Abstract
Dracunculiasis was rediscovered in Chad in 2010 after an apparent absence of 10 years. In April 2012 active village-based surveillance was initiated to determine where, when, and how transmission of the disease was occurring, and to implement interventions to interrupt it. The current epidemiologic pattern of the disease in Chad is unlike that seen previously in Chad or other endemic countries, i.e., no clustering of cases by village or association with a common water source, the average number of worms per person was small, and a large number of dogs were found to be infected. Molecular sequencing suggests these infections were all caused by Dracunculus medinensis. It appears that the infection in dogs is serving as the major driving force sustaining transmission in Chad, that an aberrant life cycle involving a paratenic host common to people and dogs is occurring, and that the cases in humans are sporadic and incidental.
Introduction
As the Guinea Worm Eradication Program (GWEP) progresses toward its ultimate goal of global eradication, ongoing efforts now focus on the four remaining endemic countries of Chad, Ethiopia, Mali, and South Sudan.1 This report describes the recent situation in Chad, where an outbreak was detected in 2010 after 10 years in which no cases were reported. Whether the infection in Chad was reintroduced or had continued at very low levels without detection is unknown. Additionally, investigations have not established firm linkage between human cases from year to year, where over the past 3.5 years (2010–June 2013), 35 cases have occurred in 31 villages but only twice have cases occurred in the same village in successive years. Furthermore, investigations have not established common water sources of infection as has been typical elsewhere. In addition, there has not been a rapid or explosive increase in cases as might be expected of typical Guinea worm transmission. Moreover, the number of infected persons per village has been limited to a single person in 31 of the 35 cases, and the average number of worms per infected person is lower, on average, than that in other endemic areas. These unusual epidemiologic features call into question whether some aberration to the typical Guinea worm (Dracunculus medinensis) life cycle is occurring in Chad. Added to this has been the observation of even more frequent Dracunculus infections in dogs in the same geographic area in Chad where most of the human cases have occurred, which again is counter to historical reports where infections in dogs were rarely reported even when human infections were very common.2–4
These observations of human and dog infections have raised the question of possible association between the infections in people and dogs, whether dogs somehow serve as a reservoir for human infections in this special instance, and whether the dogs are infected with a distinct species of Dracunculus that is spilling over into people as a zoonosis. This further begs the question of whether some form of paratenic host, such as fish, frogs, or some other animal may be channeling the infection to both dogs and people, as is known to occur in other dracunculids.5 In many regards, the recent epidemiology of human infections in Chad, i.e., sporadic unlinked cases, suggests a zoonotic infection that includes a paratenic host, and it also could explain why control efforts in place since 2011 (albeit not fully implemented in the first year) have not reduced the annual number of human infections.
This report describes our efforts to characterize the infection in people and dogs in Chad epidemiologically and at the molecular level, and to explain the unusual recent pattern of human dracunculiasis in Chad.
Materials and Methods
Surveillance in humans.
Beginning before the outbreak in 2010, as a part of precertification activities, the Chad GWEP, with support from the World Health Organization (WHO), began disseminating information by radio broadcasts, posters, and person to person about the availability of a monetary reward (circa US $100) for reports of cases of the disease. Increased awareness about such rewards led to the detection of the first human case in April 2010, which marked the beginning of the current outbreak. In March 2011 the ministry of health in Chad requested The Carter Center to help its GWEP establish an active village-based surveillance system (multiple household-by-household searches for cases each week and immediate reporting of patent cases or of patients with suspected signs/symptoms of dracunculiasis) in at-risk areas (all communities in the catchment areas of health centers having one or more villages reporting cases in 2010 and 2011) to detect and confirm cases of the disease more promptly, and to implement interventions to prevent contamination of sources of drinking water. Training of nearly 2,000 male and female village volunteers, and of about 100 supervisory staff began in October 2011. The surveillance system became fully operational in April 2012. Efforts were made to collect all emergent adult female worms from patients whenever possible and preserve them in alcohol to allow subsequent microscopic examination of the morphology of the worms and molecular testing. All human cases were interviewed using a structured questionnaire, including travel and residence during the preceding year, water sources used, and food habits, especially eating of under/uncooked foods. The geographic coordinates of their current village of residence at the time of worm emergence were determined and plotted.
Surveillance in dogs.
Beginning in 2011 there were a few rumors of cases of worms in dogs that sounded very much like emerging Guinea worms. Concurrent with the start of the active village-based surveillance system in April 2012, dogs with worms emerging from their skin (Figure 1 ) began to be observed by program staff. These dogs were investigated more systematically, and as GWEP and Carter Center technical staff made routine visits to each village, village volunteers and village supervisors were alert to any reports of emergent worms in local dogs. Any such emergent worms in dogs were noted and when possible collected and preserved in alcohol for subsequent examination just as for worms from humans. A structured questionnaire also was developed to interview dog owners regarding whether their dog had emergent worm(s), types of foods eaten, sources of drinking water, and travel of the dog away from the village of residence. Particular attention was paid to similarity or commonality in food and drinking water between people and dogs. Dog owners were interviewed within the endemic zone, as established by the presence of human infection, and outside the at-risk zone (see map, Figure 2 ), to establish whether infections in dogs occurred in a larger geographic area than did the human infections.
Figure 1.
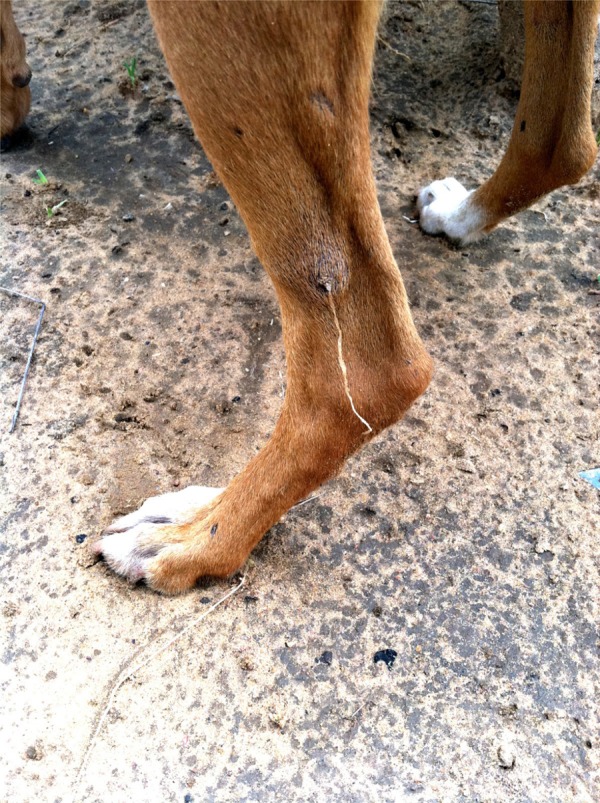
Photograph of a dog with an emergent worm on left hind leg (Photo by Chad GWEP).
