1.37) |
| Entamoeba spp. | 5 | 1.4% (0.4–3.1%) | 8 | 2.2% (0.9–4.3%) | 0.61 (0.20–1.90) | 0.59 (0.18–1.99) |
| Blastocystis hominis | 23 | 6.2% (4.0–9.2%) | 28 | 7.6% (5.1–10.9%) | 0.80 (0.45–1.42) | 0.78 (0.42–1.46) |
| Giardia lamblia | 0 | NA | 1 | 0.3% (0.3–1.5%) | NA | NA |
| Trichomonas hominis | 1 | 0.3% (0.3–1.5%) | 0 | NA | NA | NA |
| Helminths | 28 | 7.6% (5.1–10.8%) | 30 | 8.2% (5.6–11.5%) | 0.92 (0.54–1.57) | 1.06 (0.55–2.05) |
| Clonorchis sinensis | 1 | 0.3% (0.3–1.5%) | 2 | 0.5% (0.1–2.0%) | 0.49 (0.04–5.48) | NA |
| Ascaris lumbricoides | 2 | 0.5% (0.1–1.9%) | 5 | 1.4% (0.4–3.2%) | 0.39 (0.08–2.04) | 0.10 (0.01–1.44) |
| Trichuris trichiura | 8 | 2.2% (0.9–4.2%) | 13 | 3.6% (1.9–6.0%) | 0.60 (0.25–1.47) | 0.93 (0.34–2.58) |
| Hookworm | 17 | 4.6% (2.7–7.3%) | 15 | 4.1% (2.3–6.7%) | 1.13 (0.56–2.30) | 1.28 (0.55–2.97) |
| Intestinal parasites | 55 | 14.9% (11.4–19.0%) | 61 | 16.7% (13.0–20.9%) | 0.88 (0.59–1.30) | 0.90 (0.57–1.43) |
HIV = human immunodeficiency virus; PC = pulmonary tuberculosis case; HC = healthy control; CI = confidence interval, OR = odds ratio, NA = not applicable.
Adjusted for sociodemographic characteristics, health conditions, hygienic habits, and labor in farmlands.
Effect of participant characteristics on parasitic infection rates.
Overall infection rates of intestinal parasites stratified by characteristics between two groups were compared, and comparisons were adjusted by characteristics to control for confounding (Table 3). However, there was no single characteristic affecting the overall infection rates of intestinal parasites between two groups. For each group, the risk factors for overall infections of intestinal parasites were identified (Table 4). For the PC group, female sex (adjusted OR = 2.05, 95% CI = 1.01–4.17), BMI ≤ 19 (adjusted OR = 3.02, 95% CI = 1.47–6.20), and anemia (adjusted OR = 2.43, 95% CI = 1.17–5.03) were the risk factors associated with overall infections. For the HC group, annual labor time in farmlands > 2 months (adjusted OR = 4.50, 95% CI = 2.03–10.00) was only the risk factor associated with overall infections.
Table 3.
Comparison of overall infection rates for intestinal parasites stratified by characteristics of participants in a study of intestinal parasite co-infection among pulmonary tuberculosis cases without HIV infection in a rural county in China*
| Variable | PC group (n = 369) | HC group (n = 366) | OR (95% CI) | Adjusted OR (95% CI)† | ||||
|---|---|---|---|---|---|---|---|---|
| No. | No. infections | Infection rate (95% CI) | No. | No. infections | Infection rate (95% CI) | |||
| Sex | ||||||||
| M | 249 | 31 | 12.4% (8.6–17.2%) | 232 | 38 | 16.4% (11.8–21.8%) | 0.73 (0.44–1.21) | 0.63 (0.34–1.15) |
| F | 120 | 24 | 20.0% (13.2–28.3%) | 134 | 23 | 17.2% (11.2–24.6%) | 1.21 (0.64–2.27) | 1.43 (0.66–3.07) |
| Age (years) | ||||||||
| ≤ 60 | 164 | 20 | 12.2% (7.6–18.2%) | 187 | 29 | 15.5% (10.6–21.5%) | 0.76 (0.41–1.40) | 0.92 (0.44–1.91) |
| > 60 | 205 | 35 | 17.1% (12.2–22.9%) | 179 | 32 | 17.9% (12.6–24.3%) | 0.94 (0.56–1.60) | 0.88 (0.48–1.63) |
| Education | ||||||||
| Elementary school and above | 185 | 26 | 14.0% (9.4–19.9%) | 206 | 34 | 16.5% (11.7–22.3%) | 0.83 (0.48–1.44) | 0.83 (0.43–1.59) |
| Illiterate | 184 | 29 | 15.8% (10.8–21.8%) | 160 | 27 | 16.9% (11.4–32.6%) | 0.92 (0.52–1.63) | 0.90 (0.44–1.84) |
| Marital status | ||||||||
| Other | 81 | 8 | 9.9% (4.4–18.5%) | 67 | 12 | 17.9% (9.6–29.2%) | 0.50 (0.19–1.31) | 0.51 (0.14–1.82) |