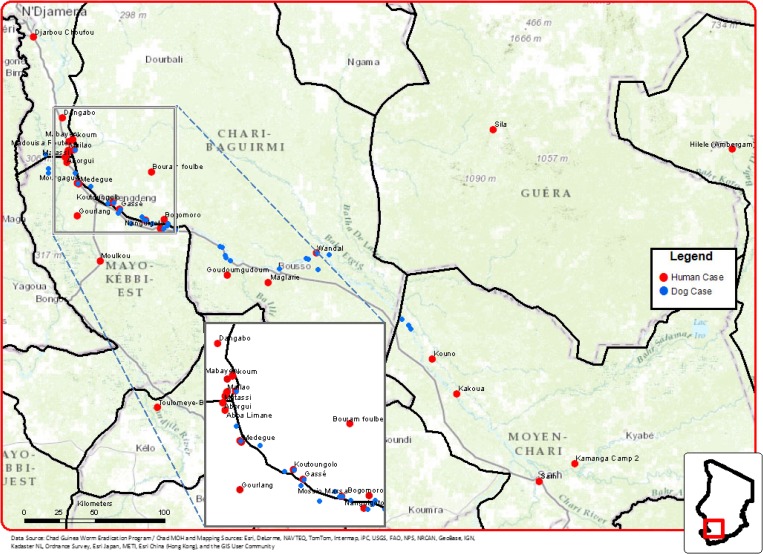
Figure 2.
Map of the Guinea worm-endemic area in Chad, noting villages reporting cases of dracunculiasis in humans 2010–2013* (red dots) and dogs 2012–2013* (blue dots) (* provisional: January–June, 2013).
The parasite.
All emergent female worms collected from people between 2010 to June 2013, and emergent female worms collected from dogs during April 2012 to June 2013, were submitted to the Centers for Disease Control and Prevention (CDC) for microscopic and molecular evaluation. Initially, worms were evaluated through polymerase chain reaction (PCR) amplification and DNA sequence analysis of the 18S small subunit ribosomal RNA locus. This method was previously described to distinguish D. medinensis from Dracunculus insignis,6 a closely related species that occurs in wildlife in North America. This method amplifies 1.8 Kb of the gene, with a difference in both total length (1,819 bases long in D. medinensis, and 1,821 in D. insignis) and at eight positions (= difference of 0.44%). To increase resolution, a mitochondrial target from the cytochrome c oxidase subunit 1 gene (COX1) was added to the evaluation, which increased the capacity to discriminate D. medinensis from D. insignis. All worms from Chad were subjected to evaluation of both genes. To confirm the species identity of the specimens recovered from people and dogs in Chad, DNA from female worms or larvae was amplified as previously described and sequenced in both forward and reverse directions6,7; the resulting sequences were assembled and compared with GenBank references using the basic local alignment search tool (BLAST) algorithm.
Subsequently, whole genome sequencing was pursued in collaborations with the Wellcome Trust Sanger Institute and a subset of worms from humans and dogs were used for sequencing the genome to confirm the identification of worms from people and dogs in Chad. Previous work between CDC and the Sanger Institute has allowed characterization of the D. medinensis genome using an intact, frozen female worm collected in Ghana in 2001 and a version of this 103.75 Mb assembly (available at ftp://ftp.sanger.ac.uk/pub/pathogens/HGI/) was available for the comparison of the Chad isolates. Further work on genome assembly and annotation is still ongoing and will be described in full elsewhere. Also used in the comparison of human and dog worms from Chad were preserved human isolates of Guinea worms collected in Ghana and South Sudan. Preserved material of D. insignis, available at CDC, was also sequenced as a comparative out-group. The whole genome libraries for this study were generated using the Illumina platform (Illumina, San Diego, CA), and each library originated from single adult female worm sections. Genomic DNA was isolated using a Promega Wizard genomic DNA purification kit (A1120) (Promega Corporation, Madison, WI) with minor alterations to the manufacturer's protocol. After RNase A treatment genomic DNA was used for preparation of amplification free Illumina paired end libraries using the protocol described previously,8 based on the methods described by Kozarewa and others,9 except for two low concentration libraries that were amplified by PCR using Illumina primer PE1.0, the appropriate Sanger indexed primer and KAPA HiFi HotStart ready mix (KAPABIOSYSTEMS, Boston, MA). All genomic libraries are detailed in Table 1 . The SNPs were called by mapping reads to the reference genome assembly with SMALT v0.7.4 with indexing parameters “-k 13 -s 2” and mapping parameters “-i 470 -r 0 -x -m 85.” this mapping was then realigned around indel positions and single nucleotide polymorphisms (SNPs) called and filtered using the Genome Analysis Toolkit (GATK) v.2.0.10 The SNP calling used the Unified Genotyper tool with parameters –stand_emit_conf 10.0 and –stand_call_conf 3.11 The SNP calls matching any of the following filter conditions were removed, to eliminate calls supported by a sufficient number of high-quality, well-mapping reads on both strands, or those supported by base calls only near the ends of reads: QD < 2.0, MQ < 4.0, FS > 60.0, HaplotypeScore > 13.0, MQRankSum < −12.5, ReadPosRankSum < −8.0. See GATK documentation for full details of these parameters. Pairwise SNP distances between samples were calculated and visualized as a two-dimensional multidimensional scaling using PLINK v.1.02.12
Table 1.
Genomic libraries
| Sample ID | Parasite species | Host species | Geographic location | Mean insert size (base pairs) | Total yield (kilobases) | ENA sample accession no. | % of reads mapping | Ave coverage depth | PCR-free or no. of cycles |
|---|---|---|---|---|---|---|---|---|---|
| Din88_31_297853_Ca_F | D. insignis | ferret | Canada | 281 | 24,902,671 | ERS201842 | 8.17*† | 12.40 | no PCR |
| Dmed10_14_297853_S_H | D. medinensis | human | South Sudan | 246 | 13,690,682 | ERS201830 | 17.32* | 20.09 | 18 cycles |
| Dmed11_1_297853_Ch_H | D. medinensis | human | Chad | 351 | 7,984,128 | ERS201824 | 87.53 | 33.49 | no PCR |
| Dmed12_38_297853_Ch_D | D. medinensis | dog | Chad | 363 | 5,192,789 | ERS201836 | 89.28 | 43.36 | no PCR |
| Dmed12_58_297853_Ch_H | D. medinensis | human | Chad | 390 | 2,966,654 | ERS201828 | 85.90 | 23.34 | 8 cycles |
| DmedGCW_297853_G_H | D. medinensis | human | Ghana | 324 | 6,493,629 | ERS201834 | 88.18 | 30.02 | no PCR |
All reads were 100 bp paired end Illumina reads.
Low mapping percentage caused by bacterial contamination in these sequencing libraries.
Low mapping percentage caused by bacteria contamination and high divergence from the D. medinensis reference genome.
PCR = polymerase chain reaction. ENA = European Nucleotide Archive.
Paratenic hosts.
A limited survey of potential paratenic hosts was undertaken during the collective fish harvesting period near the end of the dry season, June, 2013, which is also the middle of the transmission season. During a 7-day period, over 200 fish of roughly 20 different species, 28 frogs comprising two different species, and 2 large monitor (water) lizards were examined for the presence of Dracunculus larvae using standard procedures for looking at muscle and viscera.
Results
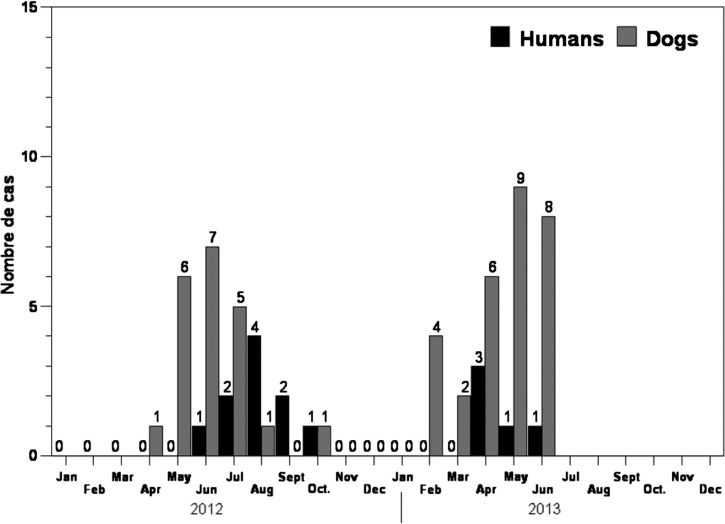
In 2010–2012, there were 30 human cases of Guinea worm disease (GWD) reported in 26 villages, including only two villages with a case in consecutive years (Table 2). There were five cases reported during January–June 2013 from five new villages not previously reporting cases during 2010–2012 (Figure 2). This resulted in an average of 1.15 infected persons per affected village. This is fewer patients on average per affected village than in Ghana and Ethiopia in 2010 and Mali in 2011 where the average was two (and formerly even higher). Furthermore, in 2010–2012 fewer worms per person (1.27) occurred in Chad than in Ethiopia (1.96) or Mali (1.7). The male/female ratio of cases was 18 of 17, and the age distribution < 15 yr/15 yr+ was 13 of 22. Peak emergence of worms was April–September in humans and dogs (Figure 3). The majority of cases occurred along a 150 km long stretch of the Chari River (Figure 2) and serves to define the at-risk zone. Although the at-risk zone includes villages along both sides of the Chari River, there is no evidence to suggest that transmission occurs in the river proper. Instead, it is the lagoons formed as the river recedes during the end of the dry season and small traditional ponds in these communities where local transmission occurs. Surveys of people and interviews with dog owners in other areas of Chad, including along the Logone and Molkou Rivers to the west of the Chari River and in communities north and east of the Chari River, indicated an absence of infections in dogs and extremely few cases in people in these areas.
Table 2.
Villages in Chad reporting cases of dracunculiasis, 2010–2013*
| Village no. | Village | District | Cases | |||
|---|---|---|---|---|---|---|
| Number contained†/Number reported | ||||||
| 2010 | 2011 | 2012 | 2013* | |||
| 1 | Nanguigoto | Guelendeng | 0/2 | 0/0 | 0/0 | 0/0 |
| 2 | Mouraye | Massenya | 0/1 | 0/0 | 0/0 | 0/0 |
| 3 | Matassi | Mandalia | 0/1 | 0/0 | 0/0 | 0/0 |
| 4 | Abba Limane | Guelendeng | 0/1 | 0/0 | 0/0 | 0/0 |
| 5 | Aborgui | Massenya | 0/1 | 0/0 | 0/0 | 0/0 |
| 6 | Molkou‡ | Guelendeng | 0/1 | 0/0 | 0/0 | 0/0 |
| 7 | Kakoua | Sarh | 0/1 | 0/0 | 0/0 | 0/0 |
| 8 | Sila | Melfi | 0/2 | 0/0 | 0/0 | 0/0 |
| 9 | Toulomeye-Bardai | Bere | 1/1 | 0/0 | 0/0 | |
| 10 | Wandal | Bousso | 0/1 | 0/0 | 0/0 | |
| 11 | Mailao marba | Mandelia | 1/1 | 0/0 | 0/0 | |
| 12 | Mossio Vill. cluster§ | Bousso | 0/1 | 2/2 | 0/0 | |
| 13 | Goudoumgudoum‡ | Bousso | 0/2 | 0/0 | 0/0 | |
| 14 | Darkou | Mandelia | 0/1 | 0/0 | 0/0 | |
| 15 | Akoum-Mabaye§ | Mandelia | 1/1 | 0/1 | 0/0 | |
| 16 | Camp Sara Matassi | Mandelia | 0/1 | 0/0 | 0/0 | |
| 17 | Manglarie | Bousso | 1/1 | 0/0 | 0/0 | |
| 18 | Mourgagué | Guelendeng | 0/1 | 0/0 | ||
| 19 | Hilele (Ambergan) | Aboudeia/Salamat | 0/1 | 0/0 | ||
| 20 | Bouram Foulbe‡ | Massenya | 1/1 | 0/0 | ||
| 21 | Dangabo | Mandelia | 0/1 | 0/0 | ||
| 22 | Kouno Center | Bousso | 0/1 | 0/0 | ||
| 23 | Kamanga 2 Camp | Kyabe | 0/1 | 0/0 | ||
| 24 | Sarh Town | Sarh | 1/1 | 0/0 | ||
| 25 | Miskine Banana | Mandelia | 1/1 | |||
| 26 | Koutoungolo | Massenya | 1/1 | |||
| 27 | Gasse | Massenya | 1/1 | |||
| 28 | Gourlong | Guelendeng | 1/1 | |||
| 29 | Djarbou Choufou | Mandelia | 0/1 | |||
| TOTAL | 0/10 | 4/10 | 4/10 | 4/5 | ||
Provisional: January–June 2013.