| Married | 288 | 47 | 16.3% (12.2–21.1%) | 299 | 49 | 16.4% (12.4–21.1%) | 1.00 (0.64–1.54) | 1.02 (0.62–1.68) |
| BMI | ||||||||
| > 19 | 160 | 14 | 8.8% (4.9–14.2%) | 228 | 35 | 15.4% (10.9–20.7%) | 0.53 (0.27–1.02) | 0.57 (0.27–1.21) |
| ≤ 19 | 209 | 41 | 19.6% (14.4–25.6%) | 138 | 26 | 18.8% (12.7–26.4%) | 1.05 (0.61–1.82) | 1.23 (0.66–2.31) |
| Anemia | ||||||||
| No | 287 | 33 | 11.5% (8.0–15.8%) | 283 | 37 | 13.1% (9.4–17.6%) | 0.86 (0.52–1.42) | 0.84 (0.47–1.48) |
| Yes | 82 | 22 | 26.8% (17.6–37.8%) | 83 | 24 | 28.9% (19.5–39.9%) | 0.90 (0.46–1.78) | 0.73 (0.29–1.82) |
| No. cutting boards in kitchen | ||||||||
| > 1 | 20 | 2 | 10.0% (1.2–31.7%) | 25 | 3 | 12.0% (2.5–31.2%) | 0.81 (0.12–5.42) | NA |
| 1 | 349 | 53 | 15.2% (11.6–19.4%) | 341 | 58 | 17.0% (13.2–21.4%) | 0.87 (0.58–1.31) | 0.88 (0.55–1.39) |
| No. cooking knives in kitchen | ||||||||
| > 1 | 116 | 16 | 13.8% (8.1–21.4%) | 117 | 12 | 10.2% (5.4–17.2%) | 1.40 (0.63–3.11) | 1.23 (0.47–3.22) |
| 1 | 253 | 39 | 15.4% (11.2–20.5%) | 249 | 49 | 19.7% (14.9–25.2%) | 0.74 (0.47–1.18) | 0.77 (0.45–1.34) |
| Washed fruits and vegetables before eating raw | ||||||||
| Often or always | 181 | 20 | 11.0% (6.9–16.5%) | 212 | 27 | 12.7% (8.6–18.0%) | 0.85 (0.46–1.58) | 0.97 (0.48–1.96) |
| Never or occasionally | 188 | 35 | 18.6% (13.3–24.9%) | 154 | 34 | 22.1% (15.8–29.4%) | 0.81 (0.48–1.37) | 0.91 (0.46–1.80) |
| Ever walked barefoot | ||||||||
| No | 162 | 20 | 12.4% (7.7–18.4%) | 200 | 26 | 13.0% (8.7–18.5%) | 0.94 (0.50–1.76) | 0.98 (0.49–1.97) |
| Yes | 207 | 35 | 16.9% (12.1–22.7%) | 166 | 35 | 21.1% (15.1–28.1%) | 0.76 (0.45–1.28) | 0.95 (0.45–2.02) |
| Washed hands before meals | ||||||||
| Often or always | 222 | 26 | 11.7% (7.8–16.7%) | 262 | 34 | 13.0% (9.2–17.6%) | 0.89 (0.52–1.53) | 0.86 (0.47–1.58) |
| Never or occasionally | 147 | 29 | 19.7% (13.6–27.0%) | 104 | 27 | 26.0% (17.8–35.5%) | 0.70 (0.38–1.27) | 1.01 (0.41–2.51) |
| Washed hands after defecating | ||||||||
| Often or always | 201 | 24 | 11.9% (7.8–17.2%) | 260 | 35 | 13.5% (9.6–18.2%) | 0.87 (0.50–1.52) | 0.88 (0.48–1.63) |
| Never or occasionally | 168 | 31 | 18.4% (12.9–25.2%) | 106 | 26 | 24.5% (16.7–33.8%) | 0.70 (0.39–1.26) | 1.00 (0.41–2.40) |
| Raised pets | ||||||||
| No | 145 | 14 | 9.7% (5.4–15.7%) | 146 | 19 | 13.0% (8.0–19.6%) | 0.71 (0.34–1.48) | 0.72 (0.30–1.75) |
| Yes | 224 | 41 | 18.3% (13.5–24.0%) | 220 | 42 | 19.1% (14.1–24.9%) | 0.95 (0.59–1.53) | 1.03 (0.59–1.81) |
| Raised poultry or livestock | ||||||||
| No | 128 | 11 | 8.6% (4.4–14.8%) | 132 | 16 | 12.1% (7.1–18.9%) | 0.68 (0.30–1.53) | 0.97 (0.34–2.71) |
| Yes | 241 | 44 | 18.3% (13.6–23.7%) | 234 | 45 | 19.2% (14.4–24.9%) | 0.94 (0.59–1.49) | 0.91 (0.53–1.56) |
| Annual labor time in farmlands, months | ||||||||
| ≤ 2 | 268 | 32 | 11.9% (8.3–16.4%) | 182 | 20 | 11.0% (6.8–16.4%) | 1.10 (0.61–1.99) | 1.11 (0.58–2.15) |
| > 2 | 101 | 23 | 22.8% (15.0–32.2%) | 184 | 41 | 22.3% (16.5–29.0%) | 1.03 (0.58–1.84) | 0.59 (0.26–1.31) |
| Ever labored barefoot in farmlands | ||||||||
| No | 241 | 26 | 10.8% (7.2–15.4%) | 221 | 29 | 13.1% (9.0–18.3%) | 0.80 (0.46–1.41) | 0.86 (0.46–1.60) |
| Yes | 128 | 29 | 22.6% (15.7–30.9%) | 145 | 32 | 22.1% (15.6–29.7%) | 1.03 (0.58–1.83) | 1.03 (0.45–2.36) |
HIV = human immunodeficiency virus; PC = pulmonary tuberculosis case; HC = healthy control; CI = confidence interval, OR = odds ratio; BMI = body mass index; NA = not applicable.
Adjusted for sociodemographic characteristics, health conditions, hygienic habits, and labor in farmlands.
Table 4.
Risk factors for infection with any and all intestinal parasites, stratified by study groups, in a study of intestinal parasite co-infection among pulmonary tuberculosis cases without HIV infection in a rural county in China*
| Variable | PC group (n = 369) | HC group (n = 366) | ||
|---|---|---|---|---|
| OR (95% CI) | Adjusted OR (95% CI)† | OR (95% CI) | Adjusted OR (95% CI)† | |
| Sex | ||||
| M | 1.00 | 1.00 | 1.00 | 1.00 |
| F | 1.76 (0.98–3.15) | 2.05 (1.01–4.17) | 1.06 (0.60–1.87) | 1.22 (0.60–2.46) |
| Age (years) | ||||
| ≤ 60 | 1.00 | 1.00 | 1.00 | 1.00 |
| > 60 | 1.48 (0.82–2.68) | 1.59 (0.75–3.35) | 1.19 (0.68–2.06) | 1.58 (0.77–3.24) |
| Education | ||||
| Elementary school and above | 1.00 | 1.00 | 1.00 | 1.00 |
| Illiterate | 1.14 (0.64–2.03) | 0.77 (0.37–1.60) | 1.03 (0.59–1.79) | 0.70 (0.34–1.44) |
| Marital status | ||||
| Other | 1.00 | 1.00 | 1.00 | 1.00 |
| Married | 1.78 (0.80–3.94) | 2.00 (0.78–5.15) | 0.90 (0.45–1.80) | 0.82 (0.36–1.86) |
| BMI | ||||
| > 19 | 1.00 | 1.00 | 1.00 | 1.00 |
| ≤ 19 | 2.54 (1.33–4.86) | 3.02 (1.47–6.20) | 1.28 (0.73–2.24) | 1.35 (0.72–2.54) |
| Anemia | ||||
| No | 1.00 | 1.00 | 1.00 | 1.00 |
| Yes | 2.82 (1.54–5.18) | 2.43 (1.17–5.03) | 2.70 (1.50–4.86) | 1.85 (0.94–3.62) |
| No. cutting boards in kitchen | ||||
| > 1 | 1.00 | 1.00 | 1.00 | 1.00 |
| 1 | 1.61 (0.36–7.15) | 1.44 (0.26–8.07) | 1.50 (0.44–5.19) | 1.12 (0.23–5.52) |
| No. cooking knives in kitchen | ||||
| > 1 | 1.00 | 1.00 | 1.00 | 1.00 |
| 1 | 1.14 (0.61–2.14) | 0.84 (0.36–1.92) | 2.14 (1.09–4.21) | 1.84 (0.76–4.44) |
| Washed fruits and vegetables before eating raw | ||||
| Often or always | 1.00 | 1.00 | 1.00 | 1.00 |
| Never or occasionally | 1.84 (1.02–3.33) | 1.39 (0.54–3.58) | 1.94 (1.11–3.38) | 1.64 (0.64–4.23) |
| Ever walked barefoot | ||||
| No | 1.00 | 1.00 | 1.00 | 1.00 |
| Yes | 1.44 (0.80–2.61) | 0.75 (0.22–2.59) | 1.79 (1.02–3.12) | 0.60 (0.21–1.67) |
| Washed hands before meals | ||||
| Often or always | 1.00 | 1.00 | 1.00 | 1.00 |
| Never or occasionally | 1.85 (1.04–3.30) | 1.69 (0.36–7.92) | 2.35 (1.33–4.15) | 5.41 (0.46–63.03) |
| Washed hands after defecating | ||||
| Often or always | 1.00 | 1.00 | 1.00 | 1.00 |
| Never or occasionally | 1.67 (0.94–2.97) | 1.71 (0.28–10.29) | 2.09 (1.18–3.69) | 0.74 (0.07–7.65) |
| Raised pets | ||||
| No | 1.00 | 1.00 | 1.00 | 1.00 |
| Yes | 2.10 (1.10–4.00) | 1.21 (0.59–2.51) | 1.58 (0.88–2.84) | 1.17 (0.60–2.27) |
| Raised poultry or livestock | ||||
| No | 1.00 | 1.00 | 1.00 | 1.00 |
| Yes | 2.38 (1.18–4.78) | 1.51 (0.69–3.31) | 1.73 (0.93–3.19) | 1.26 (0.64–2.50) |
| Annual labor time in farmlands, months | ||||
| ≤ 2 | 1.00 | 1.00 | 1.00 | 1.00 |
| > 2 | 2.17 (1.20–3.94) | 1.99 (0.92–4.30) | 2.32 (1.30–4.15) | 4.50 (2.03–10.00) |
| Ever labored barefoot in farmlands | ||||
| No | 1.00 | 1.00 | 1.00 | 1.00 |
| Yes | 2.42 (1.36–4.33) | 1.52 (0.67–3.43) | 1.87 (1.08–3.26) | 0.93 (0.42–2.08) |
HIV = human immunodeficiency virus; PC = pulmonary tuberculosis case; HC = healthy control; OR = odds ratio; CI = confidence interval; BMI = body mass index.
Adjusted for sociodemographic characteristics, health conditions, hygienic habits, and labor in farmlands.
Effect of anti-MTB treatment on parasitic infection rates.