Transmission from a patient with dracunculiasis is contained if all of the following conditions are met: 1) the disease is detected < 24 hours after worm emergence; 2) the patient has not entered any water source since the worm emerged; 3) a health system staff or volunteer has managed the patient properly, by cleaning and bandaging the lesion(s) until the worm has been fully removed manually and by providing health education to discourage the patient from contaminating any water source (if two or more emerging worms are present, transmission is not contained until the last worm is removed); and 4) the containment process, including verification of dracunculiasis, is validated by a supervisor within 7 days of emergence of the worm. All of these criteria must be achieved for each emerged worm for the case to be considered contained.
Cases of Guinea worm disease (GWD) (7) linked to Mossio village cluster.
= Endemic villages.
Figure 3.
Graph showing the month of appearance of Guinea worms in people and dogs for 2012 and 2013* (* provisional: January–June, 2013).
Moreover, the current epidemiology of Guinea worm disease in Chad is different from the epidemiology that was seen in Chad itself in the 1990s. No cases were seen in Chari Baguirmi Region after four cases were counted there during the case search in 1993, whereas 15 (43%) of the 35 cases in humans and 42 (75%) of the 56 cases in dogs in the current outbreak were in Chari Baguirmi. The average number of infected persons per affected village in Chad was 3.0 during village-based surveillance in 1995–1997 (even after discarding one village that had 66 cases in 1996), versus 1.15 in the current outbreak. In the 1990s, the season of peak transmission in western Chad, which is the area of the current outbreak, was January–March; now it is April–September (Figure 3). No dogs with emerging Guinea worms were reported in Chad before the current outbreak.
Between March 2012 and June 2013, 56 infected dogs were detected, all from the two administrative regions of Chari Baguirmi and Mayo Kebbi Est (Figure 2), the same two regions where 12 of 15 human cases occurred in 2012–June 2013 (Table 3), and also where most human cases in 2011 and 2010 occurred. However, most cases in dogs did not come from the same villages where the human cases were reported, although there was co-occurrence in six villages. Overall, the incidence of infection in dogs was four times that of humans over the same period within the same administrative regions (Table 3; Figure 3). Moreover, in January–June 2013, dogs yielded 7.6 times as many worms as humans (53 versus 7) (Table 3).
Table 3.
Summary of cases of Guinea worm in people and dogs in Chad, 2012–June 2013
| 2012 | Jan–June 2013 | Total 2012–June 2013 | ||||
|---|---|---|---|---|---|---|
| People | Dogs | People | Dogs | People | Dogs | |
| No. cases | 10 | 27* | 5 | 29 | 15 | 56 |
| No. worms | 11 | 40 | 7 | 53† | 18 | 93 |
| No. worms/case | 1.1 | 1.5 | 1.4 | 1.8 | 1.2 | 1.7 |
| Range | 1–2 | 1–6 | 1–3 | 1–9 | 1–3 | 1–9 |
Although not defined, we use the same case definition for infections in dogs as that for people, i.e., regardless of how many worms emerge a subject is counted only once as a case during the calendar year.
Represents 38 collected worms and 15 observed but not collected worms.
The interviews with dog owners within and outside the endemic zone yielded several noteworthy observations. First, only owners who lived within the endemic zone were aware of emergent worms in dogs, including their own or others in the village. Dog owners outside the endemic zone, including along other major rivers such as the Logone, were not aware of such infections in their own or other dogs in the area during the past 10 years, suggesting that the infection in dogs is confined to the same geographic areas as the majority of the human infections—a narrow band along the Chari River. Second, dog owners in both endemic and non-endemic areas confirmed that dogs and people typically drank water from the same sources (i.e., river, pond, bore hole); both within the household and outside the village, and that people and dogs eat some shared foods. The most commonly cited shared food was a cooked grain-based paste called “boule.” Questions however focused on other types of food, especially those that were either undercooked or eaten raw, and/or that had a close association with water, such as fish, frogs, snakes, turtles, and lizards. People generally denied eating any such foods raw, but one of the major food staples at the end of the dry season is dried or smoked fish, which is in greater abundance as a result of the annual mass harvesting (Figures 4 –6
Figure 5.

Photograph of mass drying of small fish directly on mats on the ground, Chad (Photo by Chad GWEP).
), and the occasional frog or water lizard.
Figure 4.

Photograph of mass fish harvesting in a local lagoon associated with one of the endemic villages in Chad. Not seen in this photograph is an open body of water to the left and to the right that is at least 10 times greater than the area depicted in this image (Photo by Chad GWEP).
Figure 6.

Larger fish split, gutted, and drying on elevated mats, Chad (Photo by Chad GWEP).
Dogs were also reported to eat raw or dried/smoked fish that they were able to steal. Additionally, when larger fish were gutted, the entrails were left on the ground for dogs, ducks, and chickens to scavenge (Figure 7). Dog owners were asked whether they recalled seeing dogs with worms in years past. The owners uniformly responded that this was a new occurrence, and that they were aware of worms emerging from dogs only in the past few years.
Figure 7.
Photograph of fish cleaning area with viscera (arrows) on ground and accessible to scavenging dogs. Gray specks on ground are fish scales (Photo by Chad GWEP).