Overall prevalence of intestinal parasites was calculated for the PC group and stratified by duration of anti-MTB treatment. The infection rate of PCs during first month of treatment was compared with those of PCs during the second through sixth months of treatment, in which comparisons were adjusted by characteristics to control for confounding (Table 5). Compared with PCs during the first month of treatment, PCs during the second through sixth months of treatment had similar infection rates of intestinal parasites. There was no significant trend between infection rates and duration of treatment (chi-square for trend = 0.23, P = 0.6281).
Table 5.
Comparison of overall infection rates for intestinal parasites by duration of anti-TB treatment of persons with PTB in a study of intestinal parasite co-infection among pulmonary tuberculosis cases without HIV infection in a rural county in China*
| Duration of treatment, months | Person with PTB (n = 369) | No. infections (n = 55) | Infection rate (95% CI) | OR (95% CI) | Adjusted OR (95% CI)† |
|---|---|---|---|---|---|
| 1 | 45 | 6 | 13.3% (5.0–26.8%) | 1.00 | 1.00 |
| 2 | 43 | 7 | 16.3% (6.8–30.7%) | 1.26 (0.39–4.12) | 1.68 (0.44–6.36) |
| 3 | 38 | 7 | 18.4% (7.7–34.3%) | 1.47 (0.45–4.82) | 1.26 (0.32–4.91) |
| 4 | 63 | 5 | 7.9% (2.6–17.6%) | 0.56 (0.16–1.96) | 0.41 (0.10–1.70) |
| 5 | 79 | 12 | 15.2% (8.1–25.0%) | 1.16 (0.40–3.35) | 1.59 (0.47–5.36) |
| 6 | 101 | 18 | 17.8% (10.9–26.7%) | 1.41 (0.52–3.83) | 1.36 (0.43–4.26) |
TB = tuberculosis; PTB = pulmonary tuberculosis; HIV = human immunodeficiency virus; CI = confidence interval; OR = odds ratio. Chi square for trend = 0.23, P = 0.6281.
Adjusted for sociodemographic characteristics, health conditions, hygienic habits, and labor in farmlands.
Discussion
The prevalence of intestinal parasite infections among HCs and infection spectrum of intestinal parasites for B. hominis of 7.6%, hookworm of 4.1%, T. trichiura of 3.6%, Entamoeba spp. of 2.2%, and A. lumbricoides of 1.4% in this study was different from that reported in a previous study also conducted in a rural area of central China in 2008, in which prevalence of B. hominis, hookworm, and Cryptosporidium spp. were 22.1%, 4.3%, and 3.0%, respectively.5 Although it was not possible to obviate the impact of participants' sociodemographic characteristics on the difference of infection spectrum between two studies, both findings indicated that infection rates of intestinal parasites in humans were lower in central China than in southern and western China, where infection rates were > 40%.7,8
Previous studies focusing only on the effect of helminth infections on TB suggested that helminth infections may be the risk factor for active PTB in addition to HIV infection,18 as well as have a negative influence on human immunity against TB.9,19 However, a review showed that PTB and parasitic diseases were shown to be risk factors for each other.20 Therefore, TB was hypothesized to also increase the risk of intestinal parasite infections. This hypothesis was not supported by results of this study, in which infection rates of intestinal parasites between PCs and HCs were similar. However, in a survey in Ethiopia, PTB patients had higher infection rates with Giardia intestinalis and Strongyloides stercoralis than persons without PTB.1 The prevalence of intestinal parasites was relatively low in this study, and this may be one of the factors that contribute to the nonsignificant differences in the prevalence of intestinal parasites among PCs and HCs. Contrary to similar infection rates, PCs were more likely to walk barefoot, never or occasionally wash fruits and vegetables before eating raw, and never or occasionally wash hands before meals and after defecating than HCs in this study, most of which were risk factors for intestinal parasite infections.21 Therefore, it is worthwhile to further investigate the findings of this study.
Tian and others5 estimated that the absence of differences in intestinal parasite infections between HIV-positive and HIV-negative persons might have been the result of enhanced attention to health education, which improved the health-related attitudes and behaviors of HIV-positive persons. However, health education did not show any improvement in health-related behaviors of PCs in this study because they were more likely to have poor hygiene habits than HCs. We did not find any factors that affected infection rates of intestinal parasites between PCs and HCs, which suggested that protective and risk factors for infection were possibly equal or comparable between two groups. Therefore, we estimated that PCs possibly decreased life-related behaviors that increased risks of exposure to intestinal parasites.
We found that more than two months a year of labor time in farmlands was a risk factor for intestinal parasitic infections only in HCs. Other studies also showed that labor in farmlands likely increased the risks of exposure to intestinal parasites.22 Labor time in farmlands can be regarded as an indicator of life-related behaviors in rural areas. We also found that annual labor time in farmlands was shorter in PCs than in HCs, which is consistent with the suggestion that persons with PTB should decrease activity and increase rest time during anti-MTB treatment.23 Therefore, we assumed that compared with HCs, the risk effect on infection with intestinal parasites caused by weight loss and anemia was decreased by less life-related behaviors of exposure to intestinal parasites in PCs. For PCs, women were more likely to be infected with intestinal parasites in this study. Under the precondition of shorter annual labor time in farmlands for PCs, it was assumed that as the rural tradition in China, women did more housework than men, which might increase risk of women for exposure to intestinal parasites. Similar findings were reported by Aimpun and Hshieh24 in Belize, where women had more exposure to hookworm infection than men because most women worked at home and did not wear shoes.
The common clinical presentation of TB is weight loss,25 which was seen in this study with lower BMI in PCs than HCs. According to the conclusions of a study that showed that weight loss was related to impaired immune responses in TB patients,26 the finding in this study that being underweight (BMI ≤ 19) was associated with the intestinal parasitic infections only in PCs indicated that weight loss increased the risk of parasitic infection in persons with TB. The prevalence of anemia was 22–23% in this study and it was 35% in a study also conducted in rural area of central China,27 which suggested that approximately 20–33% of the population in rural area of central China is at risk of malnutrition. We found that anemia was strongly associated with infection of intestinal parasites for PCs. However, further investigation may be needed to determine the chronologic sequela of anemia, PTB, and intestinal parasitic diseases because PTB and intestinal parasitic diseases are debilitating diseases and often spread in poor areas in populations with malnutrition.28–30
In China, the main regimen of anti-MTB treatment is combined use of isoniazid, rifampicin, pyrazinamide, ethambutol, and streptomycin or their derivatives.14 Many studies have shown that one or more of these drugs had a significant in vivo effect against Leishmania spp.,31,32 Plasmodium spp.,33,34 Toxoplasma gondii,35 Wuchereria bancrofti,36 Brugia malayi,37 Onchocerca gutturosa,38 and Onchocerca lienalis and Brugia pahangi.39 However, we did not find evidence that anti-MTB drugs effected intestinal parasites, which was consistent with the finding in this study that anti-MTB treatment did not influence the infection rates of intestinal parasites in PCs.
One limitation of this study was that excluded participants were generally younger than those included in this study because most middle age persons worked away from home in the rural county and their children who stayed in their hometowns for school went to the cities where they worked and reunited with them during the summer holidays. Currently, the main trend of population flow in China is that a large number of the middle-aged populations in rural areas migrate to the cities to seek work. There were approximately 0.23 billion migrants with average age of approximately 28 years old in China in 2011.40 Therefore, we had to accept the limitation of age difference between the excluded and included participants. However, this limitation probably did not affect findings in this study because migrant populations spend time in their places of residence and usually are not a part of rural populations.
In conclusion, although PCs had poorer hygiene habits and shorter labor time in farmlands than HCs, they had similar infection rates for intestinal protozoa and helminths with HCs. No single factor was found in this study to affect infection rates between two groups. Infection of PCs was associated with female sex, low BMI, and anemia, and infection of HCs was associated only with more labor time in farmlands. Anti-MTB treatment did not influence infection rates of PCs.