Getting an accurate assessment of the number of dogs in a village was difficult, but each dog was attributed to a specific household, in as much as there were no dogs identified as “stray.” Rates of dog ownership in small rural villages in the endemic zone are not known, but thought to be < 20%, and the number of dogs per household generally varied between 1 and 3 dogs, although as many as 5 or 6 was noted on occasion. However, most residents do not perceive dogs as pets. Although difficult to determine accurately, owners were questioned regarding the number of dogs in the household that had emergent worms, and the number of worms that emerged per dog. In 2012, among 13 households with multiple dogs, 8 (62%) had an infected dog, and in 3 (23%) households, more than one dog was infected. In those dogs with worms, slightly less than half (46%) had multiple worms, and averaged 2.4 worms per dog.
Thirty-five emergent female worms were collected from people between 2010 and June 2013. During April 2012 to June 2013, 93 emergent female worms were observed in dogs and 47 of these worms were submitted to CDC for evaluation. All 35 worms recovered from people and the 47 from dogs were examined microscopically and were indistinguishable from each other, and shared features common to Dracunculus based on observable morphologic features noted for the genus, including characteristic shape of the female tail and the presence of typical Dracunculus first-stage larvae (L1). Microscopically, the worms recovered from people and dogs in Chad could not be distinguished from worms collected from humans in other endemic countries.
Thirty-one of these specimens (14 from people, 17 from dogs) were subjected to molecular analysis at CDC. For all samples with positive DNA amplification and sequencing, BLAST results with the 18S rRNA and COX1 were matched to D. medinensis (≥ 99% similarity, E-value = 0, and highest bit scores). The resulting sequences for D. medinensis from people in Chad, Ethiopia, Mali, and South Sudan, and for dogs in Chad, were deposited in GenBank. Sequences from the 18S rRNA locus were assigned accession nos., KF770012–KF770020, respectively, and from the mitochondrial COX1 locus assigned accession nos., KF770021–KF770026, respectively. The DNA sequence analysis from the nuclear and mitochondrial loci confirmed the identification of all 31 specimens as D. medinensis.
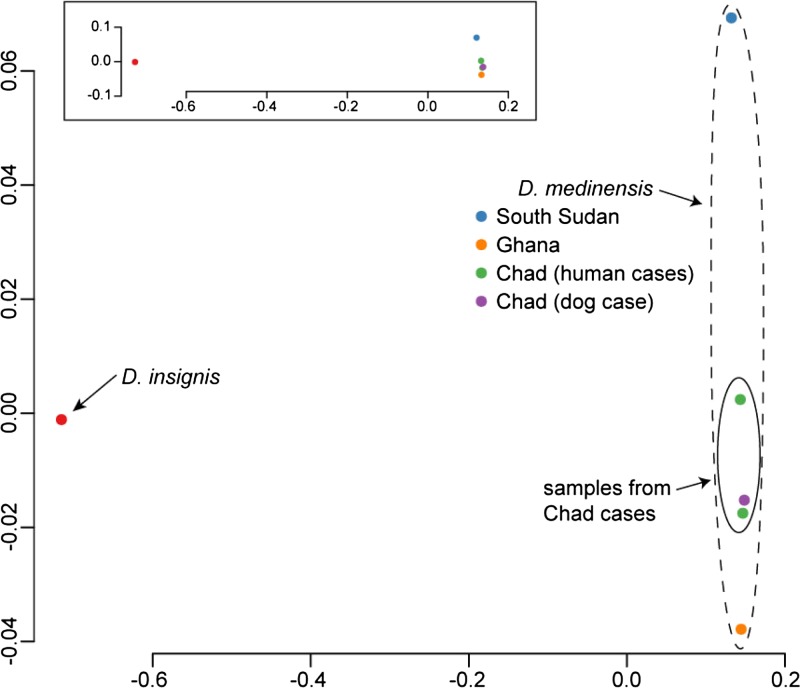
The comparison of whole genome sequences did not detect significant differences between the specimens isolated from people and dogs in Chad when compared with specimens from Ghana and South Sudan. Our whole-genome resequencing data produced an average of 27× coverage of the D. medinensis genome (Table 1), and allowed us to identify SNPs at a total of 1.3 million sites. Despite the small sample size, preliminary analysis of these variants clearly shows that D. insignis is highly divergent from the African D. medinensis samples, and that most of the variation within D. medinensis is between samples from different countries (Figure 8). In particular, although it is impossible to reach a strong conclusion about the genetic diversity present in the field from our very small sample of parasites, no significant differences were detected across the genome between the specimens from people and dogs within Chad in our data.
Figure 8.
Pairwise genetic distances between Dracunculus isolates. Data shown are a two-dimensional multidimensional scaling of single-nucleotide polymorphism (SNP) distances between isolates based on genome-wide sequencing data as described in the text. Note that the two axes of the main plot have very different scales: the inset plot shows the same data drawn with two equivalent axes.
No Dracunculus larvae were recovered from the tissues of any of the fish, frogs, or lizards examined.
Discussion
The unusual epidemiology of the 35 human cases of dracunculiasis in Chad between 2010 and 2013, including lack of clustering or link to a common water source, and preponderance of single cases in any given village over the course of the 3.5 years, suggested that the epidemiology is atypical and raises the possibility of either an unusual zoonotic species of Dracunculus previously unrecognized in Chad or elsewhere in the African region, or an unusual pattern of transmission for D. medinensis that involves a paratenic host. The emergence of an unusually large number of worms from dogs that were confirmed to be Dracunculus also supported but did not distinguish between the same two possibilities. Although there are historical reports of D. medinensis in wild animals, there is no confirmatory evidence that any naturally occurring non-human infections represent D. medinensis. Morphological distinction (and thus confirmatory diagnosis) is difficult as there are no clearly defined morphologic differences evident on emergent female worms or L1 larvae to distinguish species2,4; the most salient morphological features distinguishing species of Dracunculus are present in the male worms, which are rarely recovered. Thus, it is only recently that North American dracunculid infections of wildlife, classically considered D. medinensis, have been distinguished through molecular biology as distinct species (D. insignis and D. lutrae).6,7,13,14 To date, there have been no descriptions of other Dracunculus species in mammals in Africa except D. medinensis, nor is there evidence to date that suggests animals act as reservoir hosts for human Guinea worms. Although it is possible that infections in animals could represent aberrant infection with D. medinensis, it is also likely that they represent other, unknown locally endemic Dracunculus species. Reports attributing such animal infections to D. medinensis from countries that were never endemic for D. medinensis are especially unlikely to represent D. medinensis infection. Similarly, rare reports of human infections from countries that were never endemic for dracunculiasis also are likely of zoonotic origin and not D. medinensis.15–17
Reports of Guinea worm infections in dogs are not new, dating as far back as the 1920s, and include areas that were endemic for D. medinensis (e.g., Africa, India, Sri Lanka, Central Asian republics) and areas that have never been endemic for D. medinensis (e.g., Canada, United States, South America, China).2,4 In non-endemic areas, such infections have been attributed to a locally occurring animal species, such as D. insignis in Canada and the United States, or, on other occasions such as in China, to D. medinensis even though dracunculiasis was never endemic in that area. More likely in the latter situations, some other unrecognized animal species was responsible for the infection. In areas endemic or formerly endemic for D. medinensis, similar infections in dogs and other animals are often referred to as D. medinensis, although the identity of such worms has not been verified until this study.
The work presented in this report provides the first molecular confirmation that worms from dogs recovered in Chad are indistinguishable from those recovered from people in Chad. All worms from Chad have no detectable differences morphologically or molecularly from D. medinensis obtained from humans in other areas of Africa. It is conceivable that other species of Dracunculus that could infect dogs are also present in animals. Thus, we should be wary of calling all Guinea worms in dogs D. medinensis. Assigning all human worms to D. medinensis should also be done with caution, although there appears to be a much lower risk of zoonotic infection. During the last 86 years, there have been only three published records of human infection with Guinea worm that likely represent zoonotic infection.15–17
Although of great biologic and epidemiologic interest, the occurrence of Guinea worms in dogs in Chad poses an unknown risk to the global GWEP. In areas endemic for human infection and where Guinea worm infection in dogs has been noted previously, cases in dogs decreased as human cases were reduced, and generally disappeared before all human cases did. In a few areas, such as Bukhara, Uzbekistan, the infection persisted slightly longer and sporadically in dogs, but never reappeared in people.3,4 Hence, although there might be concern over having the same apparent parasite circulating in dogs, there is no evidence to date that human infection has ever been influenced by the infection in dogs. The general stated assumption, with which we agree, is that infections observed in dogs or other similar animals represent a spillover from people. Interesting and unusual, however, is that in Chad at least three times as many dogs as people are infected, and the relative rate of infection in dogs is high, where we documented infection in multiple dogs per village and dogs with multiple worms, sometimes as many as 5–9 worms per dog. These two observations suggest that in Chad more intense exposure to infection is occurring in dogs than in people. This also suggests that in Chad, human infections may be spilling over from the infections in dogs. Why, comparatively speaking, so many infections were noted in dogs in Chad recently is not clear, although undoubtedly the intense efforts initiated to curb the outbreak in people led to more vigilant program staff, and once sensitized by the initial cases of dogs with dracunculiasis, GWEP program staff, and residents, were extra vigilant to detecting Guinea worms in dogs. This may not fully explain the situation however, as other national programs were also equally vigilant, especially as the program got close to eradication and there was intense pressure to find and contain the last few cases. Antecedent reports of cases of infection in dogs were most numerous from Central Asian countries, India and Pakistan, and least common from sub-Saharan Africa, there being only three such cases reported in the literature from all sub-Saharan countries.2 It should also be noted that in Chad, there are no inherent differences in the association between households and their dogs relative to that in other countries that could explain this degree of infection.
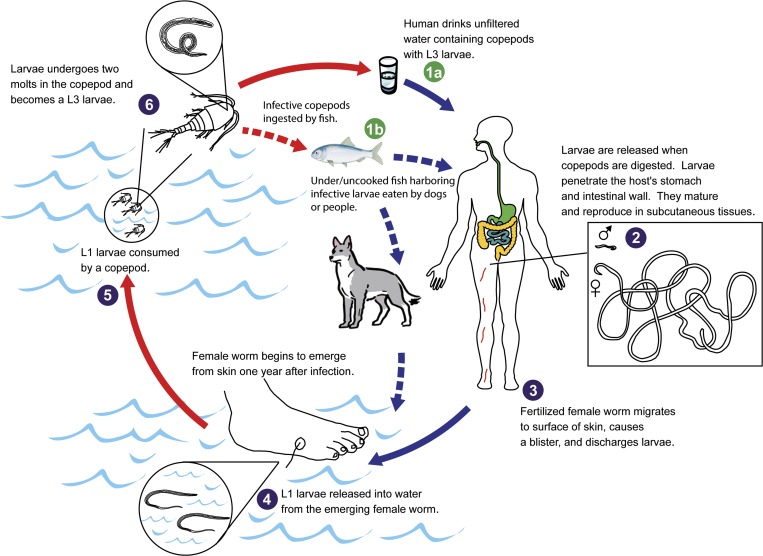
As a result of the unusual epidemiologic pattern observed in human cases, and unusually high infection rate in dogs, we felt it critical to focus more attention on the dog infections. The intent was to uncover any key insights into the transmission of the parasite that could be used to interrupt transmission in the human population. In this instance, we believe the high rate of infection in dogs and peculiar epidemiology of human infections suggests involvement of a previously unrecognized paratenic host in the life cycle in Chad. Earlier studies showed that the closely related species D. insignis (and D. lutrae) required a paratenic host,18,19 especially for animals such as dogs that drink water by lapping, which is an unlikely method of acquiring infection because copepods scatter when disturbed. It is now generally regarded that carnivorous hosts of D. insignis acquire their infection through ingestion of infected fish or frog paratenic host5; there is no reason why such infection routes could not also happen in people, under the right circumstances. This may explain also those human cases that occurred outside the at-risk (endemic) zone along the Chari River. Either they traveled through or visited the at-risk area and consumed fish or other paratenic hosts while there, or fish from the Chari River were transported and consumed outside the at-risk area, either in the places where these persons were detected with emergent Guinea worms or elsewhere. All human cases in Chad, regardless of where they occurred, appear to represent a haphazard transmission of the infection consistent with a paratenic host, and based on eating and cooking habits related to locally available aquatic animal(s) of some sort. Under/uncooked fish appear to be the most likely source for both people and dogs (Figure 9). The absence of Dracunculus larvae in a limited sample of fish and other animals in no way negates the possibility of such a role; it only serves to highlight both the infrequent occurrence and difficulty in finding such a needle in a haystack. Studies are underway to investigate this possibility and, to address it programmatically.