ACKNOWLEDGMENTS
We thank Tian-Ping Wang (Anhui Province Institute of Parasitic Disease Control) and Xu-Dong Zhao and Yan Deng (Henan Province Center for Disease Control and Prevention) for study coordination; staff at Gushi County Center for Disease Control and Prevention, and health workers at township hospitals and clinics in villages in Gushi County for assistance; and persons who participated in the study.
Footnotes
Authors' contributions: Xin-Xu Li, Jia-Xu Chen, Li-Xia Wang, and Xiao-Nong Zhou conceived and designed the study; Xin-Xu Li, Li-Guang Tian, Yu-Ping Zhang, Shuang-Pin Dong, Xue-Guang Hu, Jian Lu, Feng-Feng Wang, Yue Wang, Xiao-Mei Yin, Li-Jun He, Qiu-Ye Yan, Hong-Wei Zhang, and Bian-Li Xu conducted the study; Xin-Xu Li and Li-Guang Tian analyzed the data; Xin-Xu Li wrote the first draft of the manuscript; Jia-Xu Chen, Li-Xia Wang, and Bian-Li Xu provided constructive opinions and suggestions; Xiao-Nong Zhou provided strategic advice and assisted with editing of the manuscript; and all authors read and approved the final version of the manuscript.
Financial support: This study was supported by National Science and Technology Major Program (grant no. 2012ZX10004-220).
Disclosure: None of the authors have any conflicts of interest.
Authors' addresses: Xin-Xu Li, National Institute of Parasitic Diseases, Chinese Center for Disease Control and Prevention, Key Laboratory of Parasite and Vector Biology, Ministry of Health, World Health Organization Collaborating Centre for Malaria, Schistosomiasis and Filariasis, Shanghai 200025, China, and National Center for Tuberculosis Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China, E-mail: lixinxu@chinatb.org. Jia-Xu Chen, Li-Guang Tian, and Xiao-Nong Zhou, National Institute of Parasitic Diseases, Chinese Center for Disease Control and Prevention, Key Laboratory of Parasite and Vector Biology, Ministry of Health, World Health Organization Collaborating Centre for Malaria, Schistosomiasis and Filariasis, Shanghai 200025, China, E-mails: chenjiaxu1962@163.com, jztlg@126.com, and xiaonongzhou1962@gmail.com. Li-Xia Wang, National Center for Tuberculosis Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing 102206, China, E-mail: wanglx@chinatb.org. Yu-Ping Zhang, Shuang-Pin Dong, Xue-Guang Hu, and Jian Liu, Gushi County Center for Disease Control and Prevention, Henan Province, Gushi 465200, China, E-mails: wuhen19870103@126.com, dsp.2525@163.com, and gsxnfk@163.com. Feng-Feng Wang, Yue Wang, and Xiao-Mei Yin, Anhui Province Institute of Parasitic Disease Control, Hefei 230061, China, E-mails: wangfengahwh@163.com, bridgety@foxmail.com, and xmyahwh@126.com. Li-Jun He, Qiu-Ye Yan, Hong-Wei Zhang, and Bian-Li Xu, Henan Province Center for Disease Control and Prevention, Zhengzhou 450016, China, E-mails: helj@hncdc.com.cn, yanqiuye@126.com, zhanghw@hncdc.com.cn, and xubl@hncdc.com.cn.
References
- 1.Manuel Ramos J, Reyes F, Tesfamariam A. Intestinal parasites in adults admitted to a rural Ethiopian hospital: relationship to tuberculosis and malaria. Scand J Infect Dis. 2006;38:460–462. doi: 10.1080/00365540500525187. [DOI] [PubMed] [Google Scholar]
- 2.Abate E, Belayneh M, Gelaw A, Idh J, Getachew A, Alemu S, Diro E, Fikre N, Britton S, Elias D, Aseffa A, Stendahl O, Schon T. The impact of asymptomatic helminth co-infection in patients with newly diagnosed tuberculosis in north-west Ethiopia. PLoS ONE. 2012;7:e42901. doi: 10.1371/journal.pone.0042901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization . Global Tuberculosis Control: WHO Report 2011. Geneva: World Health Organization, WHO/HTM/TB/2011.16; 2011. [Google Scholar]
- 4.Disease Control Bureau of the Ministry of Health, Chinese Center for Disease Control and Prevention . Report on the Fifth National Tuberculosis Epidemiological Survey in China, 2010. Beijing, China: Military Medical Science Press; 2011. [Google Scholar]