Figure 9.
Diagrammatic life cycle for Dracunculus medinensis, showing the typical mode of transmission (1a), which is ingestion of water containing infected copepods. Also shown is potential transmission cycle (1b) that includes ingestion of infected copepods by fish (or other aquatic vertebrates such as tadpoles), which are then eaten under/uncooked by dogs or people leading to infection.
Whether the infection in Chad was reintroduced in recent years, or had continued at very low levels without detection since 2000 remains uncertain. However, staff, including the former GWEP national coordinator and data manager (the latter is still active with the program) who participated in the eradication effort during the 1990s denied seeing, or being aware of rumors of dracunculiasis in dogs anywhere in Chad. Similarly, elder residents interviewed during assessments of dog/human infections in 2012–2013 in samples of villages along The Chari River and other major waterways paralleling the Chari River denied knowing of dracunculiasis in dogs. Of importance, the search for cases of GWD in 167 villages of Bousso District during 1993–1994, where the current epicenter of infections is located, did not reveal any cases of human dracunculiasis. Moreover, the mass harvesting of fish at the end of the dry season (May–June) by community residents in large lagoons and ponds along the margins of the Chari and Logone Rivers is generations-old according to residents and does not by itself explain the apparently recent occurrence of the peculiar modality of infection in humans or dogs in Chad.
Successful application of modern molecular tools has proven useful in answering a long-standing question regarding the correct identification of Dracunculus that appear in man and animals in the same geographic area. This information has been extremely useful to the GWEP in Chad, and will help ongoing efforts to eliminate human Guinea worm infections from the country. At this point, we do not know whether other peculiar situations such as this will occur, however it will only be through such detailed study that the correct understanding of the species and epidemiology will be learned and appropriate adjustments made in the eradication program. As part of the ongoing Dracunculus genome project, sequence data for a larger set of D. medinensis specimens from Chad and elsewhere are being generated, and a more in-depth genetic analysis of this expanded sample collection will be possible.
Finally, we conclude that dracunculiasis can be prevented and transmission interrupted, even in this peculiar epidemiologic setting. This will likely require additional interventions directed at preventing infection of fish or other paratenic hosts and dogs. The Government of Chad has begun consideration of a range of options including preventing transmission of infection from fish or other paratenic host to dogs and people, such as by safe disposal of fish entrails and thorough cooking of fish. Any additional control measures would be undertaken in concert with existing interventions currently in place to prevent contamination of water sources and aimed at preventing human infection, namely filtering unprotected drinking water through cloth and pipe filters, containment of cases within 24 hr of worm emergence, and applications of ABATE larvicide to contaminated water sources where appropriate.
ACKNOWLEDGMENTS
We thank The Carter Center technical advisors Kristen Grenon, Amélie Cardon, Nicole Weber, Bronwyn Nichol, and Katie Schlaudt, their drivers, field supervisors, and village volunteers whose tireless efforts under difficult conditions contributed to the establishment and operation of the surveillance system generating the information that led to our understanding of dracunculiasis transmission in Chad. We also thank Neloumta Lucienne, data manager for the GWEP and Ngarodjel Djimadoumadji, former national GWEP Coordinator, MOH, Chad, for their contributions to the GWEP. We are grateful also for assistance by WHO Office in N’Djamena in facilitating the shipment of specimens to CDC. We thank members of Wellcome Trust Sanger Institute DNA Pipelines department for generating and sequencing the illumina libraries, particularly Neil Marriot and Dave Willey; and members of the Wellcome Trust Sanger Institute Parasite Genomics team for making a prerelease version of the D. medinensis reference genome available for this work. We thank Blaine Mathison and DPDx in the Division of Parasitic Diseases and Malaria, CDC, for assistance in creating the life cycle illustration in the paper.
Footnotes
Financial support: The Parasitic Diseases Branch, Division of Parasitic Diseases, CDC, serves as a WHO Collaborating Center on Dracunculiasis Eradication, and as such, received a small award from the NTD Department, WHO, in support of these activities. During 2008–2012, The Carter Center's work to eradicate Guinea worm disease has been made possible by financial and in-kind contributions from Next Generation Fund of the Hugh J. Andersen Foundation; Apple Computer, Inc.; Arab Fund for Economic and Social Development; Atlanta Woman's Club; BASF Corporation; Canadian International Development Agency; Chevron Corporation; Children's Investment Fund Foundation UK; Crawford Family Foundation; Delta Medical Supplies; Edgar O. Dixon Charitable Trust; Elfenworks Foundation; First Congregational Church; Foundation Source; Bill & Melinda Gates Foundation; General Electric Company; Girl Scouts of America Brownie Troop 861; Global Aviation Holdings; Global Health Education Consortium, Inc.; Google, Inc.; Robert and Shirley Harris Family Foundation; Harris myCFO Foundation; Conrad N. Hilton Foundation; John C. and Karyl Kay Hughes Foundation; John P. Hussman Foundation, Inc.; Johns Hopkins University; Johnson & Johnson; Kendeda Fund; Leslie Family Foundation; John D. and Catherine T. MacArthur Foundation; McKenna Foundation; Mid-Continent University; Monsanto Company; Mount Pleasant Lutheran Church; National Democratic Institute for International Affairs; OPEC Fund for International Development; Roman Catholic Diocese of Joliet; Government of Saudi Arabia; Saudi Fund for Development; JV Schiro Zavela Foundation; S.H.O.D. LLC; Stahl Family Foundation; St. Thomas Aquinas Parish; Sultanate of Oman; HH General Sheikh Mohamed bin Zayed Al Nahyan, Crown Prince of Abu Dhabi, in honor of HH Sheikh Khalifa bin Zayed, President of the United Arab Emirates; UNICEF; United Kingdom Department for International Development; U.S. Agency for International Development; U.S. Centers for Disease Control and Prevention; United Nations World Food Programme; Vanguard Charitable Endowment Program; Vestergaard Frandsen; Women's Leadership Foundation; YKK Corporation; and many generous individuals. The Wellcome Trust Sanger Institute is supported by the Wellcome Trust through grant 098051.