- 5.Tian LG, Chen JX, Wang TP, Cheng GJ, Steinmann P, Wang FF, Cai YC, Yin XM, Guo J, Zhou L, Zhou XN. Co-infection of HIV and intestinal parasites in rural area of China. Parasit Vectors. 2012;5:36. doi: 10.1186/1756-3305-5-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tian LG, Wang TP, Lv S, Wang FF, Guo J, Yin XM, Cai YC, Dickey MK, Steinmann P, Chen JX. HIV and intestinal parasite co-infections among a Chinese population: an immunological profile. Infect Dis Poverty. 2013;2:18. doi: 10.1186/2049-9957-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mofid LS, Bickle Q, Jiang JY, Du ZW, Patrick E. Soil-transmitted helminthiasis in rural south-west China: prevalence, intensity and risk factor analysis. Southeast Asian J Trop Med Public Health. 2011;42:513–526. [PubMed] [Google Scholar]
- 8.Tang N, Luo NJ. A cross-sectional study of intestinal parasitic infections in a rural district of west China. Can J Infect Dis Med Microbiol. 2003;14:159–162. doi: 10.1155/2003/721930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Resende Co T, Hirsch CS, Toossi Z, Dietze R, Ribeiro-Rodrigues R. Intestinal helminth co-infection has a negative impact on both anti-Mycobacterium tuberculosis immunity and clinical response to tuberculosis therapy. Clin Exp Immunol. 2007;147:45–52. doi: 10.1111/j.1365-2249.2006.03247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vasil'ev AV, Shenderova RI, Ginzburg ZI, Vasil'ev VI. Tuberculosis of the lungs complicated by opisthorchiasis under conditions of the extreme north, 1989. [in Russian] Probl Tuberk. 1989;6:41–44. [PubMed] [Google Scholar]
- 11.Kashuba EA, Rusakova LI. Anthelmintic therapy of opisthorchiasis in patients with active tuberculosis [in Russian] Probl Tuberk. 1992;3–4:33–36. [PubMed] [Google Scholar]
- 12.Ferreira AP, Aguiar AS, Fava MW, Correa JO, Teixeira FM, Teixeira HC. Can the efficacy of Bacille Calmette-Guerin tuberculosis vaccine be affected by intestinal parasitic infections? J Infect Dis. 2002;186:441–442. doi: 10.1086/341656. author reply 442–443. [DOI] [PubMed] [Google Scholar]
- 13.Elias D, Akuffo H, Pawlowski A, Haile M, Schon T, Britton S. Schistosoma mansoni infection reduces the protective efficacy of BCG vaccination against virulent Mycobacterium tuberculosis. Vaccine. 2005;23:1326–1334. doi: 10.1016/j.vaccine.2004.09.038. [DOI] [PubMed] [Google Scholar]
- 14.Disease Control Bureau of the Ministry of Health, Department of Medical Administration of the Ministry of Health, Chinese Center for Disease Control and Prevention . Guidelines for Implementing the National Tuberculosis Control Program in China, 2008. Beijing, China: Peking Union Medical College Press; 2009. [Google Scholar]
- 15.Lu Z, Zhong N. Internal Medicine. Beijing, China: People's Medical Publishing House; 2008. [Google Scholar]
- 16.Leelayoova S, Taamasri P, Rangsin R, Naaglor T, Thathaisong U, Mungthin M. In-vitro cultivation: a sensitive method for detecting Blastocystis hominis. Ann Trop Med Parasitol. 2002;96:803–807. doi: 10.1179/000349802125002275. [DOI] [PubMed] [Google Scholar]
- 17.Katz N, Chaves A, Pellegrino J. A simple device for quantitative stool thick-smear technique in Schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo. 1972;14:397–400. [PubMed] [Google Scholar]
- 18.Elias D, Mengistu G, Akuffo H, Britton S. Are intestinal helminths risk factors for developing active tuberculosis? Trop Med Int Health. 2006;11:551–558. doi: 10.1111/j.1365-3156.2006.01578.x. [DOI] [PubMed] [Google Scholar]
- 19.Elias D, Britton S, Kassu A, Akuffo H. Chronic helminth infections may negatively influence immunity against tuberculosis and other diseases of public health importance. Expert Rev Anti Infect Ther. 2007;5:475–484. doi: 10.1586/14787210.5.3.475. [DOI] [PubMed] [Google Scholar]
- 20.Li XX, Zhou XN. Co-infection of tuberculosis and parasitic diseases in humans: a systematic review. Parasit Vectors. 2013;6:79. doi: 10.1186/1756-3305-6-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng Q, Chen Y, Zhang HB, Chen JX, Zhou XN. The control of hookworm infection in China. Parasit Vectors. 2009;2:44. doi: 10.1186/1756-3305-2-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo JD, Shen GJ, Wu WD, Li QY, Yin XM, Zhou L, Ji H. Distribution and risk factors of Ascaris infection in Anhui Province [in Chinese] Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2004;16:456–459. [Google Scholar]
- 23.National Center for Tuberculosis Control and Prevention of Chinese Center for Disease Control and Prevention Health Promotion Toolkit of Chinese Tuberculosis Control and Prevention. 2005. http://tb.chinacdc.cn/chinatb/chinatb1/index.htm Available at.
- 24.Aimpun P, Hshieh P. Survey for intestinal parasites in Belize, Central America. Southeast Asian J Trop Med Public Health. 2004;35:506–511. [PubMed] [Google Scholar]
- 25.Rathman G, Sillah J, Hill PC, Murray JF, Adegbola R, Corrah T, Lienhardt C, McAdam KP. Clinical and radiological presentation of 340 adults with smear-positive tuberculosis in The Gambia. Int J Tuberc Lung Dis. 2003;7:942–947. [PubMed] [Google Scholar]
- 26.Mahuad C, Bozza V, Pezzotto SM, Bay ML, Besedovsky H, del Rey A, Bottasso O. Impaired immune responses in tuberculosis patients are related to weight loss that coexists with an immunoendocrine imbalance. Neuroimmunomodulation. 2007;14:193–199. doi: 10.1159/000110646. [DOI] [PubMed] [Google Scholar]
- 27.Tian LG, Cheng GJ, Chen JX, Cai YC, Guo J, Tong XM, Liu Q, Zhou XN. Survey on co-infection with HIV and intestinal parasites in high prevalence areas of HIV/AIDS, China [in Chinese] Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2012;24:168–172. [PubMed] [Google Scholar]
- 28.Gupta KB, Gupta R, Atreja A, Verma M, Vishvkarma S. Tuberculosis and nutrition. Lung India. 2009;26:9–16. doi: 10.4103/0970-2113.45198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pawlowski ZS. Implications of parasite-nutrition interactions from a world perspective. Fed Proc. 1984;43:256–260. [PubMed] [Google Scholar]
- 30.Amare H, Gelaw A, Anagaw B, Gelaw B. Smear positive pulmonary tuberculosis among diabetic patients at the Dessie referral hospital, Northeast Ethiopia. Infect Dis Poverty. 2013;2:6. doi: 10.1186/2049-9957-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mendez S, Traslavina R, Hinchman M, Huang L, Green P, Cynamon MH, Welch JT. The antituberculosis drug pyrazinamide affects the course of cutaneous leishmaniasis in vivo and increases activation of macrophages and dendritic cells. Antimicrob Agents Chemother. 2009;53:5114–5121. doi: 10.1128/AAC.01146-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Conti R, Parenti F. Rifampin therapy for brucellosis, flavobacterium meningitis, and cutaneous leishmaniasis. Rev Infect Dis. 1983;5((Suppl 3)):S600–S605. doi: 10.1093/clinids/5.supplement_3.s600. [DOI] [PubMed] [Google Scholar]
- 33.Pukrittayakamee S, Viravan C, Charoenlarp P, Yeamput C, Wilson RJ, White NJ. Antimalarial effects of rifampin in Plasmodium vivax malaria. Antimicrob Agents Chemother. 1994;38:511–514. doi: 10.1128/aac.38.3.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aditya NP, Patankar S, Madhusudhan B. Assessment of in vivo antimalarial activity of rifampicin, isoniazide, and ethambutol combination therapy. Parasitol Res. 2010;106:1481–1484. doi: 10.1007/s00436-010-1789-y. [DOI] [PubMed] [Google Scholar]
- 35.Araujo FG, Khan AA, Remington JS. Rifapentine is active in vitro and in vivo against Toxoplasma gondii. Antimicrob Agents Chemother. 1996;40:1335–1337. doi: 10.1128/aac.40.6.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Debrah AY, Mand S, Marfo-Debrekyei Y, Batsa L, Albers A, Specht S, Klarmann U, Pfarr K, Adjei O, Hoerauf A. Macrofilaricidal activity in Wuchereria bancrofti after 2 weeks treatment with a combination of rifampicin plus doxycycline. J Parasitol Res. 2011;2011:201617. doi: 10.1155/2011/201617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rao R, Well GJ. In vitro effects of antibiotics on Brugia malayi worm survival and reproduction. J Parasitol. 2002;88:605–611. doi: 10.1645/0022-3395(2002)088[0605:IVEOAO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 38.Townson S, Tagboto S, McGarry HF, Egerton GL, Taylor MJ. Onchocerca parasites and Wolbachia endosymbionts: evaluation of a spectrum of antibiotic types for activity against Onchocerca gutturosa in vitro. Filaria J. 2006;5:4. doi: 10.1186/1475-2883-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Townson S, Hutton D, Siemienska J, Hollick L, Scanlon T, Tagboto SK, Taylor MJ. Antibiotics and Wolbachia in filarial nematodes: antifilarial activity of rifampicin, oxytetracycline and chloramphenicol against Onchocerca gutturosa, Onchocerca lienalis and Brugia pahangi. Ann Trop Med Parasitol. 2000;94:801–816. doi: 10.1080/00034980020027988. [DOI] [PubMed] [Google Scholar]
- 40.Department of Services and Management of Migrant Population of the National Population and Family Planning Commission . Report in 2012 on China's Migrant Population Development. Beijing, China: China Population Publishing House; 2012. [Google Scholar]