Authors' addresses: Mark L. Eberhard, Mary H. Jenks, Elizabeth A. Thiele, and Vitaliano Cama, Division of Parasitic Diseases, CDC, Atlanta, GA, E-mails: meberhard@cdc.gov, uwq1@cdc.gov, xas4@cdc.gov, and vcama@cdc.gov. Ernesto Ruiz-Tiben, Donald R. Hopkins, Adam Weiss, and P. Craig Withers, The Carter Center, Atlanta, GA, E-mails: eruizti@emory.edu, sdsulli@emory.edu, adam.j.weiss@emory.edu, and cwither@emory.edu. Corey Farrell, Fernand Toe, The Carter Center, N’Djamena, Chad, E-mails: clfarrell2@gmail.com and fernandtoe@gmail.com. James A. Cotton, Nancy Holroyd, and Zahra Hance, Wellcome Trust Sanger Institute, Wellcome Trust Genome Campus, Cambridge, UK, E-mails: james.cotton@sanger.ac.uk, neh@sanger.ac.uk, and za1@sanger.ac.uk. Mahamat Ali Tahir and Tchonfienet Moundai, Ministry of Public Health, N’Djamena, Chad, E-mails: mahamat_tahir2@yahoo.fr and tchomcalvin@yahoo.fr.
References
- 1.Hopkins DR, Ruiz-Tiben E, Weiss A, Withers P, Jr, Eberhard M, Roy S. Dracunculiasis eradication: and now South Sudan. Am J Trop Med Hyg. 2013;89:5–10. doi: 10.4269/ajtmh.2013.13-0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muller R. Dracunculus and dracunculiasis. Adv Parasitol. 1971;9:73–151. doi: 10.1016/s0065-308x(08)60160-8. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization . WHO/CDS/CEE/DRA/99.9. Geneva: World Health Organization; 1998. Dracunculiasis eradication in Uzbekistan: country report. [Google Scholar]
- 4.Cairncross S, Muller R, Zagaria N. Dracunculiasis (Guinea worm disease) and the eradication initiative. Clin Microbiol Rev. 2002;15:223–246. doi: 10.1128/CMR.15.2.223-246.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson RC. Nematode Parasites of Vertebrates: Their Development and Transmission. Second edition. Wallingford, UK: CABI Publishing; 2000. Chapter 6.2: The Superfamily Dracunculoidea. [Google Scholar]
- 6.Bimi L, Freeman AR, Eberhard ML, Ruiz-Tiben E, Pieniazek NJ. Differentiating Dracunculus medinensis from D. insignis, by sequence analysis of the 18S rRNA gene. Ann Trop Med Parasitol. 2005;99:511–517. doi: 10.1179/136485905X51355. [DOI] [PubMed] [Google Scholar]
- 7.Elasser SC, Floyd R, Hebert PDN, Albrecht I. Species identification of North American guinea worms (Nematoda: Dracunculus) with DNA barcoding. Mol Ecol Resources. 2009;9:707–712. doi: 10.1111/j.1755-0998.2008.02393.x. [DOI] [PubMed] [Google Scholar]
- 8.Laing R, Kikuchi T, Martinelli A, Tsai IJ, Beech RN, Redman E, Holroyd N, Bartley DJ, Beasley H, Britton C, Curran D, Devaney E, Gilabert A, Hunt M, Jackson F, Johnston S, Kryukov I, Li K, Morrison AA, Reid AJ, Sargison N, Saunders G, Wasmuth JD, Wolstenholme A, Berriman M, Gilleard JS, Cotton JA. The genome and transcriptome of Haemonchus contortus, a key model parasite for drug and vaccine discovery. Genome Biol. 2013;14:R88. doi: 10.1186/gb-2013-14-8-r88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kozarewa I, Ning Z, Quail MA, Sanders MJ, Berriman M, Turner DJ. Amplification-free Illumina sequencing-library preparation facilitates improved mapping and assembly of (G+C)-biased genomes. Nat Methods. 2009;6:291–295. doi: 10.1038/nmeth.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, Garimella K, Altshuler D, Gabriel S, Daly M, DePristo MA. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20:1297–1303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DePristo M, Banks E, Poplin R, Garimella K, Maguire J, Hartl C, Philippakis A, del Angel G, Rivas MA, Hanna M, McKenna A, Fennell T, Kernytsky A, Sivachenko A, Cibulskis K, Gabriel S, Altshuler D, Daly M. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet. 2011;43:491–498. doi: 10.1038/ng.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC. PLINK: a toolset for whole-genome association and population-based linkage analysis. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wijova M, Moravec F, Jorak A, Modry D, Lukes J. Phylogenetic position of Dracunculus medinensis and some related nematodes inferred from 18S rRNA. Parasitol Res. 2005;96:133–135. doi: 10.1007/s00436-005-1330-x. [DOI] [PubMed] [Google Scholar]
- 14.Wijova A, Moravec F, Horak A, Lukes J. Evolutionary relationships of Spirurina (Nematoda: Chromadorea: Rhabditida) with special emphasis on dracunculoid nematodes inferred from SSU rRNA gene sequences. Int J Parasitol. 2006;36:1067–1075. doi: 10.1016/j.ijpara.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Hashikura T. One case of Filaria medinensis in Korea. Jap Med World. 1927;7:145–146. [Google Scholar]
- 16.Kobayashi A, Kataruta A, Hamada A, Suzuki T, Hataba Y, Tashiro N, Yoshida A. Human case of dracunculiasis in Japan. Am J Trop Med Hyg. 1986;35:159–161. doi: 10.4269/ajtmh.1986.35.159. [DOI] [PubMed] [Google Scholar]
- 17.Wang Z, Jisuan D, Wang X. Dracunculiasis discovered for the first time in China. Chin J Zoon. 1995;11:15–17. [Google Scholar]
- 18.Crichton VFJ, Beverley-Burton M. Observations on the seasonal prevalence, pathology and transmission of Dracunculus insignis (Nematoda: Dracunculoidea) in the raccoon (Procyon lotor (L) in Ontario. J Wildl Dis. 1977;13:273–280. doi: 10.7589/0090-3558-13.3.273. [DOI] [PubMed] [Google Scholar]
- 19.Eberhard ML, Brandt FH. The role of tadpoles and frogs as paratenic hosts in the life cycle of Dracunculus insignis (Nematoda: Dracunculoidea) J Parasitol. 1995;8:792–793. [PubMed] [Google Scholar]